Abstract
Radiotherapy may cause central or peripheral nervous system complications, reported examples being myelopathy, brachial/lumbosacral plexopathies or predominantly motor lumbosacral radiculopathy. In the literature some studies regard camptocormia as a paravertebral myopathy and others as of neurogenic origin. We present a patient who developed camptocormia, 42 years after radiation therapy to the para-aortic and inguinal area.
Background
Camptocormia, also known as ‘bent spine syndrome,’ is a widely recognised condition characterised by abnormal posture of the trunk with marked flexion of the thoracolumbar spine, which increases during walking and abates in recumbency. The exact aetiology of camptocormia is unknown but has been linked with disorders of the central and peripheral nervous system such as Parkinson's disease, dystonia, multiple system atrophy (MSA), as well as various primary and secondary myopathies.1 It has also been linked to non-neurological conditions such as arthritis and may be associated with certain drugs. There has been one case report2 in the literature regarding radiation-induced camptocormia and we present another patient who developed camptocormia after receiving radiotherapy to the para-aortic and inguinal area. In contrast to the other case report, we attempt to localise the pathology and discuss a mechanism for this condition.
Case presentation
In 1956, a 23-year-old Caucasian male patient was diagnosed with a teratoma of the right testis and subsequently underwent an orchidectomy. He received 40 Gy of fractionated adjuvant radiotherapy to the right inguinal area and 38 Gy to the para-aortic area over a period of 6 weeks. In 1998, 42 years later, he noticed an insidious onset of bending of his spine. His symptoms gradually worsened leading to his presentation to the neurology clinic in 2012 at the age of 79.
This patient had the typical features of camptocormia: increasing forward flexion of the spine as he walked but normal position of his spine when supine. He reported some stiffness in the morning accompanied by a mild back ache, but no sensory symptoms. He also reported shortness of breath on exertion which could not be attributed to any other cause other than his posture. There was no family history of neuromuscular disease. The patient had no other significant medical history and was not taking any medications such as neuroleptics that have been associated with camptocormia.
On examination, the patient had a profoundly stooped posture when he walked (video 1). He was able to straighten himself by pushing/climbing up his legs. His knees were bent on standing upright as an apparent compensatory mechanism (figure 1A). There was no associated weakness of head flexion or extension. In the spine, he had marked wasting of the lower thoracic and lumbar paraspinal muscles (figure 1B). He was able to lie flat without difficulty (figure 1C). Upper limb examination revealed normal tone, power and reflexes. There was no focal muscle wasting in the legs. He had mildly reduced power on hip flexion but otherwise hip extension and power in his legs were normal. His knee jerks were present, ankle reflexes absent and plantars were down going. Sensation including vibration, proprioception, temperature, pain and fine touch were all normal. There were no extrapyramidal signs and no bowel or bladder disturbances.
Figure 1.
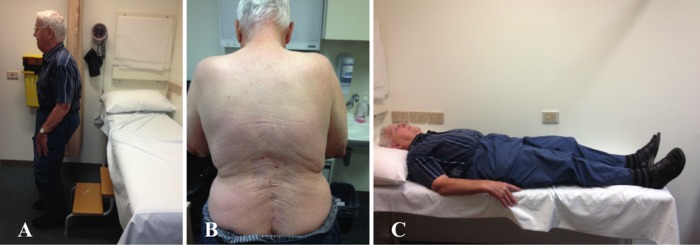
(A) Forward flexion of the spine compensated by bending of the knees when standing. (B) Marked atrophy of the paraspinal muscles. (C) Normal position of the spine when lying down.
Patient lying and sitting. Progressive forward flexion of the spine is demonstrated whilst walking.
Investigations
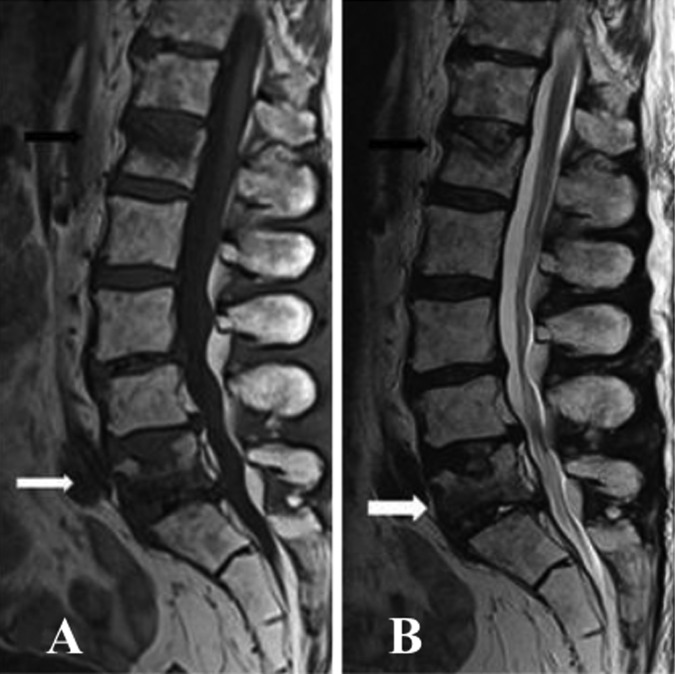
Blood tests including creatine kinase, thyroid stimulating hormone, antinuclear antibodies, C reactive protein and erythrocyte sedimentation rate were normal. Spinal MRI showed increased T2 signal throughout the lumbar vertebrae in keeping with postradiotherapy change. Old compression fractures of the L1 and L5 vertebral body were noticed (figure 2A,B). There was atrophy and an almost complete fatty replacement of the posterior paraspinal muscles between T11/T12 and S3/S4 levels wasseen in an axial T2 image (figure 3A) and coronal T1 image (figure 4). Fatty atrophy of the paraspinal muscles was shown to be persistent 6 months after the initial imaging in an axial T2 image (figure 3B).
Figure 2.
(A and B) Sagittal T2 and T1 images of the lumbar spine demonstrated increased signals throughout the lumbar vertebrae in keeping with postradiotherapy change. Mild to moderate reduction in height of L1 vertebral body (white arrow) and L5 vertebral body (black arrow) with superior end-plate involvement would be the result of old compression fractures.
Figure 3.
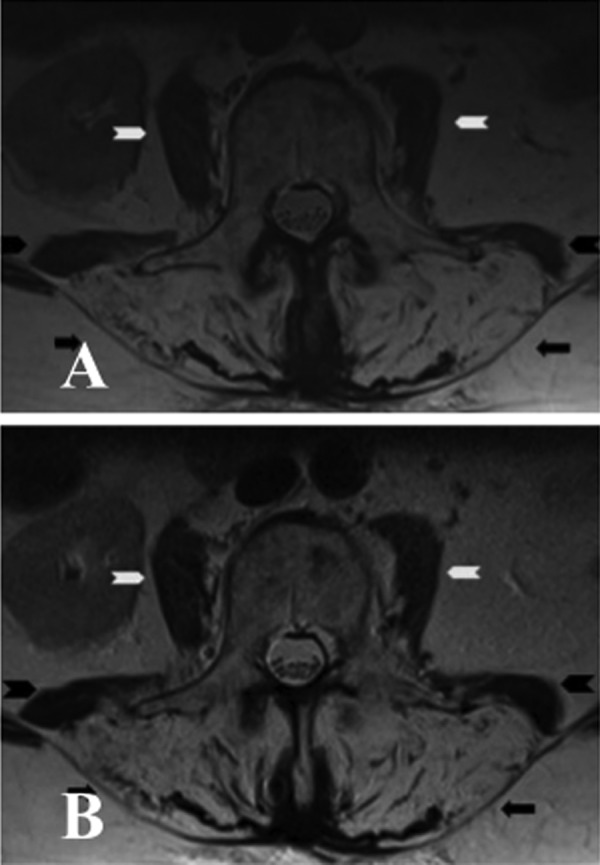
(A) Axial T2 image of the lumbar spine showed marked atrophy of paraspinal muscles (black arrow) bilaterally with increased T2 signals in keeping with fatty replacement. There was also moderate atrophy of psoas (white arrow head) and quadratus lumborum (black arrow head) on both sides. (B) Axial T2 image of the same patient performed 6 months after the initial MRI confirmed persistent fatty atrophy of the paraspinal muscles (black arrow) bilaterally. There was a further very mild increase of atrophy of quadratus lumborum (black arrow head) on both sides. Both psoas (white arrow) did not show any significant change.
Figure 4.
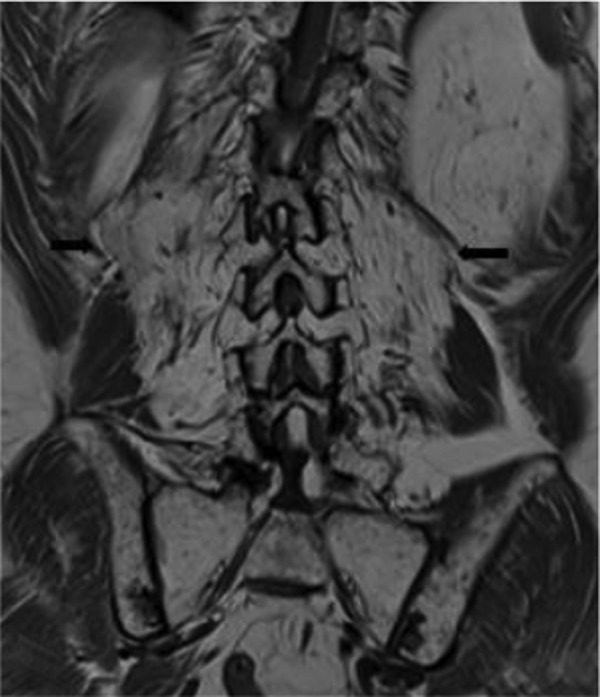
Coronal T1 image showed marked atrophy of paraspinal muscles (black arrow) bilaterally with increased T1 signal in keeping with fatty replacement.
Electromyographic (EMG) studies of paraspinal and lower limb muscles were performed. Sampling of paraspinal muscles at T12, L3 and L5 levels demonstrated paucity of motor unit recruitment associated with spontaneous muscle activity in the form of fibrillation potentials and doublets. There were no typical myokymic discharges and no myopathic units. EMG sampling of lower limb muscles only revealed very mild scattered neurogenic changes.
Lower limb somatosensory-evoked potentials (SSEP) with stimulation of both tibial nerves demonstrated a satisfactory spinal response at the L4 level but poor response on the scalp. By contrast, upper limb SSEP with stimulation of the median nerve evoked a normal cortical response.
Discussion
Our patient exhibited typical features of camptocormia which occurred 42 years after the radiotherapy to the para-aortic area and inguinal regions. Clinical examination, MRI and EMG studies suggest that the pathology is limited to the neuromuscular structures in his back, consistent with a postirradiation aetiology.
The relative preservation of the limb muscles suggests that the pathology predominantly involves the paraspinal muscles, intramuscular nerve fibres or the posterior primary rami, while sparing the nerve roots and lumbosacral plexus. The EMG findings of fibrillation potentials and doublets in the paraspinal muscles are likely to reflect severe end-stage muscle damage, but not specific for mechanism of injury either from a neurogenic or myopathic cause. We cannot exclude a severe postirradiation myopathy or a mixed pathology.
Lower limb SSEP studies demonstrated a preserved response at the L4 level but impaired response on the scalp. In contrast, upper limb SSEP studies revealed normal cortical responses. These findings suggest an abnormality in the dorsal column between the lumbar and the cervical cord. A possible explanation includes a mild radiation-induced injury to the posterior column. We acknowledge that the results may also be influenced by the patient’s age.
Camptocormia following irradiation is very rare with other case report published in the literature.2 This report described a 78-year-old patient who developed camptocormia, 7 years postradiotherapy for follicular lymphoma. We reviewed this article and submit that the patient described did not have typical camptocormia. He had atrophy and weakness in not only the lumbar paraspinal muscles, but also in cervical and the lower limbs. Apart from a stooped posture, this patient also had head drop. He had received widespread irradiation to axillary, subclavicular, mediastinal, spleen, lumboaortic lymph nodes which probably caused wasting and weakness of the entire paraspinal muscles.
In this report, we attempt to localise the pathology and discuss the probable mechanism of injury in patients with postirradiation camptocormia. The clinical signs and neurophysiological changes in our patient suggest a predominant involvement of the paraspinal rather than limb muscles. This suggests that the brunt of the pathology is to the lumbar posterior primary rami, paraspinal muscles or a combination of the above.
To our knowledge there is no documented neuropathological data on patients with postirradiation camptocormia. We may infer a comparable pathophysiological process from case series reports of extended-field radiotherapy for Hodgkin's lymphoma in the neck resulting in marked cervical paraspinal muscle atrophy and weakness.3 Electrophysiological and histopathological findings in this series suggested a combination of both neurogenic and myopathic aetiology, with the latter being more dominant. It has been demonstrated that mature muscle fibres are usually resistant to radiation whereas the blood vessels and capillaries are more susceptible to radiation injury. This leads to the hypothesis that radiation induced myopathy occurred through vascular damage.3
Patients with postirradiation camptocormia should be distinguished from patients presenting primarily with lower limb wasting and weakness following irradiation to thoracolumbar and inguinal areas.4 Neuropathological and electrophysiological studies in these patients suggest involvement of the preganglionic nerve roots, hence the term postirradiation lumbosacral radiculopathy.4
The current standard treatment for a testicular teratoma is a radical orchidectomy without any radiatiotherapy.5 For a germ cell tumour radiotherapy of 18–20 Gy may show a beneficial outcome after a partial orchidectomy.6 Noting the radiation tolerance to the spinal cord to be ∼40 Gy,4 7 our patient probably received a radiation dose in excess by today’s standard.
Learning points.
In patients presenting with camptocormia it is important to examine their medical history for radiotherapy to the lumbosacral area.
Consider the long-term consequences when prescribing high-dose radiation therapy to the paraspinal region.
Irradiation induced neuropathy or myopathy may present many years or decades after exposure.
Acknowledgments
We would like to acknowledge Dr Victor Gordon for his assistance in interpretation of the SSEP and EMG and Dr Kenneth K Lau for constructing the legends for the MRI.
Footnotes
Contributors: LK was involved in the primary writing of the case report and organising of the research project. LDP-D participated in the organisation of the supplementary material and final collaboration of report. DT contributed in the editing/reviewing of the manuscript and YCL was the supervisor for project, editing/reviewing of the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Finsterer J, Strobl W. Presentation, etiology, diagnosis, and management of camptocormia. Eur Neurol 2010;2013:1–8 [DOI] [PubMed] [Google Scholar]
- 2.Psimaras D, Maisonobe T, Delanian S, et al. Late onset radiation-induced camptocormia. J Neurol 2011;2013:1723–5 [DOI] [PubMed] [Google Scholar]
- 3.Furby A, Behin A, Lefaucheur JP, et al. Late-onset cervicoscapular muscle atrophy and weakness after radiotherapy for Hodgkin disease: a case series. J Neurol Neurosurg Psychiatry 2010;2013:101–4 [DOI] [PubMed] [Google Scholar]
- 4.Bowen J, Gregory R, Squier M, et al. The post-irradiation lower motor neuron syndrome neuronopathy or radiculopathy? Brain 1996;2013(Pt 5):1429–39 [DOI] [PubMed] [Google Scholar]
- 5.Carver BS, Al-Ahmadie H, Sheinfeld J. Adult and pediatric testicular teratoma. Urol clin North Am 2007;2013:245–51 [DOI] [PubMed] [Google Scholar]
- 6.Giannarini G, Dieckmann KP, Albers P, et al. Organ-sparing surgery for adult testicular tumours: a systematic review of the literature. Eur Urol 2010;2013:780–90 [DOI] [PubMed] [Google Scholar]
- 7.Wara WM, Phillips TL, Sheline GE, et al. Radiation tolerance of the spinal cord. Cancer 1975;2013:1558–62 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient lying and sitting. Progressive forward flexion of the spine is demonstrated whilst walking.