Abstract
Background. The synergy between herpes simplex virus type 2 (HSV-2) and human immunodeficiency virus type 1 (HIV-1) is well known, but lack of knowledge about the epidemiology of HSV-2 acquisition in HIV-1-discordant couples hampers development of HSV-2 prevention interventions that could reduce HIV-1 transmission.
Methods. HIV-1-discordant couples were enrolled in Nairobi, Kenya, and followed for up to 2 years. HSV-2 status was determined using HerpeSelect HSV-2 ELISA. Correlates of prevalence and incidence were assessed.
Results. Of 469 HIV-1-discordant couples, at baseline, 353 (75.3%) were affected by HSV-2, of which 189 (53.5%) were concordantly HSV-2 seropositive and 164 (46.5%) were HSV-2-discordant. Prevalence was lowest among HIV-1-uninfected men (39.9%) compared to HIV-1-infected women (64.8%), HIV-1-infected men (66.7%), and HIV-1-uninfected women (68.5%). During follow-up, HSV-2 seroincidence was 14.9 per 100 person-years. Incidence was 1.6-fold higher among females compared to males (95% confidence interval [CI], 1.00–2.48) and 2.5-fold higher in HIV-1-infected compared to uninfected women (95% CI, 1.12–5.74). At least 30% of incident HSV-2 infections originated from an outside partner.
Conclusions. The high HSV-2 prevalence and incidence in HIV-1-discordant couples in sub-Saharan Africa suggest HSV-2 treatment and prevention could be an effective targeted strategy to reduce HSV-2 and HIV-1 transmission in this high-risk population.
Keywords: HSV-2, herpes, HIV, discordant, serodiscordant, couples, genital ulcer disease, Kenya, incidence, prevalence, transmission, prevention, Africa, antiviral, seroconvert, ELISA
Herpes simplex virus type 2 (HSV-2) is the most common cause of genital ulcer disease in the world, and sub-Saharan Africa bears a heavy burden of infections [1, 2]. Manifestations of HSV-2 infection range from asymptomatic or nonulcerative symptoms to periodic outbreaks of painful ulcers [3, 4]. There is a strong synergistic relationship between HSV-2 and human immunodeficiency virus type 1 (HIV-1), resulting in greater shedding and elevated infectiousness of both viruses [5–10]. HSV-2 can be managed with episodic antiviral medication (eg, acyclovir, valacyclovir, or famciclovir) to abort or reduce the duration of outbreaks or with daily suppressive treatment to prevent outbreaks and reduce asymptomatic viral shedding [11]. Although suppressive HSV-2 treatment reduces plasma HIV-1 viral load, a recent randomized trial failed to show a benefit of suppressive acyclovir therapy in reducing HIV-1 transmission [12]. However, daily suppressive therapy with valacyclovir decreases herpes transmission to sexual partners [13]. Prevention of new HSV-2 infections may be an effective HIV-1 prevention strategy, particularly among HIV-1-discordant couples in which either member of the couple is HSV-2 seronegative.
While estimates vary due to population characteristics, 10%–25% of new HIV-1 infections in sub-Saharan Africa occur in HIV-1-discordant couples [14–17]. In this region, HSV-2 prevalence in one or both members of HIV-1-discordant couples is as high as 80% [18]. Yet there is limited knowledge about the timing and risk factors for HSV-2 acquisition in this high-risk population. Given the importance of HSV-2 in mediating HIV-1 transmission, we sought to better understand HSV-2 transmission in HIV-1-discordant couples by investigating its prevalence and incidence among stable HIV-1-discordant heterosexual couples in Nairobi, Kenya.
METHODS
Study Participants
Between 2007 and 2009, HIV-1-discordant couples were recruited from voluntary counseling and testing (VCT) centers in Nairobi and followed quarterly for up to 2 years. At enrollment and follow-up visits, clinicians collected blood specimens and assessed sociodemographic, behavioral, and biological characteristics by questionnaire [19, 20]. Participants were asked if there had been a separation or breakup of the relationship at each visit.
Eligible participants were ≥18 years old, had sexual intercourse with their study partner ≥3 times in the 3 months prior to screening, and planned to remain with their partner for the 2-year duration of the study. Additionally, at enrollment, the HIV-1-infected partner could not have a history of AIDS (World Health Organization [WHO] stage IV), be on antiretroviral therapy, or be enrolled in other HIV studies. Female participants who were pregnant at enrollment were excluded.
Written informed consent was obtained from all participants. Approval for the study was obtained from the Institutional Review Board at the University of Washington and the Ethics and Research Committee at Kenyatta National Hospital and the University of Nairobi.
HSV-2 Testing
HSV-2 status was assessed by HerpeSelect HSV-2 ELISA (Focus Diagnostics, Cypress, California) on blood plasma. Assays were conducted following manufacturer's guidelines with the following exception: an index value of ≥3.5, as opposed to manufacturer recommended cutoff of ≥1.1, was used to determine seropositivity in order to increase specificity [21–23] and for consistency with previous clinical trials [12, 24, 25]. An index value of 1.1–3.4 was defined as equivocal. Equivocal results may indicate true negatives with higher index values or true positives with low antibody levels. They may also indicate early HSV-2 infection. To investigate the effect of alternative cutoffs, we repeated analyses using cutoffs of ≥2.2 and ≥2.7, proposed by others to balance sensitivity and specificity [26, 27]. Baseline HSV-2 status was assessed for all participants at enrollment, and follow-up HSV-2 status was determined for those with negative or equivocal results at enrollment. Date of HSV-2 seroconversion was defined as the midpoint between the last negative and first positive visits. Participants who were HSV-2 seropositive and reported symptoms of genital ulcer disease at any study visit were treated with acyclovir according to Kenyan national guidelines.
Statistical Methods
We developed predictive models using logistic regression to identify couples at higher likelihood of being HSV-2-concordantly negative or discordant compared to couples that were concordant positive. We fit separate logistic regression models for being concordant negative and discordant. Given the cross-sectional nature, we did not seek to investigate causal relationships. The objective of these models was to aid identification of couples that they could be targeted for HSV-2 prevention. We used forward stepwise selection using a threshold of P < .20 for adding a covariate to the model.
We calculated HSV-2 incidence stratified by gender, HIV-1 status, demographic characteristics, and self-reported risk factors. Cox proportional hazards regression was used to investigate incident HSV-2 infection. We postulated a priori that gender would modify the associations between factors of interest and HSV-2 infection and therefore reported all analyses separately by gender. Univariate models were fit for each factor of interest. Adjusted associations were estimated by fitting separate models for each factor of interest that also included potential confounders selected a priori based on biological relevance to HSV-2 infection (HIV-1-status, age, and partner's HSV-2 status). All statistical analysis was performed using Stata (StataCorp, College Station).
RESULTS
Participant Characteristics
A total of 469 stable HIV-1-discordant couples were enrolled, of which 301 (64%) had a female HIV-1-infected partner. Couples reported cohabiting for a median of 5 years (interquartile range [IQR], 2–10), and nearly all couples (97%) were married. Median age at sexual debut was 18 years for both females (IQR, 16–19) and males (IQR, 16–20) (Table 1). A total of 20 (4%) men reported other sexual partners in the month before enrollment, compared to only 3 (0.6%) women. Fewer than a quarter (24.3%) of couples reported any unprotected sex with their study partner in the month before enrollment. (Table 2)
Table 1.
Baseline Characteristics of Study Participants
| Discordant Couples, no. (%) or median (IQR) |
||
|---|---|---|
| Females (N = 469) | Males (N = 469) | |
| HSV-2 serostatus | ||
| Negative | 124 (26.4) | 186 (39.7) |
| Equivocal | 35 (7.5) | 51 (10.9) |
| Positive | 310 (66.1) | 232 (49.5) |
| HIV-1-infected | 301 (64.2) | 168 (35.8) |
| Age | 29 (24, 34) | 35 (30, 41) |
| Married to partner | 446 (95.5) | 454 (96.8) |
| Male uncircumcised | – | 100 (21.4) |
| Years living together | 5 (2, 10) | 5 (2, 10) |
| Do not earn an income | 327 (69.7) | 71 (15.1) |
| Less than primary education | 116 (24.7) | 70 (14.9) |
| Informal Residence | 244 (52.1) | 238 (50.8) |
| Sexual debut ≤15 y old | 84 (18.0) | 107 (22.9) |
| Life-time sexual partners | 3 (2, 4) | 5 (3, 9) |
| Sex acts in the past montha | 5 (3, 7) | 5 (3, 7) |
| Any unprotected sexb | 114 (24.3) | 114 (24.3) |
| Outside sexual partner(s) | 3 (0.6) | 20 (4.3) |
| No. of childrena | 1 (1, 2) | 1 (1, 2) |
| Don't desire future childrena | 257 (55.0) | 221 (47.3) |
| Hormonal contraceptive use | 92 (19.7) | – |
| Lifetime STI history | 116 (25.4) | 196 (43.0) |
Hormonal contraception was assessed at the time of enrollment. “Informal residence” indicates residence in an informal or “slum” settlement. Age at sexual debut was dichotomized to early (age ≤15) or later (age >15), based on the 25th percentile of the cohort distribution.
Abbreviations: HIV, human immunodeficiency virus; HSV, herpes simplex virus; IQR, interquartile range; STI, sexually transmitted infection.
a With study partner.
b With study partner in the past 3 months.
Table 2.
Predictive Models Constructed to Identify Couples at Increased Likelihood of Being HSV-2-concordant Negative or HSV-2-discordant, Relative to HSV-2-concordant Positive Couples
| HSV-2-Concordant Negative Couples |
HSV-2-Discordant Couples |
|||||||
|---|---|---|---|---|---|---|---|---|
| OR | (95% CI) | aORa | (95% CI) | OR | (95% CI) | aORa | (95% CI) | |
| Younger age | 1.12** | (1.07–1.17) | 1.15** | (1.08–1.23) | 1.06** | (1.03–1.09) | 1.05** | (1.01–1.09) |
| Female HIV-1-infected | 2.50** | (1.42–4.38) | 1.78 | (.92–3.44) | 1.97** | (1.20–3.21) | 1.54 | (.90–2.63) |
| Years living together | 0.93** | (.88–.97) | 1.10* | (1.00–1.21) | 0.94** | (.91–.98) | ||
| Formal Residence | 1.58 | (.94–2.66) | 1.79 | (.94–3.39) | 0.77 | (.48–1.23) | ||
| Female partner sexual debut >15 y old | 2.90* | (1.24–6.78) | 2.84* | (1.03–7.85) | 0.92 | (.52–1.61) | ||
| Male partner sexual debut >15 y old | 0.90 | (.50–1.65) | 1.24 | (.70–2.21) | ||||
| No unprotected sexb | 1.45 | (.76–2.79) | 1.71 | (.77–3.80) | 0.74 | (.44–1.24) | ||
| No outside partners | 6.24 | (.80–48.50) | 6.75 | (.47–96.13) | 1.34 | (.49–3.62) | ||
| Desire additional childrenc | 2.72** | (1.59–4.66) | 2.04* | (1.00–4.13) | 0.59 | (1.00–2.55) | ||
| Fewer no. of children c | 1.48** | (1.19–1.83) | 1.27 | (.91–1.78) | 1.34** | (1.13–1.59) | 1.22* | (1.01–1.48) |
| No hormonal contraceptive use | 3.42** | (1.39–8.43) | 3.35* | (1.24–9.06) | 0.96 | (.54–1.71) | ||
| Male circumcised | 1.39 | (.73–2.62) | 1.77 | (.83–3.80) | 1.41 | (.79–2.51) | 1.68 | (.89–3.18) |
| CD4 count | 1.06 | (.95–1.18) | 1.10 | (1.00–1.21) | ||||
| Decreasing viral load | 1.26* | (1.01–1.57) | 1.28 | (.97–1.70) | 1.24* | (1.03–1.50) | 1.27* | (1.03–1.56) |
Couples in which either or both partners had equivocal HSV-2 results were excluded from this analysis. Age is based on the age of the oldest partner in the couple, and the OR for age is per year younger. The OR for CD4 count is per 100 cells/µL. The OR for viral load is per log10 RNA copies/mL decrease.
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; HIV, human immunodeficiency virus; HSV, herpes simplex virus; OR, odds ratio.
a Model selected based on forward stepwise selection.
b With study partner in the past 3 months.
c With study partner.
*P < .05.
**P < .01.
HSV-2 Prevalence in HIV-1-discordant Couples
Of the 938 participants from HIV-1-discordant couples at enrollment, 542 (58%) were HSV-2 positive, 310 (33%) were negative, and 86 (9%) had equivocal HSV-2 results. Among females, 310 (66%) were HSV-2 positive compared to 232 (49%) males. There was a significant interaction between gender and HIV-1 status, with the lowest prevalence among HIV-1-uninfected men (39.9%) and higher prevalence among HIV-1-infected women (64.8%, odds ratio [OR], 2.83; 95% confidence interval [CI], 1.99–4.04), HIV-1-infected men (66.7%, OR, 3.10; 95% CI, 2.02–4.76), and HIV-1-uninfected women (68.5%, OR, 3.26; 95% CI, 2.12–5.01). Correlates of HSV-2 prevalence are shown in Supplementary Table 1.
Effect of Alternate Index Value Cutoffs on HSV-2 Prevalence
We reclassified participants according to alternative index value cutoffs to define seropositivity and found modest effects. Among females, prevalence increased to 68.2% (index value ≥2.7) and 71.0% (index value ≥2.2). Among males, prevalence increased to 52.5% (index value ≥2.7) and 54.8% (index value ≥2.7). At the couple level, the proportion of couples affected by HSV-2 was 78% using a cutoff of ≥2.7% and 79% with a cutoff of ≥2.2.
Correlates of Couples Being HSV-2 Concordant Negative or Discordant
Of 469 couples, 353 (75%) were affected by HSV-2. Both partners were HSV-2-seropositive in 189 (40%) couples, 164 (35%) couples were HSV-2-discordant, and 116 (25%) couples were HSV-2-concordant seronegative. In 81 (17%) couples, one or both partners had equivocal HSV-2 results. We developed separate predictive models to identify couples that were HSV-2-concordant negative and HSV-2-discordant compared to couples that were HSV-2-concordant positive. In the final model for concordant negative couples, younger age (aOR, 1.15, 95% CI, 1.08–1.23), years of cohabitation (aOR, 1.10, 95% CI, 1.00–1.21), older age (>15 years) at female's first sex (aOR , 2.84, 95% CI, 1.03–7.85), desire for additional children (aOR, 2.04, 95% CI, 1.00–4.13), and not using hormonal contraceptives (aOR, 3.35, 95% CI, 1.24–9.06) were significantly associated with a couple being concordant negative vs concordant positive. The final model for HSV-2 discordance was similar but included fewer covariates, with only younger age (aOR, 1.05, 95% CI, 1.01–1.09), few number of children (aOR, 1.22, 95% CI, 1.01–1.48), and lower HIV-1 viral load in the HIV-1-infected partner (aOR, 1.27 per log10 RNA copies/mL, 95% CI, 1.03–1.56) significantly associated with HSV-2 discordancy.
HSV-2 Incidence
A total of 396 individuals from HIV-1-discordant couples were initially HSV-2 negative or equivocal, of which 382 had subsequent plasma samples and were followed for up to 2 years to assess HSV-2 incidence (101 HIV-1-infected women, 53 HIV-1-uninfected women, 52 HIV-1-infected men, and 176 HIV-1-uninfected men). During follow-up, 90 (35%) couples reported any unprotected sex with their study partner, which occurred more commonly in couples with an HIV-1-infected female partner (OR, 2.94; 95% CI, 1.85–4.68). A larger proportion of men than women reported any outside sexual partners during follow-up (12% vs 6%; OR = 0.46; 95% CI, .21–1.01), with no significant difference between HIV-1-infected and uninfected men (8% vs 13%, respectively; OR, 0.57; 95% CI, .19–1.72) or women (5% vs 8%, respectively; OR, 0.64; 95% CI, .16–2.48).
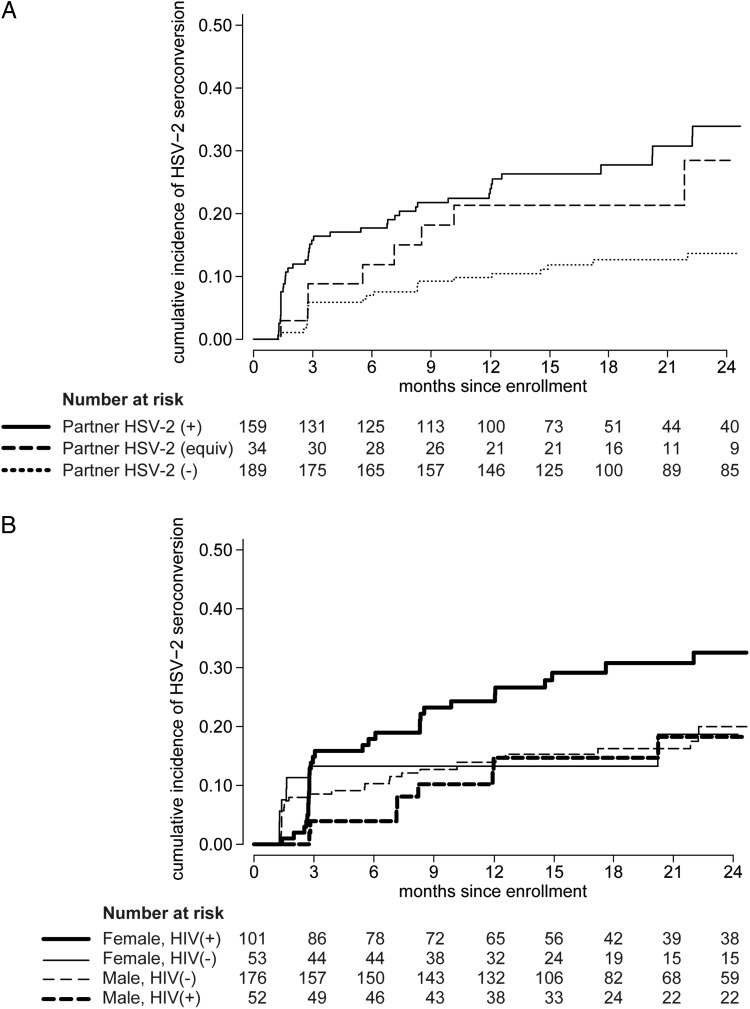
Overall, we observed 37 HSV-2 seroconversions in 423.3 person-years (8.5 per 100 person-years) among those initially HSV-2 seronegative and 39 seroconversions in 89.3 person-years (43.7 per 100 person-years) among those initially HSV-2 equivocal, for an overall incidence of 14.8 per 100 person-years (95% CI, 11.8–18.6 per 100 person-years). Incidence was higher among females than males (19.5 vs 11.9 per 100 person-years; hazard ratio [HR] = 1.58; 95% CI, 1.00–2.47). The incidence was 8.2 per 100 person-years among individuals with a partner who was HSV-2 seronegative at enrollment compared to 17.9 per 100 person-years among those with an initially HSV-2 equivocal partner (HR, 2.09; 95% CI, .94–4.68, P = .07) and 24.1 per 100 person-years among those with an initially HSV-2 positive partner (HR, 2.72; 95% CI, 1.65–4.51; Figure 1A). There was a trend indicating interaction between gender and HIV-1 status (P = .10), with the highest rate of HSV-2 seroconversion among HIV-1-infected women and roughly equivalent lower rates among HIV-1-uninfected women (aHR, 0.50; 95% CI, .23–1.10, P = .08) and HIV-1-infected (aHR, 0.36; 95% CI, .15–.84) and uninfected men (aHR, 0.47; 95% CI, .27–.81), after adjusting for age (Figure 1B).
Figure 1.
Cumulative incidence of HSV-2 infection. Participants in stable HIV-1-discordant relationships were tested for HSV-2 at enrollment and followed quarterly for 2 years. Incidence of HSV-2 seroconversion was assessed for those who were HSV-2 negative or equivocal at enrollment. A, Kaplan Meier curves show the cumulative incidence of HSV-2 seroconversion for those with ( ) an initially HSV-2 seropositive partner, (
) an initially HSV-2 seropositive partner, (

 ) an initially HSV-2 equivocal partner, and (• • •) an initially HSV-2 seronegative partner. B, Kaplan Meier curves show the cumulative incidence of HSV-2 seroconversion for (
) an initially HSV-2 equivocal partner, and (• • •) an initially HSV-2 seronegative partner. B, Kaplan Meier curves show the cumulative incidence of HSV-2 seroconversion for ( ) HIV-1-infected women, (—) uninfected women, (– – –) uninfected men, and (
) HIV-1-infected women, (—) uninfected women, (– – –) uninfected men, and (

 ) infected men. Abbreviations: HIV-1, human immunodeficiency virus type 1; HSV-2, herpes simplex virus type 2.
) infected men. Abbreviations: HIV-1, human immunodeficiency virus type 1; HSV-2, herpes simplex virus type 2.
We found small variations in HSV-2 incidence with lower positivity cutoffs. Using a cutoff index value of ≥2.7, incidence was 16.1 per 100 person-years (95% CI, 12.9–20.2 per 100 person-years), whereas a cutoff of ≥2.2 resulted in an incidence of 14.7 per 100 person-years (95% CI, 11.6–18.7 per 100 person-years). At the manufacturer recommended cutoff of ≥1.1, the incidence was 14.2 per 100 person-years (95% CI, 11.0–18.3 per 100 person-years).
Source of HSV-2 Infections
We classified the source of new HSV-2 infections as outside the HIV-1-discordant couple by identifying incident infections in participants with an initially HSV-2 seronegative partner. Overall, 23 (30%) of 76 new HSV-2 infections occurred in those with a partner who was HSV-2 seronegative at enrollment. Of 38 men who acquired HSV-2, only 3 (8%) had a partner who was initially HSV-2 seronegative. The female partners of these men had equivocal results at the end of follow-up, indicating these 3 men acquired HSV-2 from an outside partner and subsequently transmitted the infection to their partners. In sharp contrast, of 38 women who acquired HSV-2, 20 (53%) had an initially HSV-2 seronegative partner, and of these, only 2 had partners who were HSV-2 seropositive or equivocal at the end of follow-up. Only 1 of the 2 male seroconversions and 2 of the 20 female seroconversions due to an outside partner could be accounted for by a reported separation of the discordant couple. We conducted a sensitivity analysis to address concerns that the observed gender difference in the source of HSV-2 infection was due to differences in baseline HSV-2 prevalence. We assumed the extreme scenario that the source of infection was from the study partner for all women who acquired HSV-2 and had a partner who was initially HSV-2 positive or equivocal. We then varied the proportion of new infections attributable to an outside partner among men who acquired HSV-2 and had an initially HSV-2 positive or equivocal partner. To achieve the observed results if there was no true gender difference, >49% of infections in males with an initially positive or equivocal partner would have to be from an outside partner.
Correlates of Incident HSV-2 Infection Among Females and Males
After adjusting for age, baseline HIV-1 status, and partner's baseline HSV-2 status, females who had an equivocal HSV-2 result at baseline were >3-fold more likely to experience an HSV-2 seroconversion compared to those who were initially seronegative (aHR, 3.13; 95% CI, 1.55–6.31; Table 3). This association was even stronger among males (aHR, 4.04; 95% CI, 2.07–7.89). Having a partner who was HSV-2 seropositive at enrollment put men at a 9-fold higher risk (aHR, 9.35; 95% CI, 2.83–30.93) and women at 2.5-fold higher risk (aHR, 2.49; 95% CI, 1.25–4.99) of acquiring HSV-2 during follow-up. Interestingly, men with a partner who was initially HSV-2 equivocal were also at significantly higher risk of acquiring HSV-2 (aHR, 9.86; 95% CI, 2.30–40.65), whereas there was no association for females (aHR = 0.99; 95% CI, .29–3.33, P = .98). Among women, those who were HIV-1-infected acquired HSV-2 at a 2.5-fold higher rate than uninfected women (aHR = 2.53; 95% CI, 1.12–5.74). Among men, those who reported any unprotected sex with their study partner during follow-up were 2-fold more likely to acquire HSV-2 compared to those who reported no unprotected sex (aHR, 2.06; 95% CI, 1.04–4.10). There were no appreciable differences in the correlates of HSV-2 incidence when we excluded those with equivocal results at baseline or used alternate cutoff values for positivity (data not shown).
Table 3.
Correlates of HSV-2 Incidence Among Members of HIV-1-discordant Couples, by Gender
| Females |
Males |
|||||||
|---|---|---|---|---|---|---|---|---|
| HR | (95% CI) | aHRa | (95% CI) | HR | (95% CI) | aHRa | (95% CI) | |
| Equivocal HSV-2 at baseline | 3.64** | (1.92–6.91) | 3.13** | (1.55–6.31) | 5.38** | (2.82–10.27) | 4.04** | (2.07–7.89) |
| Partner HSV-2 serostatus | ||||||||
| Negative | 1 | ref | 1 | ref | ||||
| Equivocal | 0.93 | (.28–3.15) | 0.99 | (.29–3.33) | 9.86** | (2.35–41.31) | 9.68** | (2.30–40.65) |
| Positive | 2.06* | (1.05–4.03) | 2.49** | (1.25–4.99) | 9.65** | (2.94–31.69) | 9.35** | (2.83–30.93) |
| HIV-1-infected | 1.85 | (.85–4.03) | 2.53* | (1.12–5.74) | 0.87 | (.40–1.89) | 0.91 | (.41–2.03) |
| Age | 1.02 | (.97–1.07) | 1.03 | (.98–1.08) | 1.02 | (.98–1.07) | 1.01 | (.97–1.06) |
| Male uncircumcised | – | – | – | – | 0.57 | (.20–1.61) | 0.67 | (.24–1.90) |
| Years living together | 1.01 | (.95–1.07) | 0.99 | (.91–1.07) | 1.03 | (.99–1.08) | 1.03 | (.97–1.10) |
| Do not earn an income | 1.70 | (.80–3.59) | 1.64 | (.76–3.52) | 0.95 | (.40–2.28) | 1.00 | (.41–2.42) |
| Less than primary education | 2.18* | (1.10–4.33) | 1.80 | (.87–3.75) | 0.22 | (.03–1.63) | 0.17 | (.02–1.27) |
| Informal Residence | 0.83 | (.43–1.60) | 0.81 | (.42–1.56) | 1.11 | (.59–2.10) | 0.89 | (.46–1.72) |
| Sexual debut ≤15 y old | 1.56 | (.65–3.73) | 1.36 | (.56–3.29) | 0.43 | (.15–1.20) | 0.45 | (.16–1.30) |
| Life-time sexual partners | 1.00 | (.85–1.17) | 0.97 | (.80–1.16) | 1.01 | (.99–1.04) | 1.00 | (.97–1.03) |
| Any unprotected sexb | 1.00 | (.47–2.12) | 1.17 | (.53–2.57) | 2.23* | (1.15–4.34) | 2.06* | (1.04–4.10) |
| No. of childrenc | 1.17 | (.91–1.51) | 1.21 | (.88–1.68) | 1.09 | (.87–1.37) | 1.06 | (.82–1.36) |
| Don't desire additional childrenc | 1.01 | (.53–1.91) | 0.77 | (.38–1.54) | 1.04 | (.55–1.98) | 0.73 | (.37–1.45) |
| Hormonal contraceptive use | 0.77 | (.30–1.98) | 0.54 | (.20–1.45) | – | – | – | – |
| Lifetime STI history | 1.43 | (.73–2.80) | 1.27 | (.62–2.62) | 1.51 | (.79–2.91) | 1.26 | (.65–2.43) |
| Partner received acyclovird | NA | NA | NA | NA | 1.66 | (.50–5.47) | 1.63 | (.49–5.42) |
Hormonal contraception was assessed at the time of enrollment. The OR for age is per year. It was not possible to calculate risk estimates for acyclovir among females due to small sample size.
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio; HSV, herpes simplex virus; NA, not applicable; OR, odds ratio; STI, sexually transmitted infection.
a Based on a multivariate model including the factor of interest plus HIV-1 status, age, and partner's HSV-2 status.
b With study partner during follow-up.
c With study partner.
d Restricted to those with a partner who was HSV-2 positive at baseline.
*P < .05.
**P < .01.
HIV-1 Seroconversion
Among the 469 initially HIV-1-uninfected partners in HIV-1-discordant relationships, we observed 12 HIV-1 seroconversions (1.5 per 100 person-years). Of the 12 participants who acquired HIV-1, 4 (33%) were HSV-2 negative at enrollment. Of these, 2 (50%) also experienced an HSV-2 seroconversion, both of which occurred at the same visit as the HIV-1 seroconversion. One of the HIV-1/HSV-2 seroconverters, a female, had a partner who was initially HSV-2 positive, whereas the other, a male, had a partner who was initially HSV-2 negative and was HSV-2 equivocal at the end of follow-up.
DISCUSSION
We found high HSV-2 seroprevalence among HIV-1-discordant couples, with one or both partners HSV-2 positive in 75% of couples, compared to only 51% of couples in a previous population-based estimate from Kenya [18]. We also observed a large number of new HSV-2 infections in the HIV-1-discordant couples, with highest incidence in those with a partner who was initially HSV-2 seropositive (24.3 per 100 person-years). However, we also observed an appreciable incidence among those with an initially seronegative partner (8.2 per 100 person-years), indicating outside sexual partnerships for one or both members of the couple. Estimates of HSV-2 incidence in sub-Saharan Africa are limited, coming primarily from male circumcision trials. Previous studies from Kenya, Uganda, and South Africa found HSV-2 incidence in HIV-1-uninfected ranging from 2 to 6 per 100 person-years among males [28–30] and 6 per 100 person-years among females [31]. The >2-fold higher HSV-2 incidence we found among HIV-1-discordant couples indicates they are at considerably higher risk of new HSV-2 infections compared to the general population.
Participants reported high rates of condom use and experienced a low rate of HIV-1 seroconversion (1.5 per 100 person-years). Yet the high incidence of HSV-2, combined with a relatively high pregnancy rate (10 per 100 person-years), reported previously from this cohort [19], indicate condom use was likely lower than reported. Risk-reduction strategies used by these couples appear to have contributed to lower rates of HIV-1 transmission, but there remains evidence of risky behavior, with particular concern regarding sexual partners outside of the HIV-1-discordant relationship. Incident and prevalent HSV-2 infections place these couples at elevated risk of HIV-1 transmission over the course of their relationships, highlighting opportunities for interventions to reduce the joint risks of HSV-2 and HIV-1 acquisition.
We used a higher cutoff (index value of ≥3.5) to define an HSV-2 seropositive result, compared to the manufacturer-recommended cutoff of 1.1. This results in higher specificity, which is particularly important when one must be confident that a subject is truly infected with HSV-2 [21]. However, this gain in specificity comes at the expense of sensitivity. Our study demonstrated that participants with equivocal results (1.1–3.4) at baseline were 3- to 4-fold more likely to experience an HSV-2 seroconversion than those who were HSV-2 negative at baseline. It is likely that equivocal results were either due to recent HSV-2 acquisition or to transient low antibody levels. Those with equivocal results almost never regressed to have negative HSV-2 results (8%), but there was an equal proportion who went on to become HSV-2 seropositive (46%) and who remained equivocal (46%). From our data, it remains unclear whether persons with persistently equivocal HSV-2 results are truly infected with HSV-2 or if this is the result of cross-reacting antibodies [21, 32]. Given these finding, caution should be used when interpreting equivocal HSV-2 results, with the choice of how to categorize equivocal results dependent on study design and consequences of misclassification or bias due to exclusion. Additional confirmation or use of a more precise assay may reduce ambiguity.
We observed gender differences in HSV-2 acquisition. Among men, the strongest risk factor for HSV-2 acquisition was the partner's HSV-2 serostatus. Interestingly, men with an HSV-2 positive or an equivocal partner both had a 9-fold elevated risk of acquiring HSV-2. In contrast, women with an HSV-2 seropositive partner had a 3.5-fold higher risk, whereas those with an equivocal partner had the same acquisition risk as those with an HSV-2 seronegative partner. These differences are likely due to transmission dynamics within these couples. Despite the fact that men were more likely than women to report outside partners, we found that the source of new HSV-2 infections originated from outside partners in a much larger proportion of women. Although it is possible that gender differences in baseline HSV-2 seroprevalence account for some of this difference, our sensitivity analysis indicates this is unlikely to fully explain this finding. Also interesting was the finding that after adjusting for partner's baseline HSV-2 serostatus, HIV-1-infected women were 2.5-fold more likely to acquire HSV-2 compared to HIV-1-uninfected women, whereas there was no difference among men. Couples in which the female partner was HIV-1-infected were almost 3-fold more likely to report unprotected sex during follow-up compared to couples with an HIV-1-infected male partner, which when combined with higher male-to-female HSV-2 transmission [33], may explain the higher HSV-2 infection rate in HIV-1-infected women. Collectively, these factors put HIV-1-infected women at particularly high risk of acquiring HSV-2, which in turn elevates their risk of transmitting HIV-1 to an uninfected male partner.
Introduction of HSV-2 from sexual partners outside of HIV-1-discordant relationships raises concerns about risk perceptions in these couples. The low HIV-1 transmission rate within these couples and high reported condom use are promising indications that risk reduction counseling was effective. However, our findings highlight potential unintended consequences. Messages about the risk of transmission may lead some couples to use condoms consistently or to practice abstinence within the relationship but also to seek outside sexual partners that are perceived to be lower risk. This increases the risk of introducing other sexually transmitted infections (STI) into the relationship and of transmitting HIV-1 to outside partners. Individual and couple counseling should emphasize risk reduction both inside and outside of HIV-1-discordant relationship.
This study benefited from a relatively large sample size and extended follow-up; however, a number of limitations exist. Participants were recruited only from HIV-1 testing centers in Nairobi, which is a major urban center. This could affect generalizability, as HIV-1-discordant couples from rural areas or from other settings may differ from our study population. Self-reported sexual risk behavior may underestimate the frequency of unprotected sex and outside sexual partners and may limit our ability to assess these factors. The higher cutoff for positivity used in the HSV-2 assay resulted in a number of participants with equivocal results. Although we were able to determine that equivocal results at baseline were associated with HSV-2 incidence, we were unable to determine the true HSV-2 status of those with persistent equivocal results.
In conclusion, our observation of high HSV-2 incidence among HIV-1-discordant couples highlights the need for comprehensive STI treatment and prevention programs, particularly among HIV-1-discordant couples. This should include evidence-based interventions such as condoms, frequent testing, couple counseling to avoid intercourse during outbreaks, and encouraging HSV-2 status disclosure among partners [34, 35]. The high prevalence of HSV-2 in these couples puts them at elevated risk of HIV-1 transmission. Attention should be paid to identifying HIV-1-discordant couples who are HSV-2-concordant negative or discordant given that new acquisition of HSV-2 in these couples, either from within or outside the couple, represents a particularly high-risk situation [36]. Incident HSV-2 is associated with 2–3-fold higher rates of HSV-2 reactivation and asymptomatic shedding compared to prevalent infection [37–39], likely explaining the elevated risk of HIV-1 infection following recent HSV-2 infection [36]. Suppressive valacyclovir therapy reduces HSV-2 transmission among immunocompetent HSV-2-discordant couples [13]. Although previous trials of HSV-2 suppression to prevent HIV-1 transmission failed to demonstrate efficacy [12], prevention of HSV-2 within HIV-1-discordant couples may be an effective targeted intervention. The highest burden of HSV-2, both in terms of baseline prevalence and incidence during follow-up, was among HIV-1-infected women, and there was also a high incidence among women with an HSV-2 seronegative partner. This highlights the need for female controlled interventions, such as the tenofovir microbicide, which was shown to decrease the risk of HSV-2 infection [40, 41].
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was supported by National Institutes of Health (NIH) R01 AI0684316 and R24 TW007988 from Fogarty International Center (FIC) through Vanderbilt University. In addition, C. F. received support from NIH K24 AI087399, A. M. and R. B. were scholars in the University of Washington International AIDS Research and Training Program (IARTP) funded by FIC D43 TW000007, and B. L. G. was supported by an NIH/FIC postdoctoral fellowship T32 AI07140. R. Y. C. received support from the International Research Scientist Development Award K01 TW008406. This publication was made possible with help from the University of Washington Center for AIDS Research (CFAR), an NIH funded program (P30 AI027757), which is supported by the following NIH Institutes and Centers (NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health, Fogarty International Center, or Vanderbilt University.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes. 2004;11(Suppl 1):24–35A. [PubMed] [Google Scholar]
- 2.Looker KJ, Garnett GP, Schmid GP. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull World Health Organ. 2008;86:805–12. doi: 10.2471/BLT.07.046128. A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strick LB, Wald A, Celum C. Management of herpes simplex virus type 2 infection in HIV type 1-infected persons. Clin Infect Dis. 2006;43:347–56. doi: 10.1086/505496. [DOI] [PubMed] [Google Scholar]
- 4.Carney O, Ross E, Bunker C, Ikkos G, Mindel A. A prospective study of the psychological impact on patients with a first episode of genital herpes. Genitourin Med. 1994;70:40–5. doi: 10.1136/sti.70.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 6.Schacker T, Ryncarz AJ, Goddard J, Diem K, Shaughnessy M, Corey L. Frequent recovery of HIV-1 from genital herpes simplex virus lesions in HIV-1-infected men. JAMA. 1998;280:61–6. doi: 10.1001/jama.280.1.61. [DOI] [PubMed] [Google Scholar]
- 7.Schacker T, Zeh J, Hu H, Shaughnessy M, Corey L. Changes in plasma human immunodeficiency virus type 1 RNA associated with herpes simplex virus reactivation and suppression. J Infect Dis. 2002;186:1718–25. doi: 10.1086/345771. [DOI] [PubMed] [Google Scholar]
- 8.Mbopi-Keou FX, Gresenguet G, Mayaud P, et al. Interactions between herpes simplex virus type 2 and human immunodeficiency virus type 1 infection in African women: opportunities for intervention. J Infect Dis. 2000;182:1090–6. doi: 10.1086/315836. [DOI] [PubMed] [Google Scholar]
- 9.Baeten JM, McClelland RS, Corey L, et al. Vitamin A supplementation and genital shedding of herpes simplex virus among HIV-1-infected women: a randomized clinical trial. J Infect Dis. 2004;189:1466–71. doi: 10.1086/383049. [DOI] [PubMed] [Google Scholar]
- 10.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–53. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 11.Cernik C, Gallina K, Brodell RT. The treatment of herpes simplex infections: an evidence-based review. Arch Int Med. 2008;168:1137–44. doi: 10.1001/archinte.168.11.1137. [DOI] [PubMed] [Google Scholar]
- 12.Celum C, Wald A, Lingappa JR, et al. Acyclovir and Transmission of HIV-1 from Persons Infected with HIV-1 and HSV-2. N Engl J Med. 2010;362:427–39. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corey L, Wald A, Patel R, et al. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med. 2004;350:11–20. doi: 10.1056/NEJMoa035144. [DOI] [PubMed] [Google Scholar]
- 14.Chemaitelly H, Shelton JD, Hallett TB, Abu-Raddad LJ. Only a fraction of new HIV infections occur within identifiable stable discordant couples in Sub-Saharan Africa. AIDS. 2013;27:251–60. doi: 10.1097/QAD.0b013e32835ad459. [DOI] [PubMed] [Google Scholar]
- 15.Dunkle KL, Stephenson R, Karita E, et al. New heterosexually transmitted HIV infections in married or cohabiting couples in urban Zambia and Rwanda: an analysis of survey and clinical data. Lancet. 2008;371:2183–91. doi: 10.1016/S0140-6736(08)60953-8. [DOI] [PubMed] [Google Scholar]
- 16.Carpenter LM, Kamali A, Ruberantwari A, Malamba SS, Whitworth JA. Rates of HIV-1 transmission within marriage in rural Uganda in relation to the HIV sero-status of the partners. AIDS. 1999;13:1083–9. doi: 10.1097/00002030-199906180-00012. [DOI] [PubMed] [Google Scholar]
- 17.Robinson NJ, Mulder D, Auvert B, Whitworth J, Hayes R. Type of partnership and heterosexual spread of HIV infection in rural Uganda: results from simulation modeling. Int J STD AIDS. 1999;10:718–25. doi: 10.1258/0956462991913394. [DOI] [PubMed] [Google Scholar]
- 18.Mugo N, Dadabhai SS, Bunnell R, et al. Prevalence of herpes simplex virus type 2 infection, human immunodeficiency virus/herpes simplex virus type 2 coinfection, and associated risk factors in a national, population-based survey in Kenya. Sex Transm Dis. 2011;38:1059–66. doi: 10.1097/OLQ.0b013e31822e60b6. [DOI] [PubMed] [Google Scholar]
- 19.Guthrie BL, Choi RY, Bosire R, et al. Predicting pregnancy in HIV-1-discordant couples. AIDS Behav. 2010;14:1066–71. doi: 10.1007/s10461-010-9716-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guthrie BL, Lohman-Payne B, Liu AY, et al. HIV-1-specific enzyme-linked immunosorbent spot assay responses in HIV-1-exposed uninfected partners in discordant relationships compared to those in low-risk controls. Clin Vaccine Immunol. 2012;19:1798–805. doi: 10.1128/CVI.00179-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golden MR, Ashley-Morrow R, Swenson P, Hogrefe WR, Handsfield HH, Wald A. Herpes simplex virus type 2 (HSV-2) Western blot confirmatory testing among men testing positive for HSV-2 using the focus enzyme-linked immunosorbent assay in a sexually transmitted disease clinic. Sex Trans Dis. 2005;32:771–7. doi: 10.1097/01.olq.0000175377.88358.f3. [DOI] [PubMed] [Google Scholar]
- 22.Laeyendecker O, Henson C, Gray RH, et al. Performance of a commercial, type-specific enzyme-linked immunosorbent assay for detection of herpes simplex virus type 2-specific antibodies in Ugandans. J Clin Microbiol. 2004;42:1794–6. doi: 10.1128/JCM.42.4.1794-1796.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith JS, Bailey RC, Westreich DJ, et al. Herpes simplex virus type 2 antibody detection performance in Kisumu, Kenya, using the Herpeselect ELISA, Kalon ELISA, Western blot and inhibition testing. Sex Transm Infect. 2009;85:92–6. doi: 10.1136/sti.2008.031815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Celum C, Wald A, Hughes J, et al. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:2109–19. doi: 10.1016/S0140-6736(08)60920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mujugira A, Morrow RA, Celum C, et al. Performance of the Focus HerpeSelect-2 enzyme immunoassay for the detection of herpes simplex virus type 2 antibodies in seven African countries. Sex Transm Infect. 2011;87:238–41. doi: 10.1136/sti.2010.047415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lingappa J, Nakku-Joloba E, Magaret A, et al. Sensitivity and specificity of herpes simplex virus-2 serological assays among HIV-infected and uninfected urban Ugandans. Int J STD AIDS. 2010;21:611–6. doi: 10.1258/ijsa.2009.008477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sobngwi-Tambekou J, Taljaard D, Lissouba P, et al. Effect of HSV-2 serostatus on acquisition of HIV by young men: results of a longitudinal study in Orange Farm, South Africa. J Infect Dis. 2009;199:958–64. doi: 10.1086/597208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tobian AA, Serwadda D, Quinn TC, et al. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med. 2009;360:1298–309. doi: 10.1056/NEJMoa0802556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta SD, Moses S, Parker CB, Agot K, Maclean I, Bailey RC. Circumcision status and incident herpes simplex virus type 2 infection, genital ulcer disease, and HIV infection. AIDS. 2012;26:1141–9. doi: 10.1097/QAD.0b013e328352d116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tobian AA, Kigozi G, Redd AD, et al. Male circumcision and herpes simplex virus type 2 infection in female partners: a randomized trial in Rakai, Uganda. J Infect Dis. 2012;205:486–90. doi: 10.1093/infdis/jir767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashley-Morrow R, Nollkamper J, Robinson NJ, Bishop N, Smith J. Performance of focus ELISA tests for herpes simplex virus type 1 (HSV-1) and HSV-2 antibodies among women in ten diverse geographical locations. Clin Microbiol Infect. 2004;10:530–6. doi: 10.1111/j.1469-0691.2004.00836.x. [DOI] [PubMed] [Google Scholar]
- 33.Mertz GJ, Benedetti J, Ashley R, Selke SA, Corey L. Risk factors for the sexual transmission of genital herpes. Ann Int Med. 1992;116:197–202. doi: 10.7326/0003-4819-116-3-197. [DOI] [PubMed] [Google Scholar]
- 34.Wald A, Langenberg AG, Link K, et al. Effect of condoms on reducing the transmission of herpes simplex virus type 2 from men to women. JAMA. 2001;285:3100–6. doi: 10.1001/jama.285.24.3100. [DOI] [PubMed] [Google Scholar]
- 35.Wald A, Krantz E, Selke S, Lairson E, Morrow RA, Zeh J. Knowledge of partners’ genital herpes protects against herpes simplex virus type 2 acquisition. J Infect Dis. 2006;194:42–52. doi: 10.1086/504717. [DOI] [PubMed] [Google Scholar]
- 36.Reynolds SJ, Risbud AR, Shepherd ME, et al. Recent herpes simplex virus type 2 infection and the risk of human immunodeficiency virus type 1 acquisition in India. J Infect Dis. 2003;187:1513–21. doi: 10.1086/368357. [DOI] [PubMed] [Google Scholar]
- 37.Wald A, Zeh J, Selke S, Ashley RL, Corey L. Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med. 1995;333:770–5. doi: 10.1056/NEJM199509213331205. [DOI] [PubMed] [Google Scholar]
- 38.Benedetti J, Corey L, Ashley R. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann Int Med. 1994;121:847–54. doi: 10.7326/0003-4819-121-11-199412010-00004. [DOI] [PubMed] [Google Scholar]
- 39.Koelle DM, Benedetti J, Langenberg A, Corey L. Asymptomatic reactivation of herpes simplex virus in women after the first episode of genital herpes. Ann Int Med. 1992;116:433–7. doi: 10.7326/0003-4819-116-6-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cates W., Jr After CAPRISA 004: time to re-evaluate the HIV lexicon. Lancet. 2010;376:495–6. doi: 10.1016/S0140-6736(10)61200-7. [DOI] [PubMed] [Google Scholar]
- 41.Andrei G, Lisco A, Vanpouille C, et al. Topical tenofovir, a microbicide effective against HIV, inhibits herpes simplex virus-2 replication. Cell Host Microbe. 2011;10:379–89. doi: 10.1016/j.chom.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.