Abstract
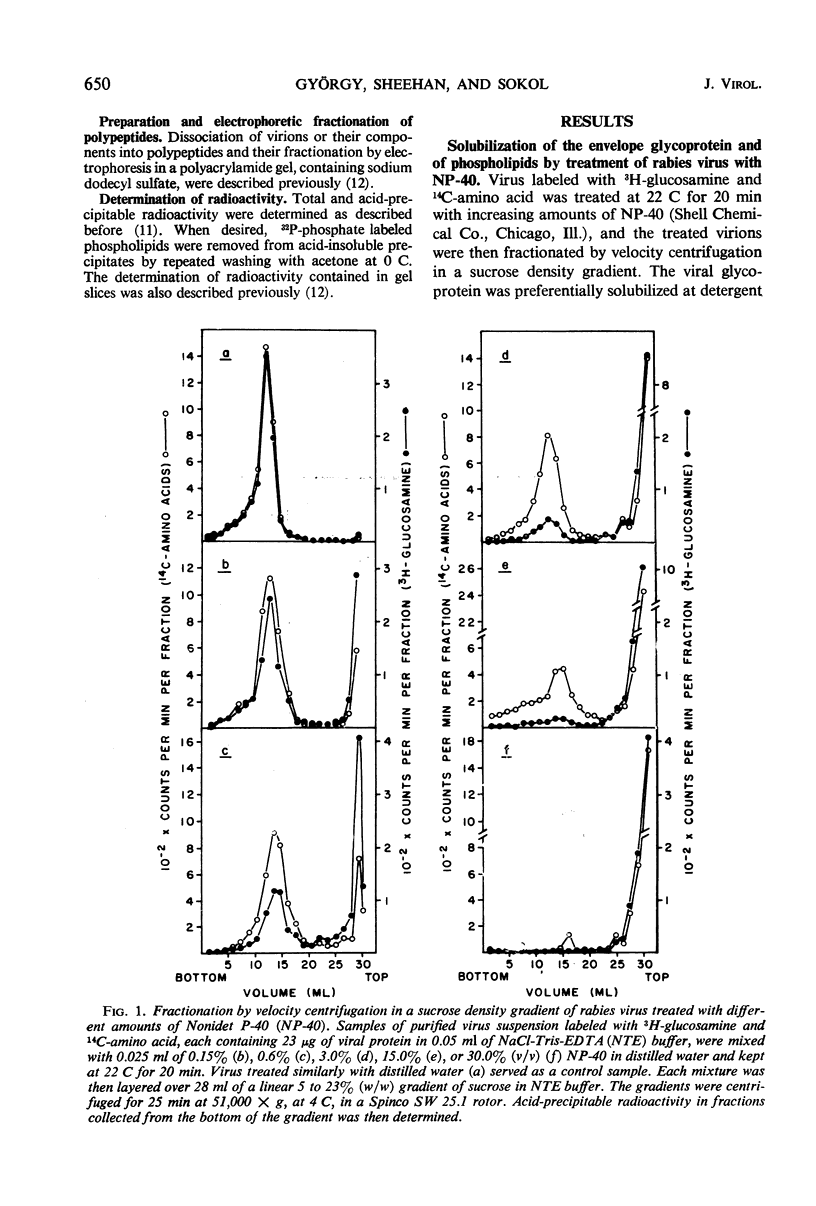
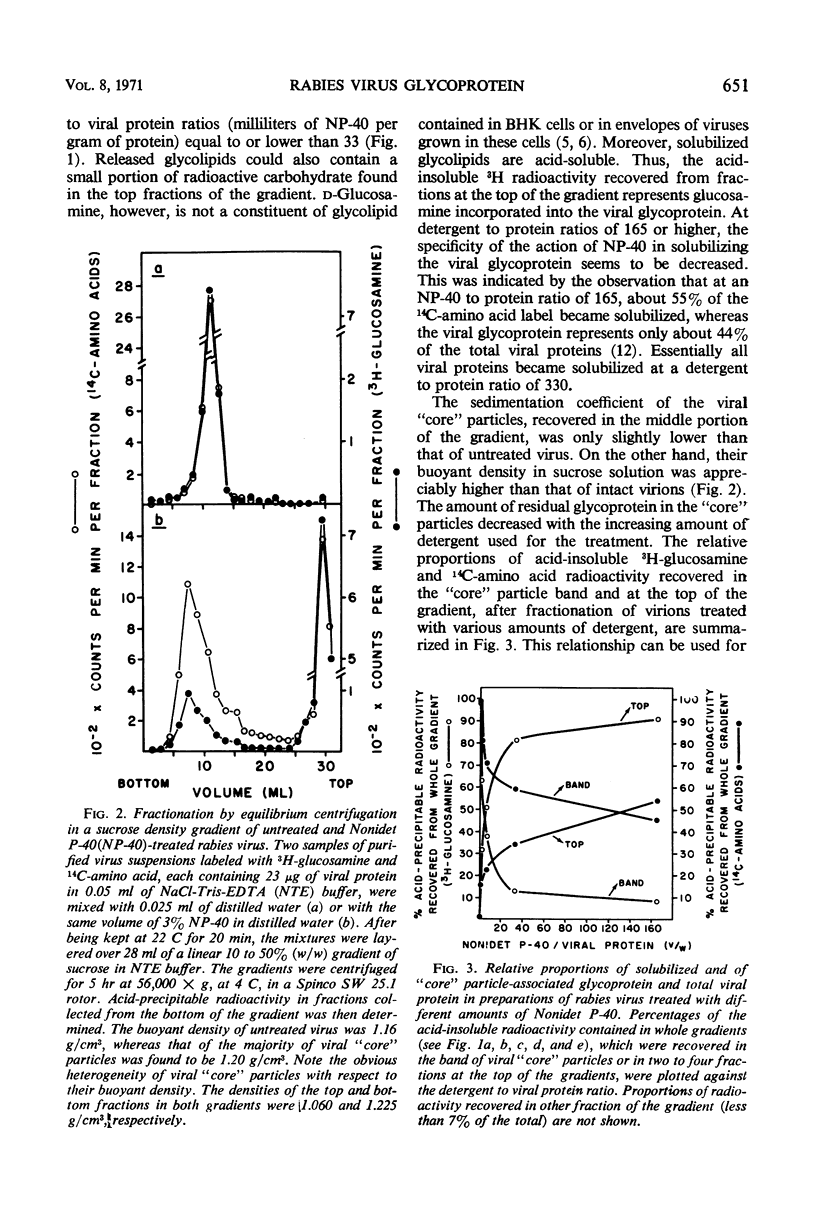
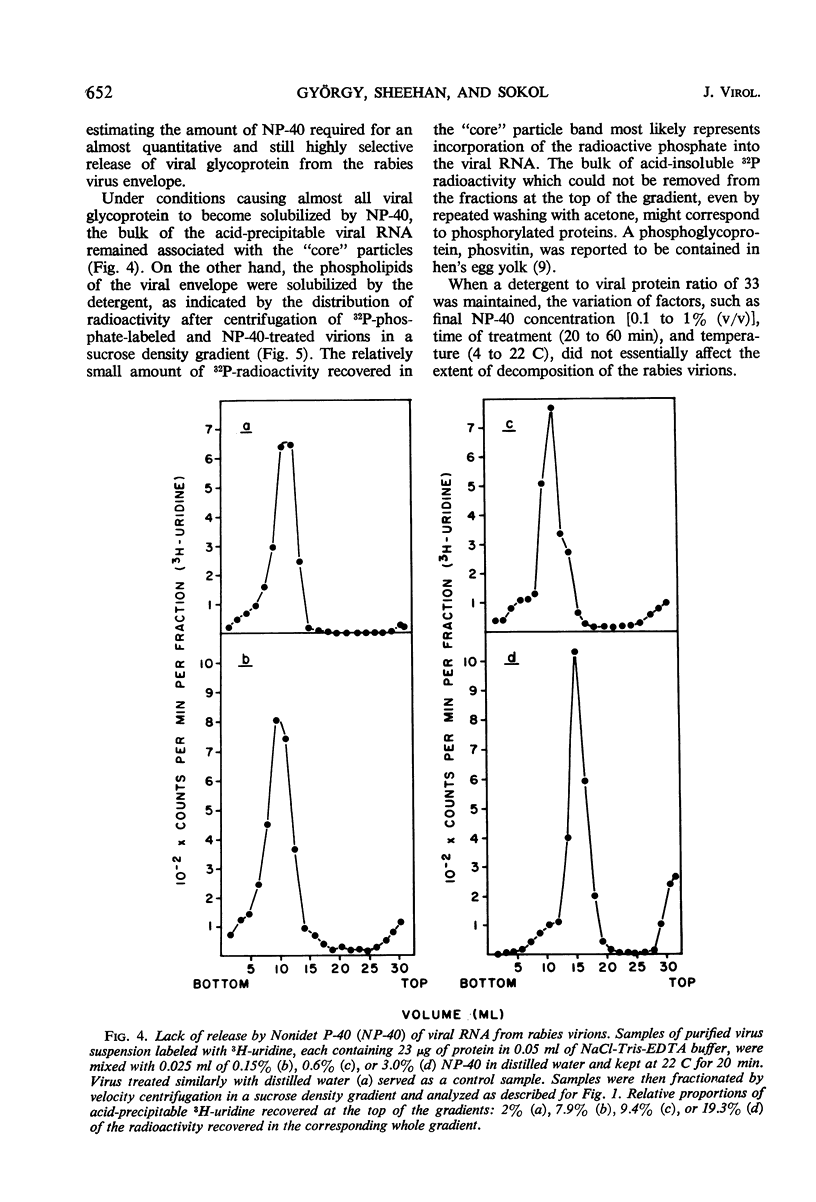
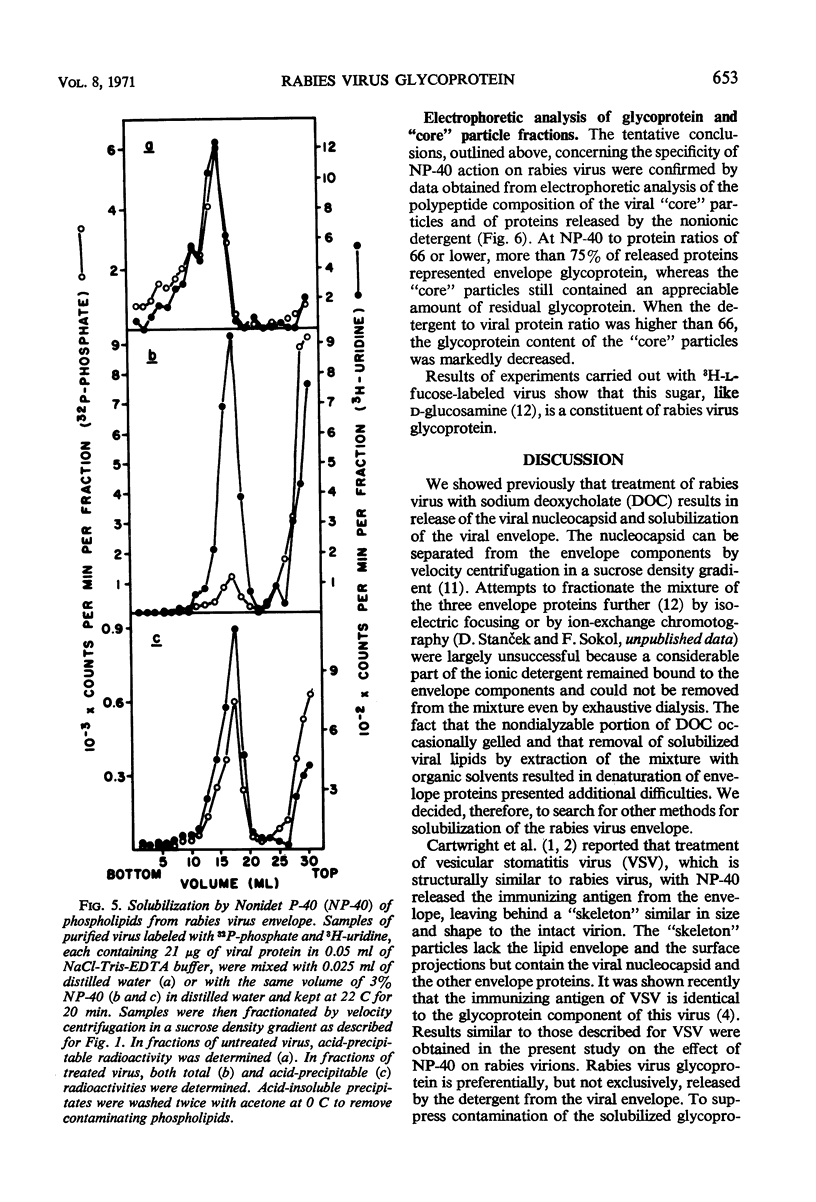
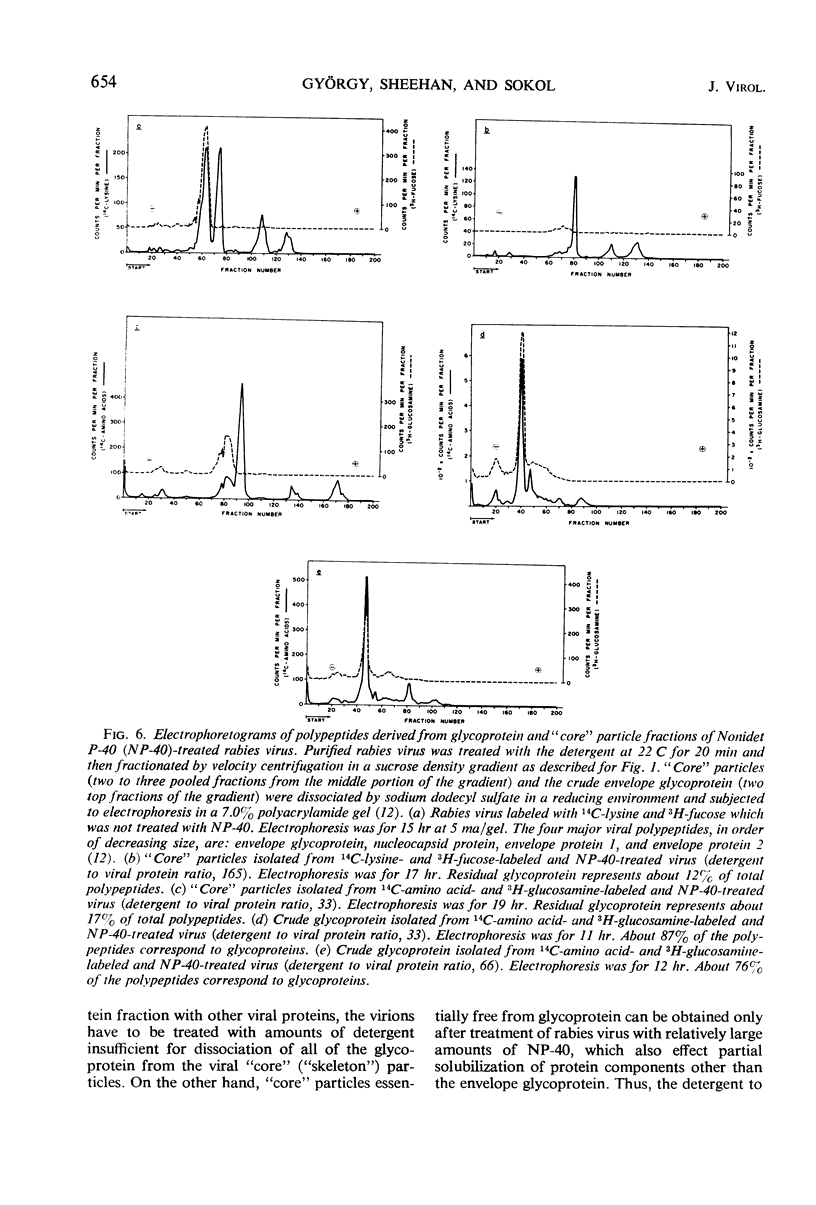
Treatment of rabies virus with the nonionic detergent Nonidet P-40 resulted in solubilization of viral lipids and in a preferential release of the envelope glycoprotein. The other viral proteins and the viral ribonucleic acid remained associated in “core” particles sedimenting at a rate similar to that of intact virions. After fractionation of treated virus by velocity centrifugation in a sucrose density gradient, the amount of residual glycoprotein recovered in the “core” particle fraction and the extent of contamination of the glycoprotein fraction by other viral components were dependent on the ratio of detergent to viral protein used.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cartwright B., Smale C. J., Brown F. Dissection of vesicular stomatitis virus into the infective ribonucleoprotein and immunizing components. J Gen Virol. 1970 Apr;7(1):19–32. doi: 10.1099/0022-1317-7-1-19. [DOI] [PubMed] [Google Scholar]
- Cartwright B., Talbot P., Brown F. The proteins of biologically active sub-units of vesicular stomatitis virus. J Gen Virol. 1970 Jun;7(3):267–272. doi: 10.1099/0022-1317-7-3-267. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. II. Immunological comparisons of viral antigens. J Virol. 1970 Jul;6(1):20–27. doi: 10.1128/jvi.6.1.20-27.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Glycolipid content of vesicular stomatitis virus grown in baby hamster kidney cells. J Virol. 1971 Mar;7(3):416–417. doi: 10.1128/jvi.7.3.416-417.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Glycosphingolipids of plasma membranes of cultured cells and an enveloped virus (SV5) grown in these cells. Proc Natl Acad Sci U S A. 1970 May;66(1):57–64. doi: 10.1073/pnas.66.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwert E., Wiktor T. J., Sokol F., Koprowski H. Hemagglutination by rabies virus. J Virol. 1968 Dec;2(12):1381–1392. doi: 10.1128/jvi.2.12.1381-1392.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Shainkin R., Perlmann G. E. Phosvitin, a phosphoglycoprotein. I. Isolation and characterization of a glycopeptide from phosvitin. J Biol Chem. 1971 Apr 10;246(7):2278–2284. [PubMed] [Google Scholar]
- Sokol F., Kuwert E., Wiktor T. J., Hummeler K., Koprowski H. Purification of rabies virus grown in tissue culture. J Virol. 1968 Aug;2(8):836–849. doi: 10.1128/jvi.2.8.836-849.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol F., Schlumberger H. D., Wiktor T. J., Koprowski H. Biochemical and biophysical studies on the nucleocapsid and on the RNA of rabies virus. Virology. 1969 Aug;38(4):651–665. doi: 10.1016/0042-6822(69)90184-6. [DOI] [PubMed] [Google Scholar]
- Sokol F., Stancek D., Koprowski H. Structural proteins of rabies virus. J Virol. 1971 Feb;7(2):241–249. doi: 10.1128/jvi.7.2.241-249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]



