Abstract
Background
We explored the association between anti-tuberculosis drug pharmacokinetics and treatment outcomes among pulmonary tuberculosis (TB) patients in Botswana.
Methods
Consenting TB outpatients had blood collected 1, 2, and 6 hours after simultaneous isoniazid, rifampin, ethambutol, and pyrazinamide ingestion. Maximum serum concentrations (Cmax) and areas under the serum concentration-time curve (AUC0-6 h) were determined. Clinical status was monitored throughout treatment.
Results
Of 225 participants, 36 (16%) experienced a poor treatment outcome (treatment failure or death); 155 (69%) were HIV-infected. Compared with published standards, low isoniazid Cmax occurred in 84 (37%); rifampin in 188 (84%); ethambutol in 87 (39%); and pyrazinamide in 11 (5%) patients. Median rifampin and pyrazinamide levels differed significantly by HIV status and CD4 cell count (HIV-CD4) categories. Only pyrazinamide pharmacokinetics were significantly associated with treatment outcome; low pyrazinamide Cmax was associated with a higher risk of documented poor treatment outcome than normal Cmax (50% vs. 16%; p<0.01). HIV-infected patients with CD4 <200 cells/μL had higher risk of poor treatment outcome (27%) than HIV-uninfected patients (11%) or HIV-infected patients with CD4 ≥ 200 cells/μL (12%); p=0.01. Adjusting for HIV infection and CD4, patients with low pyrazinamide Cmax were thrice more likely to have poor outcomes than patients with normal pyrazinamide Cmax [adjusted risk ratio = 3.38, 95% confidence interval (1.84 – 6.22)].
Conclusions
Lower-than-expected anti-tuberculosis drug Cmax occurred frequently and low pyrazinamide Cmax was associated with poor treatment outcome. Exploring the global prevalence and significance of these findings may suggest modifications in treatment regimens that could improve TB cure rates.
Keywords: tuberculosis, HIV/AIDS, pharmacokinetics, treatment outcome
Introduction
Tuberculosis (TB) is the leading cause of mortality for people living with HIV (PLWH), particularly in sub-Saharan Africa.1,2 Utilizing the DOTS strategy2 many countries have successfully increased their TB cure and completion rates, yet the persistence of HIV/AIDS and the development of multi-drug resistant strains of Mycobacterium tuberculosis continue to threaten global TB control efforts. Currently, the foundation of treatment for persons with uncomplicated TB is a six-month regimen that consists of two months of isoniazid, rifampin, ethambutol, and pyrazinamide followed by four months of isoniazid and rifampin. A series of clinical trials3,4 demonstrated this regimen’s efficacy in curing >95% of adults with pulmonary TB, however, these trials were performed before the appearance of HIV infection.
Anti-TB drug pharmacokinetics are known to be influenced by patient age, sex, and ethnicity; gastrointestinal infections and disorders; drug-drug interactions; and drug formulations.5,6,7,8 HIV infection, for reasons that are likely multi-factorial, has also been associated with lower concentrations of anti-TB drugs.9,10
11,12 Perhaps in part because of these differences PLWH who develop TB may also be at higher risk of treatment failure, treatment default, TB recurrence, and death from TB compared to non-HIV infected TB patients.13,14,15 Irrespective of HIV status, potential sequelae of inadequate anti-TB drug concentrations include prolonged infectiousness, increased risk of relapse and death, and the development of drug-resistant M. tuberculosis.16,17
Few studies 16,17,18 have prospectively assessed anti-TB drug pharmacokinetics among persons with TB and HIV in relation to TB treatment outcomes – an association that may be important to TB control efforts in the era of HIV/AIDS. We sought to determine the frequency and clinical significance of low blood concentrations of isoniazid, rifampin, ethambutol and pyrazinamide among persons diagnosed with TB in Botswana, where an estimated 60%–86% of TB patients are infected with HIV.19,20,21
Methods
Participants and Procedures
This study was conducted by the BOTUSA TB Project.* Detailed participant criteria and study procedures have been described.22
In brief, from June of 1997 through June of 2000, outpatients at Princess Marina Hospital’s Extension 2 outpatient clinic in Gaborone who were >17 years old were screened for participation in the study if they had recently received a diagnosis of pulmonary TB and had been receiving directly observed therapy (DOT) for at least seven days. Further eligibility for the enrollment included having a history of cough for ≥2 weeks and abnormal chest radiographic findings, providing consent for HIV testing, and having either sputum smear microscopy results showing acid-fast bacilli (AFB) or sputum cultures positive for M. tuberculosis. HIV infection status was determined by two ELISA tests run in parallel (Welcozyme, Burroughs Wellcome, Great Britain and Detect, Biochem Immunosystems, France). Discordant results were confirmed by a Western blot (LiaTek, Organon Technik, Germany). Neither ELISA test discriminated between HIV-1 and HIV-2. All study participants provided written consent. CD4+ T-lymphocyte cell counts (CD4) were performed in Botswana by FASCount (Becton Dickson, San Jose, California, USA). Patients having non-tuberculous mycobacteria (NTM) on culture, or who did not have an HIV or CD4 count test result, were excluded.
Single-component anti-TB drugs were provided by the Botswana Ministry of Health (MOH) and were administered by DOT seven days per week in accordance with Botswana National Tuberculosis Program (BNTP) guidelines.23 The Botswana MOH’s daily dose guidelines for anti-TB drugs are outlined in Table 1. Patients were hospitalized and fasted for ≥ 8 hours prior to witnessed simultaneous ingestion of all anti-TB drugs. Blood samples were drawn 1, 2, and 6 hours after ingestion, and serum was separated then frozen at −70°C within one hour of the blood draw. Patients were then discharged to home, to continue anti-TB treatment as outpatients. Per BNTP guidelines, outpatient treatment entailed daily DOT with two months of isoniazid, rifampin, ethambutol, and pyrazinamide followed by four months of isoniazid and rifampin, regardless of HIV status.23
Table 1.
Botswana Ministry of Health daily dose guidelines for antituberculosis drugs.
| Dose by body weight, μg | ||
|---|---|---|
| Drug | Weight 30–50 kg | Weight >50 kg |
| Isoniazid | 300 | 400 |
|
| ||
| Rifampin | 450 | 600 |
|
| ||
| Ethambutol | 1000 | 1200 |
|
| ||
| Pyrazinamide | 1500 | 2000 |
Frozen serum specimens were transported to National Jewish Medical and Research Center where pharmacokinetic analyses were performed using validated high-performance liquid chromatography (for isoniazid and rifampin) or gas chromatography (for ethambutol and pyrazinamide) with mass spectrometry. Anti-TB drug pharmacokinetics for the first 138 patients were analyzed immediately (between 1997 and 1998); these results have been reported previously.22 Due to logistical and fiscal constraints, immediate pharmacokinetic analysis of all remaining patients’ specimens was not possible. Their sera were stored frozen (−70°C) at a CDC laboratory (Lawrenceville, GA) until 2006 when additional funding was secured for their analysis. Comparison of drug concentrations obtained from 1997 to 1998 and those analyzed in 2006 showed no pharmacokinetic differences indicative of specimen degradation. National Jewish personnel were blinded to patients’ identification and outcome information.
Pharmacokinetics and treatment outcome measurement
Consistent with published reference ranges that were determined and validated worldwide24 and used in the analysis by Tappero, et al.,22 the maximum serum drug concentration (Cmax) in each sample was dichotomized as either normal or low. The values used to define low concentrations were as follows: isoniazid, <3 μg/mL (300-mg dose) or < 4 μg/mL (400-mg dose); rifampin, <8 μg/mL (weight-adjusted dose, 450mg or 600mg); ethambutol, <2 μg/mL (median dose, 21 mg/kg); and pyrazinamide, <35 μg/mL (median dose, 35 mg/kg). The area under the serum concentration-time curve from drug ingestion to final measurement at six hours (AUC 0-6 h) was considered as a continuous variable. Isoniazid acetylation was defined as slow if the half-life was ≥ 2 hours and fast if the half-life was <2 hours.
A poor primary treatment outcome was defined as either treatment failure or death during anti-TB treatment. Following BNTP guidelines, treatment failure was defined as either a positive AFB smear or mycobacterial culture at month five of TB treatment, or a clinician’s assessment that there was clinical worsening at month six compared with the status at diagnosis. Death during anti-TB treatment was defined as death due to any cause while receiving anti-TB medications or within one week of stopping TB treatment. Because of inability to monitor patients beyond treatment completion, relapse/re-infection was not included in our poor treatment outcome definition.
Statistical analysis
Preliminary analyses examined Cmax and AUC 0-6 h values of individual drugs by treatment outcomes and considered patients’ baseline demographic and medical characteristics as potential confounders. Baseline data involved detailed patient medical and social history; specific variables included gender; age; prior TB; smoking; body mass index; recent diarrhea; chest radiograph; tuberculin skin test; sputum AFB smears and mycobacterial cultures; serologic tests to assess anemia, immune, hepatic, and renal function, and nutritional status..
Further analyses stratified patients into three categories by HIV status and CD4 cell count (HIV-CD4): not HIV-infected; HIV-infected with CD4 ≥ 200 cells/μL; and HIV-infected with CD4 <200 cells/μL. In addition to continuous measures for Cmax and AUC 0-6 h, we examined drug Cmax values as dichotomized variables based on high and low concentrations from previously published ranges.25,26,27,28 Categorical variables were analyzed using a chi-square test or Fisher’s exact test when cell sizes were <5. Continuous variables were analyzed using the non-parametric Kruskal-Wallis test to compare distributions. Only variables significantly associated with poor treatment outcome in contingency table analyses were considered for multivariable analysis. Adjustment for potential confounding of the relationship between pharmacokinetic parameters and treatment outcome was performed using a Mantel-Haenszel weighted summary estimate of the risk ratio, with calculation of exact confidence intervals. A p-value of <0.05 was considered statistically significant for all analyses.
Results
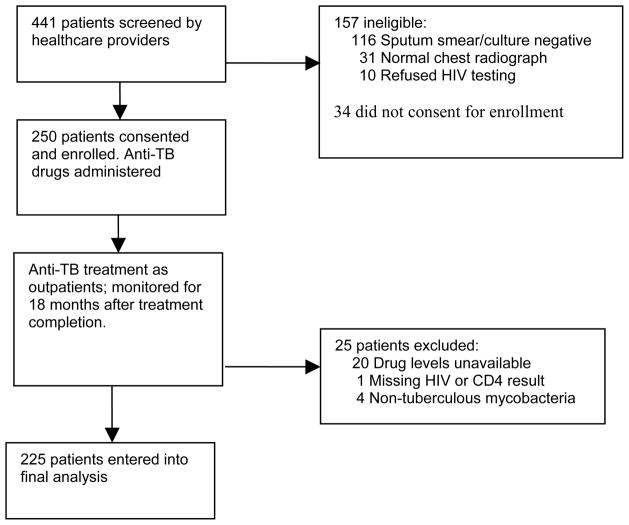
During the enrollment period 441 consecutive patients with recently diagnosed TB were screened for participation in the study. Of these 157 did not meet all of the eligibility criteria, and 34 refused enrollment (Figure 1). The remaining 250 patients gave informed written consent and were enrolled. An additional 21 patients were excluded from the final analysis because of missing HIV, CD4, or pharmacokinetic data, and four were excluded because NTM were cultured from sputum. Thus, our analysis included 225 patients, 155 (69%) of whom were infected with HIV.
Figure 1.
Study profile. HIV, human immunodeficiency virus; TB, tuberculosis.
Demographic, diagnostic and laboratory characteristics of patients are shown in Table 2. Seventeen (8%) patients were lost to follow-up before treatment completion. Those lost to follow-up were demographically and clinically similar to patients for whom treatment outcome was known.
Table 2.
Demographic, diagnostic and laboratory characteristics of 225 enrolled adults with tuberculosis, Gaborone, Botswana, 1997–2000.
| Characteristic | HIV uninfected (n=70) | HIV infected with CD4 cell count ≥ 200 cells/μL (n=71) | HIV infected with CD4 cell count <200 cells/μL (n=84) |
|---|---|---|---|
| Male sex, % | 73 | 52 | 65 |
| Median age (range), years | 32 (18–87) | 30 (21–59) | 33 (20–72) |
| Prior treatment for TB, % of patients | 10 | 16 | 10 |
| Tobacco smoker, % of patients | 40 | 37 | 37 |
| Body Mass Index, median (range), kg/m2 | 18.2 (14.4–29) | 19.1(13.3–29.5) | 18.4 (15.0–24.9) |
| Diarrhea in previous 14 days, % of patients | 8 | 24 | 22 |
| Positive AFB smear result at enrollment, % of patients | 99 | 92 | 93 |
| Culture positive for | 89 | 90 | 79 |
| Mycobacterium tuberculosis at enrollment, % of patients | |||
| TST reaction size, median (range), mm | 10 (0–30) | 11 (0–25) | 0 (0–29) |
| Cavitary disease, % of patients | 49 | 37 | 13 |
| CD4+ lymphocyte count, median (range), cells/μL | 606 (324–1327) | 318 (201–984) | 90 (1–198) |
| Total leukocyte count, median (range), 103 cells/μL | 8.9 (2.7–21) | 8 (3–26.1) | 5.8 (2.2–15.0) |
| Hemoglobin, median (range), g/dL | 11.9 (7.2–17.5) | 10.8 (6.9–15.4) | 10.4 (5.5–17.2) |
| Platelet count, median (range), 103 cells | 427 (86–996) | 382 (127–781) | 323 (85–663) |
| Albumin level, median (range), g/dL | 35.2 (24.5–68) | 32 (23.6–41) | 31.1 (22.3–41.1) |
| AST level, median (range), U/L | 23 (10–261) | 25 (13–122) | 36.0 (9–475) |
| ALT level, median (range), U/L | 20 (7–354) | 16 (7–82) | 20.5 (4–366) |
| Creatinine level, median (range), μmol/L | 74 (33–393) | 75 (29–138) | 82 (10–486) |
NOTE. AFB, acid-fast bacilli; ALT, alanine aminotransferase; AST, aspartamine aminotransferase; TST, tuberculin skin test
Pharmacokinetics
Study patients fasted for a median of 12 hours prior to drug ingestion. Fasting time did not differ by HIV-CD4 categories. Moreover, there was no significant difference in milligram per kilogram dosages of anti-TB drugs administered or in the proportion of fast isoniazid acetylators (46%) across HIV-CD4 categories.
Table 3 shows Cmax results by HIV-CD4 categories. Median pyrazinamide Cmax differed across HIV-CD4 categories (P<0.04, 2df), and was significantly lower among patients with CD4 counts <200 compared with those having CD4 counts ≥ 200, whether HIV-infected with CD4 ≥ 200 (p=0.03, 1df) or not HIV-infected (p=0.03, 1df). Median rifampin Cmax also differed across HIV-CD4 categories (p<0.04, 2df); however, significance was observed only among HIV-infected patients, for whom median rifampin Cmax was lower in those with CD4 counts <200 than in those with CD4 counts ≥ 200 (p=0.01, 1df), with no significant difference between HIV uninfected patients and HIV-infected patients with CD4 <200 (p=0.17, 1df) or HIV-infected patients with CD4 ≥ 200 (p=0.25, 1df).
Table 3.
Maximum serum drug concentrations (Cmax) by human immunodeficiency virus (HIV) status and CD4 cell count among 225 adults with tuberculosis, Gaborone, Botswana, 1997–2000.
| Drug | HIV uninfected (n=70) | HIV infected with CD4 cell count ≥ 200 cells/μL (n=71) | HIV infected with CD4 cell count <200 cells/μL (n=84) | Pa |
|---|---|---|---|---|
| Isoniazid | 4.1 (1.3–10.3) | 4.2 (0.9–10.8) | 4.3 (0.35–9.0) | .80 |
| Rifampin | 4.6 (1.2–13.4) | 5.7 (1.1–15.0) | 4.4 (0.7–12.7) | <.04 |
| Ethambutol | 2.2 (1.0–7.2) | 2.4 (0.8–5.1) | 2.1 (0.4–6.9) | 0.50 |
| Pyrazinamide | 52.3 (29.9–84.4) | 49.9 (29.4–108) | 46.9 (25.8–119) | <.04 |
P values based on 2 degrees of freedom with use of the Kruskal-Wallis test to compare all 3 HIV status and CD4 count categories.
When pharmacokinetic parameters were dichotomized as high and low (see Methods), overall, a low Cmax of isoniazid was measured in 84 (37%); low rifampin in 188 (84%); low ethambutol in 87 (39%); and low pyrazinamide in 11 (5%) of all patients.
Several drugs’ AUC 0-6 h were positively associated with various baseline laboratory characteristics, but none were associated with treatment outcome (data available upon request).
Treatment and Study Outcomes
The distribution of median number of DOT doses received (a proxy for treatment adherence) was similar across HIV-CD4 categories (median 186; range 32–373). Overall, 36 (15%) of patients with known or documented outcomes experienced a poor treatment outcome (Table 4).
Table 4.
Antituberculosis treatment and clinical outcomes by human immunodeficiency virus (HIV) status and CD4 cell count among 225 adults with tuberculosis, Gaborone, Botswana, 1997–2000.
| Proportion (%) of patients | ||||
|---|---|---|---|---|
| Outcome | HIV uninfected | HIV infected with CD4 cell count ≥ 200 cells/μL | HIV infected with CD4 cell count <200 cells/μL | P |
| Poor treatment outcome | 7/66 (11) | 8/67 (12) | 21§/77 (27) | .01† |
|
| ||||
| Treatment failure | 6/61 (10) | 7/58 (12) | 11/61 (18) | .40† |
|
| ||||
| Died during treatment | 1/66 (2) | 1/67 (2) | 12/77 (16) | <.0001‡ |
Two patients in this category experienced both treatment failure and, following extended treatment, death during treatment. For this reason the numerator for “poor treatment outcome” for persons who were HIV infected with a CD4 count <200 cells/μL does not equal the sum of the numerators of “treatment failure” and “died during treatment.”
P value based on 2 degrees of freedom with use of the χ2 test to compare all 3 HIV status and CD4 cell count categories
P value based on Fisher’s exact test to compare all 3 HIV status and CD4 cell count categories
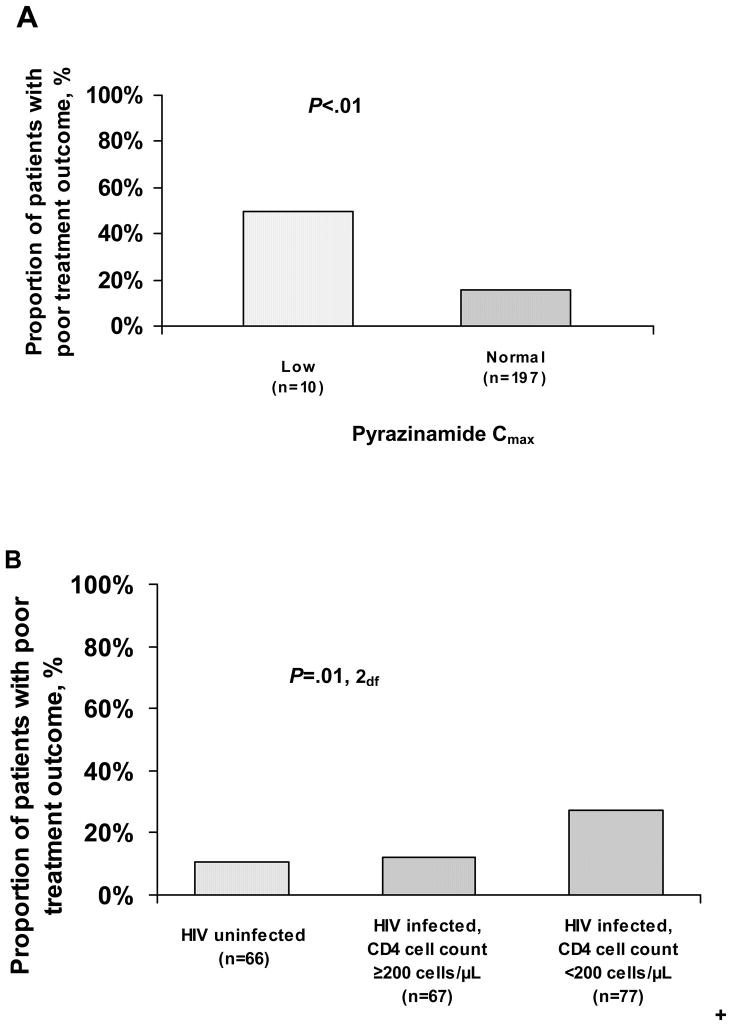
In contingency table analyses, only low pyrazinamide Cmax and HIV-infection with CD4 counts <200 cells/μL were significantly associated with poor treatment outcome (Figure 2). The risk of documented poor outcome among patients with low pyrazinamide Cmax was three times greater than the risk of poor outcome among patients having normal pyrazinamide levels (50.0% versus 15.7%; p<0.01). The risk of poor treatment outcome among patients infected with HIV and having CD4 count <200 was more than two times higher than either HIV- infected patients with CD4 ≥ 200 (27.3% versus 11.9%; p=0.02, 1df) or patients not infected with HIV (27.3% versus 10.6%; p=0.01, 1df) (Table 4 and Figure 2B). No other demographic, laboratory, or pharmacokinetic variables were significantly associated with poor treatment outcome.
Figure 2.
Proportion of patients with poor tuberculosis treatment outcome by maximum serum concentration (Cmax) of pyrazinamide (n=207; A) and human immunodeficiency virus (HIV) status and CD4 cell count category (n=210; B). P values based on χ2 tests comparing patients with either low or normal serum pyrazinamide Cmax values (A) and comparing patients across all 3 HIV status and CD4 cell count categories (B). Individual pairwise comparisons for HIV status and CD4 cell count categories were as follows: HIV-infected patients with CD4 cell count <200 cells/μL versus HIV-infected patients with CD4 cell count ≥ 200 cells/μL (P=.02, 1 degree of freedom [df]), HIV-infected patients with CD4 cell count <200 cells/μL versus HIV uninfected patients (P=.01, 1df); HIV-infected patients with CD4 cell count ≥ 200 cells/μL versus HIV uninfected patients (P=.81, 1df).
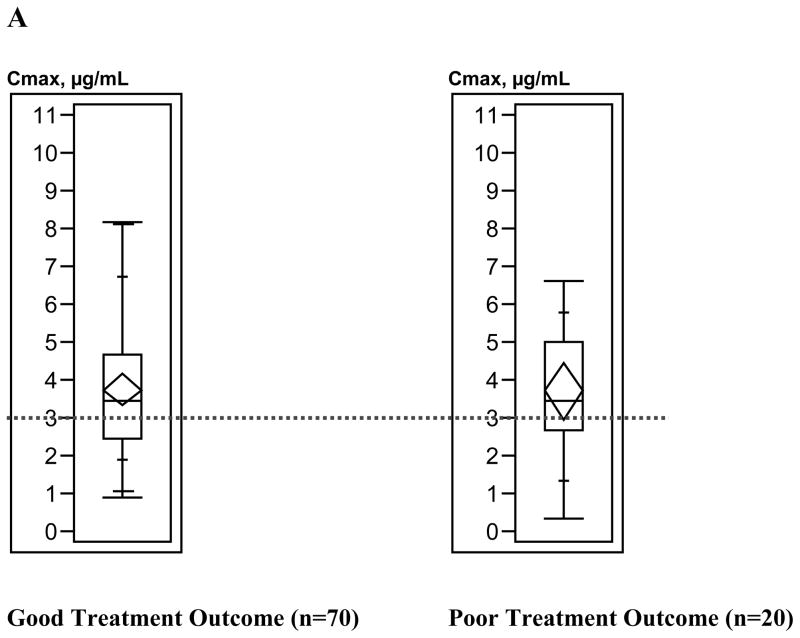
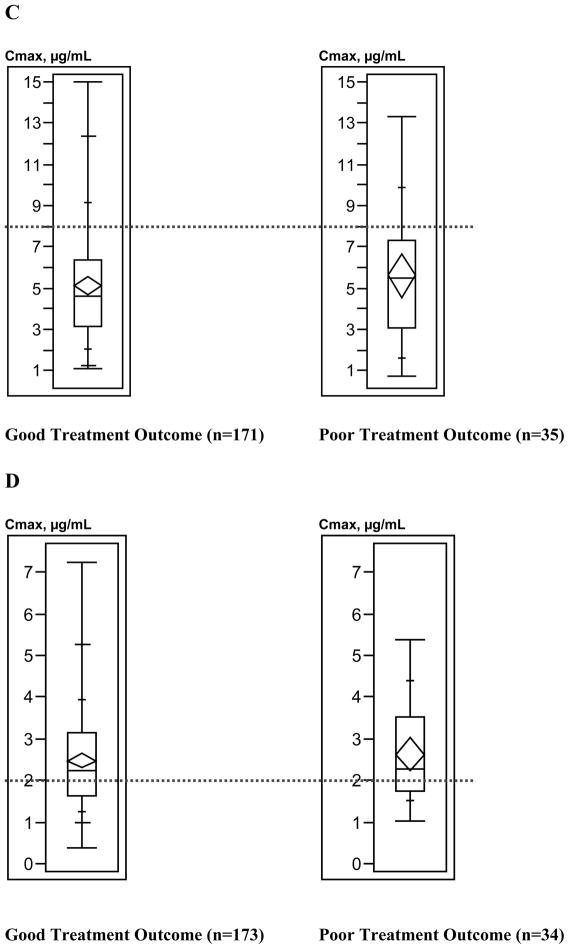
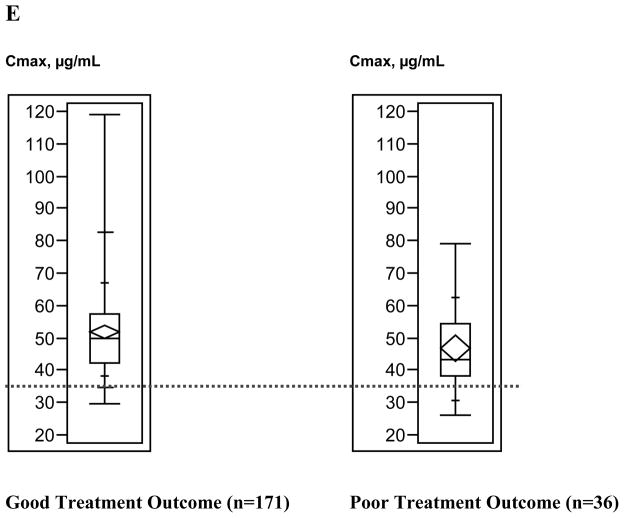
Figure 3 depicts the relationship between treatment outcome and anti-TB drug Cmax values. Generally, the upper ranges of Cmax values were higher among patients with good treatment outcomes compared with patients with poor treatment outcomes; Kruskal-Wallis analysis demonstrated that only pyrazinamide Cmax values differed significantly by treatment outcome (p=0.03); results for other anti-TB drug concentrations were not significant (isoniazid p= 0.59, rifampin p=0.32, ethambutol p=0.45).
Figure 3.
Box plots of antituberculosis drug maximum serum concentrations (Cmax) ranges by tuberculosis treatment outcome. A, Isoniazid (300-mg dose). B, Isoniazid (400-mg dose). C, Rifampin. D, Ethambutol. E, Pyrazinamide. Box lengths illustrate interquartile ranges, with the top of each box representing the 75th percentile, the bottom of each box representing the 25th percentile, and the horizontal line within each box indicating the 50th percentile (median) of the distribution. The diamond within each box represents the mean and 95% confidence interval around the mean. The vertical whisker lines extend from the minimum value to the maximum value. The dotted horizontal lines demarcate the Cmax below which each drug concentration was defined as low. Total sample sizes for individual drugs do not equal 225, because treatment outcome and Cmax could not be determined for all patients.
Since median pyrazinamide Cmax differed significantly by HIV-CD4 category and pyrazinamide Cmax and HIV-CD4 were both associated with treatment outcome, we examined the risk ratio for poor outcome for low versus normal pyrazinamide Cmax across HIV-CD4 categories (Table 5). Risk ratio (RR) estimates for low pyrazinamide were similar across HIV-CD4 categories. The association between low pyrazinamide and poor treatment outcome was strongest (RR = 4.06, 95% confidence interval: 2.72, 6.06) among patients who were HIV-infected with CD4 < 200, although the differences across HIV-CD4 categories were not statistically significant (homogeneity p=0.61). The Mantel-Haenszel summary RR for low pyrazinamide across HIV-CD4 categories was 3.38 (95% confidence interval: 1.84, 6.22).
Table 5.
Risk ratio for poor treatment outcome among adults with tuberculosis with low versus normal pyrazinamide maximum serum concentrations, adjusted for human immunodeficiency virus (HIV) and CD4 cell count category, Gaborone, Botswana, 1997–2000.
| Variable | Risk ratio (95% confidence interval) |
|---|---|
| Unadjusted | 3.18 (1.58–6.39) |
| Stratified by HIV-CD4 | |
| Not HIV-infected | 3.44 (0.59–20.26) |
| HIV-infected CD4 ≥ 200 | 2.21 (0.35–13.88) |
| HIV-infected CD4 <200 | 4.06 (2.72–6.06) |
| Adjusted§ | 3.38 (1.84–6.22) |
Mantel-Haenszel risk ratio for poor outcome for low pyrazinamide versus normal pyrazinamide concentration, adjusted for HIV status and CD4 cell count category
Discussion
In this study, poor treatment outcomes occurred in 16% of adult pulmonary TB patients, or nearly one in six adults, despite use of standard anti-TB drug dosages via daily DOT. The failure of global TB control strategies such as DOTS to guarantee good treatment outcomes in certain populations is multi-factorial. Our data suggests that low blood pyrazinamide concentrations may contribute to poor treatment outcomes in resource-limited, HIV-prevalent settings.
Although infrequent, we found that low pyrazinamide Cmax values appear to increase the risk of poor treatment outcome among TB patients, regardless of HIV infection. Low Cmax values for anti-TB drugs, particularly for rifampin, have been documented previously and have been postulated to influence treatment outcome and/or acquired drug resistance.7,8,9,10,17 To our knowledge, however, this is the first study linking low pyrazinamide Cmax with poor treatment outcome. The reason for pyrazinamide’s possible relationship to treatment outcome may reflect its presumed antimycobacterial effects. In contrast to most other anti-TB drugs, pyrazinamide’s ability to sterilize tissues enables routine treatment to be six months, instead of 9–12 months.29,30 The exact mechanism by which this effect occurs is not known, but the failure to achieve a sufficiently high concentration to achieve the sterilizing effect may account for the higher risk of treatment failure and death observed. Another possible explanation for our findings is that because pyrazinamide has been shown to have minimal absorption variability relative to other first-line anti-TB drugs31 it is most likely to be associated with treatment outcomes. Although our sample size is large relative to similar pharmacokinetic studies, its statistical power may be insufficient to reveal any associations between the more variably absorbed non-pyrazinamide anti-TB drugs and treatment outcome. Finally, pyrazinamide has also been shown to have a synergistic relationship with rifampin.32
Despite the fact that the majority of study patients went on to complete treatment or be cured, even while experiencing lower-than-expected Cmax values, persistently low drug concentrations could still facilitate the development of drug-resistant M. tuberculosis. As treatment progresses, patients may experience increased risk for developing resistance to anti-TB drugs for which their serum concentrations are low, manifesting as delayed smear conversion, treatment failure, or relapse. Unfortunately, we could not address these questions as drug-susceptibility testing was not performed on patients who remained culture-positive after 6 months of treatment, and repeat drug concentrations were not obtained throughout TB treatment.
Factors that influence anti-TB drug pharmacokinetics continue to be identified, many of which occur simultaneously (e.g., gastrointestinal infections and HIV) or are unavoidable (e.g., sex, age, isoniazid acetylator phenotype). It is almost certain that once in their home environments, patients experience anti-TB drug pharmacokinetics significantly different from those measured in controlled clinical trials.25,26,27,28 In this study we measured drug pharmacokinetics early in treatment and under ideal conditions (i.e., monitored and fasting) and at a point in treatment when a pharmacokinetic steady-state had been reached. Thus, the findings we report may actually underestimate the true frequency at which pharmacokinetic aberrations occurred once participants returned home. Performing therapeutic anti-TB drug monitoring throughout treatment would have addressed this question, but because of expense and limited access, it is not commonly performed in resource-limited nations. Another option to ensure anti-TB drug pharmacokinetic goals is to increase standard drug doses for patients at risk of lower-than-expected levels. However, adverse effects have typically limited the extent to which this can safely occur. The fundamental question may thus be: are current anti-TB drug doses and pharmacokinetic norms applicable to outpatients and PLWHA specifically, and across global populations generally? Other investigators have already raised this question.13
This study has several important limitations. First, relatively few patients had low pyrazinamide Cmax values, and small frequency changes would have had important statistical consequences. As such, our findings require further investigation. However, this study is among the largest to date that specifically examined anti-TB drug pharmacokinetics and treatment outcomes. Secondly, antiretrovirals were not widely available in Botswana during the study period, and many participants met the criteria for having AIDS and likely experienced morbidity and mortality attributable to their HIV infection. Since autopsies were not performed on decedents, misclassification bias and overestimation of TB-attributed outcomes may have occurred. However, the relationship between low pyrazinamide and poor treatment outcome was observed for each strata of HIV-CD4 category, and thus was not limited to HIV-related deaths. Moreover, our definitions of death during treatment and the composite unsuccessful or “poor” treatment outcome are in accordance with WHO guidelines.33 Third, the treatment failure rate we observed exceeded rates reported in similar populations.16,17,34 DOT eliminates patient non-adherence as a possible etiology, but sub-standard anti-TB drug quality and/or laboratory smear processing errors cannot be ruled out. Fourth, since the our study did not rely solely on culture-confirmed “cure” for a designation of good treatment outcome, its findings might not be directly comparable to other studies that do define treatment outcome on culture confirmation. Finally, we measured anti-TB drug pharmacokinetics only once during anti-TB treatment and patients’ pharmacokinetic profiles may have changed as their health status changed. This study was not designed to determine the specific etiologies (e.g., malabsorption) of observed pharmacokinetic aberrations, only their epidemiologic associations.
TB disables and kills millions of people every year, and HIV infection and anti-TB drug resistance threaten to reverse decades of TB control progress. Chemotherapy regimens against M. tuberculosis are a vital component of TB elimination strategies, but our study is a reminder that their effectiveness should not be taken for granted. This study also indicates a need to re-examine pyrazinamide’s role in anti-TB treatment and whether treatment should be extended to 9 months in PLWH with CD4 counts <200 cells/μL. The standard anti-TB treatment regimens established prior to the HIV epidemic may simply not be effective for all people, particularly those most at risk for TB today: people in resource-limited settings and those infected with HIV. We encourage others to investigate norms of anti-TB drug pharmacokinetics in diverse populations, and particularly in PLWH both taking and not taking antiretrovirals.
Acknowledgments
The authors thank the Botswana National Tuberculosis Programme, Peter McElroy, and Michael Aidoo for their assistance.
Financial support. National Institutes of Health (NIH) grants NIH K08 A134238, NIH R01 A134238, and NIH D43 TW00003-14, NIH reference data from NIH R01 AI37845, and the Centers for Disease Control and Prevention.
Appendix: Pharmacokinetic analysis of samples
Serum concentrations of isoniazid and rifampin were determined using a validated high performance liquid chromatography (HPLC) assay. Samples were measured using a system consisting of a ThermoFinnegan P4000 HPLC pump (San Jose, CA) with model AS1000 fixed-volume autosampler, a model UV2000 ultraviolet detector, a Gateway Series e computer (Poway, CA), and the Chromquest HPLC data management system. The plasma standard curve for isoniazid ranged from 0.5 μg/mL–30 μg/mL. The six point standard curves for isoniazid ranged from 0.5 μg/mL–20 μg/mL, with linearity extending well above this range. The absolute recovery of isoniazid from serum was 61%, as determined by comparing peak height counts across four serum curves to an unextracted solvent curve. The within-day precision, or % of the coefficient of variation (CV) of validation quality control (QC) samples was 1%–6%, and the overall validation precision was 6–10%. QC sample concentrations were 13 μg/mL, 6 μg/mL and 0.8 μg/mL. The plasma standard curve for rifampin ranged from 0.5 μg/mL–30μg/mL. The six-point standard curves for the rifampin ranged from 0.5 μg/mL–50 μg/mL, with linearity extending well above this range. The absolute recovery of rifampin from serum was 95.5%. The within-day precision of validation QC samples was 2.4%–4.6% CV, and the overall validation precision was 6.3%–7.1%. QC sample concentrations were 26 μg/mL, 8 μg/mL, and 3μg/mL.
Serum concentrations of ethambutol and pyrazinamide were measured using a validated assay on a gas chromatograph (Model 6890) with a mass selective detector (Model 5973, Hewlett Packard, Wilmington, DE, USA). The linear range of the serum standard curves for ethambutol was from 0.05 μg/mL–10μg/mL and the absolute recovery of ethambutol from serum was 95.8%. The within-day precision of validation QC samples was 2.2%–4.1% CV, and the overall validation precision was 2.8%–3.3%. The linear concentration range for pyrazinamide analysis was 0.5 μg/mL–100 μg/mL, with 100% recovery from serum. Inter-day and intraday precision values for quality control samples (16μg/mL, 32μg/mL, 64μg/mL) were 2.2%–3.2% and 2.8%–3.3% CV, respectively.
Footnotes
A collaborative effort of the U.S. Centers for Disease Control and Prevention (CDC); the Botswana National Tuberculosis Program (BNTP) of the Ministry of Health (MOH), Republic of Botswana; Princess Marina Hospital, Gaborone, Botswana; National Health Laboratory and National Tuberculosis Laboratory, Gaborone, Botswana; National Jewish Medical and Research Center, USA; the University of California, San Francisco (UCSF); and the University of California, Berkeley (UCB). The institutional review boards of the Botswana MOH, CDC, UCSF and UCB approved the protocol.
Contributors
S Chideya and CA Winston prepared and analyzed the data, and wrote the report. JW Tappero, CA Peloquin, WZ Bradford, PC Hopewell, AL Reingold, TA Kenyon, and TL Moeti contributed to study design, coordination, implementation, analysis and writing. CD Wells contributed to data analysis and writing. CA Peloquin performed pharmacokinetic analyses.
Potential conflicts of interest. All authors declare no conflict of interest with respect to this study.
Contributor Information
Sekai Chideya, Centers for Disease Control and Prevention, Atlanta, GA.
Carla A. Winston, Centers for Disease Control and Prevention, Atlanta, GA
Charles A. Peloquin, National Jewish Medical and Research Center, Denver, CO
William Z. Bradford, University of California, San Francisco and University of California, Berkeley
Philip C. Hopewell, University of California, San Francisco
Charles D. Wells, Centers for Disease Control and Prevention, Atlanta, GA
Arthur L. Reingold, University of California, Berkeley
Thomas A. Kenyon, Centers for Disease Control and Prevention, Atlanta, GA and The BOTUSA Project
Themba L. Moeti, National Tuberculosis Programme, Ministry of Health, Republic of Botswana, Gaborone, Botswana
Jordan W. Tappero, Centers for Disease Control and Prevention, Atlanta, GA and The BOTUSA Project
References
- 1.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Inter Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. [Accessed June 15, 2007];TB/HIV. http://www.who.int/tb/hiv/faq/en/index.html.
- 3.Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946–1986, with relevant subsequent publications. Int J Tuber Lung Dis. 1999;3(10):S231–S279. [PubMed] [Google Scholar]
- 4.Iseman MD. Tuberculosis therapy: past, present and future. Eur Resp J. 2002;20(Suppl 36):87s–94s. doi: 10.1183/09031936.02.00309102. [DOI] [PubMed] [Google Scholar]
- 5.Holdiness M. Clinical pharmacokinetics of the antituberculosis drugs. Clinical Pharmacokinetics. 1984;9:511–544. doi: 10.2165/00003088-198409060-00003. [DOI] [PubMed] [Google Scholar]
- 6.Pillai G, Fourie PB, Padayatchi N, Onyebujoh PC, McIlleron H, et al. Recent bioequivalence studies on fixed-dose combination antituberculosis drug formulations available on the global market. Int J Tuberc Lung Dis. 1999;3(11 Suppl 3):S309–316. [PubMed] [Google Scholar]
- 7.McIlleron H, Wash P, Burger A, Norman J, Folb PI, Smith P. Determinants of rifampin, isoniazid, pyrazinamide and ethambutol pharmacokinetics in a cohort of tuberculosis patients. Antimicrob Agents Chemother. 2006;50:1170–1177. doi: 10.1128/AAC.50.4.1170-1177.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkins JJ, Langdon G, McIlleron H, Pillai G, Smith PJ, Simonsson USH. Variability in the population pharmacokinetics of pyrazinamide in South African tuberculosis patients. Eur J Clin Pharmacol. 2006;62:727–735. doi: 10.1007/s00228-006-0141-z. [DOI] [PubMed] [Google Scholar]
- 9.Patel KB, Belmonte R, Crowe HM. Drug malabsorption and resistant tuberculosis in HIV-infected patients. N Eng J Med. 1995;332:336–337. doi: 10.1056/NEJM199502023320518. [DOI] [PubMed] [Google Scholar]
- 10.Peloquin CA, Nitta AT, Burman WJ, Brudney KF, Miranda-Massari JR, et al. Low antituberculosis drug concentrations in patients with AIDS. Ann Pharmacother. 1996;30:919–925. doi: 10.1177/106002809603000901. [DOI] [PubMed] [Google Scholar]
- 11.Sahai J, Gallicano K, Swick L, Tailor S, Garber G, et al. Reduced plasma concentrations of antituberculosis drugs in patients with HIV infection. Ann Int Med. 1997;127:289–293. doi: 10.7326/0003-4819-127-4-199708150-00006. [DOI] [PubMed] [Google Scholar]
- 12.Gurumurthy P, Ramachandran G, Hemanth Kumar AK, et al. Malabsorption of rifampin and isoniazid in HIV-infected patients with and without tuberculosis. Clin Infect Dis. 2004;38:280–3. doi: 10.1086/380795. [DOI] [PubMed] [Google Scholar]
- 13.Harries AD, Hargreaves NJ, Salaniponi FM. Design of regimens for treating tuberculosis patients with HIV infection, with particular reference to sub-Saharan Africa. Int J Tuberc Lung Dis. 2001;5(12):1109–1115. [PubMed] [Google Scholar]
- 14.World Health Organization. TB/HIV: A clinical manual. 2. World Health Organization; Geneva, Switzerland: 2004. [Google Scholar]
- 15.Chaisson RE, Clermont HC, Holt EA, et al. Six-month supervised intermittent tuberculosis therapy in Haitian patients with and without HIV infection. Am J Resp Crit Care Med. 1996;154(4):1034–1038. doi: 10.1164/ajrccm.154.4.8887603. [DOI] [PubMed] [Google Scholar]
- 16.Weiner M, Burman W, Vernon A, Benator D, Peloquin CA, et al. Low isoniazid concentrations and outcome of tuberculosis treatment with once-weekly isoniazid and rifapentine. Am J Respir Crit Care Med. 2003;167:1341–1347. doi: 10.1164/rccm.200208-951OC. [DOI] [PubMed] [Google Scholar]
- 17.Weiner M, Benator D, Burman W, Peloquin CA, Khan A, et al. Association between acquired rifamycin resistance and the pharmacokinetics of rifabutin and isoniazid among patients with HIV and tuberculosis. Clin Infect Dis. 2005;40:1481–1491. doi: 10.1086/429321. [DOI] [PubMed] [Google Scholar]
- 18.Mehta JB, Shantaveerapa H, Byrd RP, et al. Utility of rifampin blood levels in the treatment and follow-up of active pulmonary tuberculosis in patients who were slow to respond to routine Directly Observed Therapy. Chest. 2001;120:1520–1524. doi: 10.1378/chest.120.5.1520. [DOI] [PubMed] [Google Scholar]
- 19.Nelson LJ, Talbot EA, Mwasekaga MJ, Ngirubiu PK, Mwansa RA, Notha M, Wells CD. Anti-Tuberculosis Drug Resistance and Anonymous HIV Surveillance Among Tuberculosis Patients—Botswana. Lancet. 2005;366:488–90. doi: 10.1016/S0140-6736(05)67062-6. [DOI] [PubMed] [Google Scholar]
- 20.Talbot EA, Burgess DCH, Hone NM, Iademarco MF, Mwasekaga MJ, Moffat HJ, Moeti TL, Mwansa RA, Letsatsi P, Gokhale NT, Kenyon TA, Wells CW. Tuberculosis Serodiagnosis in a Predominantly HIV-infected Population of Hospitalized Patients with Cough, Botswana 2002. Clinical Infectious Diseases. 2004;39:e1–7. doi: 10.1086/421388. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. [Accessed May 23, 2007];TB Country Profiles. http://www.who.int/globalatlas/predefinedreports/tb/PDF_Files/bwa.pdf.
- 22.Tappero JW, Bradford WZ, Agerton TB, Hopewell P, Reingold AL, et al. Serum concentrations of antimycobacterial drugs in patients with pulmonary tuberculosis in Botswana. Clin Infect Dis. 2005;41:461–469. doi: 10.1086/431984. [DOI] [PubMed] [Google Scholar]
- 23.Botswana National Tuberculosis Programmme. Botswana National Tuberculosis Guidelines. 5. 1995. [Google Scholar]
- 24.Peloquin CA. Antituberculosis drugs: pharmacokinetics. In: Heifets L, editor. Drug susceptibility in the chemotherapy of mycobacterial infections. Boca Raton, FL: CRC Press; 1991. pp. 59–88. [Google Scholar]
- 25.Peloquin CA, Bulpitt AE, Jaresko GS, Jellife RW, James GT, et al. Pharmacokinetics of pyrazinamide under fasting conditions, with food, and with antacids. Pharmacotherapy. 1998;18:1205–1211. [PubMed] [Google Scholar]
- 26.Peloquin CA, Bulpitt AE, Jaresko GS, Jellife RW, James GT, et al. Pharmacokinetics of ethambutol under fasting conditions, with food, and with antacids. Antimicrob Agents Chemother. 1999;43:568–572. doi: 10.1128/aac.43.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peloquin CA, Namdar R, Dodge RR, Nix DE. Pharmacokinetics of isoniazid under fasting conditions, with food, and with antacids. Int J Tuber Lung Dis. 1999;3:703–710. [PubMed] [Google Scholar]
- 28.Peloquin CA, Namdar R, Singleton MD, Nix DE. Pharmacokinetics of rifampin under fasting conditions, with food, and with antacids. Chest. 1999;115:12–18. doi: 10.1378/chest.115.1.12. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Mitchison DA. The curious characteristics of pyrazinamide: a review. Int J Tuber Lung Dis. 2003;7:6–21. [PubMed] [Google Scholar]
- 30.Mitchison DA. The diagnosis and therapy of tuberculosis during the past 100 years. Am J Resp Crit Care Med. 2005;171:695–706. doi: 10.1164/rccm.200411-1603OE. [DOI] [PubMed] [Google Scholar]
- 31.Peloquin CA, Jaresko GS, Yong CL, Keung AC, Bulpitt AE, Jelliffe RW. Population pharmacokinetic modeling of isoniazid, rifampin, and pyrazinamide. Antimicrob Agents Chemother. 1997;41:2670–2679. doi: 10.1128/aac.41.12.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grosset J. The sterilizing value of rifampin and pyrazinamide in experimental short-course chemotherapy. Tubercle. 1978;59:287–297. [PubMed] [Google Scholar]
- 33.World Health Organization. Global tuberculosis control: surveillance, planning, and financing. Geneva: World Health Organization; 2005. [Google Scholar]
- 34.Johnson JL, Okwera A, Vjecha MJ, et al. Risk factors for relapse in human immunodeficiency virus type 1 infected adults with pulmonary tuberculosis. Int J Tuberc Lung Dis. 1997;1(5):446–453. [PubMed] [Google Scholar]