Abstract
A 51-year-old woman with dermatomyositis (DM) on chronic immunosuppressive therapy was hospitalised for evaluation of haematuria. Surprisingly, abdominal imaging demonstrated pneumoperitoneum and pneumatosis intestinalis (PI). Her abdominal examination and white cell count were normal, but she subsequently developed nausea and fever. Owing to concern for perforation, a hemicolectomy was performed. Pathology revealed PI without inflammatory, ischaemic or neoplastic features, and she recovered uneventfully. Her immunosuppressive therapy was discontinued. Six months later, a follow-up CT of the abdomen revealed recurrence of PI. As she was asymptomatic, she was managed conservatively with resolution of PI on subsequent imaging. PI is characterised by the presence of gas within the wall of the intestine. Its aetiology is often unclear but this case highlights the association between PI and both immunosuppressive therapy and DM. A review of PI in patients with DM suggests that clinically stable patients may be observed, while avoiding surgical intervention.
Background
Pneumatosis intestinalis (PI), the presence of gas within the wall of the gastrointestinal tract, is a radiological and pathological finding and not necessarily a diagnosis in itself.1 Depending on the nature and severity of the underlying aetiology, PI's manifestations can range from asymptomatic to life threatening. Although underlying conditions such as infection, ischaemia, autoimmune diseases and use of immunosuppressants are linked to the development of PI, its aetiology is not always clearly established for a particular patient. Thorough assessment for factors that suggest a benign aetiology may help avoid over-aggressive management. In the setting of an autoimmune disease, a careful history including review of medications and past gastrointestinal manifestations, an examination for features of superimposed vasculitis or flare of autoimmune disease and close observation during a trial of conservative measures may guide management.
Case presentation
A 51-year-old woman was diagnosed with anti-Jo-1 negative dermatomyositis (DM) in 2006 based on clinical findings of a heliotrope rash and Gottron's sign dermatitis, lower and upper girdle weakness and elevation of creatine phosphokinase and aldolase. She was initially treated successfully with high-dose mycophenolate mofetil (MMF), hydroxychloroquine and prednisone. Her MMF was tapered in early 2008, but she unfortunately developed a flare of symptoms manifested by lower girdle weakness and dermatitis. Therefore, in May 2008, MMF was increased to 3 g daily in conjunction with an increase in her prednisone dose to 60 mg daily with improvement in dermatitis, but persistence of other symptoms. In August 2008, she developed a left leg deep venous thrombosis, thought in part to have resulted from weakness-related decreased mobility. Two weeks after being started on warfarin, she developed gross haematuria for which she was admitted to a community hospital affiliated with our academic centre. On admission, vital signs were notable only for blood pressure of 176/107 mm Hg; there was no evidence of clinical flare of DM, such as change in muscle strength or skin abnormality; the abdominal examination was normal.
Investigations
White cell count (WCC) was 11.7 K/mm3 with 74% neutrophils, which was unchanged when compared with her WCC 1 month prior to presentation. International normalised ratio was equal to 20.4, for which she received 3 units of fresh frozen plasma and vitamin K, with resolution of gross haematuria. Urinalysis was also notable for pyuria and bacteriuria. Abdominal X-ray revealed pneumoperitoneum (figure 1) and CT of the abdomen revealed colonic PI with pneumoperitoneum (figure 2).
Figure 1.
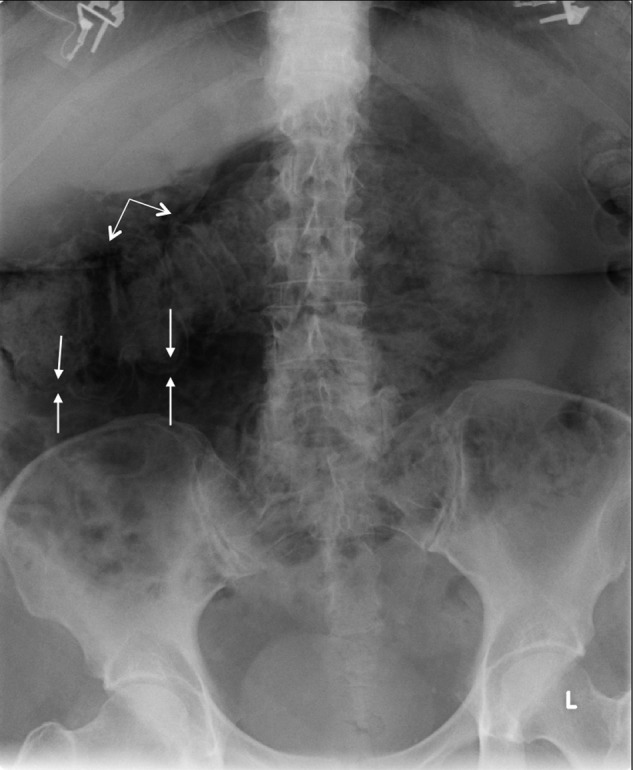
This supine abdominal X-ray shows Rigler's sign (double bowel sign) where gas outlines both sides of the transverse colon wall (pairs of arrows). The linear lucency in the subhepatic region (double-headed arrow) represents free air, which follows the liver contour in an inferolateral to superomedial orientation.
Figure 2.
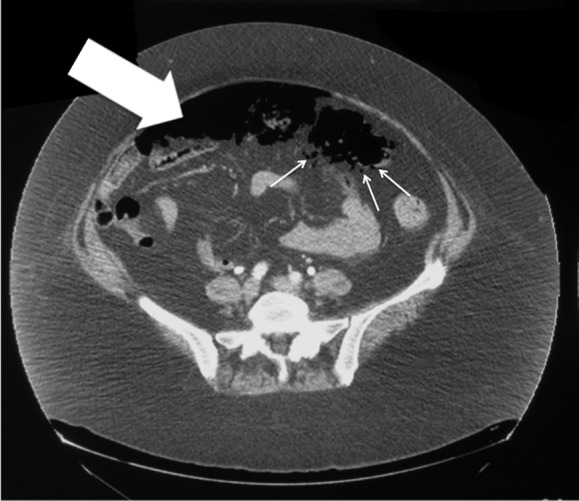
CT of the abdomen. Pneumatosis intestinalis (small arrows) and pneumoperitoneum (large arrow).
Differential diagnosis
The underlying differential diagnosis for this patient's PI included infection, autoimmune-mediated, medication-related, ischaemia, vasculitis or malignancy.
Treatment
The patient was started on empiric antibiotics for urinary tract infection on admission.
Approximately 24 h after admission, the patient developed nausea and fever and underwent an exploratory laparotomy, which did not reveal perforation, colitis or obstruction. Owing to the surgeon's concern for occult malignancy or microperforation, a right hemicolectomy was performed.
Outcome and follow-up
Pathology revealed PI without inflammatory, ischaemic, infectious or dysplastic features (figures 3 and 4). The patient recovered uneventfully. MMF was discontinued and prednisone was tapered over a period of months.
Figure 3.
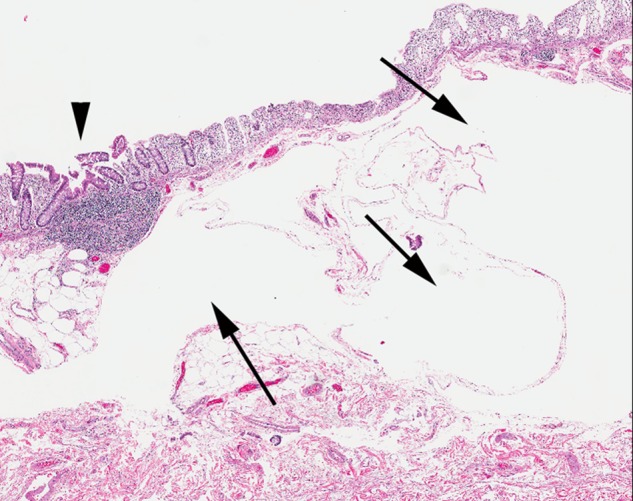
Colon histopathology. Submucosal cystic dilation typical of pneumatosis intestinalis is present (arrows). Lymphoid aggregate is normal in appearance (arrowhead) (×10, H&E).
Figure 4.
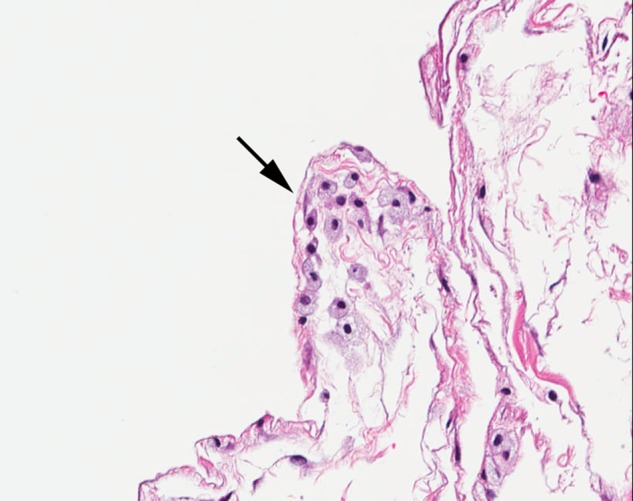
Colon histopathology. Cystic spaces within the submucosa and subserosa are focally lined by histiocytes, a classic feature of pneumatosis intestinalis (×200, H&E).
Six months later, a follow-up CT of the abdomen showed recurrence of pneumoperitoneum near the transverse colon. As the patient's vital signs were normal, abdominal examination unremarkable, and WCC was 5.6 K/mm3, the patient was managed conservatively. A CT of the abdomen several months later revealed resolution of pneumoperitoneum and no signs of PI.
Five years since this surgery, the patient has had stable disease, well-controlled on hydroxychloroquine monotherapy. Other than occasional loose stools as expected postcolectomy, she denied any gastrointestinal symptoms.
Discussion
To our knowledge, this is the second reported case of recurrent pneumoperitoneum and PI in a patient with DM.2 With the benefit of surgical pathology specimens, our patient's PI was found unrelated to infection, malignancy or vasculitis, and no frank perforation was present. This case raises an important question: should a patient with DM on immunosuppression who develops PI and pneumoperitoneum, but few other signs and symptoms, undergo operative management? The paucity of findings on laparotomy, the successful non-operative management of our patient's recurrent episode of pneumoperitoneum, and a review of the literature cast doubt on the decision to perform a laparotomy.3
PI is associated with many autoimmune disorders, most commonly systemic sclerosis, but is also reported in systemic lupus erythematosus, Sjogren's syndrome and the inflammatory myopathies, DM and polymyositis (PM).4 The aetiology of PI is often unclear though evidence exists for contribution from increased intraluminal pressure due to gastrointestinal dysmotility, overgrowth of anaerobic bacteria, retroperitoneal dissection of pulmonary gas and disruption of mucosal integrity.5 6 In an autoimmune disease, mucosal integrity may be compromised by various factors including vasculitis, ischaemic ulcers or peptic ulcer disease and immunosuppressive therapy.7 8 The inflammatory myopathies in adults are well recognised for their prominent upper gastrointestinal involvement including dysmotility affecting the pharynx and oesophagus, as well as delayed gastric emptying.9 Though lower gastrointestinal tract involvement, including vasculitis with or without PI, is more classically associated with juvenile DM,8 10 lower gastrointestinal tract dysmotility has been reported with adult inflammatory myopathies, primarily PM.11
There are nine cases of PI in adult DM12–18 and seven cases in adult PM19–25 reported in the literature. Of these, seven of the adult DM and all of the adult PM cases were managed non-operatively with resolution. Mortality was observed in only one patient who presented with abdominal pain and PI, and subsequently developed acutely worsening abdominal pain, high fever and shock. Laparotomy diagnosed a sigmoid perforation which was attributed to diverticulitis, and a sigmoid resection specimen did not reveal PI.18 Predisposing factors for PI inherent to adult DM and PM have been proposed but none have been clearly established.9 21 Based on our review of the literature, it is likely that PI in patients with inflammatory myopathies is multifactorial. All 16 previously reported cases of PI in patients with adult DM or PM were on glucocorticoid therapy, as was our patient. Glucocorticoid therapy is the most common drug associated with PI.1 Glucocorticoids are suggested to deplete lymphoid tissue within Peyer's patches with resulting mucosal disruption that enables gas diffusion into the bowel wall.14 Non-glucocorticoid immunosuppressants such as methotrexate and cyclosporine have also been suggested as possible triggers of PI in autoimmune disorders.6 Our unique case suggests that MMF may also contribute to PI in DM. In addition to compromised mucosa, increased intraluminal pressure due to dysmotility likely plays a role in the development of PI in inflammatory myopathies. Of the nine cases of PI in adult DM, three cases noted significant bowel dilatation or megacolon2 14 18 one of which required total parenteral nutrition for presumed intestinal obstruction.18 Similarly, of the cases of PI in adult PM, four of the seven cases involved ileus or significant bowel dilation.22–25 Though bowel dilation was not noted on imaging for our patient, disturbances of transit may occur without bowel dilation.26
When PI is an incidental radiological finding in adult DM or PM, its significance needs to be considered in the context of the patient's symptoms, physical examination findings, disease activity, immunosuppressive therapy and risk factors for underlying ischaemia, infection and malignancy. PI may have a better prognosis in DM and PM than in other autoimmune disorders.20 Though our findings suggest that PI in adult DM and PM can frequently be managed conservatively, surgical intervention must be considered in patients with progressive symptoms, physical examination findings consistent with peritonitis or radiographic findings of inflammation or neoplasia.
Learning points.
Though infrequent, pneumatosis intestinalis (PI) is seen in dermatomyositis (DM) and polymyositis (PM) and generally has a good prognosis.
In DM and PM, chronic immunosuppressive therapy and intestinal dysmotility are associated with PI.
PI with or without pneumoperitoneum in DM and PM frequently do not require surgical intervention once an acute intra-abdominal emergency has been excluded.
Footnotes
Contributors: The idea for the case report was conceived by JM and ECVR. YZ saw the patient in follow-up in JM's rheumatology clinic. YZ performed the literature search and wrote the article with feedback from JM and ECVR. ECVR chose the best radiological images. WT selected the best pathological images and aided in their interpretation. ECVR is the guarantor.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Heng Y, Schuffler MD, Haggitt RC, et al. Pneumatosis intestinalis: a review. Am J Gastroenterol 1995;2013:1747–58 [PubMed] [Google Scholar]
- 2.Morris-Stiff GJ, Williams RJ. Pneumatosis cystoides intestinalis in a patient with dermatomyositis. J R Soc Med 1999;2013:366–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsueh K-C, Tsou S-S, Tan K-T. Pneumatosis intestinalis and pneumoperitoneum on computed tomography: beware of non-therapeutic laparotomy. World J Gastrointest Surg 2011;2013:86–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dovrish Z, Arnson Y, Amital H, et al. Pneumatosis intestinalis presenting in autoimmune diseases. Ann N Y Acad Sci 2009;2013:199–202 [DOI] [PubMed] [Google Scholar]
- 5.Gagliardi G, Thompson IW, Hershman MJ, et al. Pneumatosis coli: a proposed pathogenesis based on study of 25 cases and review of the literature. Int J Colorectal Dis 1996;2013:111–8 [DOI] [PubMed] [Google Scholar]
- 6.St Peter SD, Abbas MA, Kelly KA. The spectrum of pneumatosis intestinalis. Arch Surg 2003;2013:68–75 [DOI] [PubMed] [Google Scholar]
- 7.Cabrera GE, Scopelitis E, Cuellar ML, et al. Pneumatosis cystoides intestinalis in systemic lupus erythematosus with intestinal vasculitis: treatment with high dose prednisone. Clin Rheumatol 1994;2013:312–16 [DOI] [PubMed] [Google Scholar]
- 8.Mamyrova G, Kleiner DE, James-Newton L, et al. Late-onset gastrointestinal pain in juvenile dermatomyositis as a manifestation of ischemic ulceration from chronic endarteropathy. Arthritis Rheum 2007;2013:881–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebert EC. Review article: the gastrointestinal complications of myositis. Aliment Pharmacol Ther 2010;2013:359–65 [DOI] [PubMed] [Google Scholar]
- 10.Fischer TJ, Cipel L, Stiehm ER. Pneumatosis intestinalis associated with fatal childhood dermatomyositis. Pediatrics 1978;2013:127–30 [PubMed] [Google Scholar]
- 11.Hughes AJ, Ferguson I, Rankin E, et al. Polymyositis as a cause of total gut failure. Ann Rheum Dis 2002;2013:305–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasquier E, Wattiaux MJ, Peigney N. First case of pneumatosis cystoides intestinalis in adult dermatomyositis. J Rheumatol 1993;2013:499–503 [PubMed] [Google Scholar]
- 13.Selva-O'Callaghan A, Martínez-Costa X, Solans-Laque R, et al. Refractory adult dermatomyositis with pneumatosis cystoides intestinalis treated with infliximab. Rheumatology (Oxford) 2004;2013:1196–7 [DOI] [PubMed] [Google Scholar]
- 14.Nosho K, Hatakeyama K, Hamamoto Y, et al. A case of pneumatosis cystoides intestinalis with dermatomyositis in which EUS was useful for the diagnosis. Int J Colorectal Dis 2005;2013:473–4 [DOI] [PubMed] [Google Scholar]
- 15.Saito M, Tanikawa A, Nakasute K, et al. Additive contribution of multiple factors in the development of pneumatosis intestinalis: a case report and review of the literature. Clin Rheumatol 2007;2013:601–3 [DOI] [PubMed] [Google Scholar]
- 16.Sbidian E, Pruvost C, Zagdanski AM, et al. [Pneumatosis cystoides intestinalis complicating paraneoplastic dermatomyositis]. Ann Dermatol Venereol 2008;2013:668–71 [DOI] [PubMed] [Google Scholar]
- 17.Xiao T, Xu HH, Wu J, et al. Case of pneumatosis cystoides intestinalis in adult dermatomyositis. J Dermatol 2008;2013:49–51 [DOI] [PubMed] [Google Scholar]
- 18.Sagara A, Kitagawa K, Furuichi K, et al. Three cases of pneumatosis intestinalis presenting in autoimmune diseases. Mod Rheumatol 2012;2013:610–15 [DOI] [PubMed] [Google Scholar]
- 19.Kuroda T, Ohfuchi Y, Hirose S, et al. Pneumatosis cystoides intestinalis in a patient with polymyositis. Clin Rheumatol 2001;2013:49–52 [DOI] [PubMed] [Google Scholar]
- 20.Wada Y, Murayama N, Hirose S, et al. A case of pneumatosis cystoides intestinalis in a patient with polymyositis and interstitial pneumonia. Mod Rheumatol 2004;2013:260–3 [DOI] [PubMed] [Google Scholar]
- 21.Lee SJ, Park JY, Kwon SA, et al. [A case of pneumatosis cystoids intestinalis with polymyositis]. Korean J Gastroenterol 2011;2013:249–52 [DOI] [PubMed] [Google Scholar]
- 22.Kim JY, Kwon YH, Lee SJ, et al. Pneumatosis cystoides intestinalis and partial abdominal obstruction in a patient with polymyositis. Intest Res 2011;2013:234–7 [Google Scholar]
- 23.Elkayam O, Caspi D, Flusser G. Pneumatosis intestinalis in a patient with polymyositis. Clin Exp Rheumatol 2001;2013:483. [PubMed] [Google Scholar]
- 24.Hanawa M, Toda H, Kobayashi H, et al. Interstitial pneumonia, intestinal pseudo-obstruction and pneumatosis cystoides intestinalis associated with polymyositis: report of a case. Stomach Intestine 1982;2013:1021–7 [Google Scholar]
- 25.Park YH, Kanoh T, Nishida O, et al. [A case of polymyositis with ileus-like symptoms and pneumatosis cystoides intestinalis, improved by the treatment of high-flow oxygen therapy.] Nihon Naika Gakkai Zasshi 1985;2013:808–12 [DOI] [PubMed] [Google Scholar]
- 26.Camilleri M, Hasler WL, Parkman HP, et al. Measurement of gastrointestinal motility in the GI laboratory. Gastroenterology 1998;2013:747–62 [DOI] [PubMed] [Google Scholar]


