Abstract
Duodenal varices are an unexpected source of upper gastrointestinal haemorrhage associated with high mortality. The prevalence of ectopic variceal bleeding accounts for 2–5% of all variceal bleeding; of this, only 17% occurs in the duodenum. Diagnosis is difficult, and insufficient evidence exists to demonstrate the best treatment option when haemorrhage occurs. We report the case of a 69-year-old man with a history of chronic alcoholism who presented to the emergency department (ED) with nausea, vomiting and several episodes of haematochezia. Diagnostic workup in the ED included CT with multiplanar reconstruction, which revealed a network of large tortuous blood vessels running near the second portion of the duodenum between the inferior vena cava and portal vein. The patient was emergently treated with endoscopic therapy and clipping of the vessel. This failed, and he was subsequently taken to the operating room for suture ligation of the bleeding duodenal varices.
Background
Duodenal varices are a rare complication in patients with portal hypertension. The prevalence of ectopic variceal bleeding accounts for 2–5% of all variceal bleeding; of this, only 17% of ectopic variceal bleeding occurs in the duodenum.1 Though rare, haemorrhage from duodenal varices is life-threatening, with a mortality rate of up to 40% from the initial bleeding episode.2 Diagnosis is difficult, and insufficient evidence exists to demonstrate the best treatment option when haemorrhage occurs. Here we report a case of life-threatening bleed from duodenal varices detected with multidetector helical CT with multiplanar reconstruction, and treated with surgical ligation after failed endoscopic therapy. It serves to provide further evidence of the usefulness of multidetector helical CT with multiplanar reconstruction in detecting ectopic varices. Most importantly, it reminds us that ectopic varices, although uncommon, should be considered in cases where an upper gastrointestinal bleeding source is not clearly identifiable in patients with known liver disease.
Case presentation
A 69-year-old man presented to the emergency department with nausea, vomiting and several bloody bowel movements containing bright red blood and clots. He also reported weakness, fatigue and light-headedness, as well as right-sided abdominal pain. He denied recent illness, fevers, chills, cough, shortness of breath, chest pain, dysuria, haematuria and haematemesis.
The patient's medical history included hypertension, gastro-oesophageal reflux disease and chronic back pain. His surgical history was significant for lumbar laminectomy. The patient's medications included omeprazole, ibuprofen and hydrocodone. His family history was unremarkable. The patient had a history of heavy smoking, but had quit several years prior. He denied any illicit drug use, but reported heavy alcohol intake for the past 5 years. Notably, he had quit drinking abruptly several days prior to admission.
On physical examination, the patient was afebrile, and demonstrated a heart rate of 114 bpm, blood pressure of 134/83 mm Hg and a respiratory rate of 22. He was lethargic but oriented to person, place and time. He generally appeared ill, with pale non-jaundiced skin and conjunctiva. Cardiac examiniation was notable only for tachycardia. The patient's bilateral lung fields were clear to auscultation and his abdomen was soft and mildly distended, with mild right-sided tenderness to deep palpation. No rebound tenderness, rigidity or guarding were noted. Bowel sounds were diminished. On rectal examination, dark blood and clot were noted. The extremities revealed no cyanosis, clubbing or oedema. A nasogastric (NG) tube was placed, which revealed bilious aspirate with no frank blood present; however, the aspirate was haemoccult positive.
The patient's initial laboratory data demonstrated a white cell count of 6.5×103/mm3 (normal range 4–11), haemoglobin of 9.3 g/dL (normal range 12–16) and platelet count of 112×103/mm3 (normal range 140–440). His sodium was 141 mmol/L (normal range 135–145), potassium 4.8 mmol/L (normal range 3.5–5.3), blood urea nitrogen 17 mg/dL (normal range 6–20), creatine 0.8 mg/dL (normal range 0.6–1.1), aspartate aminotransferase 134 U/L (normal range 0–37), alanine aminotransferase 63 U/L (normal range 6–37), lipase 65 U/L (normal range 16–63) and troponin T was less than 0.01 ng/mL (normal range 0.00–0.03). The patient's lactic acid level was 7.9 mmol/L (normal range 0.7–2.5). The international normalised ration (INR) was elevated at 1.6 (normal value around 1.0), and prothrombin time (PT) was 28 s (the normal range being 23–32). An emergent CT scan of the abdomen and pelvis initially revealed a mild degree of mucosal thickening in the area of the caecum, possibly reflecting colitis and mild distension of the stomach with fluid. The bowel was otherwise unremarkable.
The medical intensivists were consulted for admission. While in the ED, the patient had non-bloody emesis, and an additional episode of haematochezia passing blood and clots. His blood pressure dropped but responded to a normal saline bolus. Two units of typed and crossed packed red blood cells (PRBCs) were started, and the patient was transferred to the intensive care unit (ICU) for ongoing resuscitation and monitoring. Two units of fresh frozen plasma (FFP) and 10 mg of vitamin K were given to correct the elevated INR, esomeprazole was started, and a central venous line and arterial line were placed. The patient was also started on a Clinical Institute Withdrawal Assessment protocol. Gastroenterology and general surgery consults were emergently obtained.
Eight hours after admission, the patient's follow-up haemoglobin was 7.6 g/dL, which was concerning for ongoing gastrointestinal haemorrhage. His vitals included a blood pressure of 91/68 mm Hg, heart rate 120 bpm, respiratory rate 22 breaths/min and oxygen saturation 99% on 4 L nasal cannula. Lactic acid had increased to 9.4 mmol/L. As a bleed had not been localised, upper endoscopy was performed at the bedside. Oesophagogastroduodenoscopy revealed mild non-bleeding oesophageal varices with a normal stomach. Minimal haemosiderin-stained bile was noted in the antrum, with a large amount of bright red blood in the duodenum. Just distal to the ampulla, a subcentimeter erosion with an adherent clot was noted. The base was injected with epinephrine, and upon withdrawal of the needle, a single continuous stream of blood was observed. This was clipped, successfully achieving haemostasis.
Following the procedure, interventional radiology was consulted for a mesenteric arteriogram with possible embolisation. During this consultation, the CT was re-examined demonstrating the sequelae of portal hypertension, and noted a network of large tortuous blood vessels running near the second portion of the duodenum between the inferior vena cava and portal vein (figures 1–3). An attempt at coil embolisation of the duodenal varices was planned; however, the patient began having massive bright red blood around his NG tube and through his nares while in the ICU. He became profoundly hypotensive and tachycardic. He was therefore taken to the operating room for an emergency laparotomy, and the massive transfusion protocol was initiated.
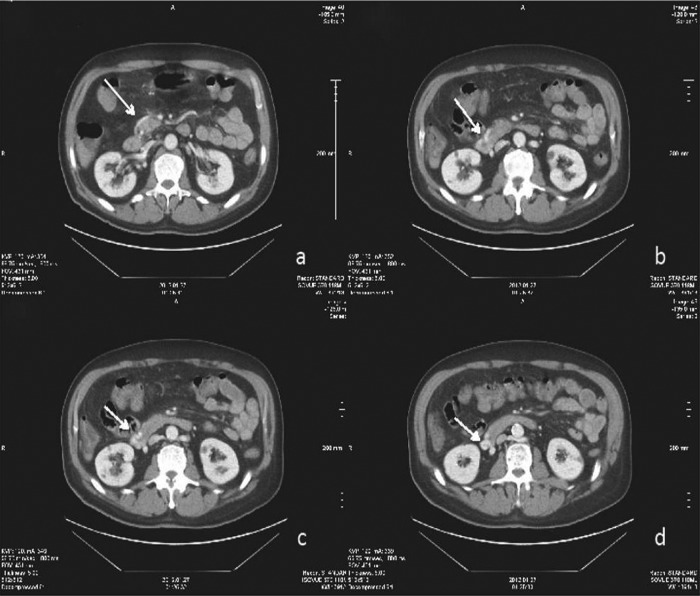
Figure 1.
Axial contrast-enhanced CT images through the junction of the second and third portions of the duodenum. White arrow denotes varix arising from the superior mesenteric vein, embedded in the wall of the duodenum.
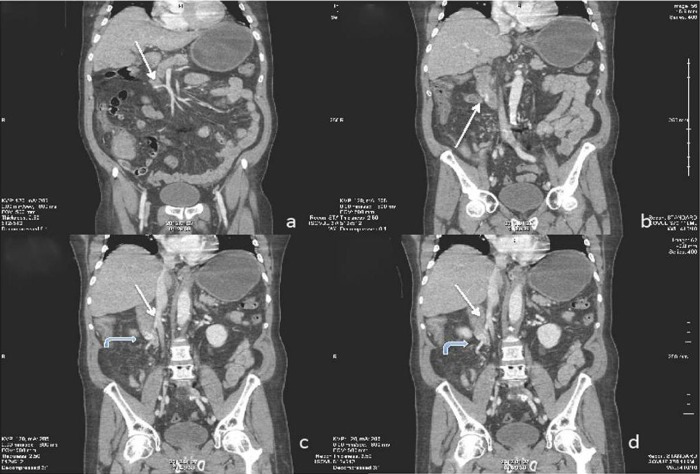
Figure 2.
Coronal reconstructed CT images through the second and third portions of the duodenum. (2A) White arrow better delineates varix originating from the superior mesenteric vein. (2B). Varix embedded in the lateral duodenal wall. Curved arrows in (2C and D) demonstrate the circuitous route the varix takes inferiorly ultimately draining into the posterior, lateral aspect of the inferior vena cava.
Figure 3.
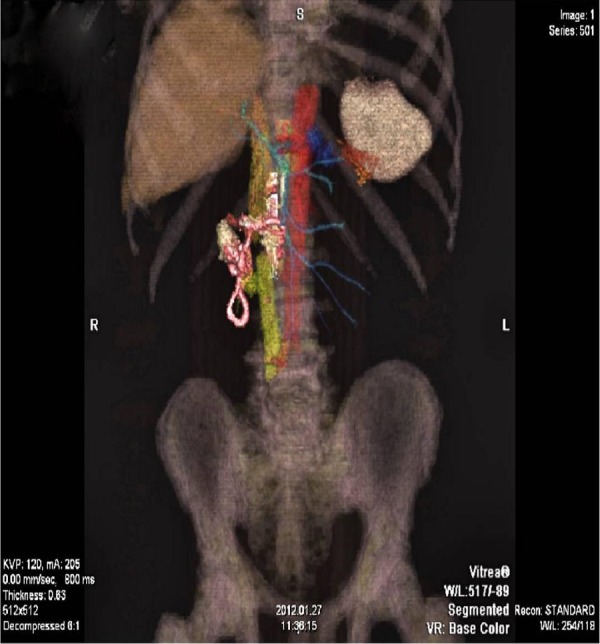
Volume rendered, colour-coded, 3D reconstructed image demonstrating a circuitous course of duodenal varix (pink). Varix arises from the superior mesenteric vein (blue) and drains into the inferior vena cava (yellow). Duodenum (white), aorta (red) for reference (Image courtesy of John Sherwood, MD).
On entry of the abdomen, clear ascitic fluid was noted, the stomach and small bowel were distended with blood and a frankly cirrhotic liver was visualised. The duodenum was then kocherized, and an enterotomy was made at the second portion of the duodenum. There was a massive efflux of old, dark blood. The surgeon's finger was inserted into the lumen of the duodenum and the endoscopically-placed clips were palpated. The enterotomy was opened further distally, allowing direct visualisation of the lumen and erosion. Profuse bleeding was identified from the bowel wall along the edges of the enterotomy and from the lesion. Clamps and ties were placed for initial control, and the lesion was oversewn with a 2-0 silk and packs were put in place. This allowed for initial control of the bleeding, and anaesthesia was allowed to catch up. The patient continued to be hypotensive with a systolic blood pressure of 80–90 mm Hg while receiving blood products, coagulation factors and pressor support.
On re-exploration, the duodenal wall was friable, and there were numerous areas of haemorrhage from the bowel wall despite numerous attempts to achieve haemostasis. The abdomen was again packed, allowing anaesthesia to continue with aggressive resuscitation. After a period of time, a final attempt was made to control the remaining bleeding vessels from the bowel wall with clamps and ties. This was successful, and no large bleeding vessels remained. The patient, however, continued to have ongoing oozing from raw surfaces and the skin edges. The abdomen was packed with lap pads, and the patient transferred back to the ICU for continued resuscitation.
In total, the operative time was approximately 90 min, and the patient received a total of 33 units of PRBCs, 25 units of FFP, 5 units of cryoprecipitate, five 6-packs of platelets and 1500 mL of crystalloid. His estimated blood loss was calculated to be 23 L total, before and during the case. Throughout the case, the patient was euthermic, and his systolic blood pressure ranged from 75 to 110 mm Hg.
Outcome and follow-up
Postoperatively, the patient was transferred back to the ICU. On arrival, the patient's INR was 1.5, with a PT of 51 s. Haemoglobin was 7.2 g/dL, platelet count 47×103/mm3 and fibrinogen 167 mg/dL. His pH was 7.33 (normal range 7.35–7.45), CO2 40 mm Hg (normal range 35–45), O2 175 mm Hg (normal range 80–90) and base deficit 4.9 mEq/L (normal range 0–2.5). Further resuscitation was continued with correction of the patient's acidosis and coagulopathy. Despite maximal attempts at optimising the patient's physiology, he continued to be hypotensive and tachycardic, consistent with ongoing blood loss. Consideration for a transjugular intrahepatic portosystemic shunt (TIPS) was made, but the patient was deemed too unstable and unlikely to survive the procedure. After a discussion with the family, surgery and the critical care team, it was determined by all that further operative intervention would be futile. The decision to withdraw care was made, and comfort measures were implemented. He expired shortly thereafter.
Discussion
Our report describes an unusual case of upper gastrointestinal haemorrhage arising from ectopic varices located in the second and third portion of the descending duodenum. The patient's initial presentation was confounded by the symptom of haematochezia, which may represent an upper gastrointestinal bleed, but is more indicative of a lower gastrointestinal source. Second, the patient's CT scan was initially interpreted with changes suggesting ischaemic colitis, which is also indicative of a lower gastrointestinal bleed. Finally, the NG aspirate did not contain frank blood, further confounding the patient's presentation as a lower gastrointestinal source. Despite this unusual presentation, an upper gastrointestinal source was not ruled out prematurely, and a timely endoscopy was performed.
Duodenal varices were first described in 1931 by Alberti et al. As these varices are outside the gastro-oesophageal junction, they are referred to as ectopic varices.3 Anatomically, they usually consist of a single vessel with associated afferent and efferent vessels forming a retroperitoneal portosystemic shunt.4 The afferent vessel arises from either the superior or the inferior pancreaticoduodenal vein or from the superior mesenteric vein, and the efferent vessel is thought to arise from one of the many retroperitoneal veins draining into the inferior vena cava.4 These communications between intestinal or retroperitoneal tributaries of the superior and inferior mesenteric veins with the inferior vena cava are called the veins of Retzius.5 Duodenal varices, therefore, are considered dilated veins of Retzius located in the pancreaticoduodenal region. The duodenal bulb is reported to be the most common location, with decreasing frequency as one travels distally down the duodenum.6 7 A more recent study, however, reported duodenal varices to commonly occur in the descending part of the duodenum, occasionally coexisting with varices in the oesophagus, stomach and duodenal bulb.8
Duodenal varices most frequently result from cirrhosis of the liver, portal vein thrombosis or obstruction of the splenic vein and inferior vena cava.6 Other rare aetiologies include schistosomiasis, venoocclusive disease, congenital malformations, postoperative shunt thrombosis and intra-abdominal adhesions related to prior surgery.6 9 A diagnosis is usually based on findings obtained by an endoscopy or an angiography. The diagnosis can be quite difficult; however, as duodenal varices are difficult to identify on an endoscopy due to their serosal and submucosal location.10 Furthermore, a conventional endoscopy is unable to reach the distal site of the duodenum, and an angiography occasionally fails to depict duodenal varices due to inappropriate catheter cannulation to collateral vessels.11 The pathology may also not be recognised as it is so rare, or may be mistaken for other more common forms of pathology, such as duodenal ulcers. Capsule endoscopy allows for the possibility to see small intestinal mucosa, and is reported to be sensitive in detecting obscure gastrointestinal bleeding.12 A CT angiography also has been reported as efficient in detecting duodenal varices.5 The multislice helical CT has also been shown to be effective in depicting duodenal varices as a cause of massive upper gastrointestinal bleeding, along with recent reports of the effectiveness of the multidetector helical CT with multiplanar reconstruction, suggesting that this may play an increasingly important role in the detection of ectopic intestinal varices in patients with cirrhosis and gastrointestinal bleeding.13 14
Treatment of variceal haemorrhage in the upper gastrointestinal tract is largely standardised.1 15 However, there is insufficient evidence to demonstrate the best treatment option for duodenal variceal bleeding due to the limited number of reports of duodenal varices cases, and the lack of randomised control trials relating to the management of this condition. After initial resuscitation, there are several treatment options available. These include vasoactive medications, endoscopic injection sclerotherapy and variceal ligation, TIPS procedure, endovascular obliteration and surgical interventions such as surgical variceal ligation and sclerotherapy, surgical shunt placement or segmental bowel resection.1 Endoscopic treatment is one of the most reasonable options, as it can provide diagnosis as well as the potential for less invasive intervention and treatment in the form of sclerotherapy and ligation.15 However, the efficacy of endoscopic variceal ligation is short-term and limited to small varices, and endoscopic injection sclerotherapy appears to be less effective against duodenal varices and also has a risk of perforation after the procedure.1 16 17 TIPS is a means of effectively treating acute variceal haemorrhage in patients refractory to endoscopic treatment, and in patients whose gastrointestinal varices are difficult to access by endovascular intervention, or who are poor surgical candidates.1 9 It is effective in decompressing an elevated portosystemic pressure gradient, but decreases hepatic clearance by reducing blood flow through the portal vein, and promotes hepatic encephalopathy by increasing portosystemic shunt blood flow.15–18 TIPS with concomitant endovascular embolisation have also been reported to be effective, with the added benefit of addressing the portal hypertension as well as the source of the haemorrhage.9 19 Embolisation of bleeding duodenal varices using percutaneous transhepatic or balloon-occluded retrograde transvenous obliteration has been described in several case reports and is an accepted alternative for the management of life-threatening bleeding.20–22 This was the initial endovascular consideration in our patient in order to expedite the patient's stability prior to TIPS. However, this method is difficult to perform in patients with multiple afferent vessels, and is only a temporary measure as it does not alter portal pressures, allowing new collaterals to develop or pre-existing varices to recanalize.20 23 Surgical shunt placement or surgical variceal ligation may be necessary to control haemorrhage in patients that sclerotherapy has failed.2 Although surgical ligation and sclerotherapy is more invasive than other procedures, it allows for the possibility to achieve curative effects on duodenal varices, especially in patients with reserved liver function and complicated shunt vessels.14
Learning points.
Although rare, duodenal varices should remain in the differential diagnosis of upper gastrointestinal haemorrhage, especially in individuals with portal hypertension or known liver disease.
The diagnosis remains challenging. However, with the aid of multislice helical CT scans with multiplanar reconstruction, in addition to endoscopy, this rare and unexpected source of haemorrhage can be detected.
As demonstrated in this case, the treatment of ectopic variceal bleeding is problematic, and likely requires a multidisciplinary approach combining gastroenterology, interventional radiology and surgery.
Acknowledgments
We thank Dr Lisa Miller, attending surgeon, and Dr John Ebersole, radiologist, for their knowledge and guidance in the preparation of this manuscript.
Footnotes
Contributors: JVL and DMB were the primary authors and data collectors for the manuscript. LHB and EAS significantly revised the manuscript for publication purposes.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Norton ID, Andrews JC, Kamath PS. Management of ectopic varices. Hepatology 1998;2013:1154–8 [DOI] [PubMed] [Google Scholar]
- 2.Khouqueer F, Morrow C, Jordan P. Duodenal varices as a cause of massive upper gastrointestinal bleeding. Surgery 1987;2013:548–52 [PubMed] [Google Scholar]
- 3.Alberti W. Uber den roentgenologischen Nachweis von Varizen in Buolbus duodeni. Fortschro Geb Roentgenstr 1931;2013:60–5 [Google Scholar]
- 4.Hashizume M, Tanoue K, Ohta M, et al. Vascular anatomy of duodenal varices: angiographic and histopathological assessments. Am J Gastroenterol 1993;2013:1942–5 [PubMed] [Google Scholar]
- 5.Ibukuro K, Tsukiyama T, Mori K, et al. Veins of Retzius at CT during arterial portography: anatomy and clinical importance. Radiology 1998;2013:793–800 [DOI] [PubMed] [Google Scholar]
- 6.Amin R, Alexis R, Korzis J. Fatal ruptured duodenal varix: a case report and review of literature. Am J Gastroenterol 1985;2013:13–18 [PubMed] [Google Scholar]
- 7.Tanaka T, Kato K, Taniguchi I, et al. A case of ruptured duodenal varices and review of the literature. Jpn J Surg 1988;2013:595–600 [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Yang J, Yang Y. Clinical characteristics and endoscopic treatment with cyanoacrylate injection in patients with duodenal varices. Scandinavian J Gastroenterol 2009;2013:1012–16 [DOI] [PubMed] [Google Scholar]
- 9.Haskal ZJ, Scott M, Rubin RA, et al. Intestinal varices: treatment with transjugular intrahepatic portosystemic shunt. Radiology 1994;2013:183–7 [DOI] [PubMed] [Google Scholar]
- 10.Sukigara M, Koyama I, Komazaki T, et al. Bleeding varices located in the second portion of the duodenum. Jpn J Surg 1987;2013:130–5 [DOI] [PubMed] [Google Scholar]
- 11.Perchik L, Max T. Massive haemorrhage from varices of the duodenal loop in a cirrhotic patient. Radiology 1963;2013:641–5 [Google Scholar]
- 12.Pennazio M, Santucci R, Rondonotti E, et al. Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: report of 100 consecutive cases. Gastroenterology 2004;2013:643–53 [DOI] [PubMed] [Google Scholar]
- 13.Weishaupt D, Pfammatter T, Hilfiker PR, et al. Detecting bleeding duodenal varices with multislice helical CT. AJR Am J Roentgenol 2002;2013:399–401 [DOI] [PubMed] [Google Scholar]
- 14.Yoshida S, Watabe H, Omata M. Usefulness of multi-detector helical CT with multiplanar reconstruction for depicting the duodenal varices with multiple collateral shunt vessels. Hepatol Int 2010;2013:775–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanley AJ, Hayes PC. Portal hypertension and variceal haemorrhage. Lancet 1997;2013:1235–9 [DOI] [PubMed] [Google Scholar]
- 16.Yoshida Y, Imai Y, Nishikawa M, et al. Successful endoscopic injection sclerotherapy with n-butyl-2-cyanoacrylate following the recurrence of bleeding soon after endoscopic ligation for ruptured duodenal varices. Am J Gastroenterol 1997;2013:1227–9 [PubMed] [Google Scholar]
- 17.Tsuji H, Okano H, Fujino H, et al. A case of endoscopic injection sclerotherapy for a bleeding duodenal varix. Gastroenterol Jpn 1989;2013:60–4 [DOI] [PubMed] [Google Scholar]
- 18.Sahagun G, Benner KG, Saxon R, et al. Outcome of 100 patients after transjugular intrahepatic porto-systemic shunt for variceal haemorrhage. Am J Gastroenterol 1997;2013:1444–52 [PubMed] [Google Scholar]
- 19.Lopera JE, Arthurs B, Scheuerman C, et al. Bleeding duodenal: varices treatment by TIPS and transcatheter embolization. Cardiovasc Intervent Radiol 2008;2013:431–4 [DOI] [PubMed] [Google Scholar]
- 20.Menu Y, Gayet B, Nahum H. Bleeding duodenal varices: diagnosis and treatment by percutaneous portography and transcatheter embolization. Gastrointest Radiol 1987;2013:111–13 [DOI] [PubMed] [Google Scholar]
- 21.Sonomura T, Horihata K, Yamahara K, et al. Ruptured duodenal varices successfully treated with balloon-occluded retrograde transvenous obliteration: usefulness of microcatheters. AJR Am J Roentgenol 2003;2013:725–7 [DOI] [PubMed] [Google Scholar]
- 22.Kaji T, Tamura J, Matsumoto M, et al. Superior mesenteric varices and splenic renal shunt with portal hypertension: a case report of liver cirrhosis with complications. J Int Med Res 1993;2013:58–65 [DOI] [PubMed] [Google Scholar]
- 23.Ozaki CK, Hansen M, Kadir S. Transhepatic embolization of superior mesenteric varices in portal hypertension. Surgery 1989;2013:446–8 [PubMed] [Google Scholar]