Abstract
Background
Hyperglycemia is among the potent factors that may induce or facilitate apoptosis. TNF-Related Apoptosis-Inducing Factor (TRAIL) is known for its apoptotic and immunomodulatory effects that have recently been correlated with diabetes. We examined serum-soluble TRAIL (sTRAIL) and high-sensitivity CRP (hs-CRP) levels and their association with various distinct parameters in type 2 diabetic nephropathy patients with diabetic foot disease.
Material/Methods
Twenty-two diabetic nephropathy patients with foot ulcers were enrolled in our study. Patients had been diagnosed with diabetes at age 24±10.58 years. Circulating sTRAIL and Hs-CRP levels were compared with control values, and possible correlations were investigated with parameters such as age, Wagner’s Grade (WG), BMI, HbA1c, and creatinine.
Results
Serum sTRAIL levels were significantly reduced in the patient group, compared to healthy subjects. High HsCRP levels correlated with age, and WGS correlated with BMI and creatinine levels.
Conclusions
Significantly suppressed sTRAIL levels in diabetic nephropathy patients with foot ulcers compared to healthy controls suggest a protective role for TRAIL in the disease setting.
Keywords: soluble TRAIL, type-2 diabetes mellitus, diabetic nephropathy, diabetic foot, hsCRP, HbA1c, Wagner grading system
Background
Type 2 diabetes (T2D), affecting approximately 6% of Western populations, is associated with a high risk for development of skin complications. The most prominent of these are the “diabetic foot ulcers” resulting from defective wound healing, in which impaired angiogenesis is considered as the main contributing factor [1]. Hyperglycemia is a well-recognized major causative factor in development of such complications, but growing evidence also points to inflammation as a significant factor [2]. Angiopathy of capillaries in glomeruli is known to develop secondary to longstanding diabetes, leading to diabetic nephropathy (DN), the major cause of morbidity and mortality in patients with T2D [3].
Progressive renal cell depletion through apoptotic cell death was recently correlated with DN. Hyperglycemia is known to induce or facilitate apoptosis through activation of cell death effector pathways. Death ligands from the TNF superfamily trigger apoptosis in various different types of cells through activation of the related caspases, central activators, and effectors, via the so-called “death receptors” on the cell surface [4]. TNF-Related Apoptosis-Inducing Ligand (TRAIL) is a relatively recently defined member of the TNF superfamily, possessing some unique features not found in the other family members. Accordingly, it exerts a generally selective apoptotic effect on tumor cells but not on normal cells. Furthermore, its wide expression in human tissues suggests yet other roles of this significant ligand [5]. In accordance with its definition as a molecule with multiple roles, it can interact with 4 different membrane receptors and a soluble osteoprotegerin receptor in humans, unlike other TNF family members, which usually have just 1 or 2 functional receptors. The transmembrane receptors are TRAIL-R1 (DR4) and TRAIL-R2 (DR5), which function as death receptors, and TRAIL-R3 (DcR1) and TRAIL-R4 (DcR2), which are decoy receptors that are not capable of triggering apoptosis [6–9]. Although TRAIL is a trans-membrane molecule, membrane-bound TRAIL can be cleaved from the cell surface to form a soluble trimeric ligand, which maintains the proapoptotic activity [10].
TRAIL was correlated with both destructive and protective roles in diabetes progression, but other TNF family members are mainly known for their disruptive effects on pancreatic beta cells [11]. Although strong evidence of its protective role in diabetes allows the possibility of preventive/therapeutic use of TRAIL in diabetes, its exact role in this disease has not yet been defined [11,12]. According to recent research, an early protective role is attributed to TRAIL in the onset of disease in type 1 diabetes, whereas a modulating role in vascular complications is suggested for both in type 1 and type 2 diabetes [4,11–16]. Thus, its precise role in diabetes and its association with the related secondary complications is yet to be clarified.
We thus aimed to determine possible changes in serum sTRAIL and high-sensitivity CRP (hsCRP) levels in a cohort of insulin-using type 2 diabetic nephropathy patients with diabetic feet, compared to healthy subjects. Possible correlations were investigated between these markers and values of: HbA1c, creatinine, Wagner Grading System (WGS), and body mass index (BMI), as well as sedimentation rate, preprandial glucose levels, and age.
Material and Methods
Patients and controls
Twenty-two type 2 diabetic patients with diabetic nephropathy, presented with macroalbuminuria (>300 mg albumin/24 hours or ACR >34 mg/mmol [300 mg/g]), who also had diabetic feet, were enrolled in our study. Patients were referred to Antalya Education and Research Hospital during the period January–September 2012 (mean age, 52±12.91 years; 36.3% male). None of the patients had any autoimmune diseases or cancer. Patients had been diagnosed with diabetes at 24±10.58 years of age. The control group consisted of healthy volunteers (n=20; 9 male, 11 female; mean age, 37 years).
All patients gave their informed, written consent. The study was approved by the Local Independent Ethics Committee, and was carried out in accordance with the guidelines of the Declaration of Helsinki.
Laboratory tests
In vitro quantitative determination of soluble TNF-Related Apoptosis-Inducing Ligand (sTRAIL) in serum samples was performed with a human soluble TRAIL/Apo2L ELISA kit (Diaclone, France). Spectrophotometric absorbance values at 450 nm were used to acquire sTRAIL concentrations (pg/ml).
Serum hs-CRP levels were measured using an hs-CRP assay (Behring Latex-Enhanced utilizing Behring Nephelometer BN-100; Behring Diagnostics, Westwood, MA, USA). The sensitivity range of the assay was 0.04–5.0 mg/L.
Statistical analysis
The Statistical Package for the Social Sciences 13.0 software for Windows (SPSS Inc., Chicago, IL) and GraphPad Prism version 5 (La Jolla, CA) were used for data plotting and statistical analysis. A non-parametric unpaired t test was used to evaluate sTRAIL levels in patient groups versus controls. Spearman’s Rho correlation analysis was performed to determine possible associations between different parameters.
Results
The patient group presented with the following values: Hs-CRP: 151.23±21.82 mg/L, WGS: 3.47±0.34, BMI: 35.74±8.03, creatinine: 1.95±0.171mg/dL, sedimentation rate: 79.77±3.54, pre-prandial blood glucose level: 176.32±16.38mg/dL, and WBC: 12.61±0.99.
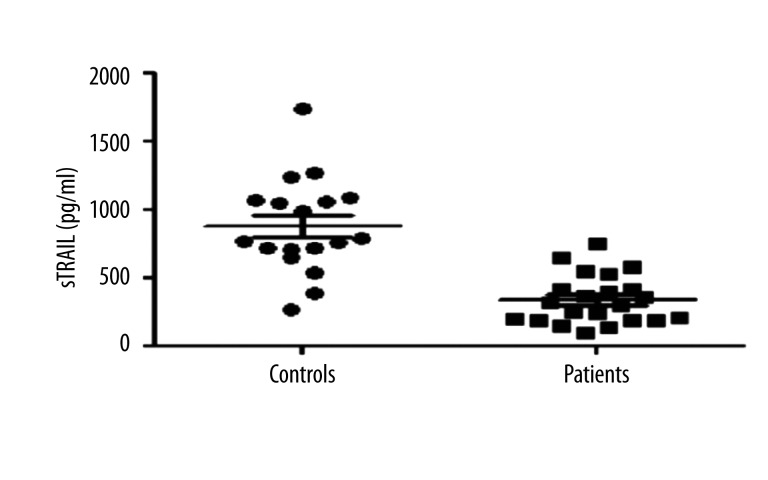
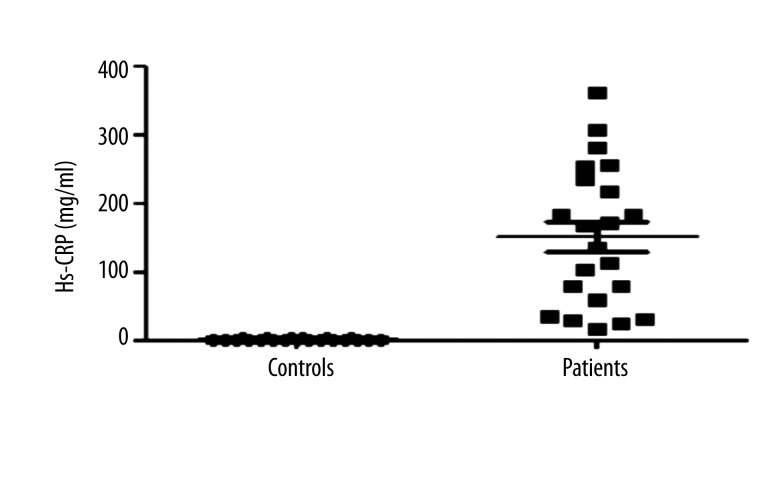
The mean serum sTRAIL level in the patient group was 338.29±38.15 pg/mL (mean±SEM). The control group had a mean sTRAIL value of 875.96±82.41 pg/mL (mean ±SEM). The difference between the circulating sTRAIL levels of the patient and control groups was statistically significant (p<0.001) (Figure 1). In contrast to low sTRAIL levels, high Hs-CRP levels were evident in patients (151.23±21.82 mg/L) (Figure 2).
Figure 1.
Scatter dot plots of circulating sTRAIL levels (pg/ml) in 22 insulin-using type 2 diabetic nephropathy patients with diabetic feet.
Figure 2.
Hs-CRP levels in healthy controls and diabetic foot patients.
Spearman’s correlation test revealed a positive correlation between HsCRP and age (p<0.001). WGS positively correlated with BMI and creatinine levels (p<0.05). sTRAIL or HbA1c levels did not correlate with clinical presentations of T2D, thus particularly the late complication of diabetic foot. Accordingly, sTRAIL levels did not significantly correlate with Hs-CRP, HbA1c, BMI, Wagner Grading system, creatinine values, sedimentation rate, or pre-prandial glucose levels.
Discussion
The global number of people with diabetes is expected to reach 380 million by the year 2025 [2]. Both type 1 diabetes (T1D) and type 2 diabetes (T2D) are associated with increased risk of macro- and micro-vascular complications. Micro-vascular complications are the main cause of blindness (diabetic retinopathy), end-stage renal disease (diabetic nephropathy), and foot amputation due to diabetic foot (diabetic neuropathy) in these patients [2]. Diabetic foot involves ulceration, which is a major complication of diabetes mellitus, the severity of which is classified from 1 to 5 according to the Wagner Grading System (WGS). The annual incidence of ulcers is estimated at between 2.5% and 10.7% in diabetic patients in developed countries and the annual incidence of amputation for any reason is reported as between 0.25–1.8% [13].
TNF-Related Apoptosis-Inducing Ligand (TRAIL) is a relatively recently identified TNF superfamily member, which has been attributed with apoptotic, antiinflammatory, protective, and even proliferative roles in different settings on human cells. Under normal physiological conditions, a toxic role is highly unlikely for this molecule in normal human tissues, as it is fairly widely expressed, in contrast to the other members of the TNF superfamily, such as TNF-alpha and FasL [5]. However, TRAIL induced normal parenchymal cell apoptosis in an inflammatory setting, an example to its differing actions under certain conditions [14]. Accordingly, TRAIL-TRAIL receptor interactions have recently been associated with a variety of diseases, and many reports have found variable levels of circulating sTRAIL in cancer, as well as in cardiac, renal, and even allergic diseases [15–18]. TRAIL has recently been associated with progressive atherosclerosis as an independent risk factor [19,20]. Furthermore, TRAIL’s soluble decoy receptor, osteoprotegerin (OPG), is considered as a surrogate marker for peripheral artery disease, and sTRAIL levels negatively correlated with CRP levels [21,22].
TRAIL has also been associated with diabetes, with many reports claiming a destructive role in disease progression, as well as a protective role suggested by yet many other studies [23,24]. In accordance with the claimed protective role, newly diagnosed, non-drug using T2D patients had lower levels of circulating sTRAIL compared to healthy individuals in a previous study by our group [25]. We found a 989.6 pg/mL (n=22) mean sTRAIL level in the patient group and 1800 pg/mL (n=20) in the control group. In the present study, sTRAIL levels were also decreased to 338.29 pg/mL in 22 patients, in comparison with the 875.96 pg/mL in healthy controls. While the patients involved in the previous study were newly diagnosed with T2D, cases included in the present study were at a relatively advanced stage of the disease, with diabetic nephropathy and foot ulcers. However, many studies emphasize that the exact role of TRAIL and its receptors in diabetes is yet to be identified clearly [11,12], TRAIL has already been attributed with predominant protective roles in diabetes. Our findings are in accordance with this hypothesis, as we found lower mean sTRAIL levels in T2D patients with advanced disease compared to healthy subjects, and also compared to the levels measured in the newly diagnosed type 2 diabetic patients.
We found no correlation between circulating sTRAIL levels and the clinical/biochemical parameters HbA1c, creatinine, BMI, WGS, sedimentation rate, preprandial glucose levels, or age. However, Hs-CRP levels were high (151.23±21.82 mg/L) in the patient group, where sTRAIL levels were decreased significantly. The finding that sTRAIL levels are lower in patients with nephropathy and foot ulcers compared to healthy controls and to the newly diagnosed T2D patients supports the anti-inflammatory effect attributed to TRAIL and its suggested modulatory role in vascular complications. In contrast, HsCRP increased with age, and positive correlations were evident between WGS and BMI, and WGS and creatinine levels, as expected.
Conclusions
To the best of our knowledge, this is the first study to assess serum sTRAIL and HbA1c levels in T2D patients with the late complication of diabetic foot. We investigated evidence for possible correlations between serum sTRAIL and Hs-CRP levels, and HbA1c, BMI, WGS, creatinine, pre-prandial glucose, and sedimentation rate. The sTRAIL levels did not correlate with any of these markers. Further studies are required to assess whether TRAIL and/or any of its receptors has a distinct role in the pathogenesis of T2D, and, thus, if sTRAIL could serve as a marker for T2D and/or its complications.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Source of support: Departmental sources
References
- 1.Aghdam SY, Eming SA, Willenborg S, et al. Vascular endothelial insulin/IGF-1 signaling controls skin wound vascularization. Biochem Biophys Res Commun. 2012;421:197–202. doi: 10.1016/j.bbrc.2012.03.134. [DOI] [PubMed] [Google Scholar]
- 2.Kaul K, Hodgkinson A, Tarr JM, et al. Is inflammation a common retinal-renal-nerve pathogenic link in diabetes? Curr Diabetes Rev. 2010;6:294–303. doi: 10.2174/157339910793360851. [DOI] [PubMed] [Google Scholar]
- 3.Roopakala MS, Pawan HR, Krishnamurthy U, et al. Evaluation of high sensitivity C-reactive protein and glycated hemoglobin levels in diabetic nephropathy. Saudi J Kidney Dis Transpl. 2012;23:286–89. [PubMed] [Google Scholar]
- 4.Lorz C, Benito-Martin A, Boucherot A, et al. The death ligand TRAIL in diabetic nephropathy. J Am Soc Nephrol. 2008;19:904–14. doi: 10.1681/ASN.2007050581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LeBlanc HN, Ashkenazi A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 2003;10:66–75. doi: 10.1038/sj.cdd.4401187. [DOI] [PubMed] [Google Scholar]
- 6.Pan G, O’Rourke K, Chinnaiyan AM, et al. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–13. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 7.Walczak H, Degli-Esposti MA, Johnson RS, et al. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 1997;16:5386–97. doi: 10.1093/emboj/16.17.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu GS, Burns TF, Zhan Y, et al. Molecular cloning and functional analysis of the mouse homologue of the KILLER/DR5 tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor. Cancer Res. 1999;59:2770–75. [PubMed] [Google Scholar]
- 9.Degli-Esposti MA, Dougall WC, Smolak PJ, et al. The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity. 1997;7:813–20. doi: 10.1016/s1074-7613(00)80399-4. [DOI] [PubMed] [Google Scholar]
- 10.Benito-Martin A, Ucero AC, Santamaria B, et al. Transcriptomics illustrate a deadly TRAIL to diabetic nephropathy. Nefrologia. 2009;29:13–19. doi: 10.3265/Nefrologia.2009.29.1.13.1.en.full.pdf. [DOI] [PubMed] [Google Scholar]
- 11.Dirice E, Kahraman S, Elpek GO, et al. TRAIL and DcR1 expressions are differentially regulated in the pancreatic islets of STZ- versus CY-applied NOD mice. Exp Diabetes Res. 2011;2011:625813. doi: 10.1155/2011/625813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaccarezza M, Delbello G, Zauli G. A role of the TRAIL-TRAIL receptor system in the pathogenesis of diabetes. Acta Biomed. 2007;78(Suppl 1):262–67. [PubMed] [Google Scholar]
- 13.Hunt DL. Diabetes: foot ulcers and amputations. Clin Evid (Online) 2011:2011. [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchez-Nino MD, Benito-Martin A, Goncalves S, et al. TNF superfamily: a growing saga of kidney injury modulators. Mediators Inflamm. 2010:2010. doi: 10.1155/2010/182958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bisgin A, Kargi A, Yalcin AD, et al. Increased serum sTRAIL levels were correlated with survival in bevacizumab-treated metastatic colon cancer. BMC Cancer. 2012;12:58. doi: 10.1186/1471-2407-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liabeuf S, Barreto DV, Barreto FC, et al. The circulating soluble TRAIL is a negative marker for inflammation inversely associated with the mortality risk in chronic kidney disease patients. Nephrol Dial Transplant. 2010;25:2596–602. doi: 10.1093/ndt/gfq042. [DOI] [PubMed] [Google Scholar]
- 17.Yalcin AD, Bisgin A, Kargi A, Gorczynski RM. Serum-soluble TRAIL levels in patients with severe persistent allergic asthma: its relation to omalizumab treatment. Med Sci Monit. 2012;18(3):PI11–15. doi: 10.12659/MSM.882504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kargi A, Yalcin AD, Erin N, et al. IL8 and serum soluble TRAIL levels following anti-VEGF monoclonal antibody treatment in patients with metastatic colon cancer. Clin Lab. 2012;58:501–5. [PubMed] [Google Scholar]
- 19.Kawano N, Mori K, Emoto M, et al. Association of serum TRAIL levels with atherosclerosis in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2011;91:316–20. doi: 10.1016/j.diabres.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 20.Michowitz Y, Goldstein E, Roth A, et al. The involvement of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in atherosclerosis. J Am Coll Cardiol. 2005;45:1018–24. doi: 10.1016/j.jacc.2004.12.065. [DOI] [PubMed] [Google Scholar]
- 21.Ziegler S, Kudlacek S, Luger A, Minar E. Osteoprotegerin plasma concentrations correlate with severity of peripheral artery disease. Atherosclerosis. 2005;182:175–80. doi: 10.1016/j.atherosclerosis.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 22.Kiechl S, Schett G, Wenning G, et al. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation. 2004;109:2175–80. doi: 10.1161/01.CIR.0000127957.43874.BB. [DOI] [PubMed] [Google Scholar]
- 23.Lamhamedi-Cherradi SE, Zheng S, Tisch RM, Chen YH. Critical roles of tumor necrosis factor-related apoptosis-inducing ligand in type 1 diabetes. Diabetes. 2003;52:2274–78. doi: 10.2337/diabetes.52.9.2274. [DOI] [PubMed] [Google Scholar]
- 24.Cheung SS, Metzger DL, Wang X, et al. Tumor necrosis factor-related apoptosis-inducing ligand and CD56 expression in patients with type 1 diabetes mellitus. Pancreas. 2005;30:105–14. doi: 10.1097/01.mpa.0000148515.77497.4b. [DOI] [PubMed] [Google Scholar]
- 25.Bisgin A, Yalcin AD, Gorczynski RM. Circulating soluble tumor necrosis factor related apoptosis inducing-ligand (TRAIL) is decreased in type-2 newly diagnosed, non-drug using diabetic patients. Diabetes Res Clin Pract. 2012;96:e84–86. doi: 10.1016/j.diabres.2012.02.028. [DOI] [PubMed] [Google Scholar]