Abstract
Objective
Striatum-based circuits have been implicated in both major depressive disorder (MDD) and anhedonia, a symptom that reflects deficits of reward processing. Yet adolescents with MDD often exhibit a wide range of anhedonia severity. Addressing this clinical phenomenon, we aimed to use intrinsic functional connectivity (iFC) to study striatum-based circuitry in relation to categorical diagnosis of MDD and anhedonia severity.
Method
A total of 21 psychotropic medication–free adolescents with MDD and 21 healthy controls (HC), group-matched for age and sex, underwent resting-state functional magnetic resonance imagining (fMRI) scans. Voxelwise maps indicating correlation strengths of spontaneous blood-oxygenation-level–dependent (BOLD) signals among 6 bilateral striatal seeds (dorsal caudate, ventral caudate, nucleus accumbens, dorsal-rostral putamen, dorsal-caudal putamen, ventral-rostral putamen) and the remaining brain regions were compared between groups. Relationships between striatal iFC and severity of MDD and anhedonia were examined in the MDD group. Analyses were corrected for multiple comparisons.
Results
Adolescents with MDD manifested increased iFC between all striatal regions bilaterally and the dorsomedial prefrontal cortex (dmPFC), as well as between the right ventral caudate and the anterior cingulate cortex (ACC). MDD severity was associated with iFC between the striatum and midline structures including the precuneus, posterior cingulate cortex, and dmPFC. However, distinct striatal iFC patterns involving the pregenual ACC, subgenual ACC, supplementary motor area, and supramarginal gyrus were associated with anhedonia severity.
Conclusions
Although MDD diagnosis and severity were related to striatal networks involving midline cortical structures, distinct circuits within the reward system were associated with anhedonia.
Keywords: depression, functional connectivity, functional magnetic resonance imagining (fMRI), intrinsic functional connectivity (iFC)
Adolescent major depressive disorder (MDD) is a profoundly disabling illness, yet its pathophysiology and underlying neural circuitry remain poorly defined. It has become evident that the inherent heterogeneity of psychiatric disorders has been a major impediment to the development of reliable biomarkers. Therefore, the field has emphasized the importance of investigating specific symptoms along the continuum of severity. Anhedonia, a core symptom of MDD that reflect deficits in reward processing, has been the target of such investigations.1-3 Although anhedonia is highly prevalent among depressed adolescents,4 its extent is quite variable which results in contrasting MDD phenotypes.5,6 Notably, converging evidence, including recent data from the Treatment of Resistant Depression in Adolescents (TORDIA) multisite trial, indicates that anhedonia may represent a negative prognostic predictor for suicide and treatment response.7-9 In our previous work, we identified specific neurobiological correlates of anhedonia involving the immune system2 and the major inhibitory neurotransmitter γ-aminobutyric acid (GABA).3 The current study extends this line of work by investigating striatal circuitry in adolescents with MDD as it relates to anhedonia severity.
Converging evidence suggests that anhedonia reflects disturbances in reward circuitry tied to the mesolimbic striatum–based system underlying reward processing.10,11 Because anhedonia is a core symptom of MDD, functional magnetic resonance imagining (fMRI) studies have used pleasant stimuli (images) or reward-related tasks to study MDD. Broadly, the most consistent findings have been hypoactivation of the ventral and dorsal striatum along with altered activation (both hyper- and hypoactivation) in the medial prefrontal cortex (PFC), specifically the dorsomedial PFC (dmPFC) and the perigenual (pg) and subgeneual (sg) anterior cingulate cortex (ACC).12-18 However, most of these studies did not account for interindividual differences in anhedonia severity among the examined population. Here, we used a seed-based approach to investigate resting-state functional connectivity (RSFC) and to illuminate striatum-based circuitry related to adolescent MDD and anhedonia. RSFC identifies functional networks based on patterns of correlation in low-frequency fluctuations of blood-oxygenation-level–dependent (BOLD) signals during rest, referred to as intrinsic functional connectivity (iFC).19-22 The absence of a task minimizes potential floor, ceiling, and practice effects and allows recruitment of subjects who would not otherwise be able to perform a cognitive task satisfactorily (e.g., younger or more severely ill subjects). There have been multiple RSFC investigations in adult MDD and a few in pediatric MDD populations. These studies confirm task-based fMRI findings of alterations in fronto-striatal-limbic circuits along with medial wall abnormalities in both adults23-27 and adolescents with MDD.28-30 However, findings have been conflicting as to whether MDD is associated with increased31-34 or decreased17,24,29,35 iFC along these circuits. This inconsistency may be related to the techniques used, as these studies have not directly examined striatal circuitry. Indeed, a recent study of striatal circuitry in adults with MDD reported decreased iFC between the ventral striatum and the sgACC but increased iFC between the dorsal caudate and dorsolateral PFC.36
Building upon these observations, our aims were to study striatum-based iFC in adolescents with MDD and its relationships to severity of illness and anhedonia. We used a previously validated set of 6 bilateral striatal seeds consisting of the dorsal and ventral caudate, the nucleus accumbens (NAc), and the dorsal (caudal and rostral) and ventral putamen.37-40 Based on prior resting-state and task-based fMRI findings in pediatric and adult MDD, 12-21 we hypothesized the following: that, compared to healthy controls (HC), psychotropic-medication-free adolescents with MDD would exhibit altered iFC along frontostriatal circuits involving both the dmPFC and ACC (specifically decreased iFC with ventral striatum seeds and increased iFC with dorsal caudate seeds, per prior seed-based striatal findings in adult MDD36); and that, in the MDD group, anhedonia severity would be associated with strength of striatal iFC with brain regions identified in the group comparison, particularly the dmPFC and ACC. Furthermore, in light of substantial data linking the sgACC and pgACC with reward circuitry, we expected that these regions would be separately identified in relation to anhedonia severity. Analyses were repeated using a set of 3 bilateral striatal seeds corresponding to the entire caudate, putamen, and NAc, with results provided in Supplement 1, available online.
Method
Study Participants
We enrolled 21 adolescents with MDD (aged 12–19 years, mean 17.1 ± 2.5, 12 female and 9 male) and 21 HC (aged 13–19, mean 16.3 ± 1.4, 12 female and 9 male), group-matched for age, sex, and handedness. Fourteen subjects in each group were also enrolled in a prior proton MR spectroscopy study of GABA in adolescents with MDD and anhedonia.3 Subjects with MDD were recruited from the New York University (NYU) Child Study Center, from the Bellevue Hospital Center Department of Psychiatry, and through local advertisements in the New York (NY) metropolitan area. Healthy control (HC) subjects were recruited from the greater NY metropolitan area through local advertisements and from the families of NYU staff. This study was approved by the NYU School of Medicine Institutional Review Board and the NYU University Committee on Activities Involving Human Subjects. Before baseline clinical evaluations, study procedures were explained to subjects and parents. Participants aged 18 years and older provided informed consent; those less than 18 years provided assent and a parent-provided informed consent.
Inclusion and Exclusion Criteria
Exclusion criteria for all subjects consisted of the presence of any significant medical or neurological disorder, IQ < 80, claustrophobia, or any MRI contraindication as assessed by a standard safety screening form, a positive urine toxicology test, or a positive urine pregnancy test in females.
All adolescents with MDD met the DSM-IV-TR diagnosis of MDD with current episode ≥ 8 weeks duration, raw severity score ≥ 40 (T score ≥ 63) on the Children's Depression Rating Scale–Revised (CDRS-R), and psychotropic-medication–free status ≥ 3 months.
Exclusionary diagnoses included a lifetime history of bipolar disorder, schizophrenia, pervasive developmental disorder, panic disorder, obsessive-compulsive disorder, conduct disorder, or Tourette's disorder; or a substance-related disorder in the past 12 months. A current diagnosis of posttraumatic stress disorder or an eating disorder was also exclusionary.
HC subjects did not meet criteria for any current or past DSM-IV-TR diagnoses and had never received psychotropic medication.
Clinical Assessments
Subjects and parents were interviewed by a board-certified child and adolescent psychiatrist (V.G., C.A.) at the NYU Child Study Center. Diagnoses were established using the Schedule for Affective Disorders and Schizophrenia for School-Aged Children, Present and Lifetime Version (K-SADS-PL),41 a semistructured interview completed with subjects and parents. Additional assessments included the CDRS-R and the Beck Depression Inventory, 2nd edition (BDI-II).42 IQ was estimated with the Kaufman Brief Intelligence Test43 or the Wechsler Abbreviated Scale of Intelligence.44 Urine toxicology and pregnancy tests were administered on the day of the scan.
Severity of MDD Episode
The severity of MDD episodes was determined from CDRS-R scores.
Anhedonia
The anhedonia score (range 1–13) for each subject was computed, as in our previous work,3 by summing the responses associated with anhedonia on a self-rated questionnaire and a clinician-rated scale: the self-rated BDI-II (0–3 points for item 4: “loss of pleasure” and 0-3 points for item 12: “loss of interest”); and the clinician-rated CDRS-R (1–7 points for item 2: “difficulty having fun”). Thus, clinician- and self-rated assessments each contributed equally to the computed anhedonia score (0–6 points from the BDI-II and 1–7 points from the CDRS-R). Such an approach has been previously used to assess anhedonia severity in others' and our laboratories.1,3,7,45,46
Data Acquisition
Imaging data were acquired on a Siemens Allegra 3.0T scanner at the NYU Center for Brain Imaging. For each participant, a high-resolution T1-weighted anatomical image (magnetization prepared rapid acquisition gradient-echo [MPRAGE]; repetition time [TR] = 2,500 ms; echo time [TE] = 3.93 ms; inversion time [TI] = 600 ms; flip angle = 8°; 176 slices; field of view [FOV] = 256 × 256 mm2; voxel size = 1×1×1 mm3) was acquired. Resting-state fMRI data were acquired using an echo planar imaging (EPI) sequence (197 whole-brain volumes; TR = 2,000 ms; effective TE = 25 ms; flip angle = 90°; 39 contiguous 3-mm oblique axial slices parallel to the AC-PC; matrix = 64×64; FOV = 192×192 mm2; voxel size = 3×3×3 mm3). Participants were asked to relax with their eyes open while the word “Relax” was displayed.
Data Analysis
Image Preprocessing
Consistent with prior work, we used a combination of AFNI (http://afni.nimh.nih.gov/afni) and the FMRIB software library tool (FSL, www.fmrib.ox.ac.uk).47-49 Resting-state data preprocessing comprised slice time correction for interleaved slice acquisition, 3D motion correction, despiking, spatial smoothing (using a 3D spatial filter implemented in FSL with full width at half maximum (FWHM) = 6 mm), mean-based intensity normalization of all volumes by the same factor, temporal bandpass filtering (0.009–0.1 Hz) and linear and quadratic detrending. Linear registration of high-resolution structural images to the Montreal Neurological Institute MNI152 template with 2×2×2 mm3 resolution was carried out using the FSL tool FLIRT, and was then refined using FNIRT nonlinear registration.50 Linear registration of each participant's functional data to his or her high-resolution structural image was also carried out using FLIRT.
Nuisance Signal Regression
As described elsewhere,39 to control for motion, physiological nuisance signals (e.g., cardiac and respiratory fluctuations), and the large-scale global neural signal(s) present in all voxels throughout the cortex,51 we regressed the pre-processed data on the following 9 nuisance covariates: white matter, cerebrospinal fluid, 6 motion parameters, and the global signal. The resultant 4-dimensional residual time-series were transformed into MNI152 2-mm standard space and used for subsequent participant-level correlation analyses.
As there remains controversy regarding whether to correct for the global signal,21,51-53 and how to do so without introducing artifactual findings,54 we repeated analyses without global signal correction. These results, which are consistent with our primary findings, are presented in Supplement 1 and Table S2, available online.
Selection of Regions of Interest
We used 6 bilateral striatal seeds as described by Di Martino et al. (2008).37 Each seed region of interest (ROI) was approximately spherical (volume = 257×1 mm3 voxels, radius = ∼4 mm). These were located (in MNI152 space) in the following: nucleus accumbens (NAc; ±9, 9, −8); ventral caudate (VC; ±10, 15, 0); dorsal caudate (DC; ±13, 15, 9), dorsal caudal putamen (DCP; ±28, 1, 3); dorsal rostral putamen (DRP; ±25, 8, 6), and ventral rostral putamen (VRP; ±20, 12, −3). These seed ROI were defined based on anatomical and functional subdivisions of the striatum,55,56 and their iFC patterns have been replicated independently.38-40,57 Secondary analyses, focusing on broader striatal divisions, used a set of 3 anatomically defined ROI consisting of the right and left caudate, putamen, and NAc as defined by the Harvard-Oxford Structural Atlas;58 the results of these analyses were largely confirmatory and are available in Supplement 1, Table S1, and Figures S1–S3, available online.
Subject-Level iFC Analysis
For each participant, we first resampled the 4-dimensional EPI residuals to 1×1×1 mm3 and applied seed masks to the resampled data to obtain representative time series from the seed ROI. Each extracted seed ROI time series was then used to calculate the correlation between it and that of every other voxel in the EPI residuals data in native (i.e., acquisition) space to derive iFC maps. The resultant participant-level correlation maps were Fisher z transformed to Z-value maps and transformed into MNI152 2 mm3 standard space for group-level analyses.
Group-Level iFC Analysis and Brain–Behavior Associations
Analyses of group-level iFC for adolescents with MDD versus HC were carried out using a random-effects least-squares model implemented in FSL (nuisance covariates: age, sex, full-scale IQ). This group-level analysis produced thresholded Z-score maps of positive and negative iFC for each striatal ROI and for each condition. Direct voxelwise condition comparisons of the 2 groups (HC, MDD) produced thresholded Z-score maps of those voxels that showed significant iFC differences between the groups for each ROI.
To assess dimensional relationships between striatal iFC and anhedonia, voxel-wise regression analyses were carried out in the MDD group, with anhedonia scores as the covariate of interest. Since severity of MDD episode and anhedonia scores were significantly correlated, all analyses controlled for CDRS-R scores with the anhedonia question omitted to account for the nonspecific influences of depressive episode severity. Resulting relationships between iFC and our clinical variables are thus partial correlations. The HC group was excluded from this analysis because of the limited range of anhedonia scores.
For all analyses, cluster-level corrections for multiple comparisons were performed using Gaussian random field theory (Z > 2.3; cluster significance: p < .008 corrected; p < .008 was selected to take into account the number of independent seed regions used (0.008 = 0.05/6). Six, as opposed to 12, seed ROIs were considered, given the high degree of correlated activity between homotopic seed regions59,60). Scatterplots found in Figures 2 and 3, and in S4, available online, demonstrating the iFC relationships for all significant clusters, are for illustrative purposes only and were not used for calculating r values.
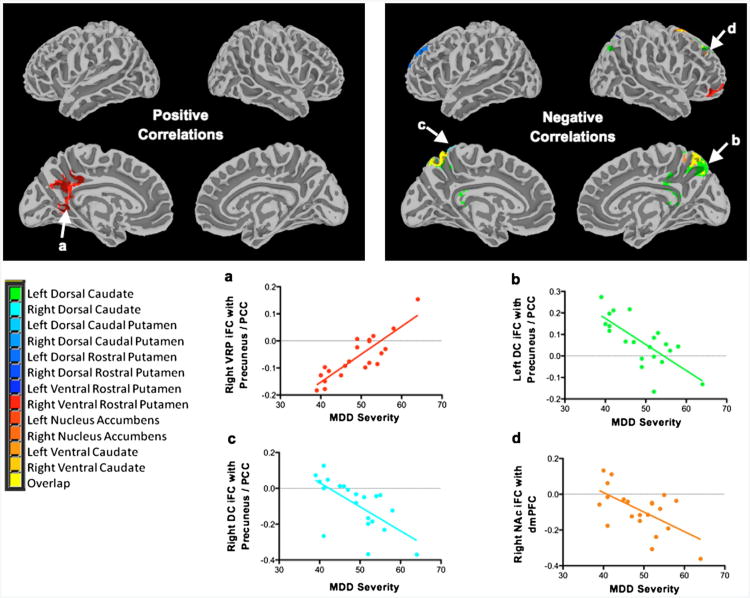
Figure 2.
Intrinsic functional connectivity (iFC) correlations with major depressive disorder (MDD) severity and associated plots. Note: Maps showing regions with iFC (left) positively correlated with MDD severity and (right) negatively correlated with MDD severity. Plots a–d below demonstrate these relationships. Display threshold: Z > 2.3. DC = dorsal caudate; dmPFC = dorsomedial prefrontal cortex; NAc = nucleus accumbens; PCC = posterior cingulate cortex; VRP = ventral rostral putamen.
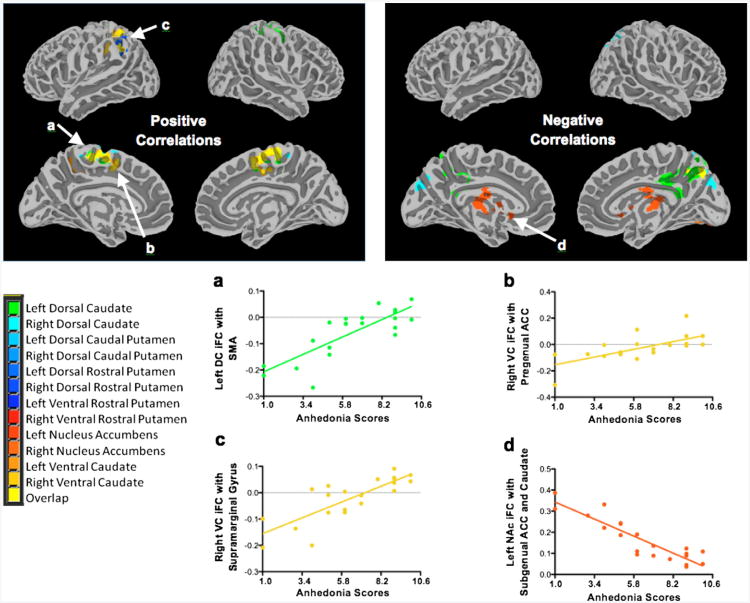
Figure 3.
Intrinsic functional connectivity (iFC) correlations with anhedonia scores and representative plots. Note: Maps showing regions with iFC (left) positively correlated with anhedonia severity and (right) negatively correlated with anhedonia severity. Plots a–d below demonstrate several of these relationships. Additional plots are available in Figure S4, available online. Display threshold: Z > 2.3. ACC = anterior cingulate cortex; DC = dorsal caudate; NAc = nucleus accumben; SMA = supplementary motor area; VC = ventral caudate.
Results
Participants
Demographic and clinical characteristics are summarized in Table 1. One subject with MDD had been treated with escitalopram for 6 months but was medication-free for 9 months before scanning. All other subjects were psychotropic-medication-naive. Nineteen subjects with MDD (90%) had experienced only 1 episode of depression, with length of episode ranging from 4 to 30 months, and 2 patients reported having 2 distinct episodes.
Table 1. Demographic and Clinical Characteristics of Adolescents With Major Depressive Disorder (MDD) and Healthy Controls.
| Characteristic | MDD Subjects n = 21 | Healthy Controls n = 21 |
|---|---|---|
| Age, y (Range) | 17.1 ± 2.5 (12–19) | 16.3 ± 1.4 (13–19) |
| Gender (female/male), n (%) | 12/9 (57/43) | 12/9 (57/43) |
| Ethnicity (white/African American/Hispanic/Asian/other), n (%) | 10/3/6/0/2 (48/14/29/0/10)a | 10/5/1/2/3 (48/24/5/10/14)a |
| Illness history | ||
| Current episode duration, mo (Range) | 13.6 ± 7.9 (4–30) | 0 |
| No. of MDD episodes (n) | 1 (n = 19), 2 (n = 2) | 0 (n = 21) |
| History of suicide attempts (Range) | 0.2 ± 0.5 (0–2) | 0 |
| Medication-naive/medication-free, n (%) | 20/1 (95/5) | 21/0 (100/0) |
| CDRS-R (Range) | 48.9 ± 6.8 (39–64) | 19.1 ± 2.2 (17–27) |
| BDI-II (Range) | 25.3 ± 12.5 (11 –51) | 1.9 ± 2.4 (0–9) |
| BSSI (Range) | 5.6 ± 9.7 (0–37) | 0.1 ± 0.2 (0–1) |
| MASC (Range) | 50.0 19.5 (11–85) | 31.2 13.1 (6–52) |
| Anhedonia scores (Range) | 6.3 ± 2.7 (1–10) | 1.3 ± 0.6 (1 –3) |
| Current comorbidity | ||
| ADHD, n (%) | 3 (14) | 0 |
| Any anxiety disorder, n (%) | 10 (48) | 0 |
| GAD, n (%) | 8 (38) | 0 |
Note: ADHD = attention-deficit/hyperactivity disorder; BDI-II = Beck Depression Inventory, 2nd ed.; BSSI = Beck Scale for Suicidal Ideation; CDRS-R = Children's Depression Rating Scale–Revised; GAD = generalized anxiety disorder; MASC = Multidimensional Anxiety Scale for Children.
Respective percentages (may not add up to 100% because of rounding).
Anhedonia scores were positively correlated with MDD severity scores, as indexed by CDRS-R scores (r = 0.68, p < .001). Because our anhedonia scale included the anhedonia-related item from the CDRS-R, we retested the correlation between anhedonia scores and the CDRS-R computed without the anhedonia item. The correlation remained significant (r = 0.56, p = .008).
Movement
Head movement during resting-state scans was approximated using 5 estimators: mean head displacement, maximum head displacement, number of micromovements (> 0.1 mm), head rotation,61 and mean framewise displacement (FD).62 These estimators were calculated from the 6 translation and rotation parameters of 3-dimensional motion correction during data preprocessing. Independent-samples t tests were conducted to compare these estimators between MDD and HC groups, and no significant differences were found for any of the movement measures (all p > 0.5; Table 2). Although motion was relatively low in the sample used for the present work and was unrelated to diagnostic status or our covariates of interest (i.e., MDD severity and anhedonia), we repeated our analyses with mean FD62 as a nuisance covariate at the group level to rule out any motion sensitivities for our findings (Table S3 and Figures S5–S7, available online). Nearly all findings remained unchanged; a notable exception was the relationship between anhedonia and NAc/sgACC iFC, which fell below threshold. Reassuring overall, we recommend some caution regarding the sgACC finding until replicated in future work. Given the relatively low occurrence of motion in the present sample, we avoided use of higher-order regression models for motion correction at the individual subject level.63
Table 2. Intrinsic Functional Connectivity (iFC) Group Comparisons (Major Depressive Disorder [MDD] vs. Controls) and Correlations With MDD and Anhedonia Severity (MDD Only).
| Intrinsic Functional Connectivity | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Region | Seed | Connectivity Peak | Cluster Size | Peak (MNI) | Peak Z | p | ||
|
| ||||||||
| X | Y | Z | ||||||
| MDD > Controls | ||||||||
| Caudate | Right DC | dmPFC | 2,430 | −12 | 42 | 38 | 4.83 | 2.3×810−7 |
| Right VC | dmPFC and ACC | 5,911 | −18 | 40 | 36 | 4.82 | 1.07×10−11 | |
| Putamen | Left DRP | dmPFC | 903 | 8 | 26 | 52 | 4.10 | 4.66×10−4 |
| Right DRP | Inferior frontal gyrus | 951 | −60 | 28 | 14 | 4.06 | 2.81×10−3 | |
| Right VRP | dmPFC and paracingulate gyrus | 2,618 | 6 | 24 | 48 | 4.25 | 1.79×10−7 | |
| Nucleus Accumbens | Right NAc | dmPFC | 2,945 | −22 | 46 | 26 | 4.69 | 5.96×10−8 |
| Controls > MDD | ||||||||
| Caudate | Left DC | Superior temporal lobe | 1,614 | −66 | −26 | 12 | 4.51 | 1.×10−5 |
| Left DC | Occipital cuneal cortex | 771 | 8 | −78 | 30 | 3.78 | 5.27×10−3 | |
| Left VC | Postcentral gyrus | 656 | −62 | −22 | 16 | 4.08 | 6.06×10−3 | |
| Right DC | Occipital cuneal cortex | 1,750 | −10 | −90 | 26 | 4.48 | 1.×10−5 | |
| Right DC | Occipital lingual gyrus | 877 | −26 | −60 | −2 | 3.88 | 3.36×10−3 | |
| Right VC | Occipital cuneal cortex | 2,799 | −22 | −68 | 24 | 4.59 | 9.54×10−7 | |
| Right VC | Occipital fusiform cortex | 1,646 | −28 | −78 | −14 | 4.13 | 1.82×10−4 | |
| Putamen | Left DRP | Occipital fusiform cortex | 867 | 36 | −72 | −18 | 4.45 | 6.42×10−4 |
| Right DRP | Occipital fusiform cortex | 1,300 | 36 | −78 | −18 | 4.55 | 2.87×10−4 | |
| Right DRP | Lateral occipital cortex | 1,029 | 34 | −80 | 12 | 4.57 | 1.65×10−3 | |
| Right DRP | Lateral occipital cortex | 988 | −40 | −90 | 2 | 4.71 | 2.18×10−3 | |
| Right VRP | Occipital fusiform cortex | 1,172 | −24 | −74 | −10 | 3.89 | 5.64×10−4 | |
| Right VRP | Occipital cuneal cortex | 3,591 | 18 | −80 | 24 | 4.72 | 1.×10−9 | |
| Nucleus Accumbens | Right NAc | Middle temporal gyrus | 1,277 | 50 | −54 | −2 | 4.14 | 3.63×10−4 |
| MDD: Positive CDRS-R Correlations | ||||||||
| Putamen | Right VRP | Precuneus and PCC | 889 | −2 | −56 | 32 | 4.19 | 3.46×10 |
| MDD: Negative CDRS-R Correlations | ||||||||
| Caudate | Left DC | Precuneus and PCC | 1,716 | 6 | −66 | 62 | 4.29 | 1.70×10−5 |
| Right DC | Precuneus and PCC | 656 | −4 | −62 | 62 | 4.38 | 2.×10−3 | |
| Nucleus Accumbens | Right NAc | dmPFC | 652 | 24 | 12 | 50 | 4.36 | 1.74×10−3 |
| MDD: Positive Anhedonia Correlations | ||||||||
| Caudate | Left DC | Supplementary motor area | 2,248 | 10 | 2 | 52 | 4.18 | < .001 |
| Left VC | Supplementary motor area | 679 | 10 | 2 | 52 | 4.14 | < .001 | |
| Left VC | Precuneus | 620 | −30 | −52 | 52 | 3.88 | < .001 | |
| Right DC | Middle frontal gyrus | 600 | −18 | −16 | 54 | 4.92 | .001 | |
| Right VC | Pregenual ACC | 607 | 16 | −6 | 44 | 4.06 | .004 | |
| Right VC | Supramarginal gyrus | 648 | −54 | −38 | 42 | 3.58 | < .001 | |
| Putamen | Right DRP | Supramarginal gyrus | 431 | −52 | −46 | 34 | 4.01 | .003 |
| MDD: Negative Anhedonia Correlations | ||||||||
| Nucleus Accumbens | Left NAc | Subgenual ACC and left caudate | 939 | −8 | 10 | 14 | 4.70 | < .001 |
| Right NAc | Occipital fusiform cortex | 391 | 24 | −70 | −12 | 3.82 | .005 | |
|
| ||||||||
| Motion Estimators | ||||||||
|
| ||||||||
| Estimator | MDD Mean | MDD SD | Control Mean | Control SD | p | |||
|
| ||||||||
| Mean relative displacement (mm) | 0.039 | 0.006 | 0.044 | 0.009 | >.6 | |||
| Max relative displacement (mm) | 0.353 | 0.053 | 0.443 | 0.141 | >.5 | |||
| No. of micro movements | 17.857 | 5.084 | 19.810 | 5.619 | >.7 | |||
| Mean relative rotation (degrees) | 0.011 | 0.001 | 0.012 | 0.002 | >.6 | |||
| Mean framewise displacement (mm) | 0.72 | 0.016 | 0.88 | 0.019 | >.5 | |||
Note: ACC = anterior cingulate cortex; CDRS-R = Children's Depression Rating Scale–Revised; DC = dorsal caudate; DCP = dorsal caudal putamen; dmPFC = dorsomedial prefrontal cortex; DRP = dorsal rostral putamen; MNI = Montreal Neurological Institute; NAc = nucleus accumbens; PCC = posterior cingulate cortex; VC = ventral caudate; VRP = ventral rostral putamen.
Primary Hypothesis Testing: Striatal iFC Group Comparisons
MDD Group versus HC
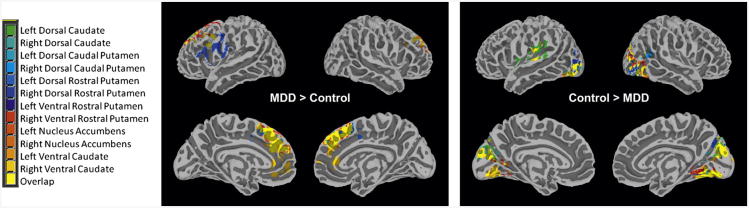
Consistent with our hypothesis, group analyses revealed that adolescents with MDD manifested increased iFC between striatal seeds and the dmPFC, regardless of the hemisphere in which the seed was located. Decreased iFC in the MDD group was identified between striatal seeds and mainly the occipital cortex. Additional findings are detailed below, based on seed locations, as well as in Table 2 and Figure 1. Similar findings from our secondary analysis are presented in Table S1 and Figure S1, available online. Baseline connectivity maps for each seed region (based on the HC group) and overlays of findings for each seed are available in Figure S8, available online.
Figure 1.
Intrinsic functional connectivity (iFC) group comparison between adolescents with major depressive disorder (MDD) and healthy controls. Note: Maps showing regions with (left) increased iFC in MDD versus controls, and (right) increased iFC in controls versus MDD. Significant iFC with each seed is color coded, with regions functionally connected with more than 1 seed indicated in yellow. Display threshold: Z > 2.3.
Caudate
Adolescents with MDD compared to HC exhibited increased iFC between the right ventral caudate and the ACC, including both the pregenual (pgACC) and subgenual (sgACC) regions of the ACC. Adolescents with MDD exhibited decreased iFC between the left dorsal caudate and the superior temporal lobe, as well as between the left ventral caudate and the postcentral gyrus.
Putamen
Within the putamen, adolescents with MDD also manifested increased iFC between the right ventral rostral putamen and the paracingulate gyrus, and between the right dorsal rostral putamen and the inferior frontal gyrus.
Nucleus Accumbens
Relative to HC, adolescents with MDD exhibited decreased iFC between the right NAc and the middle temporal gyrus.
Associations of Striatal iFC With Clinical Variables in the MDD Group
MDD Severity
As indexed by CDRS-R scores, MDD severity was positively correlated with iFC strength between the right ventral rostral putamen seed and the precuneus/posterior cingulate cortex (PCC). Interestingly, negative correlations with MDD severity were observed for iFC strength between the bilateral dorsal caudate and the precuneus/PCC. Negative correlations between MDD severity and iFC were also observed between the right NAc seed and the dmPFC. Findings are detailed in Table 2 and presented in Figure 2. Severity findings from our secondary analysis were largely consistent and are presented in Table S1 and Figure S2, available online.
Anhedonia Severity
As noted, because anhedonia scores were positively correlated with the severity of current depressive episode, we carried out analyses adjusted at the cluster level for CDRS-R scores with the anhedonia question omitted. The majority of anhedonia-related findings were in the caudate. Specifically, anhedonia scores were positively correlated with iFC strength of the ventral and dorsal caudate seeds with the supplementary motor area (SMA), middle frontal gyrus, supramarginal gyrus, precuneus, and pgACC, as well as between the right dorsal rostral putamen and the supramarginal gyrus. Negative correlations were found between the left NAc and both the sgACC and the left caudate, and between the right NAc and the occipital fusiform cortex. Anhedonia correlations are detailed in Table 2 and presented in Figure 3, with additional correlation plots provided in Figure S4, available online. Our secondary analysis yielded similar results (Table S1 and Figure S3, available online).
Discussion
Our hypotheses that adolescents with MDD would manifest altered iFC along frontstriatal circuits were confirmed; however, we detected only increased iFC compared to HC. Similarly, we were able to detect distinct patterns of striatum-based circuitry that were related to illness and anhedonia severity beyond our specific hypotheses. These findings are discussed below.
Striatal Circuitry Based on a Categorical Diagnostic Approach
In group comparisons, adolescents with MDD manifested increased iFC between striatal seeds and the dmPFC bilaterally, and between the right ventral caudate seed and the ACC. Unexpectedly, we also found that adolescents with MDD manifested decreased iFC in circuits connecting the striatum with the occipital cortex.
Our finding of increased connectivity between the striatum and dmPFC/ACC implies a higher degree of coordination between these regions in adolescents with MDD. As noted, there have been reports of both increased31-34 and decreased17,24,29,35 striatum-PFC iFC in MDD patients compared to HC. However, most relevant to the current study is a recent striatal-seed iFC investigation in adult MDD reporting increased striatum-PFC iFC for dorsal striatum seeds versus decreased striatum-PFC iFC for ventral striatum seeds.36 In contradiction to this study in adult MDD, we found only increased striatal-PFC iFC, and our hypothesis that patients would exhibit decreased iFC between ventral striatum seeds and the PFC was not supported. Interestingly, we did find a negative correlation between MDD severity and iFC strength of the ventral-striatal NAc seed within the same network, suggesting that the lower the coordination between the ventral striatum and the dmPFC, the greater the illness severity. Our finding of increased increased iFC within the fronto-striatal circuits observed in adolescents with MDD compared to HC may reflect an earlier manifestation or compensatory process of the disease. As such, this finding may be used in the future to assess early stages of the disorder or at-risk individuals.
The coordinates of peak cluster activity also differ substantially between the NAc group comparison and MDD severity correlation findings, with the left dmPFC involved in group differences and the right dmPFC associated with MDD severity (Table 2). Past research has indicated that the dmPFC responds to self-referential words with both positive and negative valence bilaterally, but the right dmPFC is more involved in positive self-referential processing.64 These opposing findings may therefore be driven by functional differences in the distinct dmPFC regions detected.
Our finding of increased iFC between the striatum and both the dmPFC and the ACC in adolescents with MDD is consistent with mounting evidence implicating these specific circuits in MDD across the lifespan,10,12,13,65-67 using a widerange of imaging techniques. 3,10,39,68-71 fMRI studies further support such findings through a wide range of task paradigms 72-74 as well as diverse iFC approaches in adolescent,12,13,29,30,67 adult,27,74-76 geriatric,31,77 and postpartum patients.72 Histopathological reports have confirmed findings in the medial PFC and ACC, documenting reduction in neuron size and/or loss of glia in these regions in MDD.78-80 A possible explanation for the consistent involvement of striatum-dmPFC/ACC circuits in MDD is their critical role in the cognitive control of reward, reappraisal, mood, and reasoning: processes that contribute to key symptoms of MDD.81-83 A germane meta-analysis supports the view that these cortical midline structures, along with their striatal connections, mediate self-referential processes and constitute the core of both our sense and our feeling of self.84 Findings from a recent iFC study in adults with MDD provide additional support for this notion by identifying a “dorsal nexus” region centered around the dmPFC that exhibits increased iFC with 3 distinct networks involved in cognitive control, affect, and the default mode.27 Recent work has further identified the dmPFC as a distinct subsystem within the default mode network that is activated in spontaneous cognition involving self-referential thoughts about one's mental state and affective information, as well as those that involve spontaneous social cognition—processes that are disturbed in MDD.85-88
We also found decreased iFC between striatal seeds and the occipital cortex in adolescents with MDD compared to HC. Albeit unexpected, a large body of evidence has pointed to the possible role of the occipital lobe in MDD. Findings include changes in metabolism,89,90 white matter alterations,91,92 and increased BOLD signal in response to neutral faces93 and during a working memory task.94 Adding to this literature is a recent iFC study that examined the topological properties of brain networks in adults with MDD and documented reduced nodal centrality in the occipital lobe among other relevant visual regions in MDD.95 These findings are most likely related to the critical role of the occipital cortex in processing emotionally relevant visual stimuli.96,97 It is important to note that although occipital findings in resting-state fMRI can be affected by whether eyes are open or closed during scans,98,99 there is not a 1:1 relationship that would provide a direct explanation. Previous studies, including a key meta-analysis of emotional task-based fMRI paradigms,100 noted the presence of an occipital network linked to the PCC. In addition, a growing body of literature suggests the presence of a functional hub based in cuneus, once again arguing against a simple explanation based on eyes-open status.101
Neural Circuitry Related to Illness and Anhedonia Severity
MDD Severity
In our dimensional analysis, MDD severity was associated with iFC strength between striatal seeds and both the precuneus/PCC and the dmPFC.
Both the precuneus and PCC are considered a major connectivity hub along with the dmPFC; together, they form the midline core of the default-mode network that is involved in spontaneous cognition, self-referential processing, and affective decision-making.88,101 Multiple investigations have repeatedly implicated these circuits in MDD.16,25-27,31,32
In our study, we documented both positive and negative relationships between MDD severity and iFC strength in circuits linking the striatum and the precuneus/PCC, depending on the seed: positive correlations were identified with the right ventral putamen seed, and negative correlations with the dorsal caudate seeds. These opposite directions with different seeds suggest that the function of the ventral-putamen– based circuit differs from the caudate/NAc-based circuit. Indeed, in our prior investigation of the striatum, we concluded that a ventral-putamen– based network is involved with executive functioning, whereas the caudate is involved with cognition and reward.37
Anhedonia
When we related striatum-based iFC to anhedonia severity, distinct circuits within the neural reward system were identified. Specifically, positive associations with anhedonia were found for circuits connecting the striatum and the pgACC, SMA, and supramarginal gyrus. In addition, anhedonia was negatively associated with circuits connecting the NAc with the sgACC and caudate. Intriguingly, these circuits and regions have been previously linked to anhedonia as well as to reward processes across several neuropsychiatric disorders, indicating that the circuitry underlying anhedonia is independent of the specific MDD diagnosis.11,102-106
Furthermore, in our current investigation, we also documented 2 opposing striatum-ACC circuits that related to anhedonia severity: a positive association with ventral-caudate-pgACC iFC, and a negative association with NAc-sgACC iFC. We hypothesize that these contrasting associations represent independent circuits that participate in distinct reward processes. Supporting this notion are recent fMRI findings documenting pgACC activation during decisions that entail large versus small rewards, as opposed to sgACC activation during decisions with positive versus negative outcomes.107 Similarly, our finding of a positive association of striatum-SMA iFC with anhedonia severity fits with current literature identifying the SMA as a key region in reward processing. The SMA has often been activated along with the pgACC in reward task-based fMRI studies, particularly during the anticipation/decision phase of reward.108-110 We also found positive correlations between striatum-supramarginal-gyrus iFC and anhedonia severity; these findings are akin to a previously reported correlation between activation of this region in response to sad stimuli and anhedonia severity in adults with MDD.15 Interestingly, the supramarginal gyrus has also been implicated in adult obesity, where alterations within the reward circuitry are hypothesized to play a key role.105
Although our sample size is comparable to those of other studies of clinical populations in the functional neuroimaging literature, definitive interpretation must be deferred until findings are replicated independently. A possible limitation of the current study is the use of an anhedonia scale that was based on questions from the BDI (self-rated) and the CDRS-R (clinicianrated). However, this approach has been used in many other investigations in both adults and adolescents, including in the multisite Treatment of Resistant Depression in Adolescents (TORDIA) trial, and seems to adequately assess anhedonia severity in MDD populations.3,7,46 Importantly, Leventhal et al. (2006) demonstrated that a similar scoring approach based on self-administered questionnaires correlated with other several anhedonia measures (e.g., the Snaith-Hamilton Pleasure Scale).111 Furthermore, in our study we were able to capture a wide range of anhedonia severity (1–10) in a moderately to severely depressed population. Future studies should use measures that are more sensitive and applicable to both patients and controls, to fully explore the nature of anhedonia neurobiology quantitatively. Similarly, the present work relied on task-independent approaches. Although this is an attractive option because of the relative ease of data collection and the benefits detailed above, future work should include task-activation probes with demonstrated utility in the examination of reward circuitry during distinct phases of a pleasurable activity (i.e., anticipatory versus attainment phases). Simultaneous assessment of neurotransmitters such as GABA and glutamate would also have enhanced our understanding of the involved circuits, particularly in light of our recent finding of a negative relationship between ACC GABA concentrations and anhedonia severity.3 Although the present work focused on striatal connectivity because of the sizeable literature implicating striatal dysfunction in MDD and anhedonia, it is not our intention to dissuade others from examining alternative circuits. In fact, our findings suggest the need to expand neural models of MDD, as our analyses revealed alterations in regions such as the occipital cortex and precuneus/PCC—prominent functional hubs in the brain.101
Our investigation of striatal iFC in medication-free adolescents with MDD revealed a consistent pattern of altered iFC between striatal seeds and the dmPFC/ACC, as well as the visual cortex, in adolescents with MDD. However, when we examined striatal circuits as they related to severity of depressive episode and anhedonia, we were able to distinguish specific connections. Although the precuneus along with the midline core of the default mode network (i.e., the PCC and dmPFC) was related to MDD severity, striatal circuits connecting to the SMA, pgACC, and sgACC were instead related to anhedonia severity. Importantly, our findings suggest that several previously established striatal networks, including cortical associations, motor, and limbic, are involved in the phenomenology of adolescent MDD (comprehensively reviewed by Choi et al.).112 Our findings suggest that distinct circuits may contribute to different aspects of MDD. Consistent with prior work,3,20 this study further emphasizes that assessing symptoms as dimensions in addition to binary categories can enrich our understanding of the underlying neurobiology of psychiatric disorders.
Supplementary Material
Figure S1: Intrinsic functional connectivity (iFC) secondary analysis group comparison between adolescents with major depressive disorder (MDD) and healthy controls. Note: Maps showing regions with (left) increased iFC in MDD versus controls, and (right) increased iFC in controls versus MDD. Significant iFC with each seed is color coded, with regions functionally connected with more than 1 seed indicated in yellow. Display threshold: Z > 2.3.
Figure S2: Intrinsic functional connectivity (iFC) secondary analysis correlations with major depressive disorder (MDD) severity and corresponding plots. Note: Maps showing regions with iFC negatively correlated with MDD severity. Plots a–b demonstrate these relationships. Display threshold: Z > 2.3.
Figure S3: Intrinsic functional connectivity (iFC) correlations with anhedonia scores and corresponding plots (secondary analysis). Note: Maps showing regions with iFC positively correlated with anhedonia severity. Plots a–c demonstrate these relationships. Display threshold: Z > 2.3. ACC = anterior cingulate cortex; SMA = supplementary motor area.
Figure S4: Additional plots for intrinsic functional connectivity (iFC) primary analysis correlations with anhedonia scores. Note: DC = dorsal caudate; DRP = dorsal rostral putamen; NAc = nucleus accumbens; SMA = supplementary motor area; VC = ventral caudate.
Figure S5: Intrinsic functional connectivity (iFC) controlled for mean framewise displacement (FD) group comparison between adolescents with major depressive disorder (MDD) and healthy controls. Note: Maps controlled for mean FD showing regions with (left) increased iFC in MDD versus controls, and (right) increased iFC in controls versus MDD. Significant iFC with each seed is color coded, with regions functionally connected with more than 1 seed indicated in yellow. Display threshold: Z > 2.3.
Figure S6: Intrinsic functional connectivity (iFC) correlations with major depressive disorder (MDD) severity controlled for mean framewise displacement (FD). Note: Maps controlled for mean FD showing regions with iFC (left) positively correlated with MDD severity and (right) negatively correlated with MDD severity. Display threshold: Z > 2.3.
Figure S7: Intrinsic functional connectivity (iFC) correlations with anhedonia scores controlled for mean framewise displacement (FD). Note: Maps controlled for mean FD showing regions with iFC (left) positively correlated with anhedonia severity and (right) negatively correlated with anhedonia severity. Display threshold: Z > 2.3.
Figure S8: Baseline and individual seed intrinsic functional connectivity (iFC) maps. Note: Maps showing baseline iFC connectivity for each seed region based on the control group, as well as group comparisons, major depressive disorder (MDD) severity correlations, and anhedonia severity correlations for each seed. Display threshold: Z > 2.3. ANHED = anhedonia severity correlations; CDRSR = MDD severity; CT = control; DC = dorsal caudate; dcP = dorsal caudal putamen; drP = dorsal rostral putamen; L = left; NAc = nucleus accumbens; R = right; VC = ventral caudate; vrP = ventral rostral putamen.
Table S1: Intrinsic Functional Connectivity (iFC) Secondary Analysis Group Comparisons (Major Depressive Disorder [MDD] vs. Controls) and Correlations With MDD and Anhedonia Severity (MDD Only)
Note: ACC = anterior cingulate cortex; CDRS-R = Children's Depression Rating Scale–Revised; dmPFC = dorsomedial prefrontal cortex; MNI = Montreal Neurological Institute; PCC = posterior cingulate cortex.
Table S2: Intrinsic Functional Connectivity (iFC) Without Global Signal Regression Group Comparisons (Major Depressive Disorder [MDD] vs. Controls) and Correlations With MDD and Anhedonia Severity (MDD Only)
Note: ACC = anterior cingulate cortex; CDRS-R = Children's Depression Rating Scale–Revised; DC = dorsal caudate; DCP = dorsal caudal putamen; dmPFC = dorsomedial prefrontal cortex; DRP = dorsal rostral putamen; MNI = Montreal Neurological Institute; NAc = nucleus accumbens; PCC = posterior cingulate cortex; VC = ventral caudate; VRP = ventral rostral putamen.
Table S3: Intrinsic Functional Connectivity (iFC) Controlled for Mean Framewise Displacement (FD) Group Comparisons (Major Depressive Disorder [MDD] vs. Controls) and Correlations With MDD and Anhedonia Severity (MDD Only)
Note: ACC = anterior cingulate cortex; CDRS-R = Children's Depression Rating Scale–Revised; DC = dorsal caudate; DCP = dorsal caudal putamen; dmPFC = dorsomedial prefrontal cortex; DRP = dorsal rostral putamen; MNI = Montreal Neurological Institute; NAc = nucleus accumbens; PCC = posterior cingulate cortex; VC = ventral caudate; VRP = ventral rostral putamen.
Acknowledgments
Findings support the incorporation of both categorical and dimensional approaches in neuropsychiatric research. J. Am. Acad. Child Adolesc.
Dr. Gabbay and Mr. Ely are with Mount Sinai School of Medicine. Mr. Li and Dr. Milham are with the Center for the Developing Brain of the Child Mind Institute. Drs. Gabbay and Milham are also with the Nathan S. Kline Institute for Psychiatric Research. Ms. Bangaru, Ms. Panzer, and Drs. Alonso and Castellanos are with the New York University Child Study Center of the New York University School of Medicine.
This study was supported by grants from the National Institutes of Health (NIH) (AT002395, AT004576, MH077072, MH077072-03S1, MH075895, and MH095807), the Chrissy Rossi National Alliance for Research on Schizophrenia and Depression Award, and generous gifts from the Leon Levy and Anita Saltz Foundations. These sources of funding had no further role in study design and conduct; in the collection, management, analysis, and interpretation of data; and in preparation, review and approval of this manuscript. Dr. Gabbay had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Supplemental material cited in this article is available online.
Disclosure: Dr. Gabbay has served as a sub-investigator on studies sponsored by Otsuka, Biovail, Shire Pharmaceuticals, Bristol-Myers Squibb, and Boehringer Ingestion, but has received no compensation for this work. She has received funding from the Tourette Syndrome Association, NIH, and Hope for Depression Research Foundation. She has received honoraria from the National Center for Complementary and Alternative Medicine (NCCAM)/NIH Mechanistic Research on CAM Natural Products, NIH Mechanisms Explaining Differences in Depressive and Anxiety Disorders Across Racial/Ethnic Groups, and the Centers of Excellence for Research on CAM. Dr. Castellanos has received funding from the National Institute of Child Health and Human Development (NICHD), the Children's Tumor Foundation, the National Institute of Mental Health (NIMH), the National Center for Research Resources, the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the Brain Research Foundation, and Autism Speaks. He has served as the Vice-Chair for the Workgroup on Attention-Deficit/Hyperactivity Disorder (ADHD) and Disruptive Behavior Disorders, DSM-5 Task Force, American Psychiatric Association, and has served as a member of the National Advisory Council on Drug Abuse, NIH, the National Advisory Council on Drug Abuse Steering Committee, and the NIH Council of Councils. He holds U.S. patent no. 8,003,406 awarded to the National Human Genome Research Institute on behalf of inventors Maximilian Muenke, Mauricio Arcos-Burgos, and F. Xavier Castellanos for “Method of Detecting and Treating Attention-Deficit/Hyperactivity Disorder;” Provisional application filed 9/9/2008 through New York University on the “Use of Carbon Monoxide as a Therapeutic Agent” on behalf of inventors Donald F. Klein and F. Xavier Castellanos. Dr. Milham has received funding from NIMH, the Child Mind Institute, Autism Speaks, and NICHD. Dr. Alonso, Mr. Ely, Mr. Li, Ms. Bangaru, and Ms. Panzer report no biomedical financial interests or potential conflicts of interest.
References
- 1.Pizzagalli DA, Jahn AL, O'Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabbay V, Ely BA, Babb J, Liebes L. The possible role of the kynurenine pathway in anhedonia in adolescents. J Neural Transm. 2012;119:253–260. doi: 10.1007/s00702-011-0685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabbay V, Mao X, Klein RG, et al. Anterior cingulate cortex γ-aminobutyric acid in depressed adolescents: relationship to anhedonia. Arch Gen Pychiatry. 2012;69:139–149. doi: 10.1001/archgenpsychiatry.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yorbik O, Birmaher B, Axelson D, Williamson DE, Ryan ND. Clinical characteristics of depressive symptoms in children and adolescents with major depressive disorder. J Clin Psychiatry. 2004;65:1654–1659. doi: 10.4088/jcp.v65n1210. quiz 1760-1651. [DOI] [PubMed] [Google Scholar]
- 5.Ryan ND, Puig-Antich J, Ambrosini P, et al. The clinical picture of major depression in children and adolescents. Arch Gen Psychiatry. 1987;44:854–861. doi: 10.1001/archpsyc.1987.01800220016003. [DOI] [PubMed] [Google Scholar]
- 6.Klein RG, Mannuzza S, Koplewicz HS, et al. Adolescent depression: controlled desipramine treatment and atypical features. Depress Anxiety. 1998;7:15–31. doi: 10.1002/(sici)1520-6394(1998)7:1<15::aid-da3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 7.McMakin DL, Olino TM, Porta G, et al. Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment-resistant depression. J Am Acad Child Adolesc Psychiatry. 2012;51:404–411. doi: 10.1016/j.jaac.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robbins DR, Alessi NE, Colfer MV. Treatment of adolescents with major depression: implications of the DST and the melancholic clinical subtype. J Affect Disord. 1989;17:99–104. doi: 10.1016/0165-0327(89)90031-1. [DOI] [PubMed] [Google Scholar]
- 9.Spijker J, de Graaf R, Ten Have M, Nolen WA, Speckens A. Predictors of suicidality in depressive spectrum disorders in the general population: results of the Netherlands Mental Health Survey and Incidence Study. Soc Psychiatry Psychiatr Epidemiol. 2010;45:513–521. doi: 10.1007/s00127-009-0093-6. [DOI] [PubMed] [Google Scholar]
- 10.Pizzagalli DA, Oakes TR, Fox AS, et al. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Mol Psychiatry. 2004;9(325):393–405. doi: 10.1038/sj.mp.4001501. [DOI] [PubMed] [Google Scholar]
- 11.Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-relateddeficits. Trends Neurosci. 2012;35:68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forbes EE, Hariri AR, Martin SL, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forbes EE, Christopher May J, Siegle GJ, et al. Rewardrelated decision-making in pediatric major depressive disorder: an fMRI study. J Child Psychol Psychiatry. 2006;47:1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forbes EE, Olino TM, Ryan ND, et al. Reward-related brain function as a predictor of treatment response in adolescents with major depressive disorder. Cogn Affect Behav Neurosci. 2010;10:107–118. doi: 10.3758/CABN.10.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Gaffrey MS, Luby JL, Repovs G, et al. Subgenual cingulate connectivity in children with a history of preschool-depression. Neuroreport. 2010;21:1182–1188. doi: 10.1097/WNR.0b013e32834127eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCabe C, Cowen PJ, Harmer CJ. Neural representation of reward in recovered depressed patients. Psychopharmacology (Berl) 2009;205:667–677. doi: 10.1007/s00213-009-1573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang TT, Simmons AN, Matthews SC, et al. Depressed adolescents demonstrate greater subgenual anterior cingulate activity. Neuroreport. 2009:20440–20444. doi: 10.1097/WNR.0b013e3283262e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 20.Chabernaud C, Mennes M, Kelly C, et al. Dimensional brain-behavior relationships in children with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012:71434–71442. doi: 10.1016/j.biopsych.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly C, Biswal BB, Craddock RC, Castellanos FX, Milham MP. Characterizing variation in the functional connectome: promise and pitfalls. Trends Cogn Sci. 2012;16:181–188. doi: 10.1016/j.tics.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mennes M, Kelly C, Colcombe S, Castellanos FX, Milham MP. The extrinsic and intrinsic functional architectures of the human brain are not equivalent. Cereb Cortex. 2013;23:223–229. doi: 10.1093/cercor/bhs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Hermens DF, Hickie IB, Lagopoulos J. A systematic review of resting-state functional-MRI studies in major depression. J Affect Disord. 2012;142:6–12. doi: 10.1016/j.jad.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Anand A, Li Y, Wang Y, et al. Antidepressant effect on connectivity of the mood-regulating circuit: an FMRI study. Neuropsychopharmacology. 2005;30:1334–1344. doi: 10.1038/sj.npp.1300725. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Yu C, Zheng H, et al. Increased neural resources recruitment in the intrinsic organization in major depression. J Affect Disord. 2010;121:220–230. doi: 10.1016/j.jad.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 26.Bluhm R, Williamson P, Lanius R, et al. Resting state default-mode network connectivity in early depression using a seed region-of-interest analysis: decreased connectivity with caudate nucleus. Psychiatry Clin Neurosci. 2009;63:754–761. doi: 10.1111/j.1440-1819.2009.02030.x. [DOI] [PubMed] [Google Scholar]
- 27.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci USA. 2011;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cullen KR, Gee DG, Klimes-Dougan B, et al. A preliminary study of functional connectivity in comorbid adolescent depression. Neurosci Lett. 2009;460:227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiao Q, Ding J, Lu G, et al. Increased activity imbalance in frontosubcortical circuits in adolescents with major depression. PloS One. 2011;6:e25159. doi: 10.1371/journal.pone.0025159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin C, Gao C, Chen C, et al. A preliminary study of the dysregulation of the resting networks in first-episode medication-naive adolescent depression. Neurosci Lett. 2011;503:105–109. doi: 10.1016/j.neulet.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 31.Kenny ER, O'Brien JT, Cousins DA, et al. Functional connectivity in late-life depression using resting-state functional magnetic resonance imaging. Am J Geriatr Psychiatry. 2010;18:643–651. doi: 10.1097/JGP.0b013e3181cabd0e. [DOI] [PubMed] [Google Scholar]
- 32.Greicius MD, Flores BH, Menon V, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamilton JP, Chen G, Thomason ME, Schwartz ME, Gotlib IH. Investigating neural primacy in major depressive disorder: multivariate Granger causality analysis of resting-state fMRI time-series data. Mol Psychiatry. 2011;16:763–772. doi: 10.1038/mp.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng DH, Shen T, Zhang J, et al. Abnormal functional connectivity with mood regulating circuit in unmedicated individual with major depression: a resting-state functional magnetic resonance study. Chin Med J (Engl) 2012;125:3701–3706. [PubMed] [Google Scholar]
- 35.Veer IM, Beckmann CF, van Tol MJ, et al. Whole brain restingstate analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci. 2010;4:41. doi: 10.3389/fnsys.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furman DJ, Hamilton JP, Gotlib IH. Frontostriatal functional connectivity in major depressive disorder. Biol Mood Anxiety Disord. 2011;1:11. doi: 10.1186/2045-5380-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Martino A, Scheres A, Margulies DS, et al. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- 38.Harrison BJ, Soriano-Mas C, Pujol J, et al. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry. 2009;66:1189–1200. doi: 10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- 39.Kelly C, de Zubicaray G, Di Martino A, et al. L-dopa modulates functional connectivity in striatal cognitive and motor networks: a double-blind placebo-controlled study. J Neurosci. 2009;29:7364–7378. doi: 10.1523/JNEUROSCI.0810-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Martino A, Kelly C, Grzadzinski R, et al. Aberrant striatal functional connectivity in children with autism. Biol Psychiatry. 2011;69:847–856. doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 42.Beck AT, Guth D, Steer RA, Ball R. Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for Primary Care. Behav Res Ther. 1997;35:785–791. doi: 10.1016/s0005-7967(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 43.Kaufman AS, Kaufman NL. Manual for the Kaufman Brief Intelligence Test. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- 44.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 45.Joiner TE, Brown JS, Metalsky GI. A test of the tripartite model's prediction of anhedonia's specificity to depression: patients with major depression versus patients with schizophrenia. Psychiatry Res. 2003;119:243–250. doi: 10.1016/s0165-1781(03)00131-8. [DOI] [PubMed] [Google Scholar]
- 46.Walter M, Henning A, Grimm S, et al. The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry. 2009;66:478–486. doi: 10.1001/archgenpsychiatry.2009.39. [DOI] [PubMed] [Google Scholar]
- 47.Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. NeuroImage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 48.Di Martino A, Shehzad Z, Kelly C, et al. Relationship between cingulo-insular functional connectivity and autistic traits in neurotypical adults. Am J Psychiatry. 2009;166:891–899. doi: 10.1176/appi.ajp.2009.08121894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mennes M, Kelly C, Zuo XN, et al. Inter-individual differences in resting-state functional connectivity predict task-induced BOLD activity. NeuroImage. 50:1690–1701. doi: 10.1016/j.neuroimage.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andersson JL, Jenkinson M, Smith SM. Non-linear optimisation TR07JA1. [Accessed December, 7 2012];FMRIB Tech Rep. 2007 Available at: http://www.fmrib.ox.ac.uk/analysis/techrep/
- 51.Leopold DA, Maier A. Ongoing physiological processes in the cerebral cortex. NeuroImage. 2012;62:2190–2200. doi: 10.1016/j.neuroimage.2011.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He H, Liu TT. A geometric view of global signal confounds in resting-state functional MRI. NeuroImage. 2012;59:2339–2348. doi: 10.1016/j.neuroimage.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saad ZS, Gotts SJ, Murphy K, et al. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2:25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heimer L, Alheid GF. Piecing together the puzzle of basal forebrain anatomy. Adv Exp Med Biol. 1991;295:1–42. doi: 10.1007/978-1-4757-0145-6_1. [DOI] [PubMed] [Google Scholar]
- 56.Drevets WC, Price JC, Kupfer DJ, et al. PET measures of amphetamine-induced dopamine release in ventral versus dorsal striatum. Neuropsychopharmacology. 1999;21:694–709. doi: 10.1016/S0893-133X(99)00079-2. [DOI] [PubMed] [Google Scholar]
- 57.Wu T, Wang L, Hallett M, Chen Y, Li K, Chan P. Effective connectivity of brain networks during self-initiatedmovement in Parkinson's disease. NeuroImage. 2011;55:204–215. doi: 10.1016/j.neuroimage.2010.11.074. [DOI] [PubMed] [Google Scholar]
- 58.Makris N, Meyer JW, Bates JF, Yeterian EH, Kennedy DN, Caviness VS. MRI-based topographic parcellation of human cerebral white matter and nuclei II. Rationale and applications with systematics of cerebral connectivity. NeuroImage. 1999;9:18–45. doi: 10.1006/nimg.1998.0384. [DOI] [PubMed] [Google Scholar]
- 59.Stark DE, Margulies DS, Shehzad ZE, et al. Regional variation in interhemispheric coordination of intrinsic hemodynamic fluctuations. J Neurosci. 2008;28:13754–13764. doi: 10.1523/JNEUROSCI.4544-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salvador R, Suckling J, Schwarzbauer C, Bullmore E. Undirected graphs of frequency-dependent functional connectivity in whole brain networks. Philos Trans R Soc Lond B Biol Sci. 2005;360:937–946. doi: 10.1098/rstb.2005.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2011;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Satterthwaite TD, Elliott MA, Gerraty RT, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. NeuroImage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fossati P, Hevenor SJ, Graham SJ, et al. In search of the emotional self: an fMRI study using positive and negative emotional words. Am J Psychiatry. 2003;160:1938–1945. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- 65.Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- 66.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 67.Forbes EE. FMRI studies of reward processing in adolescent depression. Neuropsychopharmacology. 2011;36:372–373. doi: 10.1038/npp.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bora E, Fornito A, Pantelis C, Yucel M. Gray matter abnormalities in Major depressive disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord. 2012;138:9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 69.Li L, Ma N, Li Z, et al. Prefrontal white matter abnormalities in young adult with major depressive disorder: a diffusion tensor imaging study. Brain Res. 2007;1168:124–128. doi: 10.1016/j.brainres.2007.06.094. [DOI] [PubMed] [Google Scholar]
- 70.Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 71.Gabbay V, Hess DA, Liu S, Babb JS, Klein RG, Gonen O. Lateralized caudate metabolic abnormalities in adolescent major depressive disorder: a proton MR spectroscopy study. Am J Psychiatry. 2007;164:1881–1889. doi: 10.1176/appi.ajp.2007.06122032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moses-Kolko EL, Perlman SB, Wisner KL, James J, Saul AT, Phillips ML. Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. Am J Psychiatry. 2010;167:1373–1380. doi: 10.1176/appi.ajp.2010.09081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carballedo A, Scheuerecker J, Meisenzahl E, et al. Functional connectivity of emotional processing in depression. J Affect Disord. 2011;134:272–279. doi: 10.1016/j.jad.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 74.Grimm S, Ernst J, Boesiger P, et al. Increased self-focus in major depressive disorder is related to neural abnormalities in subcortical-cortical midline structures. Hum Brain Mapp. 2009;30:2617–2627. doi: 10.1002/hbm.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bermpohl F, Walter M, Sajonz B, et al. Attentional modulation of emotional stimulus processing in patients with major depression—alterations in prefrontal cortical regions. Neurosci Lett. 2009;463:108–113. doi: 10.1016/j.neulet.2009.07.061. [DOI] [PubMed] [Google Scholar]
- 76.Tao H, Guo S, Ge T, et al. Depression uncouples brain hate circuit. Mol Psychiatry. 2013;18:101–111. doi: 10.1038/mp.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xie C, Goveas J, Wu Z, et al. Neural basis of the association between depressive symptoms and memory deficits in nondemented subjects: resting-state fMRI study. Hum Brain Mapp. 2012;33:1352–1363. doi: 10.1002/hbm.21291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hercher C, Turecki G, Mechawar N. Through the looking glass: examining neuroanatomical evidence for cellular alterations in major depression. J Psychiatr Res. 2009;43:947–961. doi: 10.1016/j.jpsychires.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 79.Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- 80.Rajkowska G, O'Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology. 2007;32:471–482. doi: 10.1038/sj.npp.1301234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 82.Levesque J, Eugene F, Joanette Y, et al. Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry. 2003;53:502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- 83.Northoff G. Psychopathology and pathophysiology of the self in depression - neuropsychiatric hypothesis. J Affect Disord. 2007;104:1–14. doi: 10.1016/j.jad.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 84.Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 85.Wagner DD, Kelley WM, Heatherton TF. Individual differences in the spontaneous recruitment of brain regions supporting mental state understanding when viewing natural social scenes. Cereb Cortex. 2011;21:2788–2796. doi: 10.1093/cercor/bhr074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. Evidence for the default network's role in spontaneous cognition. J Neurophysiol. 2010;104:322–335. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Andrews-Hanna JR. The brain's default network and its adaptive role in internal mentation. Neuroscientist. 2012;18:251–270. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fujimoto T, Takeuchi K, Matsumoto T, et al. Metabolic changes in the brain of patients with late-onset major depression. Psychiatry Res. 2008;164:48–57. doi: 10.1016/j.pscychresns.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 90.Diaconescu AO, Kramer E, Hermann C, et al. Distinct functional networks associated with improvement of affective symptoms and cognitive function during citalopram treatment in geriatric depression. Hum Brain Mapp. 2011;32:1677–1691. doi: 10.1002/hbm.21135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kumar A, Gupta RC, Albert Thomas M, Alger J, Wyckoff N, Hwang S. Biophysical changes in normal-appearing white matter and subcortical nuclei in late-life major depression detected using magnetization transfer. Psychiatry Res. 2004;130:131–140. doi: 10.1016/j.pscychresns.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 92.Huang H, Fan X, Williamson DE, Rao U. White matter changes in healthy adolescents at familial risk for unipolar depression: a diffusion tensor imaging study. Neuropsychopharmacology. 2011;36:684–691. doi: 10.1038/npp.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tang Y, Li Y, Wang N, Li H, Li H, Wang J. The altered cortical connectivity during spatial search for facial expressions in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1891–1900. doi: 10.1016/j.pnpbp.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 94.Garrett A, Kelly R, Gomez R, Keller J, Schatzberg AF, Reiss AL. Aberrant brain activation during a working memory task in psychotic major depression. Am J Psychiatry. 2011;168:173–182. doi: 10.1176/appi.ajp.2010.09121718. [DOI] [PubMed] [Google Scholar]
- 95.Zhang J, Wang J, Wu Q, et al. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol Psychiatry. 2011;70:334–342. doi: 10.1016/j.biopsych.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 96.Adolphs R. Neural systems for recognizing emotion. Curr Opin Neurobiol. 2002;12:169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- 97.Lang PJ, Bradley MM, Fitzsimmons JR, et al. Emotional arousal and activation of the visual cortex: an fMRI analysis. Psychophysiology. 1998;35:199–210. [PubMed] [Google Scholar]
- 98.Zou Q, Long X, Zuo X, et al. Functional connectivity between the thalamus and visual cortex under eyes closed and eyes open conditions: a resting-state fMRI study. Hum Brain Mapp. 2009;30:3066–3078. doi: 10.1002/hbm.20728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yan C, Liu D, He Y, et al. Spontaneous brain activity in the default mode network is sensitive to different resting-state conditions with limited cognitive load. PLoS One. 2009;4:e5743. doi: 10.1371/journal.pone.0005743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. NeuroImage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tomasi D, Volkow ND. Functional connectivity hubs in the human brain. NeuroImage. 2011;57:908–917. doi: 10.1016/j.neuroimage.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cog Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 103.Hollander E, Pallanti S, Baldini Rossi N, Sood E, Baker BR, Buchsbaum MS. Imaging monetary reward in pathological gamblers. World J Biol Psychiatry. 2005;6:113–120. doi: 10.1080/15622970510029768. [DOI] [PubMed] [Google Scholar]
- 104.Romero MJ, Asensio S, Palau C, Sanchez A, Romero FJ. Cocaine addiction: diffusion tensor imaging study of the inferior frontal and anterior cingulate white matter. Psychiatry Res. 2010;181:57–63. doi: 10.1016/j.pscychresns.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 105.Gonzalez-Hernandez JA, Scherbaum WA. Obesity-specific circuits in the human brain: exploration by Dynamic Brain Self-Reference (dynBSR) Horm Metab Res. 2006;38:777–782. doi: 10.1055/s-2006-958629. [DOI] [PubMed] [Google Scholar]
- 106.Jiang GH, Qiu YW, Zhang XL, et al. Amplitude low-frequency oscillation abnormalities in the heroin users: a resting state fMRI study. NeuroImage. 2011;57:149–154. doi: 10.1016/j.neuroimage.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 107.Rogers MA, Bellgrove MA, Chiu E, Mileshkin C, Bradshaw JL. Response selection deficits in melancholic but not nonmelancholic unipolar major depression. J Clin Exp Neuropsychol. 2004;26:169–179. doi: 10.1076/jcen.26.2.169.28086. [DOI] [PubMed] [Google Scholar]
- 108.Osaka N, Osaka M. Striatal reward areas activated by implicit laughter induced by mimic words in humans: a functional magnetic resonance imaging study. Neuroreport. 2005;16:1621–1624. doi: 10.1097/01.wnr.0000181581.18636.a7. [DOI] [PubMed] [Google Scholar]
- 109.Campos M, Breznen B, Bernheim K, Andersen RA. Supplementary motor area encodes reward expectancy in eye-movement tasks. J Neurophysiol. 2005;94:1325–1335. doi: 10.1152/jn.00022.2005. [DOI] [PubMed] [Google Scholar]
- 110.Mobbs D, Greicius MD, Abdel-Azim E, Menon V, Reiss AL. Humor modulates the mesolimbic reward centers. Neuron. 2003;40:1041–1048. doi: 10.1016/s0896-6273(03)00751-7. [DOI] [PubMed] [Google Scholar]
- 111.Leventhal AM, Chasson GS, Tapia E, Miller EK, Pettit JW. Measuring hedonic capacity in depression: a psychometric analysis of three anhedonia scales. J Clin Psychol. 2006;62:1545–1558. doi: 10.1002/jclp.20327. [DOI] [PubMed] [Google Scholar]
- 112.Choi EY, Yeo BT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 2012;108:2242–2263. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Intrinsic functional connectivity (iFC) secondary analysis group comparison between adolescents with major depressive disorder (MDD) and healthy controls. Note: Maps showing regions with (left) increased iFC in MDD versus controls, and (right) increased iFC in controls versus MDD. Significant iFC with each seed is color coded, with regions functionally connected with more than 1 seed indicated in yellow. Display threshold: Z > 2.3.
Figure S2: Intrinsic functional connectivity (iFC) secondary analysis correlations with major depressive disorder (MDD) severity and corresponding plots. Note: Maps showing regions with iFC negatively correlated with MDD severity. Plots a–b demonstrate these relationships. Display threshold: Z > 2.3.
Figure S3: Intrinsic functional connectivity (iFC) correlations with anhedonia scores and corresponding plots (secondary analysis). Note: Maps showing regions with iFC positively correlated with anhedonia severity. Plots a–c demonstrate these relationships. Display threshold: Z > 2.3. ACC = anterior cingulate cortex; SMA = supplementary motor area.
Figure S4: Additional plots for intrinsic functional connectivity (iFC) primary analysis correlations with anhedonia scores. Note: DC = dorsal caudate; DRP = dorsal rostral putamen; NAc = nucleus accumbens; SMA = supplementary motor area; VC = ventral caudate.
Figure S5: Intrinsic functional connectivity (iFC) controlled for mean framewise displacement (FD) group comparison between adolescents with major depressive disorder (MDD) and healthy controls. Note: Maps controlled for mean FD showing regions with (left) increased iFC in MDD versus controls, and (right) increased iFC in controls versus MDD. Significant iFC with each seed is color coded, with regions functionally connected with more than 1 seed indicated in yellow. Display threshold: Z > 2.3.
Figure S6: Intrinsic functional connectivity (iFC) correlations with major depressive disorder (MDD) severity controlled for mean framewise displacement (FD). Note: Maps controlled for mean FD showing regions with iFC (left) positively correlated with MDD severity and (right) negatively correlated with MDD severity. Display threshold: Z > 2.3.
Figure S7: Intrinsic functional connectivity (iFC) correlations with anhedonia scores controlled for mean framewise displacement (FD). Note: Maps controlled for mean FD showing regions with iFC (left) positively correlated with anhedonia severity and (right) negatively correlated with anhedonia severity. Display threshold: Z > 2.3.
Figure S8: Baseline and individual seed intrinsic functional connectivity (iFC) maps. Note: Maps showing baseline iFC connectivity for each seed region based on the control group, as well as group comparisons, major depressive disorder (MDD) severity correlations, and anhedonia severity correlations for each seed. Display threshold: Z > 2.3. ANHED = anhedonia severity correlations; CDRSR = MDD severity; CT = control; DC = dorsal caudate; dcP = dorsal caudal putamen; drP = dorsal rostral putamen; L = left; NAc = nucleus accumbens; R = right; VC = ventral caudate; vrP = ventral rostral putamen.
Table S1: Intrinsic Functional Connectivity (iFC) Secondary Analysis Group Comparisons (Major Depressive Disorder [MDD] vs. Controls) and Correlations With MDD and Anhedonia Severity (MDD Only)
Note: ACC = anterior cingulate cortex; CDRS-R = Children's Depression Rating Scale–Revised; dmPFC = dorsomedial prefrontal cortex; MNI = Montreal Neurological Institute; PCC = posterior cingulate cortex.
Table S2: Intrinsic Functional Connectivity (iFC) Without Global Signal Regression Group Comparisons (Major Depressive Disorder [MDD] vs. Controls) and Correlations With MDD and Anhedonia Severity (MDD Only)
Note: ACC = anterior cingulate cortex; CDRS-R = Children's Depression Rating Scale–Revised; DC = dorsal caudate; DCP = dorsal caudal putamen; dmPFC = dorsomedial prefrontal cortex; DRP = dorsal rostral putamen; MNI = Montreal Neurological Institute; NAc = nucleus accumbens; PCC = posterior cingulate cortex; VC = ventral caudate; VRP = ventral rostral putamen.
Table S3: Intrinsic Functional Connectivity (iFC) Controlled for Mean Framewise Displacement (FD) Group Comparisons (Major Depressive Disorder [MDD] vs. Controls) and Correlations With MDD and Anhedonia Severity (MDD Only)
Note: ACC = anterior cingulate cortex; CDRS-R = Children's Depression Rating Scale–Revised; DC = dorsal caudate; DCP = dorsal caudal putamen; dmPFC = dorsomedial prefrontal cortex; DRP = dorsal rostral putamen; MNI = Montreal Neurological Institute; NAc = nucleus accumbens; PCC = posterior cingulate cortex; VC = ventral caudate; VRP = ventral rostral putamen.