Endoscopic Equipment: Does it Matter ?
Visualization of small mucosal irregularities is becoming increasingly difficult. In the advent of flat adenomas in the colon to subtle lesions in the esophagus and stomach that represent the earliest signs of malignancy, the need to image better has never been more apparent. The 20% miss rate of colonic polyps is undoubtedly related to the inability to view subtle mucosal irregularities. Fortunately, there are a number of technical improvements since the fiberoptic endoscopes that not only magnify the mucosa, but also enhance the resolution of the mucosa so that subtle lesions can be easily visualized.
Resolution is defined in imaging as the ability to resolve two small dots adjacent to each other. A high resolution endoscope could find differences between two dots that look like a single dot on standard videoendoscopy. It will be undoubtedly true that there will be even higher resolution videoendoscopes in the near future.
In the age of HDTV and it’s variants, it is important to understand what is meant by the term “resolution” and exactly what it means to endoscopy. Most of the time, high-resolution endoscopy is a term that is used to reference the pixel density of the CCD in the endoscope. The CCD is the actual silicon chip that captures light and converts this energy into a digital signal. It is important to recognize that this does not mean just because you are using a high-resolution endoscope you are getting a high-resolution image. The visualization will require a high-resolution processor and a video screen that can display high-resolution images. Standard resolution endoscopes were designed to mimic television standards with resolutions of 640 pixel width and 486 lines vertical (NTSC standard in United States, Japan)) versus a 576 lines vertical (PAL standard in Europe) with pixel densities of about 367,000. High-resolution endoscopes that have recently been developed are generally thought of as those with over a million pixels. Current video endoscopes approach this but most do not exceed a million pixels with color chips. The Pentax i-Flex endoscope has a 1.25 million pixel CCD which is what is needed to be able to display in High Definition (1280 × 1024 pixels). The current generation Olympus 180 series endoscope also has 1280X1024 resolution and can display in HD mode. However, this does not mean that the endoscopist will see this in HD since the outputs from the EPK-i (Pentax) processor or the Evis Exera II (Olympus) processor include standard video outputs including RGB, Y/C, and composite outputs (NTSC) as well as HD outputs.
There are differences with endoscopes that are most commonly used in the United States since they all use a so-called “color chip.” Each pixel of these colored chips actually are created from three separate dots, each of whom represents one of the primary colors – red, green, blue, which allows the chip to present a colored image. Because of this though, the color chips are generally larger than their monochromatic counterparts and have less resolution. The highest resolution endoscopes currently available are monochromatic with a color image being generated by a color wheel with red, green, and blue filters is placed in front of the chip. As the wheel turns, the particular filter in front of the chip is recorded and that image is saved and blended with two other images using the other two primary colors to create a composite color image. This type of endoscope has not been well received because of color streaming effects when there is water or rapid motion of the endoscope.
Endoscopic Evaluation of Barrett’s Esophagus
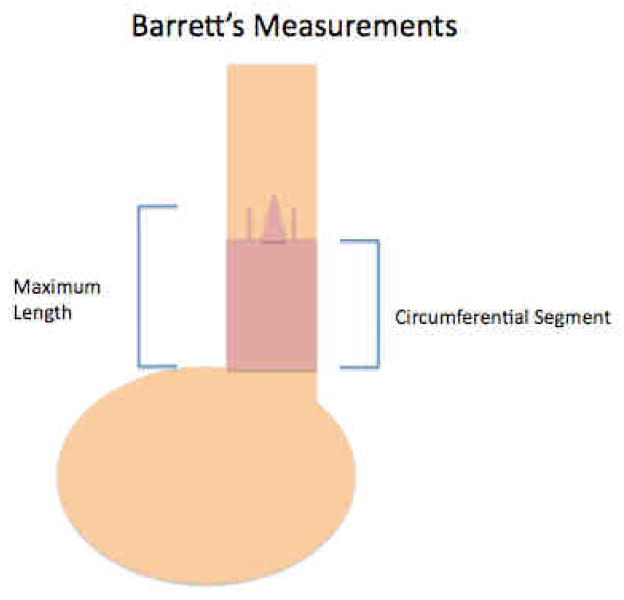
Barrett’s esophagus is an endoscopically defined condition so its description is critical to the diagnosis of any patient suspected of having the condition. Currently, it is the custom to denote the proximal extent of the Barrett’s esophagus as well as the maximal circumferential extent in terms of distances from these landmarks to the incisors (See Figure 1)
Figure 1.
This illustrates the current system of classifying the length of Barrett’s esophagus. The Maximum length and the circumferential segment length should be identified.
The Barrett’s segment is usually described as being salmon colored in appearance and must be distinct from the gastric mucosa which is also reddish in color. Since the gastroesophageal junction is difficult to define endoscopically, it is typically chosen to be at the level of the tops of the gastric folds. This can be seen in Figure 2 where the tops of the folds can be clearly demarcated.
Figure 2.
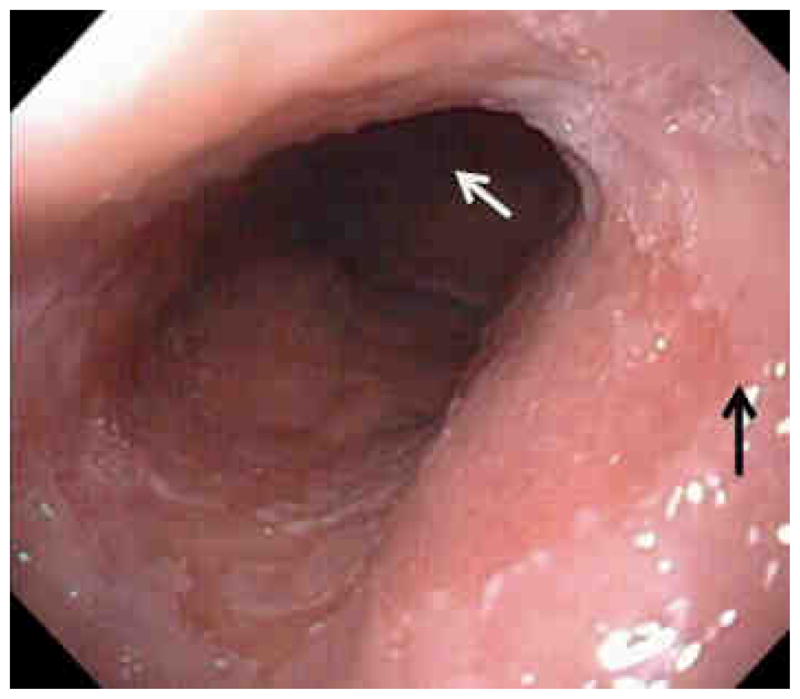
This is a typical Barrett’s esophagus segment with a proximal squamo-columnar junction at the black arrow and the distal segment at the white arrow at the tops of the gastric folds.
Once the segment length is documented, the next important step is to define if there are any mucosal abnormalities within the mucosal surface. Although there are many different techniques to enhance imaging, it is clear that what is most important is a careful white light examination as most abnormalities can be visualized with a standard endoscope. All mucosal abnormalities should be carefully biopsied. These include lesions such as ulcers, nodules, or just areas of mucosal irregularity (Figures 3 and 4).
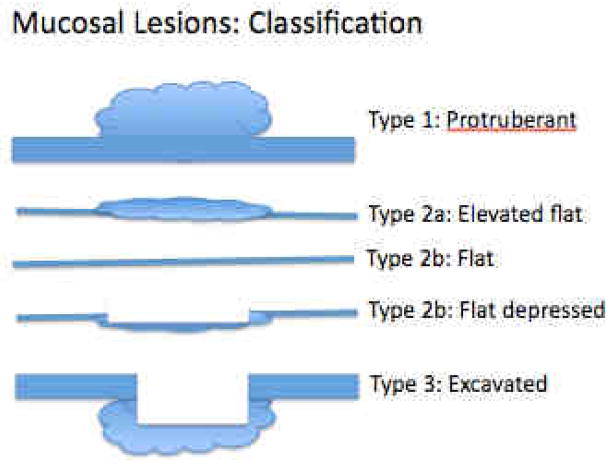
Figure 3.
Classification of lesions. The most common lesions found in Barrett’s esophagus are types 2a and 2b being slightly elevated or flat.
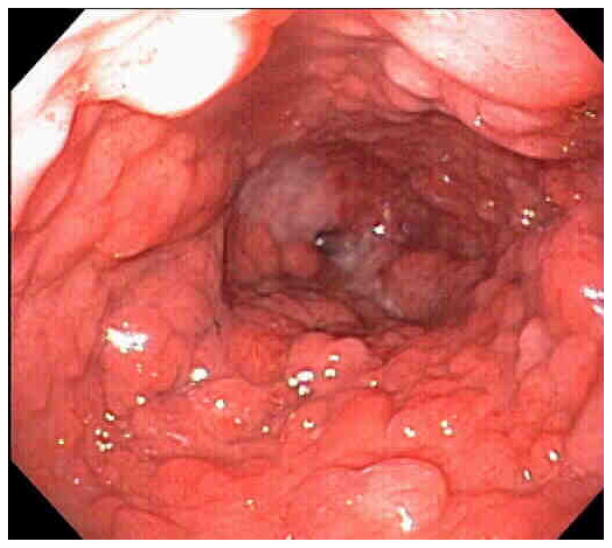
Figure 4.
This is an example of multiple Type 1 lesions that are protuberant into the lumen of the Barrett’s esophagus. This is an example of polypoid Barrett’s Esophagus. Each of these polypoid lesions were high grade dysplasia.
Mucosal abnormalities in Barrett’s esophagus must be carefully inspected. All mucosal irregularities could contain areas of dysplasia or even frank carcinoma. These abnormal areas, according to recent guidelines, should be individually targeted for histological sampling. In the setting of Barrett’s esophagus with highgrade dysplasia, these abnormalities should be mucosally resected as studies have shown that there may be as high as a 40% incidence of carcinoma occurring in these regions 1. Although white light examination is sufficient to visualize almost all abnormalities, there if often times information that can be obtained using other forms of imaging.
Chromoendoscopy
Chromoendoscopy has also been proposed since the time of fiberoptic endoscopes to enhance imaging. In the esophagus, this was primarily with iodine staining of the esophagus to detect areas of squamous neoplasia. In the case of Barrett’s esophagus, most of the effort has been spent on using either a contrast stain (indigo carmine) typically used with a magnification endoscope (45–200X) to allow visualization of mucosal patterns or an absorptive dye (methylene blue) whose increased uptake is suggestive of intestinal metaplasia. However, as the area becomes more dysplastic, methylene blue staining decreases (Figure 5).
Figure 5.
Image of the gastroesophageal junction with methylene blue staining. The areas of non-staining could be dysplastic tissue or inflammatory non-Barrett’s mucosa.
A recent meta-analysis was performed of 9 studies and 450 patients that reported on the yield of using methylene blue as a contrast agent for detection of dysplasia compared with random biopsies 2–10. The variation in the results from the studies was quite remarkable with both markedly positive and negative results. Ultimately, a meta-analysis found that the there was no significant difference between methylene blue and random biopsies in detection of dysplasia 11. In addition, studies have raised concerns that methylene blue could serve as a photosensitizing agent that might lead to DNA damage when used in Barrett’s esophagus 12. Other agents have been used for chromoendoscopy but there is not sufficient data to draw conclusions regarding their performance.
Narrow Band Imaging
Narrow band imaging (NBI) is a term applied to a specific commercial imaging technique (Olympus America, Center Valley, Pennsylvania). It involves the use of two different wavelengths of filtered light 415 and 540 nm for illumination. This is what gives the light a bluish appearance when NBI is activated. The blue illumination at 414 nm allows the imaging of the surface capillaries while the green 540 nm illumination allows visualization of vessels at a great depth (Figure 6).
Figure 6.
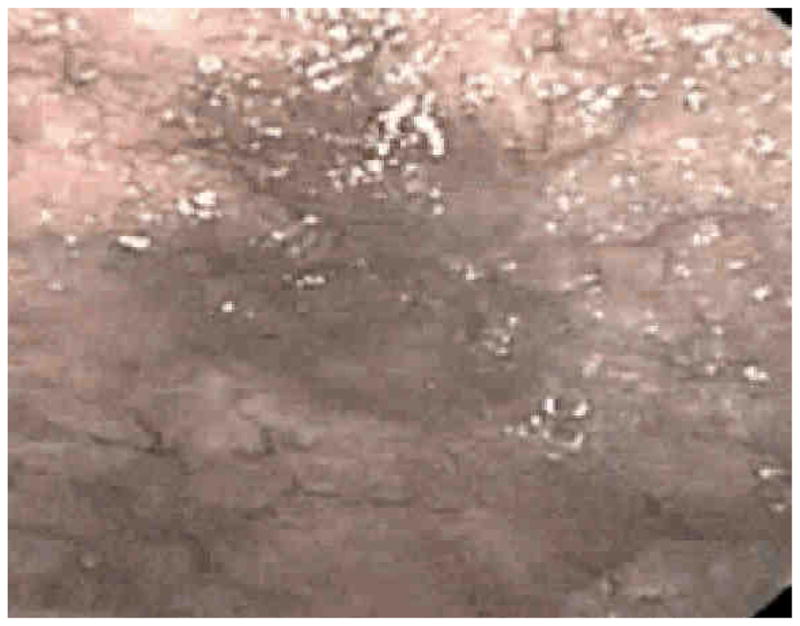
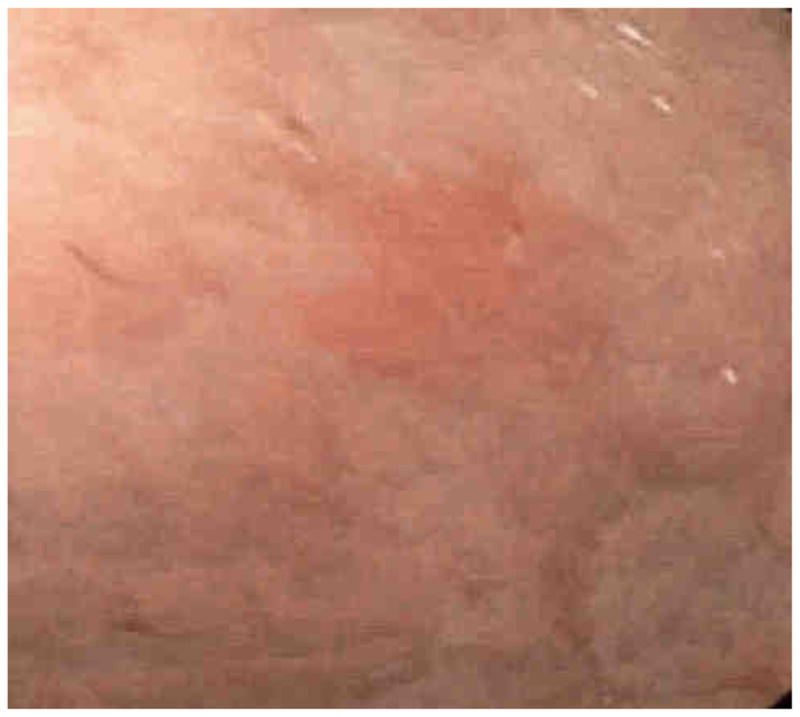
These are images of NBI of a erythematous patch seen below and then under NBI above. The borders are clearer and mucosal detail is easier to appreciate.
Multiple studies have been published looking at the values of NBI in Barrett’s esophagus. In particular, criteria have been established to examine vascular as well as mucosal abnormalities 13–21. These studies are summarized in Table I below.
Table 1.
Studies of NBI values in Barrett’s Esophagus.
| Authors Year, reference | Mean age, years | Patients, n | Totl lesions examined | Study Design | Endoscope type |
|---|---|---|---|---|---|
| Kara et al. 2006 | 65 | 63 | 161 | Cross-sectional Post hoc image evaluation |
GIF Q240Z |
| Kara et al. 2006 | 66 | 20 | 47 | Cross-sectional Real-time evaluation |
GIF Q240Z |
| Sharma et al. 2007 | 64 | 51 | 204 | Cross-sectional Image evaluation |
GIF Q240Z |
| Curvers et al. 2008 | 67 | 84 | 165 | Cross-sectional Real-time evaluation |
GIF Q240Z |
| Singh et al. 2008 | 61.9 | 109 | 1021 | Cross-sectional Post hoc image evaluation |
GIF Q240Z |
| Goda et al. 2007 | 60 | 58 | 217 | Cross-sectional Real-time evaluation |
GIF Q240Z |
| Anagnostopoulos et al. 2007 | 62.1 | 50 | 344 | Cross-sectional Real-time evaluation |
GIF Q240Z |
| Kara et al. 2005 | 66 | 28 | 36 | RCT, Real-time evaluation | GIF Q260Z |
If one does a meta-analysis on this data, we find that there is a significant advantage in using NBI particularly in detecting areas of high-grade dysplasia. A pooled sensitivity for the detection of high grade dysplasia on a per patient basis is 0.88 with a 95 percent confidence interval of 79%–93%. It is important to point out that this advantage has been established with a high resolution endoscope that is not commercially available in the United States. The GIF Q240Z and Q260Z are high resolution endoscopes primarily used in Asia and Europe and are not approved by the FDA. There is some data to suggest that the GIF 180 series endoscope that is used in the United is of benefit with NBI but this has only been found in a one study that compared this to a low resolution endoscope 22. This does it more difficult to discern whether this improvement in dysplasia detection is due to increased resolution or NBI. In addition, these studies have been done in very enriched populations with high proportions of patients with high-grade dysplasia. The pooled specificity is probably not very relevant to most practices because of this skewed population.
There are other modalities that can enhance the appearance of blood vessels although these rely post-processing techniques. The FICE system from Fujinon (Wayne, New Jersy) uses a white light source and processes the color obtained through spectral analysis which is obtained from a CCD and a prism mounted on the tip of the endoscope. The light from the endoscope always appears white unlike the blue-green light seen from the Olympus system. The FICE system is selectable for different enhancement modes that can be optimized for a specific function. There are only small series using FICE for detection of Barrett’s esophagus 23. The other system is I-Scan from Pentax (Montvale, New Jersey) which offers three levels of post-processing enhancements to emphasize the contrast, surface, and tone in addition to standard white light examination. These enhancements use the high definition image to emphasize features such as color, or the contrast, or the short wavelengths of light reflected from the surface. These enhancements have been used to increase visualization of mucosal breaks in the esophagus 24.
Magnification Techniques
Several magnification techniques have been developed to enhance the imaging in Barrett’s esophagus. It should be recognized that standard endoscopes have magnified the image from the esophagus by several fold depending on the size of the viewing monitor. This has made it much easier to appreciate mucosal detail. A variation on the standard endoscope is the endocytoscope that has can be placed on a standard endoscope processor and simply has a very powerful magnifying lens that focuses on the CCD. The endocytoscope is designed to fit through a therapeutic endoscope (outside diameter of 3.4 mm) to allow magnification of suspicious areas. The degree of magnification achieved can either be 450 X or 1100 X depending on the instrument used. In order to visualize the mucosa, a topical contrast agent such as indigo carmine or methylene blue must be applied since high magnification without contrast agents does not allow any imaging. These contrast agents allow for visualization of glandular strucures (Figure 7).
Figure 7.
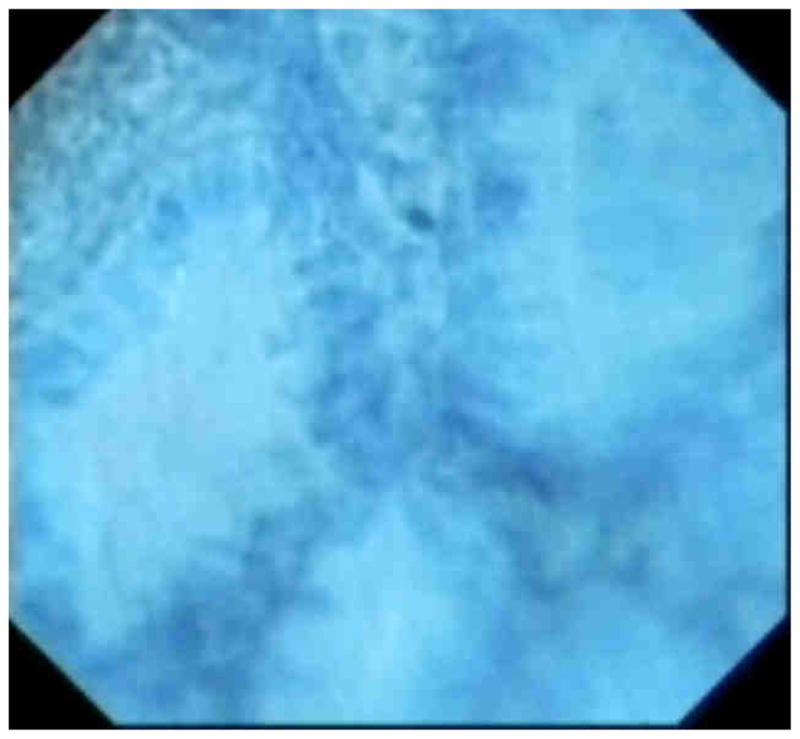
Endocytosocopy of Barrett’s crypts on endoscopy with methylene blue contrast. The crypts are regular in appearance.
Endocytoscopy has been used to define areas of dysplasia within Barrett’s esophagus but initial results have not been promising primarily due to difficulties with maintaining a stable image 25. Due to the high magnification, any motion distorts the image such as that caused by the patient’s heartbeat and esophageal motility. Nonetheless, this entity can be useful in determining areas of cancer and dysplasia in controlled situations 26.
Magnification endoscopes which have capability to zoom up to 200 fold. There is a trade-off with these magnifying endoscopes, generally, the higher the magnification the shorter the focal length of imaging meaning that the scopes become impractical to use at anything but a targeted lesion. In addition, the endoscopes tend to be of larger diameter which makes them less tolerable in the upper gastrointestinal tract. Magnification endoscopy has been used in conjunction with chromoendoscopy for over a decade in Barrett’s esophagus 27–35. As with most imaging modalities, there is a problem with inter-observer agreement (k<0.4) since there is difficulty in identifying mucosa types that are glandular in nature such as between Barrett’s esophagus and gastric cardia type tissue 36. In addition, there is a suggestion that in prospective studies, magnification endoscopy does not enhance detection of intestinal metaplasia 37.
Confocal Laser Endomicroscopy and Optical Coherence Tomography
Confocal laser endomicroscopy (CLE) and optical coherence tomography (OCT) have at their roots similar principles. Both are able to focus laser light to a specific plane deep to the surface and to magnify the appearance of microscopic structure approximately 400X. Both use light interference as the basis for their effect. In CLE, this focusing is done using a pinhole that focuses the laser light entering the pinhole to light from a specific depth. With optical coherence tomography, there is a reference light path that is used to create interference patterns with the reflected light from the surface. Adjustments in the reference path length have the effect of increase penetration into the mucosa. OCT yields an image similar to B-mode ultrasound with good resolution of layer structure while CLE offers more of a tangential imaging of the surface glandular structure. Both of these techniques allow better imaging with lateral resolution potential of about 10 micrometers 38. By giving an intravenous injection of 10% sodium fluorescein, the mucosa can be seen with CLE after 2–3 minutes. This contrast enables the confocal laser microscope to view the intravascular space where there are leaks in the capillary membranes, and actually see areas of extravasation of dye into the extravascular space. However, this type of imaging does not allow direct visualization of cells and their structure, but only the shadow of the cells that is cast because one is able to image the surrounding parenchyma because of the vascular structures present. Overall, when a disorganized pattern is seen to both the capillaries and the mucosal structure, there is reasonable certainty that there is dysplasia present (Figure 8).
Figure 8.
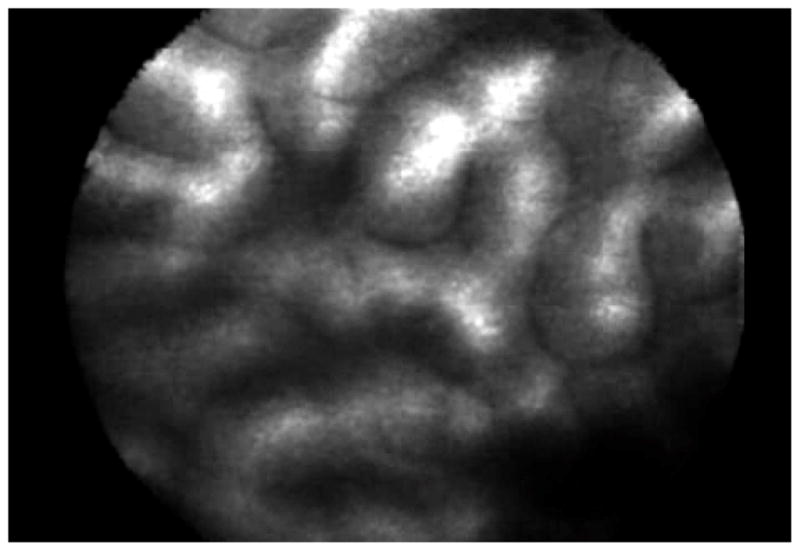
Confocal laser images. The first is of irregular glandular structure in Barrett’s esophagus with high-grade dysplasia while the bottom is of benign typical gastric mucosal glands.
The reported sensitivity of an endoscope based system of CLE is 92.9% and a specificity of 98.4% for detection of neoplasia in Barrett’s esophagus in a cohort study of 63 patients 39. This system takes advantage of the confocal principles by having a zoom feature that can focus from 0–250 microns from the surface which allows the user to scan “down” the length of a gland. Since the LCE obtains views parallel to the surface rather than the perpendicular as achieved with standard histology, this zoom may be important in trying to differentiate dysplastic lesions. The resolution of this system is also superior to the probe system (0.7 microns versus 1 micron for the probe systems) and has a wider field of view of (500 microns versus 240 microns). However, the endoscope system is integrated into an endoscope with a diameter of 12.8 mm and the confocal system acquires images continuously at 0.8 second intervals which leads to a large number of unusuable images.
The probe system has also been reported to be about 75% sensitive and 90% specific for detection of dysplasia in a small cohort study (n=38) with two endoscopists 40. This probe system is more convenient to apply since the site that is being optically investigated can be visualized with the endoscope. With the integrated endoscope system, the site being imaged is out of view during the imaging and must be estimated from the distance from a suction mark deliberately made by the endoscopist. Other advantages of the dedicated endoscope is reusability while the probe can only be used 20 times with some degradation of the imaging during re-use. The likelihood that these systems may decrease biopsies is suggested by a recent randomized controlled study with the dedicated endoscope system that indicated that with the system, much more dysplasia can be detected and being able to exclude dysplasia in other patients 41. These are promising devices that may supplant or supplement histology in the near future. However, implementation into clinical practice will be dependent on demonstration that these tools will actually improve patient outcomes in patients with dysplasia or cancer.
Spectroscopy Based Diagnostic Tools
These include devices that can analyze light that is typically scattered or undergo fluorescence from the mucosal surface. Spectroscopy does not actually give an image; it gives a quantitative assessment of the light that is reflected from the mucosal surface. This assessment can be made on the intensity of the light that is captured or by the amount of light that is detected over a period of time. Both of these methods can give very specific information of the tissue type. By using careful analysis of the spectroscopic patterns, an estimate can be made regarding the optical characteristics of the mucosa. This can include such estimates of nuclear size, crowding, degree of vascularity, as well as the organization of the intestinal glands. These technologies would incorporate the interpretation of the information into the device rather than require image interpretation by a physician. These are all “point” technologies that cannot examine an entire area but only potentially suspicious region.
One of the first spectroscopic technologies was laser induced fluorescence which can measure fluorescence from naturally occurring fluorophores such as FADH, NAD, tryptophan and collagen. Porphyrins that are blood breakdown products also form a large portion of the fluorescent peak. By analysis of the degree of autofluorescence, the determination of neoplasia can be made. Generally areas with decreased autofluorescence have an increased propensity toward neoplasia. Below are excitation-emission matrices of areas of cell cultures of keratinocytes and squamous carcinoma cells. It is clear that even in vivo, there are distinct differences in the autofluorescence pattern between benign and malignant cell lines (Figure 9). Laser induced fluorescence has been found to detect dysplasia in Barrett’s esophagus and has actually been approved by the FDA for use in colon polyps for adenoma detection 42, 43. However, this technology has not been commercialized at this point.
Figure 9.
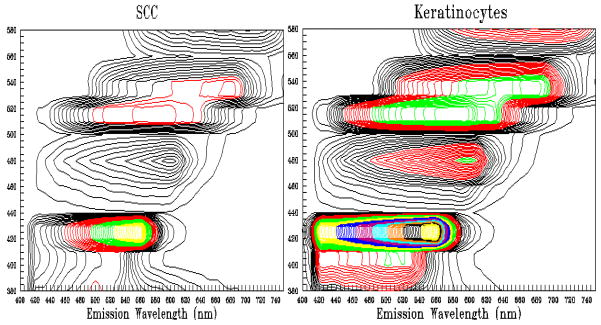
These are two autofluorescence spectroscopic images of squamous carcinoma cells in vitro and keratinocytes. They were examined using a multitude of excitation laser wavelengths and plotted against the fluorescence seen. The colors of the lines indicate the intensity of the light. As can be seen, normal keratinocytes have much greater intrinsic fluorescence than cancer cell lines which is what is typically found.
Diffuse reflectance spectroscopy examines light scattering from the surface of the mucosa has also been found to differentiate normal from neoplastic tissues throughout the gastrointestinal tract 44, 45. This technology is based on the increased scattering of light from cells that are less organized and have greater sized nucelei. Though promising, there have been refinements on examination of this light spectra with the most notable being low coherence enhanced back scattering which has been used to focus on short light path scattering phenomenon which allows the detection of nanoscale changes in cells 46. This low-coherence enhanced coherent back scattering uses nonlinear optics in its derivations which means that the degree of resolution is not limited by the wavelength of light. This theoretically can resolve nanoscale-type changes in the mucosa 47. These changes in tissue characteristics has been applied to the duodenum to detect field effects of pancreatic cancer and to the distal colon for the field effects of more proximal colon neoplasia 48, 49. This would allow identification of patients at risk of carcinoma simply by applying a probe to a more conveniently available structure. For instance, assessment of the buccal mucosa could be used to determine whether or not there is neoplastic risk in the lungs or in the esophagus. Though this is very intriguing, the need for large scale clinical trials are clear to demonstrate the ability of these technologies to function in the clinical situation.
Summary
Enhanced visualization techniques are available for Barrett’s esophagus and have promise in the detection of dysplasia and cancer. Several of these techniques such as narrow band imaging and chromoendoscopy are clinically being applied. These techniques will allow the endoscopist to screen the surface of the Barrett’s esophagus to detect areas of neoplasia. Once detected, it is hoped that either magnification techniques such as confocal laser endomicroscopy or spectroscopic techniques can be of value to allow in vivo real time diagnostic capabilities.
Acknowledgments
The author wishes to acknowledge the support of the National Cancer Institute R01CA097048, R01CA111603, and R21CA122426 and the Mayo Foundation.
Footnotes
Conflicts of Interest: Research funding from Olympus Corporation, SpectraScience, Consulting with OncoScope
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Nijhawan PK, Wang KK. Endoscopic mucosal resection for lesions with endoscopic features suggestive of malignancy and high-grade dysplasia within Barrett’s esophagus comment. Gastrointestinal Endoscopy. 2000;52:328–32. doi: 10.1067/mge.2000.105777. [DOI] [PubMed] [Google Scholar]
- 2.Canto MI, Setrakian S, Willis J, Chak A, Petras R, Powe NR, Sivak MV., Jr Methylene blue-directed biopsies improve detection of intestinal metaplasia and dysplasia in Barrett’s esophagus. Gastrointestinal Endoscopy. 2000;51:560–8. doi: 10.1016/s0016-5107(00)70290-2. [DOI] [PubMed] [Google Scholar]
- 3.Canto MI, Setrakian S, Willis JE, Chak A, Petras RE, Sivak MV. Methylene blue staining of dysplastic and nondysplastic Barrett’s esophagus: an in vivo and ex vivo study. Endoscopy. 2001;33:391–400. doi: 10.1055/s-2001-14427. [DOI] [PubMed] [Google Scholar]
- 4.Sharma P, Topalovski M, Mayo MS, Weston AP. Methylene blue chromoendoscopy for detection of short-segment Barrett’s esophagus. Gastrointest Endosc. 2001;54:289–93. doi: 10.1067/mge.2001.115728. [DOI] [PubMed] [Google Scholar]
- 5.Wo JM, Ray MB, Mayfield-Stokes S, Al-Sabbagh G, Gebrail F, Slone SP, Wilson MA. Comparison of methylene blue-directed biopsies and conventional biopsies in the detection of intestinal metaplasia and dysplasia in Barrett’s esophagus: a preliminary study. Gastrointest Endosc. 2001;54:294–301. doi: 10.1067/mge.2001.115732. [DOI] [PubMed] [Google Scholar]
- 6.Gossner L, Pech O, May A, Vieth M, Stolte M, Ell C. Comparison of methylene blue-directed biopsies and four-quadrant biopsies in the detection of highgrade intraepithelial neoplasia and early cancer in Barrett’s oesophagus. Digestive & Liver Disease. 2006;38:724–9. doi: 10.1016/j.dld.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 7.Lim CH, Rotimi O, Dexter SP, Axon AT. Randomized crossover study that used methylene blue or random 4-quadrant biopsy for the diagnosis of dysplasia in Barrett’s esophagus [see comment] Gastrointestinal Endoscopy. 2006;64:195–9. doi: 10.1016/j.gie.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 8.Lim CH, Rotimi O, Dexter SPL, Axon ATR. Randomized crossover study that used methylene blue or random 4-quadrant biopsy for the diagnosis of dysplasia in Barrett’s esophagus [see comment] Gastrointestinal Endoscopy. 2006;64:195–9. doi: 10.1016/j.gie.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 9.Horwhat JD, Maydonovitch CL, Ramos F, Colina R, Gaertner E, Lee H, Wong RKH. A randomized comparison of methylene blue-directed biopsy versus conventional four-quadrant biopsy for the detection of intestinal metaplasia and dysplasia in patients with long-segment Barrett’s esophagus. American Journal of Gastroenterology. 2008;103:546–54. doi: 10.1111/j.1572-0241.2007.01601.x. [DOI] [PubMed] [Google Scholar]
- 10.Ormeci N, Savas B, Coban S, Palabiyikoglu M, Ensari A, Kuzu I, Kursun N. The usefulness of chromoendoscopy with methylene blue in Barrett’s metaplasia and early esophageal carcinoma. Surgical Endoscopy. 2008;22:693–700. doi: 10.1007/s00464-007-9463-x. [DOI] [PubMed] [Google Scholar]
- 11.Ngamruengphong S, Sharma VK, Das A. Diagnostic yield of methylene blue chromoendoscopy for detecting specialized intestinal metaplasia and dysplasia in Barrett’s esophagus: a meta-analysis. Gastrointestinal Endoscopy. 2009;69:1021–8. doi: 10.1016/j.gie.2008.06.056. [DOI] [PubMed] [Google Scholar]
- 12.Olliver JR, Wild CP, Sahay P, Dexter S, Hardie LJ. Chromoendoscopy with methylene blue and associated DNA damage in Barrett’s oesophagus. Lancet. 2003;362:373–4. doi: 10.1016/s0140-6736(03)14026-3. [DOI] [PubMed] [Google Scholar]
- 13.Singh R, Karageorgiou H, Owen V, Garsed K, Fortun PJ, Fogden E, Subramaniam V, Shonde A, Kaye P, Hawkey CJ, Ragunath K. Comparison of high-resolution magnification narrow-band imaging and white-light endoscopy in the prediction of histology in Barrett’s oesophagus. Scandinavian Journal of Gastroenterology. 2009;44:85–92. doi: 10.1080/00365520802400818. [DOI] [PubMed] [Google Scholar]
- 14.Kara MA, Peters FP, Rosmolen WD, Krishnadath KK, ten Kate FJ, Fockens P, Bergman JJGH. High-resolution endoscopy plus chromoendoscopy or narrow-band imaging in Barrett’s esophagus: a prospective randomized crossover study. Endoscopy. 2005;37:929–36. doi: 10.1055/s-2005-870433. [DOI] [PubMed] [Google Scholar]
- 15.Kara MA, Bergman JJ, Kara MA, Bergman JJ. Autofluorescence imaging and narrow-band imaging for the detection of early neoplasia in patients with Barrett’s esophagus. Endoscopy. 2006;38:627–31. doi: 10.1055/s-2006-925385. [DOI] [PubMed] [Google Scholar]
- 16.Kara MA, Ennahachi M, Fockens P, ten Kate FJW Bergman JJGHM. Detection and classification of the mucosal and vascular patterns (mucosal morphology) in Barrett’s esophagus by using narrow band imaging. Gastrointestinal Endoscopy. 2006;64:155–66. doi: 10.1016/j.gie.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 17.Kara MA, Peters FP, Fockens P, ten Kate FJW Bergman JJGHM. Endoscopic video-autofluorescence imaging followed by narrow band imaging for detecting early neoplasia in Barrett’s esophagus [see comment] Gastrointestinal Endoscopy. 2006;64:176–85. doi: 10.1016/j.gie.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 18.Curvers W, Baak L, Kiesslich R, Van Oijen A, Rabenstein T, Ragunath K, Rey JF, Scholten P, Seitz U, Ten Kate F, Fockens P, Bergman J. Chromoendoscopy and narrow-band imaging compared with high-resolution magnification endoscopy in Barrett’s esophagus [see comment] Gastroenterology. 2008;134:670–9. doi: 10.1053/j.gastro.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Sharma P, Marcon N, Wani S, Bansal A, Mathur S, Sampliner R, Lightdale C. Non-biopsy detection of intestinal metaplasia and dysplasia in Barrett’s esophagus: a prospective multicenter study. Endoscopy. 2006;38:1206–12. doi: 10.1055/s-2006-944974. [DOI] [PubMed] [Google Scholar]
- 20.Goda K, Tajiri H, Ikegami M, Urashima M, Nakayoshi T, Kaise M, Goda K-i, Tajiri H, Ikegami M, Urashima M, Nakayoshi T, Kaise M. Usefulness of magnifying endoscopy with narrow band imaging for the detection of specialized intestinal metaplasia in columnar-lined esophagus and Barrett’s adenocarcinoma [see comment] Gastrointestinal Endoscopy. 2007;65:36–46. doi: 10.1016/j.gie.2006.03.938. [DOI] [PubMed] [Google Scholar]
- 21.Anagnostopoulos GK, Yao K, Kaye P, Hawkey CJ, Ragunath K. Magnification endoscopy with Narrow Band Imaging in Barrett’s esophagus. Journal of Clinical Gastroenterology. 2006;40:S192–3. [Google Scholar]
- 22.Wolfsen HC, Crook JE, Krishna M, Achem SR, Devault KR, Bouras EP, Loeb DS, Stark ME, Woodward TA, Hemminger LL, Cayer FK, Wallace MB. Prospective, controlled tandem endoscopy study of narrow band imaging for dysplasia detection in Barrett’s Esophagus [see comment] Gastroenterology. 2008;135:24–31. doi: 10.1053/j.gastro.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Osawa H, Yamamoto H, Yamada N, Yoshizawa M, Sunada K, Kita H, Ajibe H, Satoh K, Sugano K. Diagnosis of endoscopic Barrett’s esophagus by transnasal flexible spectral imaging color enhancement. Journal of Gastroenterology. 2009;44:1125–32. doi: 10.1007/s00535-009-0121-z. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman A, Basting N, Goetz M, Tresch A, Mudter J, Biesterfeld S, Galle PR, Neurath MF, Kiesslich R. High-definition endoscopy with i-Scan and Lugol’s solution for more precise detection of mucosal breaks in patients with reflux symptoms. Endoscopy. 2009;41:107–12. doi: 10.1055/s-0028-1119469. [DOI] [PubMed] [Google Scholar]
- 25.Pohl H, Koch M, Khalifa A, Papanikolaou IS, Scheiner K, Wiedenmann B, Rosch T. Evaluation of endocytoscopy in the surveillance of patients with Barrett’s esophagus [see comment] Endoscopy. 2007;39:492–6. doi: 10.1055/s-2007-966340. [DOI] [PubMed] [Google Scholar]
- 26.Tomizawa Y, Abdulla HM, Prasad GA, Wong Kee Song L-M, Lutzke LS, Borkenhagen LS, Wang KK. Endocytoscopy in esophageal cancer. Gastrointestinal Endoscopy Clinics of North America. 2009;19:273–81. doi: 10.1016/j.giec.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guelrud M, Herrera I, Essenfeld H, Castro J. Enhanced magnification endoscopy: a new technique to identify specialized intestinal metaplasia in Barrett’s esophagus. Gastrointestinal Endoscopy. 2001;53:559–65. doi: 10.1067/mge.2001.114059. [DOI] [PubMed] [Google Scholar]
- 28.Connor MJ, Sharma P. Chromoendoscopy and magnification endoscopy in Barrett’s esophagus Review 24 refs. Gastrointestinal Endoscopy Clinics of North America. 2003;13:269–77. doi: 10.1016/s1052-5157(03)00011-4. [DOI] [PubMed] [Google Scholar]
- 29.Kiesslich R, Mergener K, Naumann C, Hahn M, Jung M, Koehler HH, Nafe B, Kanzler S, Galle PR. Value of chromoendoscopy and magnification endoscopy in the evaluation of duodenal abnormalities: a prospective, randomized comparison. Endoscopy. 2003;35:559–63. doi: 10.1055/s-2003-40240. [DOI] [PubMed] [Google Scholar]
- 30.Meining A, Rosch T, Kiesslich R, Muders M, Sax F, Heldwein W. Inter- and intra-observer variability of magnification chromoendoscopy for detecting specialized intestinal metaplasia at the gastroesophageal junction. Endoscopy. 2004;36:160–4. doi: 10.1055/s-2004-814183. [DOI] [PubMed] [Google Scholar]
- 31.Toyoda H, Rubio C, Befrits R, Hamamoto N, Adachi Y, Jaramillo E. Detection of intestinal metaplasia in distal esophagus and esophagogastric junction by enhanced-magnification endoscopy. Gastrointestinal Endoscopy. 2004;59:15–21. doi: 10.1016/s0016-5107(03)02527-6. [DOI] [PubMed] [Google Scholar]
- 32.Rey JF, Inoue H, Guelrud M. Magnification endoscopy with acetic acid for Barrett’s esophagus. Endoscopy. 2005;37:583–6. doi: 10.1055/s-2005-861321. [DOI] [PubMed] [Google Scholar]
- 33.Ferguson DD, DeVault KR, Krishna M, Loeb DS, Wolfsen HC, Wallace MB, Ferguson DD, DeVault KR, Krishna M, Loeb DS, Wolfsen HC, Wallace MB. Enhanced magnification-directed biopsies do not increase the detection of intestinal metaplasia in patients with GERD. American Journal of Gastroenterology. 2006;101:1611–6. doi: 10.1111/j.1572-0241.2006.00622.x. [DOI] [PubMed] [Google Scholar]
- 34.Mayinger B, Oezturk Y, Stolte M, Faller G, Benninger J, Schwab D, Maiss J, Hahn EG, Muehldorfer S. Evaluation of sensitivity and inter- and intraobserver variability in the detection of intestinal metaplasia and dysplasia in Barrett’s esophagus with enhanced magnification endoscopy [see comment] Scandinavian Journal of Gastroenterology. 2006;41:349–56. doi: 10.1080/00365520510024016. [DOI] [PubMed] [Google Scholar]
- 35.Anagnostopoulos GK, Yao K, Kaye P, Hawkey CJ, Ragunath K. Novel endoscopic observation in Barrett’s oesophagus using high resolution magnification endoscopy and narrow band imaging. Alimentary Pharmacology & Therapeutics. 2007;26:501–7. doi: 10.1111/j.1365-2036.2007.03374.x. [DOI] [PubMed] [Google Scholar]
- 36.Mayinger B, Oezturk Y, Stolte M, Faller G, Benninger J, Schwab D, Maiss J, Hahn EG, Muehldorfer S, Mayinger B, Oezturk Y, Stolte M, Faller G, Benninger J, Schwab D, Maiss J, Hahn EG, Muehldorfer S. Evaluation of sensitivity and inter- and intra-observer variability in the detection of intestinal metaplasia and dysplasia in Barrett’s esophagus with enhanced magnification endoscopy. Scandinavian Journal of Gastroenterology. 2006;41:349–56. doi: 10.1080/00365520510024016. [DOI] [PubMed] [Google Scholar]
- 37.Ferguson DD, DeVault KR, Krishna M, Loeb DS, Wolfsen HC, Wallace MB. Enhanced magnification-directed biopsies do not increase the detection of intestinal metaplasia in patients with GERD. American Journal of Gastroenterology. 2006;101:1611–6. doi: 10.1111/j.1572-0241.2006.00622.x. [DOI] [PubMed] [Google Scholar]
- 38.Testoni P-A, Mangiavillano B. Optical coherence tomography in detection of dysplasia and cancer of the gastrointestinal tract and bilio-pancreatic ductal system. World Journal of Gastroenterology. 2008;14:6444–52. doi: 10.3748/wjg.14.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiesslich R, Gossner L, Goetz M, Dahlmann A, Vieth M, Stolte M, Hoffman A, Jung M, Nafe B, Galle PR, Neurath MF. In vivo histology of Barrett’s esophagus and associated neoplasia by confocal laser endomicroscopy. Clinical Gastroenterology & Hepatology. 2006;4:979–87. doi: 10.1016/j.cgh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Pohl H, Rosch T, Vieth M, Koch M, Becker V, Anders M, Khalifa AC, Meining A. Miniprobe confocal laser microscopy for the detection of invisible neoplasia in patients with Barrett’s oesophagus. Gut. 2008;57:1648–53. doi: 10.1136/gut.2008.157461. [DOI] [PubMed] [Google Scholar]
- 41.Dunbar KB, Okolo P, 3rd, Montgomery E, Canto MI. Confocal laser endomicroscopy in Barrett’s esophagus and endoscopically inapparent Barrett’s neoplasia: a prospective, randomized, double-blind, controlled, crossover trial. Gastrointestinal Endoscopy. 2009;70:645–54. doi: 10.1016/j.gie.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang KK, Densmore J. Laser induced fluorescence in the diagnosis of esophageal carcinoma. In: Lambert R, editor. Proceeding of the European SPIE. Amsterdam: Elsevier; 1995. pp. 132–4. [Google Scholar]
- 43.von Holstein CS, Nilsson AM, Andersson-Engels S, Willen R, Walther B, Svanberg K. Detection of adenocarcinoma in Barrett’s oesophagus by means of laser induced fluorescence. Gut. 1996;39:711–6. doi: 10.1136/gut.39.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Georgakoudi I, Van DJ. Characterization of dysplastic tissue morphology and biochemistry in Barrett’s esophagus using diffuse reflectance and light scattering spectroscopy Review 24 refs. Gastrointestinal Endoscopy Clinics of North America. 2003;13:297–308. doi: 10.1016/s1052-5157(03)00008-4. [DOI] [PubMed] [Google Scholar]
- 45.Badizadegan K, Backman V, Boone CW, Crum CP, Dasari RR, Georgakoudi I, Keefe K, Munger K, Shapshay SM, Sheetse EE, Feld MS. Spectroscopic diagnosis and imaging of invisible pre-cancer. Faraday Discussions. 2004;126:265–79. doi: 10.1039/b305410a. discussion 303–11. [DOI] [PubMed] [Google Scholar]
- 46.Kim YL, Liu Y, Wali RK, Roy HK, Backman V. Low-coherent backscattering spectroscopy for tissue characterization. Applied Optics. 2005;44:366–77. doi: 10.1364/ao.44.000366. [DOI] [PubMed] [Google Scholar]
- 47.Kim YL, Turzhitsky VM, Liu Y, Roy HK, Wali RK, Subramanian H, Pradhan P, Backman V. Low-coherence enhanced backscattering: review of principles and applications for colon cancer screening. Journal of Biomedical Optics. 2006;11:041125. doi: 10.1117/1.2236292. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y, Brand RE, Turzhitsky V, Kim YL, Roy HK, Hasabou N, Sturgis C, Shah D, Hall C, Backman V. Optical markers in duodenal mucosa predict the presence of pancreatic cancer [see comment] Clinical Cancer Research. 2007;13:4392–9. doi: 10.1158/1078-0432.CCR-06-1648. [DOI] [PubMed] [Google Scholar]
- 49.Roy HK, Turzhitsky V, Kim Y, Goldberg MJ, Watson P, Rogers JD, Gomes AJ, Kromine A, Brand RE, Jameel M, Bogovejic A, Pradhan P, Backman V. Association between rectal optical signatures and colonic neoplasia: potential applications for screening. Cancer Research. 2009;69:4476–83. doi: 10.1158/0008-5472.CAN-08-4780. [DOI] [PMC free article] [PubMed] [Google Scholar]