Abstract
Background
Most drug treatment patients smoke cigarettes, yet few data exist on the prevalence and outcomes of varenicline treatment among smokers with comorbid substance use and psychiatric disorders.
Methods
We reviewed all patient charts of opioid-dependent smokers prescribed varenicline between May 2006 and December 2009 in two urban methadone clinics that also provide on-site medical and psychiatric care. We assessed prevalence, adverse events, and effectiveness of varenicline treatment in this cohort.
Results
We identified 575 smokers among 690 patients (83.3%), and assessed 82 courses of varenicline treatment prescribed to 70 smokers. Both cardiovascular risk factors and psychiatric illness were highly prevalent among those prescribed varenicline: hypertension, 51%; hyperlipidemia, 23%; diabetes, 20%; depression, 53%; anxiety, 30%; psychotic disorders, 10%; bipolar disorder, 8.6%. Of 82 varenicline courses, nine (11%) were discontinued due to adverse events and two due to depressive symptoms. One patient initiated new psychiatric medications within six months of initiating varenicline, but did not discontinue varenicline. There were no reports of suicidal ideation, agitation prompting clinical intervention, or psychiatric hospitalization. There were no incident cardiac or vascular events within six months of varenicline prescription. Some (8.6%) varenicline-treated smokers quit smoking, and cessation was significantly associated with varenicline treatment duration.
Conclusions
Despite substantial comorbidity, opioid-dependent smokers receiving integrated substance abuse, medical and psychiatric care had few documented adverse events with varenicline treatment. Methadone patients will likely experience little harm and a great deal of benefit from treatment with varenicline for smoking cessation.
Keywords: Opioid dependence, Smoking cessation, Varenicline, Adverse events
1. Introduction
Both tobacco use and tobacco-related disease among persons with alcohol or drug use disorders are highly prevalent (Hser et al., 1994; Hurt et al, 1996; Lasser et al., 2000; Richter and Arnsten, 2006), but multiple studies have shown limited smoking cessation treatment provision in substance abuse treatment programs (Friedmann et al., 2008; Fuller et al., 2007; Hunt et al, 2012; Richter et al., 2004). In particular, smoking cessation pharmacotherapy is underutilized in these settings, partly because the safety of varenicline in patients with comorbid psychiatric illness has been questioned.
The psychiatric risks of varenicline are not well characterized. Reports of behavior change, agitation, depression, suicidal ideation and suicide among patients taking varenicline led to an FDA warning in February 2008. A subsequent “boxed warning” in July 2009 advised providers to monitor patients taking varenicline for the development of psychiatric symptoms, including agitation, depression, and suicidal ideation (U.S. Food and Drug Administration). While phase III trials of varenicline had stringent eligibility criteria that excluded persons with medical and psychiatric comorbidity, including substance users (Gonzales et al., 2006; Jorenby et al., 2006), the results of post-marketing surveillance demonstrating adverse psychiatric events highlight the need to evaluate varenicline safety in patients with psychiatric comorbidity and substance abuse disorders.
Varenicline may also be associated with a small increase in risk of cardiovascular events (Rigotti et al., 2010; Singh et al., 2011). In one randomized clinical trial among patients with cardiovascular disease, there were slightly higher rates of nonfatal myocardial infarction, need for coronary revascularization, and peripheral vascular disease among varenicline-treated compared to placebo participants, though these differences were not statistically significant (Rigotti et al., 2010). In addition, a meta-analysis of selected varenicline clinical trials critiqued for trial selection and statistical methods that could bias toward false-positive findings (Prochaska and Hilton, 2012; Squire, 2011; Takagi and Umemoto, 2011) described a small absolute increase in the risk of serious adverse cardiovascular events among varenicline arm participants (Singh et al., 2011). By contrast, a subsequent meta-analysis, including all published randomized trials of varenicline, and focused on the period of varenicline exposure, found no significant increase in serious cardiovascular events associated with varenicline (Prochaska and Hilton, 2012).
Because varenicline is more efficacious than bupropion (Gonzales et al., 2006; Jorenby et al., 2006) or nicotine replacement therapy in the general population (Stapleton et al., 2008), it is particularly important to determine its risk profile among substance abuse treatment patients. Cessation approaches evaluated to date among opioid-dependent smokers have included bupropion or nicotine replacement therapy in combination with behavioral therapy, but these treatments have not been effective over control conditions (Mooney et al., 2008; Reid et al., 2008; Shoptaw et al., 2002; Stein et al., 2006b). Trials are currently underway to assess varenicline's efficacy in this population, but concerns remain regarding adverse events.
Our objective was to assess prevalence, adverse events, and effectiveness of varenicline treatment among opioid-dependent smokers receiving substance abuse treatment. Our setting is uniquely suited for this study as we provide comprehensive, longitudinal primary care in combination with methadone treatment to patients with opioid dependence.
2. Methods
2.1. Study setting and participants
We reviewed paper medical charts of all patients receiving methadone maintenance treatment at two of the clinics in the Einstein Division of Substance Abuse (DoSA). DoSA offers treatment through a network of 11 closely linked clinics in the Bronx, NY. The two clinics were selected because of the high volume of patients receiving on-site primary medical care. All DoSA patients are opioid-dependent and >80% smoke cigarettes (Nahvi et al., 2006). Each DoSA clinic offers integrated on site general and HIV-related medical, gynecologic, and mental health services, co-located with substance abuse treatment. Patients receive methadone from nurses up to six times weekly, meet with counselors at least monthly for structured psychosocial assessment, and meet with medical providers for annual physical exams and mandatory visits following hospitalization, incarceration or other clinic absence. Psychosocial and clinical concerns, including changes in behavior, are discussed in weekly interdisciplinary meetings including clinic leadership, counselors, nurses, physician assistants and physicians. Subjects for this analysis included cigarette smokers prescribed varenicline for smoking cessation by DoSA providers at any point during a 3.5-year period from May 2006 through December 2009. Patients reporting varenicline prescription from outside providers were excluded from analysis because exact dates of treatment initiation and duration were unavailable.
2.2. Design, procedures, and measures
2.2.1. Data collection
A physician and trained research assistants used structured data collection forms to conduct medical record reviews. Among all patients, we extracted data regarding demographic characteristics, smoking status, and prescribed smoking cessation treatment. Ninety-four percent of charts from the two clinics were available for review. Among patients prescribed varenicline, we extracted data regarding medical and psychiatric comorbidity, concomitant substance use, methadone dose, and adverse events and tobacco use following varenicline prescription. Charts were re-reviewed to resolve discrepancies or correct omissions as necessary; 25 (36%) charts were reviewed at least twice. The research protocol was approved by the Einstein Committee on Clinical Investigations.
2.2.2. Smoking status and smoking cessation treatment
Smoking status is recorded by medical providers during annual physical exams on a standard form used in all DoSA clinics. Only one reviewed chart was missing this information. Biochemical assessment of tobacco use is not standard practice in DoSA.
Medical provider notes and patient medication lists were reviewed to determine whether nicotine replacement therapy, bupropion, or varenicline were prescribed. If varenicline was prescribed, the maximum treatment duration was estimated based on the following three categories: (1) single four-week course without refills, (2) four-week course and two refills (twelve-week course), or (3) four-week course without documentation of whether or not refills were prescribed (four weeks or more). Prescriptions were filled at commercial pharmacies of patients' choosing; pharmacy records were unavailable for verification of prescription filling.
2.2.3. Comorbidity
Self-reported and/or clinically verified medical and psychiatric diagnoses are recorded by DoSA medical providers during history and physical exam visits conducted annually with all patients. Medical providers' notes were reviewed to ascertain whether patients were receiving psychiatric treatment, and, if so, whether it was on- or off-site.
2.2.4. Substance use and methadone dose
Unannounced urine toxicology tests are routinely performed in all DoSA clinics. Urine testing is conducted using the Enzyme Multiplied Immunoassay Technique (EMIT) at a commercial laboratory, and evaluates for presence of opiates, cocaine, or benzodiazepines. We extracted data regarding: (1) number of toxicology tests performed and (2) number of tests positive for cocaine, opiates, or benzodiazepines, in the six months prior to and the six months following each varenicline prescription. Methadone dose at the time of varenicline prescription was also recorded.
2.2.5. Adverse events following varenicline prescription
We reviewed medical and psychiatric providers' notes and patient medication lists to assess adverse events: (1) in the six months following each varenicline treatment course and (2) beyond the six months following each varenicline course, but specifically attributed to varenicline. We collected data on all potential adverse events that have been reported by >5% of participants receiving varenicline in published research, including: nausea, vomiting, headache, irritability, fatigue, insomnia, difficulty concentrating, and changes in dreams. We also collected data on neuropsychiatric events outlined in the 2008 and 2009 FDA warnings regarding varenicline. To characterize neuropsychiatric events, we assessed: (1) whether varenicline was discontinued due to specific side effects; (2) whether new antidepressant, antipsychotic, anxiolytic or mood-stabilizing medications were prescribed; (3) whether new psychiatric treatment was initiated; (4) occurrence of suicidal ideation, documented on standard annual physical exam forms used throughout DoSA or medical or psychiatric progress notes; (5) incident agitation or aggression, documented in ad hoc meetings with patients and administrative, counseling, and medical staff to address disruptive behavior, or in mandatory medical visits following incarceration; (6) medical or psychiatric hospitalizations, documented in mandatory medical visits to resume methadone dosing following clinical absence or hospitalization; and (7) mortality. A physician re-reviewed charts of all patients with documented neuropsychiatric symptoms to ascertain patients' baseline clinical status, psychotropic medication history, and whether patients or providers attributed symptoms to varenicline treatment.
Following the FDA drug safety communication in June 2011 warning of potential cardiovascular risks with varenicline use, charts were re-reviewed to ascertain whether patients experienced incident cardiovascular events. These included: myocardial infarction, cardiac arrhythmia, peripheral vascular disease, cerebral vascular disease, or cardiac or vascular procedures in the six months following varenicline prescription. Cardiovascular events are documented in annual physical exams, medical progress notes, or mandatory medical visits following hospitalization. Nearly all (98.5%) charts were available for re-review.
2.2.6. Tobacco use following varenicline prescription
We ascertained self-reported tobacco use status following varenicline prescription from annual physical exam forms and provider notes. Since smokers are advised to quit one week after starting varenicline, duration of abstinence was estimated from one week after the date of varenicline prescription to the date at which tobacco abstinence was last documented in the medical record. This estimation may overestimate duration (if onset of abstinence lagged more than one week following varenicline initiation) or underestimate duration (if relapse occurred later than last date of recorded abstinence).If the chart had no documented assessment of smoking status following varenicline (n = 4), smoking was assumed.
2.3. Analysis
Analyses were performed using STATA (StataCorp, College Station, TX). Descriptive data include patient characteristics (described by means and standard deviations, medians and interquartile ranges, or percentages) and the proportion of patients experiencing each outcome. Associations between patient characteristics and smoking cessation were tested using t-tests, Wilcoxon rank sum tests, Chi-square tests, or Fisher's exact tests, as appropriate. In a subgroup of 53 patients for whom complete varenicline treatment course information was available, we evaluated the association between treatment duration and smoking cessation with Fisher's exact tests. Given that the recommended course of varenicline treatment is 12-24 weeks, we dichotomized treatment duration at either ≥12 weeks or <12 weeks. We categorized patients as having a ≥12-week duration if they were ever prescribed a 12-week course of treatment and did not discontinue treatment during the 12 weeks. We categorized patients as having a <12-week duration if: (1) they were prescribed a four-week course of treatment without refills; or (2) they were prescribed between 4 and 12 weeks of treatment, but charts documented discontinuation of treatment.
3. Results
3.1. Patient characteristics
690 patient charts were reviewed and 575 (83.3%) smokers were identified. One hundred sixty three smokers (28.3%) were prescribed smoking cessation treatment: 115 (20%) were prescribed nicotine replacement therapy, and 19 (3.3%) were prescribed bupropion. Seventy (12.2% of smokers) were prescribed varenicline, including nine patients who received two or more varenicline prescriptions, resulting in a total of 82 courses of varenicline treatment. Patients prescribed varenicline had a mean age of 50 years, and 54% were male. Forty-nine percent were prescribed nicotine replacement therapy or bupropion prior to or following varenicline treatment (Table 1).
Table 1.
Characteristics of patients prescribed varenicline, n = 70.
| n | (%) | |
|---|---|---|
| Sociodemographic characteristics | ||
| Mean age, years (SD) | 50 | (7.8) |
| Male sex | 38 | 54% |
| Race/ethnicity | ||
| African American | 14 | 20% |
| White, Hispanic | 53 | 76% |
| White, non-Hispanic | 1 | 1.4% |
| Native American | 1 | 1.4% |
| Other | 1 | 1.4% |
| Smoking cessation treatment characteristics | ||
| Other smoking cessation treatment prescribed | 34 | 49% |
| Nicotine replacement therapy | 32 | 46% |
| Bupropion | 11 | 16% |
| Clinical characteristics | ||
| Medical comorbidity | ||
| HIV | 20 | 29% |
| Hepatitis C | 43 | 61% |
| Hypertension | 36 | 51% |
| Hyperlipidemia | 16 | 23% |
| Diabetes | 14 | 20% |
| Asthma/COPD | 32 | 46% |
| Coronary artery disease | 1 | 1.4% |
| Peripheral vascular disease | 6 | 8.6% |
| Cancer | 3 | 4.3% |
| Cervical dysplasia | 4 | 5.7% |
| Psychiatric comorbidity and treatment status | ||
| Any psychiatric illness | 46 | 66% |
| Depression | 37 | 53% |
| Anxiety | 21 | 30% |
| Bipolar disorder | 6 | 8.6% |
| Psychotic disorder | 7 | 10% |
| Receiving onsite psychiatric treatment | 25 | 36% |
| Receiving any psychiatric treatment | 35 | 50% |
| Substance use characteristics | ||
| Median methadone dose, mg (IQR) | 105 | (60, 150) |
| Positive urine toxicology report in prior six months | ||
| Any opiate | 37 | 47% |
| Any cocaine | 27 | 39% |
| Any benzodiazepine | 8 | 11% |
| ≥50% tests positive for opiate | 10 | 14% |
| ≥50% tests positive for cocaine | 13 | 19% |
| ≥50% tests positive for benzodiazepine | 3 | 4.3% |
Both cardiovascular risk factors and psychiatric illness were highly prevalent: 51% had hypertension, 23% had hyperlipidemia, 20% had diabetes, 53% had preexisting depression, 30% had preexisting anxiety, 10% had preexisting psychotic disorders, and 8.6% had preexisting bipolar disease. In addition, 29% were HIV-infected.
3.2. Varenicline treatment
The Fig. 1 describes the estimated duration of varenicline exposure for the 82 varenicline treatment courses prescribed to 70 smokers. Thirty-four prescriptions (41%) were for four-week courses, while 26 (32%) were for twelve-week courses, and 22 (27%) were for four weeks or more. Assuming those prescribed four weeks or more were prescribed four-week courses, or twelve-week courses, respectively, estimated varenicline exposure ranged from 134 to 178 person-months.
Fig. 1.
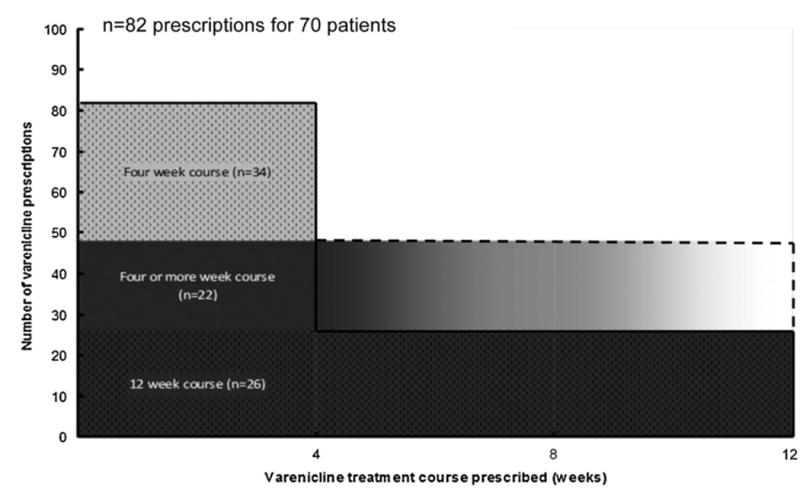
Maximum varenicline exposure.
Among the subgroup of 53 patients for whom complete treatment course information was available, the majority of patients had suboptimal (<12 weeks) durations. Among 30 patients prescribed four-week treatment courses, one received a subsequent refill for a 12-week treatment course. Two of the 18 patients initially prescribed 12-week courses discontinued treatment. Five patients whose initial treatment course was not documented discontinued treatment. Thus, 36 patients (51.4%) received <12 weeks of treatment, and only 17 patients (24.2%) received the recommended 12 or more weeks.
3.3. Adverse events following varenicline prescription
We assessed events for six months after treatment initiation for all 82 (100%) varenicline prescriptions. Patients were in close contact with medical and substance abuse providers, with a median of six [interquartile range (IQR) 3–9] medical visits and 10 (IQR 7–13) substance abuse counselor visits in the six months following each varenicline prescription.
There were 9 individuals with adverse events prompting varenicline discontinuation. Seven were neither psychiatric nor cardiovascular (3 were gastrointestinal, plus 1 each of dysguesia, palpitations, dizziness, and insomnia). Two patients discontinued varenicline due to depressive symptoms, which they attributed to varenicline. In both cases, patients had preexisting depression and/or anxiety requiring medication, and received on-site psychiatric treatment, but neither required psychotropic medication adjustments in the six months following varenicline prescription. Also in both cases, the association between varenicline and depressive symptoms was documented months to years following varenicline prescription (Table 2).
Table 2.
Adverse events following varenicline prescription.
| Time to event (days)a | Treatment course (weeks) | Preexisting psychiatric disease | |
|---|---|---|---|
| Psychiatric adverse effects prompting treatment discontinuation (n = 2)b | |||
| Worsening depression | 794 | 4–12 | Depression, anxiety, on citalopram |
| Worsening depression | 479 | 4 | Bipolar and panic disorders, prior suicide attempts |
| Psychiatric adverse effects not prompting treatment discontinuation(n =4)b | |||
| Increased auditory hallucinations | 129 | 4 | Schizophrenia, cocaine dependence, on aripiprazole, olanzapine, and citalopram |
| New antipsychotic medication | 29 | 12 | Major depressive disorder with psychotic features, cocaine dependence, on bupropion, quetiapine, trazodone |
| Irritability | 55 | 4–12 | Bipolar disorder, generalized anxiety disorder, poor psychiatric medication and appointment adherence |
| Incarceration for assault | 168 | 4 | No documented psychiatric disease; active cocaine use |
Time to documentation of event in the medical record.
No agitation prompting clinical intervention, suicidal ideation or psychiatric hospitalizations.
Three additional patients reported new psychiatric symptoms. One patient, with a prior history of psychotic disorder, reported increased auditory hallucinations, which resolved with dose titration of antipsychotic medication. One patient, with a prior history of bipolar and generalized anxiety disorders and poor treatment adherence, reported increased irritability. One patient, with a prior history of major depressive disorder with psychotic features, had a new antipsychotic medication prescribed approximately one month following varenicline prescription. In these three cases, psychiatric symptoms were not attributed to varenicline by patients or their providers, and there was no documentation of varenicline discontinuation. There were no incidents of agitation prompting clinical intervention, no reports of suicidal ideation, and no psychiatric hospitalizations. One patient, without documented prior psychiatric history, was incarcerated for assaulting a police officer immediately following his wife's death. His urine toxicology tests were positive for cocaine just prior to the event, which occurred 24 weeks following a four-week prescription for varenicline.
There were no documented incident cardiovascular, peripheral vascular, or cerebrovascular events within six months of varenicline prescription. There were five medical hospitalizations, none of which were due to cardiovascular causes. Reasons for hospitalization included nausea and dehydration, pneumonia, poorly controlled diabetes, cellulitis and abscess in the context of injection drug use, and bleeding uterine fibroids. There was one patient death, due to metastatic renal cell cancer.
3.4. Tobacco use following varenicline prescription
Six patients (8.6%) self-reported tobacco cessation following varenicline treatment. Tobacco abstinence was documented a median of 336 days (interquartile range 252-499) following varenicline prescription. There were no significant associations between tobacco cessation and age, sex, race/ethnicity, HIV status, preexisting psychiatric illness, methadone dose, or positive urine toxicology tests.
Among the subgroup of 53 patients for whom complete varenicline treatment course information was available, tobacco cessation was significantly associated with treatment duration. Of the six patients with documented smoking cessation following varenicline treatment, 5 received at least one 12-week varenicline treatment course; only one received <12 weeks. Of those with a ≥12-week treatment duration, 29.4% (95% CI 6.6–52.3%) quit, compared to only 2.8% (95% CI 0–8.4%) of those with <12 weeks (p = 0.01). There were no significant differences in age, sex, race/ethnicity, pre-existing psychiatric disease, methadone dose, or drug use between patients who did and did not receive a ≥12-week treatment course.
4. Discussion
In this sample of opioid-dependent methadone-maintained patients, tobacco use was nearly universal and a minority of smokers were prescribed varenicline. Among those prescribed varenicline, the proportion with documented treatment-emergent adverse events was very low, and comparable to that seen in the general population. In particular, despite substantial psychiatric comorbidity, psychiatric adverse events were infrequent, almost entirely limited to patients with pre-existing psychiatric disease, and not clearly attributable to varenicline. In addition, though patients had multiple cardiovascular risk factors, we observed no adverse cardiovascular events. Varenicline treatment duration was significantly associated with tobacco cessation.
U.S. Public Health Service guidelines recommend brief individual smoking cessation counseling with five components (known as the “5 A's”) at each clinical encounter. Providers are advised to systematically ask patients about tobacco use, advise smokers to quit, assess willingness to quit, assist with quitting, and arrange follow up (Fiore et al, 2008). In contrast to studies that have shown limited smoking cessation treatment provision in substance abuse treatment programs (Friedmann et al., 2008; Fuller et al., 2007; Hunt et al, 2012; Richter et al., 2004), we found that smoking status was routinely assessed in our setting and one-third of smokers were offered smoking cessation pharmacotherapy. This may reflect the fact that primary medical care services are offered at our sites, and suggests that integration of medical and substance abuse treatment can help to address the disproportionate burden of tobacco use among substance abuse treatment patients. Indeed, within an integrated system in which patients are in close contact with both medical and substance abuse treatment providers, any emergent psychiatric symptoms following smoking cessation pharmacotherapy may be promptly identified and treated.
Our findings extend prior research supporting the safety of varenicline among patients with psychiatric illness. In one prior study, psychiatric patients (with depression, bipolar disorder, and/or psychosis) receiving varenicline demonstrated no exacerbation of mental illness symptoms, and no increased frequency or intensity of varenicline-associated adverse symptoms (Stapleton et al, 2008). An open label trial of 112 smokers with stable schizophrenia also found no exacerbation of psychosis (Pachas et al., 2012). In a retrospective analysis of 78 veterans with posttraumatic stress disorder, two patients were found to have depressive episodes attributable to varenicline treatment, and four other patients had suicidal ideation or mental health-related hospitalizations not attributed to varenicline (Campbell, 2010). In another retrospective cohort of 50 veterans prescribed varenicline, four patients with underlying psychiatric illness discontinued therapy due to behavioral changes (Purvis et al., 2009). Among smokers receiving varenicline as part of a randomized trial of a behavioral intervention, patients with a history of major depression did not experience greater incident depressive symptoms compared to patients without major depression (McClure et al, 2009). Our finding of absent treatment-emergent adverse psychiatric events among persons with comorbid psychiatric illness and opioid dependence strengthens this body of evidence.
Though few patients in our sample discontinued varenicline treatment due to adverse effects, the majority did not receive the recommended 12 or more weeks of treatment. Our findings are consistent with research that demonstrates that smokers often fail to adhere to the prescribed amount or duration of nicotine replacement therapy, bupropion, or varenicline (Halperin et al., 2009; Lam et al., 2005; Paluck et al., 2006; Purvis et al., 2009; Wiggers et al., 2006). Drug users may have additional adherence challenges, including comorbid medical and psychiatric disease, limited social support, and poor self-efficacy. Methadone maintained smokers receiving nicotine patches in a prior clinical trial used patches for a mean of only 44% of treatment days (Stein et al., 2006a), and in a separate study 53% of methadone maintained smokers reported using bupropion less often than prescribed (Richter et al., 2005).
Our finding that varenicline treatment duration was significantly associated with smoking cessation suggests that adherence support may be an especially important area of intervention to promote smoking cessation among opioid-dependent smokers. Smoking cessation medication treatment duration has consistently shown to be associated with significant improvement in smoking cessation outcomes (Balmford, 2011; Blak, 2010; Catz et al., 2011; Cummings et al, 1997; Halperin et al., 2009; Hays et al., 2010; Jolicoeur et al., 2000; Lam et al., 2005; Paluck et al., 2006; Shiffman et al., 2008) with a dose dependent relationship between duration of treatment and cessation success in some studies (Blak, 2010; Cummings et al., 1997). In two large smoking cessation trials among methadone maintenance patients, adherence to nicotine patch treatment was shown to be associated with improved smoking cessation outcomes (Frosch et al., 2002; Stein et al., 2006a, 2007).
Though our study is one of the first to examine treatment-emergent events among varenicline-prescribed opioid-dependent smokers, it has some limitations. As a retrospective review, we were limited by the available documentation in medical records and were unable to assess smoking characteristics (such as cigarettes smoked/day, interest in quitting, or nicotine dependence), biochemically validated cessation, or exact dates of treatment duration in many cases. In addition, psychiatric diagnoses were abstracted from the record. Our overall assessment of adverse events may have been limited by our small sample size and short treatment duration. As this was an exploratory, hypothesis generating study, no formal sample size calculation was performed. Comparative data assessing outcomes of patients prescribed other smoking cessation treatments, or not offered treatment, are unavailable. Despite these limitations, to our knowledge, this is the largest series to date describing varenicline treatment among methadone maintained smokers.
In sum, we observed documentation of few psychiatric and no cardiovascular adverse events following varenicline prescription among opioid-dependent patients with comorbid psychiatric and medical illnesses. The rate of tobacco cessation was modest, but associated with treatment duration. Given the disproportionate prevalence and disease burden of tobacco use among substance abuse treatment patients, interventions to increase varenicline use and adherence should be developed.
Acknowledgments
Role of funding source: This work was supported by the National Center for Research Resources grant UL1 RR025750 to Dr. Nahvi, and the National Institute on Drug Abuse grants K23 DA025736 to Dr. Nahvi, and R25 DA023021 to Drs. Nahvi and Arnsten. The funding sources had no further role in the study design; data collection, analysis, or interpretation; manuscript preparation; or the decision to submit the paper for publication.
The authors thank Amanda Carter, Gwen Codner, Alain Litwin, Tanya Nahvi, Yuming Ning, Tessa Rabinowitz, Katie Segal, Lauren Sher, Division of Substance Abuse staff, and the Division of General Internal Medicine Substance Abuse Affinity Group for assistance with data collection, data management, and manuscript preparation and review.
Footnotes
Contributors: Authors Nahvi, Wu and Arnsten designed the study. Authors Nahvi and Wu conducted the statistical analysis. Dr. Nahvi conducted literature searches and summaries of previous related work, and wrote the first draft of the manuscript. All authors contributed to manuscript preparation and have approved the final manuscript.
Conflict of interest: All authors declare that they have no conflicts of interest.
References
- Balmford J. Adherence to and reasons for premature discontinuation from stop-smoking medications: data from the ITC Four-Country Survey. Nicotine Tob Res. 2011;13:94–102. doi: 10.1093/ntr/ntq215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blak BT. Evaluation of varenicline as an aid to smoking cessation in UK general practice – a THIN database study. Curr Med Res Opin. 2010;26:861–870. doi: 10.1185/03007990903526461. [DOI] [PubMed] [Google Scholar]
- Campbell AR. Mental health stability in veterans with posttraumatic stress disorder receiving varenicline. Am J Health Syst Pharm. 2010;67:1832–1837. doi: 10.2146/ajhp100196. [DOI] [PubMed] [Google Scholar]
- Catz SL, Jack LM, McClure JB, Javitz HS, Deprey M, Zbikowski SM, McAfee T, Richards J, Swan GE. Adherence to varenicline in the COMPASS Smoking Cessation Intervention Trial. Nicotine Tob Res. 2011;13:361–368. doi: 10.1093/ntr/ntr003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KM, Hyland A, Ockene JK, Hymowitz N, Manley M. Use of the nicotine skin patch by smokers in 20 communities in the United States, 1992–1993. Tob Control. 1997;6:S63–S70. doi: 10.1136/tc.6.suppl_2.s63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF, Froelicher ES, Goldstein MG, Healton CG, Henderson PN, Heyman RB, Koh HK, Kottke TE, Lando HA, Mecklenburg RE, Mermelstein RJ, Mullen PD, Orleans CT, Robinson L, Stitzer ML, Tommasello AC, Villejo L, Wewers ME. Treating Tobacco Use and Dependence. [accessed 10.09.12];Clinical Practice Guideline. 2008 http://www.ahrq.gov/clinic/tobacco/treating_tobacco_use08.pdf.
- Friedmann PD, Jiang L, Richter KP. Cigarette smoking cessation services in outpatient substance abuse treatment programs in the United States. J Subst Abuse Treat. 2008;34:165–172. doi: 10.1016/j.jsat.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frosch DL, Nahom D, Shoptaw S. Optimizing smoking cessation outcomes among the methadone maintained. J Subst Abuse Treat. 2002;23:425–430. doi: 10.1016/s0740-5472(02)00280-5. [DOI] [PubMed] [Google Scholar]
- Fuller BE, Guydish J, Tsoh J, Reid MS, Resnick M, Zammarelli L, Ziedonis DM, Sears C, McCarty D. Attitudes toward the integration of smoking cessation treatment into drug abuse clinics. J Subst Abuse Treat. 2007;32:53–60. doi: 10.1016/j.jsat.2006.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR, Varenicline Phase 3 Study, G Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. J Am Med Assoc. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Halperin AC, McAfee TA, Jack LM, Catz SL, McClure JB, Deprey TM, Richards J, Zbikowski SM, Swan GE. Impact of symptoms experienced by varenicline users on tobacco treatment in a real world setting. J Subst Abuse Treat. 2009;36:428–434. doi: 10.1016/j.jsat.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays JT, Leischow SJ, Lawrence D, Lee TC. Adherence to treatment for tobacco dependence: association with smoking abstinence and predictors of adherence. Nicotine Tob Res. 2010;12:574–581. doi: 10.1093/ntr/ntq047. [DOI] [PubMed] [Google Scholar]
- Hser YI, McCarthy WJ, Anglin MD. Tobacco use as a distal predictor of mortality among long-term narcotics addicts. Prev Med. 1994;23:61–69. doi: 10.1006/pmed.1994.1009. [DOI] [PubMed] [Google Scholar]
- Hunt JJ, Cupertino AP, Garrett S, Friedmann PD, Richter KP. How is tobacco treatment provided during drug treatment? J Subst Abuse Treat. 2012;42:4–15. doi: 10.1016/j.jsat.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, Melton LJ., 3rd Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. J Am Med Assoc. 1996;275:1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- Jolicoeur DG, Ahluwalia JS, Richter KP, Mosier M, Harris KJ, Gibson C, Moranetz CA. The use of nicotine patches with minimal intervention. Prev Med. 2000;30:504–512. doi: 10.1006/pmed.2000.0670. [DOI] [PubMed] [Google Scholar]
- Jorenby D, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR, Varenicline Phase 3 Study, G Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. J Am Med Assoc. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Lam TH, Abdullah ASM, Chan SSC, Hedley AJ, Hong Kong Council on Smoking and Health Smoking Cessation Health Centre Steering Group Adherence to nicotine replacement therapy versus quitting smoking among Chinese smokers: a preliminary investigation. Psychopharmacology (Berlin) 2005;177:400–408. doi: 10.1007/s00213-004-1971-y. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. J Am Med Assoc. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- McClure J, Swan G, Jack L, Catz S, Zbikowski S, McAfee T, Deprey M, Richards J, Javitz H. Mood, side-effects and smoking outcomes among persons with and without probable lifetime depression taking varenicline. J Gen Intern Med. 2009;24:563–569. doi: 10.1007/s11606-009-0926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney ME, Poling J, Gonzalez G, Gonsai K, Kosten T, Sofuoglu M. Preliminary study of buprenorphine and bupropion for opioid-dependent smokers. Am J Addict. 2008;17:287–292. doi: 10.1080/10550490802138814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahvi S, Richter K, Li X, Modali L, Arnsten J. Cigarette smoking and interest in quitting in methadone maintenance patients. Addict Behav. 2006;31:2127–2134. doi: 10.1016/j.addbeh.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Pachas GN, Cather C, Pratt SI, Hoeppner B, Nino J, Carlini SV, Achtyes ED, Lando H, Mueser KT, Rigotti NA, Goff DC, Evins AE. Varenicline for smoking cessation in schizophrenia: safety and effectiveness in a 12-week open-label trial. J Dual Diagn. 2012;8:117–125. doi: 10.1080/15504263.2012.663675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluck EC, McCormack JP, Ensom MHH, Levine M, Soon JA, Fielding DW. Outcomes of bupropion therapy for smoking cessation during routine clinical use. Ann Pharmacother. 2006;40:185–190. doi: 10.1345/aph.1G324. [DOI] [PubMed] [Google Scholar]
- Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. Br Med J. 2012;344:e2856. doi: 10.1136/bmj.e2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis TL, Mambourg SE, Balvanz TM, Magallon HE, Pham RH. Safety and effectiveness of varenicline in a veteran population with a high prevalence of mental illness. Ann Pharmacother. 2009;43:862–867. doi: 10.1345/aph.1L661. [DOI] [PubMed] [Google Scholar]
- Reid MS, Fallon B, Sonne S, Flammino F, Nunes EV, Jiang H, Kourniotis E, Lima J, Brady R, Burgess C, Arfken C, Pihlgren E, Giordano L, Starosta A, Robinson J, Rotrosen J. Smoking cessation treatment in community-based substance abuse rehabilitation programs. J Subst Abuse Treat. 2008;35:68–77. doi: 10.1016/j.jsat.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Richter K, Arnsten JH. A rationale and model for addressing tobacco dependence in substance abuse treatment. Subst Abuse Treat Prev Policy. 2006;1:1–23. doi: 10.1186/1747-597X-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K, McCool RM, Catley D, Hall M, Ahluwalia JS. Dual pharmacotherapy and motivational interviewing for tobacco dependence among drug treatment patients. J Addict Dis. 2005;24:79–90. doi: 10.1300/j069v24n04_06. [DOI] [PubMed] [Google Scholar]
- Richter KP, Choi WS, McCool RM, Harris KJ, Ahluwalia JS. Smoking cessation services in U.S. methadone maintenance facilities. Psychiatr Serv. 2004;55:1258–1264. doi: 10.1176/appi.ps.55.11.1258. [DOI] [PubMed] [Google Scholar]
- Rigotti NA, Pipe AL, Benowitz NL, Arteaga C, Garza D, Tonstad S. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized Trial. Circulation. 2010;121:221–229. doi: 10.1161/CIRCULATIONAHA.109.869008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Sweeney CT, Ferguson SG, Sembower MA, Gitchell JG. Relationship between adherence to daily nicotine patch use and treatment efficacy: secondary analysis of a 10-week randomized, double-blind, placebo-controlled clinical trial simulating over-the-counter use in adult smokers. Clin Ther. 2008;30:1852–1858. doi: 10.1016/j.clinthera.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Rotheram-Fuller E, Yang XW, Frosch D, Nahom D, Jarvik ME, Rawson RA, Ling W. Smoking cessation in methadone maintenance. Addiction. 2002;97:1317–1328. doi: 10.1046/j.1360-0443.2002.00221.x. [DOI] [PubMed] [Google Scholar]
- Singh S, Loke YK, Spangler JG, Furberg CD. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. Can Med Assoc J. 2011;183:1359–1366. doi: 10.1503/cmaj.110218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire EN. Varenicline: quantifying the risk. Can Med Assoc J. 2011;183:1404–1405. doi: 10.1503/cmaj.111-2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton JA, Watson L, Spirling LI, Smith R, Milbrandt A, Ratcliffe M, Sutherland G. Varenicline in the routine treatment of tobacco dependence: a pre–post comparison with nicotine replacement therapy and an evaluation in those with mental illness. Addiction. 2008;103:146–154. doi: 10.1111/j.1360-0443.2007.02083.x. [DOI] [PubMed] [Google Scholar]
- Stein MD, Anderson BJ, Niaura R. Nicotine replacement therapy: patterns of use after a quit attempt among methadone-maintained smokers. J Gen Intern Med. 2006a;21:753–757. doi: 10.1111/j.1525-1497.2006.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Anderson BJ, Niaura R. Smoking cessation patterns in methadone-maintained smokers. Nicotine Tob Res. 2007;9:421–428. doi: 10.1080/14622200701188885. [DOI] [PubMed] [Google Scholar]
- Stein MD, Weinstock MC, Herman DS, Anderson BJ, Anthony JL, Niaura R. A smoking cessation intervention for the methadone-maintained. Addiction. 2006b;101:599–607. doi: 10.1111/j.1360-0443.2006.01406.x. [DOI] [PubMed] [Google Scholar]
- Takagi H, Umemoto T. Varenicline: quantifying the risk. Can Med Assoc J. 2011;183:1404. doi: 10.1503/cmaj.111-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. Information for Healthcare Professionals: Varenicline (marketed as Chantix) and Bupropion (marketed as Zyban, Wellbutrin, and generics) [accessed 10.09.12]; http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm169986.htm.
- Wiggers L, Smets E, Oort F, de Haes H, Storm-Versloot M, Vermeulen H, Legemate D, van Loenen L, Peters R. Adherence to nicotine replacement patch therapy in cardiovascular patients. Int J Behav Med. 2006;13:79–88. doi: 10.1207/s15327558ijbm1301_10. [DOI] [PubMed] [Google Scholar]


