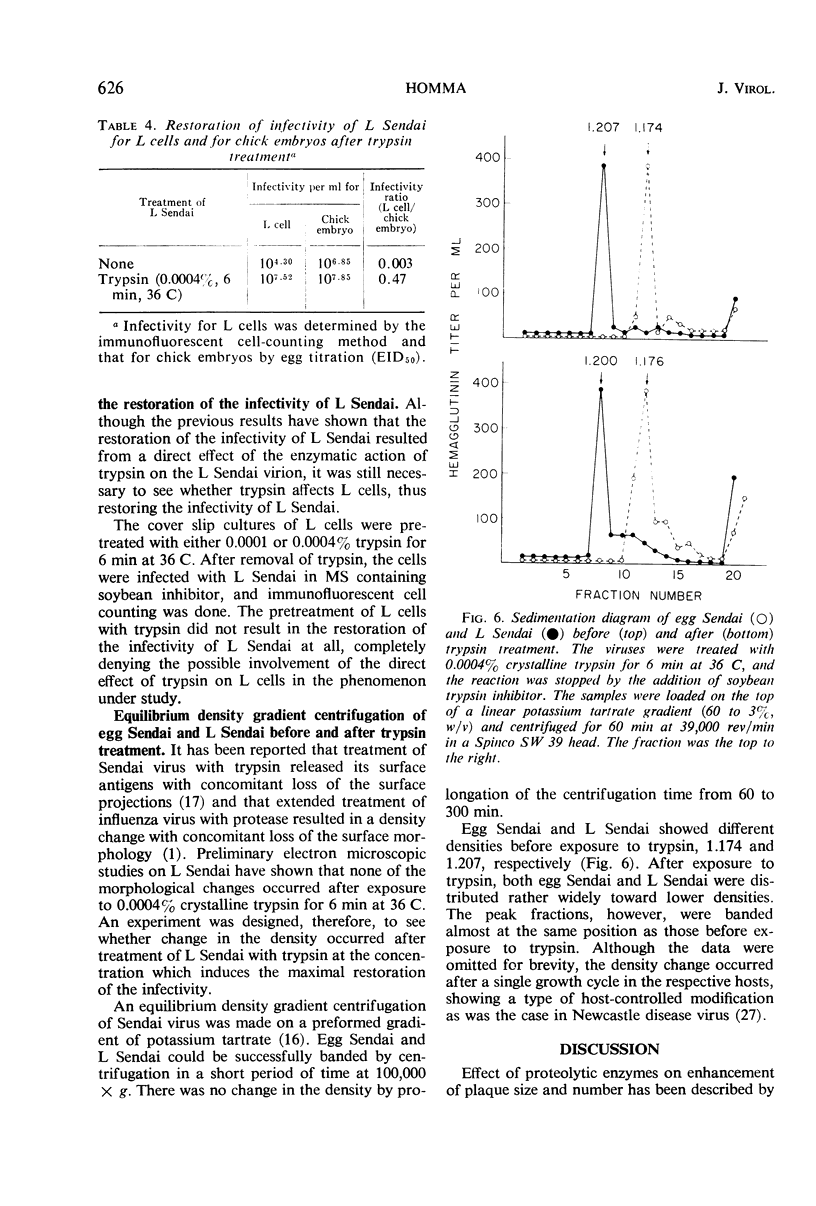
Abstract
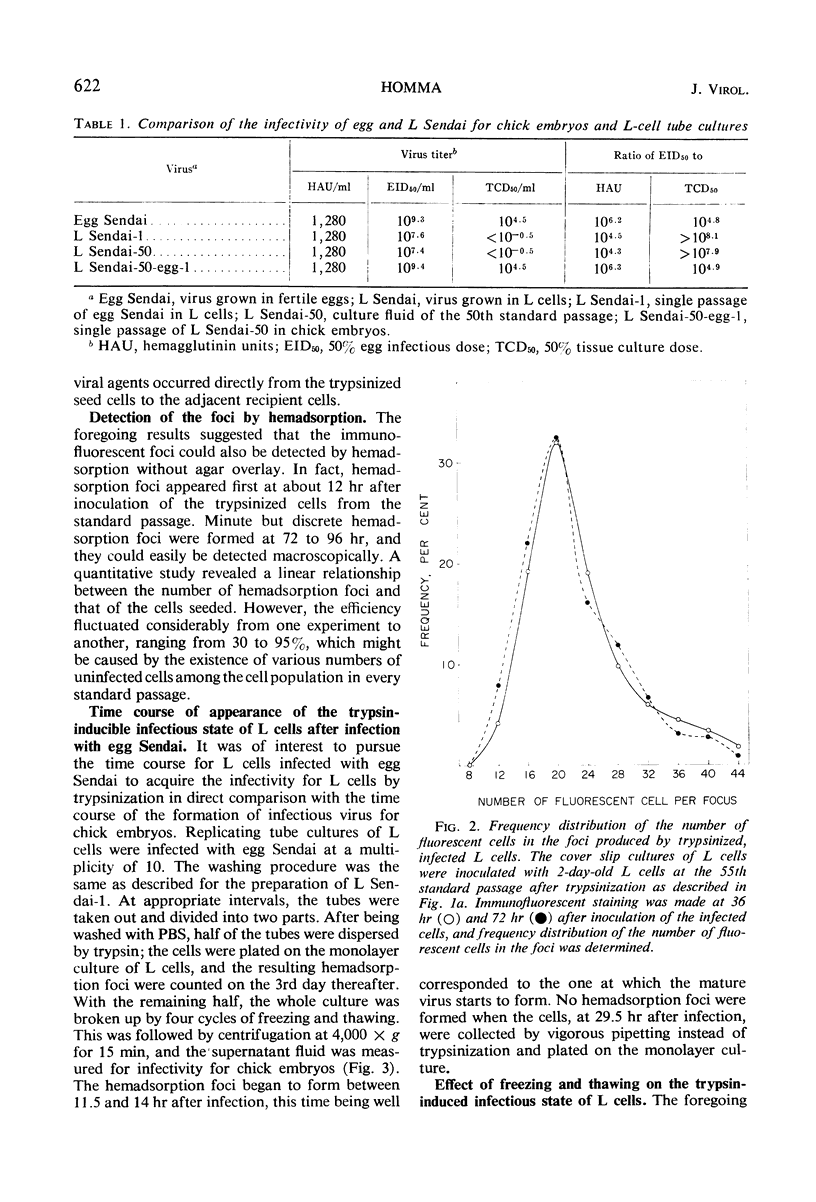
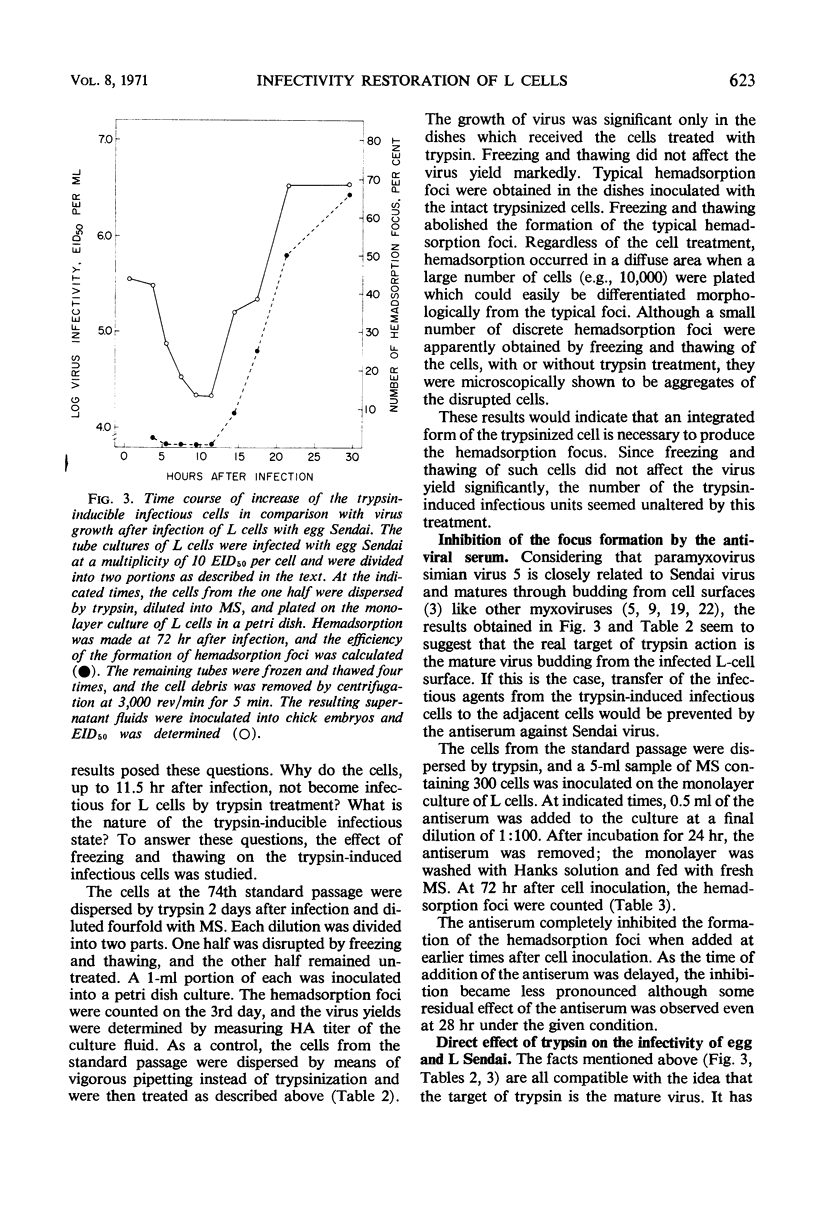
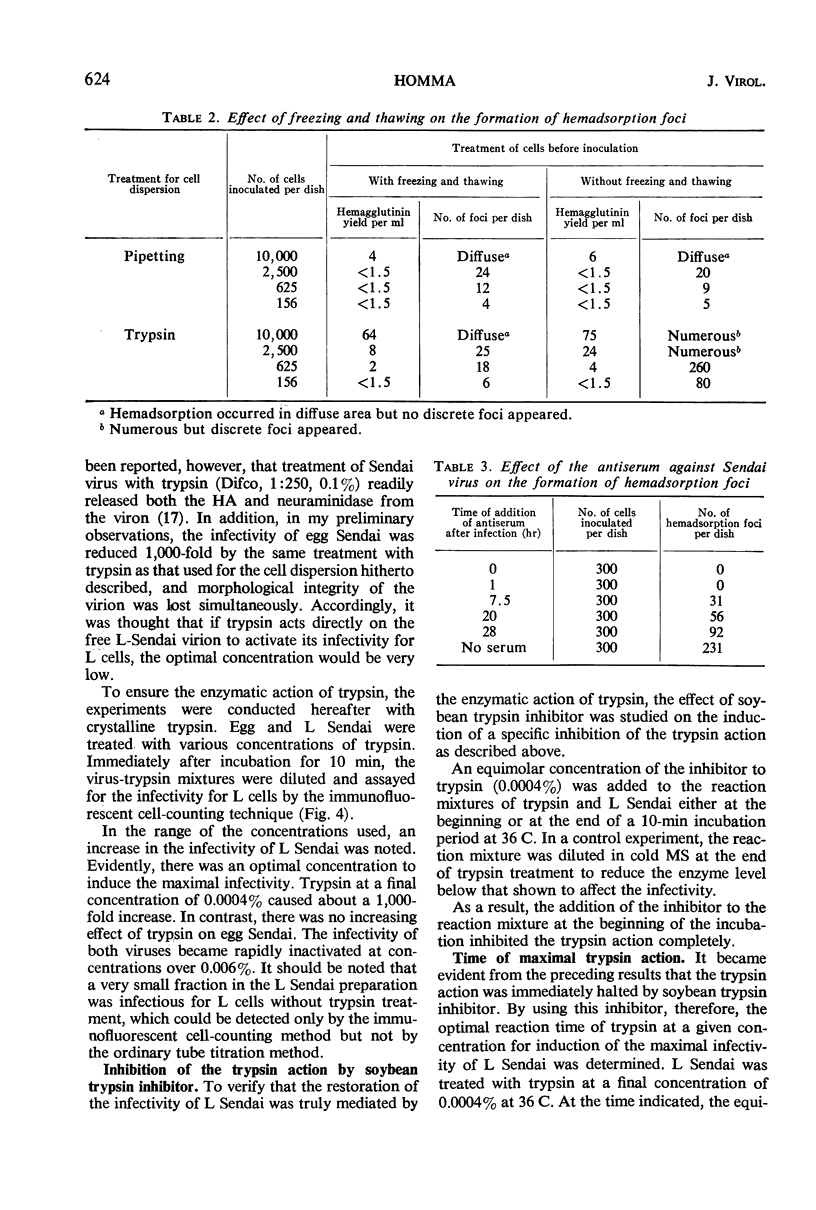
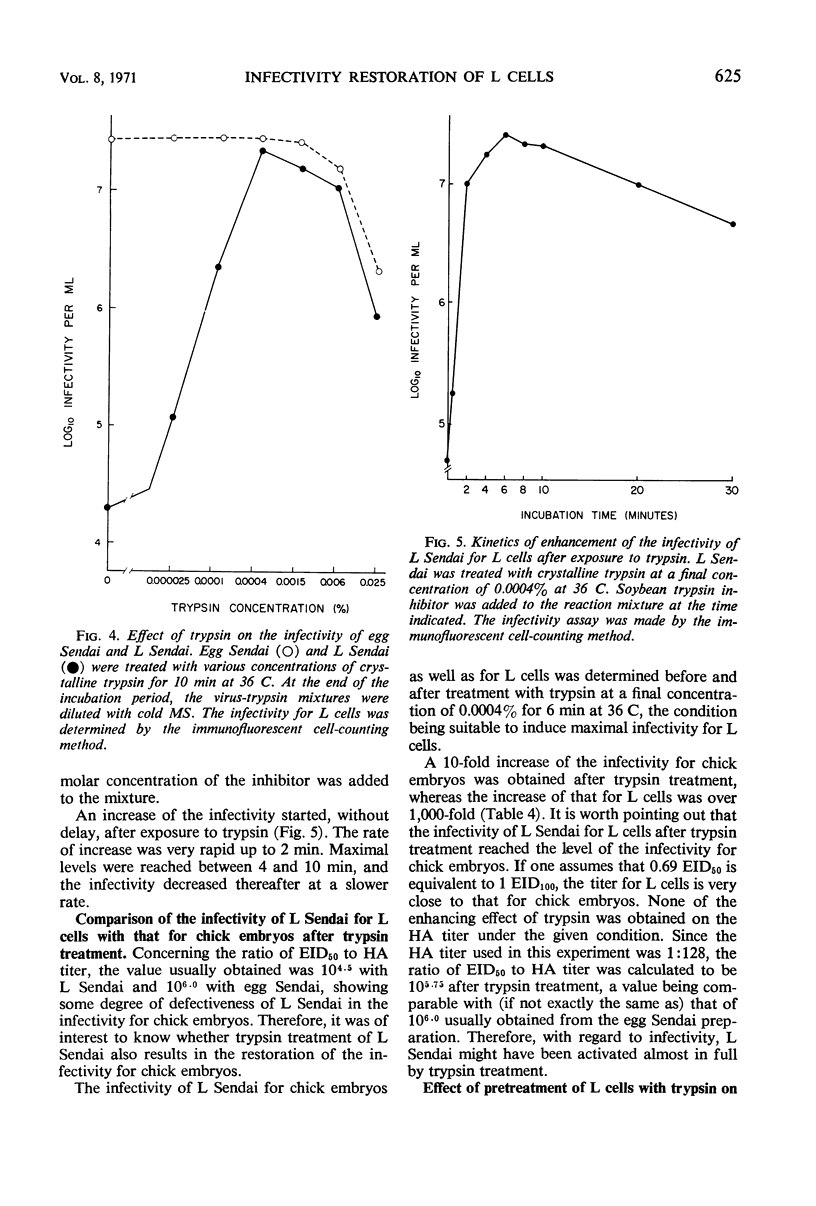
Sendai virus grown in fertile eggs (egg Sendai) infects L cells in which the synthesis of L Sendai (grown in L cells) occurs by the one-step mechanism. L Sendai is not infectious for L cells when tested by the tube titration method although it is infectious for chick embryos. When L cells infected with egg Sendai were dispersed by trypsin and plated on a monolayer culture of L cells, the viral agents spread to the adjacent recipient cells in which the synthesis of L Sendai occurred. The newly infected L cells became infectious for L cells again by trypsin treatment. Kinetic experiments suggested that the target of trypsin is the mature virus, of L Sendai nature, just budding from the L-cell surface. By using an immunofluorescent cell-counting technique, recovery of the infectivity of L Sendai for L cells due to a direct enzymatic action of trypsin was demonstrated. Under the optimal condition, the infectivity increased 1,000-fold for L cells and 10-fold for chick embryos, and both the titers could favorably be compared. No increasing effect of trypsin was observed on the infectivity of egg Sendai. Density centrifugation studies revealed a difference between egg Sendai and L Sendai in the density. Trypsin treatment which induced the maximal enhancement of L Sendai infectivity did not affect both the densities, showing that variations of Sendai virus in the infectivity for L cells and in the density are independent types of host-controlled modification.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biddle F. The action of protease on influenza A2 virus. J Gen Virol. 1968 Jan;2(1):19–28. doi: 10.1099/0022-1317-2-1-19. [DOI] [PubMed] [Google Scholar]
- Came P. E., Pascale A., Shimonaski G. Effect of pancreatin on plaque formation by influenza viruses. Arch Gesamte Virusforsch. 1968;23(4):346–352. doi: 10.1007/BF01242130. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Holmes K. V., Dales S., Choppin P. W. An electron microscopic study of moderate and virulent virus-cell interactions of the parainfluenza virus SV5. Virology. 1966 Nov;30(3):411–426. doi: 10.1016/0042-6822(66)90119-x. [DOI] [PubMed] [Google Scholar]
- DRAKE J. W., LAY P. A. Host-controlled variation in NDV. Virology. 1962 May;17:56–64. doi: 10.1016/0042-6822(62)90081-8. [DOI] [PubMed] [Google Scholar]
- Duc-Nguyen H., Rosenblum E. N. Immuno-electron microscopy of the morphogenesis of mumps virus. J Virol. 1967 Apr;1(2):415–429. doi: 10.1128/jvi.1.2.415-429.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford G. E., Klapper D. G. Effect of proteolytic enzymes on vaccinia virus replication. Arch Gesamte Virusforsch. 1969;26(4):321–333. doi: 10.1007/BF01250942. [DOI] [PubMed] [Google Scholar]
- Gifford G. E., Klapper D. G. Enhancement of vaccinia virus plaque formation by trypsin. Proc Soc Exp Biol Med. 1967 Nov;126(2):515–517. doi: 10.3181/00379727-126-32492. [DOI] [PubMed] [Google Scholar]
- HOMMA M. A particular binding of L cell-grown Sendal virus by host L cells. (Growth characteristics of myxoviruses in tissue culture. 5th). Tohoku J Exp Med. 1961 Feb 25;73:215–229. doi: 10.1620/tjem.73.215. [DOI] [PubMed] [Google Scholar]
- Howe C., Morgan C., de Vaux St Cyr C., Hsu K. C., Rose H. M. Morphogenesis of type 2 parainfluenza virus examined by light and electron microscopy. J Virol. 1967 Feb;1(1):215–237. doi: 10.1128/jvi.1.1.215-237.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIDA N., HOMMA M. A variant Sendai virus, infectious to egg embryos but not to L cells. III. Growth characteristics of myxoyiruses in tissue culture. Tohoku J Exp Med. 1960 Dec 25;73:56–69. doi: 10.1620/tjem.73.56. [DOI] [PubMed] [Google Scholar]
- ISHIDA N., HOMMA M. Host-controlled variation observed with Sendai virus grown in mouse fibroblast (L) cells. Virology. 1961 Aug;14:486–488. doi: 10.1016/0042-6822(61)90342-7. [DOI] [PubMed] [Google Scholar]
- Ito H., Morimoto Y., Iwase I., Doi Y., Sanpe T. Effect of trypsin on viral susceptibility of Vero cell cultures--cercopithecus kidney line. Jpn J Med Sci Biol. 1970 Aug;23(4):227–235. doi: 10.7883/yoken1952.23.227. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Lipids of plasma membranes of monkey and hamster kidney cells and of parainfluenza virions grown in these cells. Virology. 1969 Jun;38(2):255–268. doi: 10.1016/0042-6822(69)90367-5. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO T., MAENO K. A host-induced modification of hemagglutinating virus of Japan (HVJ, Sendai virus) in its hemolytic and cytopathic activity. Virology. 1962 Aug;17:563–570. doi: 10.1016/0042-6822(62)90156-3. [DOI] [PubMed] [Google Scholar]
- MCCREA J. F., EPSTEIN R. S., BARRY W. H. Use of potassium tartrate for equilibrium density-gradient centrifugation of animal viruses. Nature. 1961 Jan 21;189:220–221. doi: 10.1038/189220a0. [DOI] [PubMed] [Google Scholar]
- Maeno K., Yoshida T., Iinuma M., Nagai Y., Matsumoto T. Isolation of hemagglutinin and neuraminidase subunits of hemagglutinating virus of Japan. J Virol. 1970 Oct;6(4):492–499. doi: 10.1128/jvi.6.4.492-499.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPENDLOVE R. S., SCHAFFER F. L. ENZYMATIC ENHANCEMENT OF INFECTIVITY OF REOVIRUS. J Bacteriol. 1965 Mar;89:597–602. doi: 10.1128/jb.89.3.597-602.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STENBACK W. A., DURAND D. P. HOST INFLUENCE ON THE DENSITY OF NEWCASTLE DISEASE VIRUS (NDV). Virology. 1963 Aug;20:545–551. doi: 10.1016/0042-6822(63)90278-2. [DOI] [PubMed] [Google Scholar]
- Sabina L. R., Munro T. W. Effects of trypsin on replication of parainfluenza-3 virus in HEp-2 cell cultures. Can J Microbiol. 1969 Jun;15(6):577–582. doi: 10.1139/m69-098. [DOI] [PubMed] [Google Scholar]
- Seto J. T., Chang F. S. Functional significance of sialidase during influenza virus multiplication: an electron microscope study. J Virol. 1969 Jul;4(1):58–66. doi: 10.1128/jvi.4.1.58-66.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuta H., Akami M., Matumoto M. Plaque formation by sendai virus of parainfluenza virus group, type 1 on monkey, calf kidney and chick embryo cell monolayers. Jpn J Microbiol. 1971 Mar;15(2):175–183. doi: 10.1111/j.1348-0421.1971.tb00567.x. [DOI] [PubMed] [Google Scholar]
- Spendlove R. S., Lennette E. H., Knight C. O., Chin J. N. Production in FL cells of infectious and potentially infectious reovirus. J Bacteriol. 1966 Oct;92(4):1036–1040. doi: 10.1128/jb.92.4.1036-1040.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spendlove R. S., McClain M. E., Lennette E. H. Enhancement of reovirus infectivity by extracellular removal or alteration of the virus capsid by proteolytic enzymes. J Gen Virol. 1970 Aug;8(2):83–94. doi: 10.1099/0022-1317-8-2-83. [DOI] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L., Rapp F. Effects of pancreatin on the growth of reovirus. J Bacteriol. 1966 Jul;92(1):155–160. doi: 10.1128/jb.92.1.155-160.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C., Morales F., Powell J., Melnick J. L. Plaque enhancement of enteroviruses by magnesium chloride, cysteine, and pancreatin. J Bacteriol. 1966 May;91(5):1932–1935. doi: 10.1128/jb.91.5.1932-1935.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]