Abstract
Thymic positive selection is based on the interactions of T cell antigen receptors (TCRs) with self peptide–major histocompatibility complex (MHC) ligands, but the identity of selecting peptides for MHC class II–restricted TCRs and the functional consequences of this peptide specificity are not clear. Here we identify several endogenous self peptides that positively selected the MHC class II–restricted 5C.C7 TCR. The most potent of these also enhanced mature T cell activation, which supports the hypothesis that one function of positive selection is to produce T cells that can use particular self peptide–MHC complexes for activation and/or homeostasis. We also show that inhibiting the microRNA miR-181a resulted in maturation of T cells that overtly reacted toward these erstwhile positively selecting peptides. Therefore, miR-181a helps to guarantee the clonal deletion of particular moderate-affinity clones by modulating the TCR signaling threshold of thymocytes.
From the time it first expresses a T cell antigen receptor (TCR), an αβ T cell’s life-and-death ‘decisions’ are informed by the interactions of its TCRs with complexes of peptide and major histocompatibility complex (pMHC). Roles for endogenous or pathogen-derived agonist peptides in the thymus and the periphery have been appreciated for some time1, and beginning with the initial landmark discovery for MHC class I (ref. 2), endogenous self peptides have been linked to positive selection, the maintenance and homeostatic proliferation of peripheral naive T cells3,4 and the priming of effector T cell responses to antigen5-7. Several groups have suggested a connection between those particular self peptides able to promote positive selection and those that effect the peripheral maintenance of MHC class I–restricted T cells3 and MHC class II–restricted T cells4. Subsequently, a link has been drawn between the positively selecting MHC allele and the ability of self peptide–MHC complexes to enhance peripheral T cell responses5. However, it is not known whether the fairly small set of self peptides8 that trigger the positive selection of a particular T cell are also the ones that can enhance the responses of that T cell in the periphery.
Many weak nonagonist peptides, including mutant versions of antigens as well as endogenous peptides, have been identified as positively selecting ligands for MHC class I–restricted TCRs8,9. In this context, the study of MHC class II–restricted TCRs has not kept pace. It has been known for some time that the positive selection of conventional CD4+ T cells also relies on MHC10 and, critically, on specific peptides11,12, but so far none of these peptides have been identified. And although peptides that positively select MHC class I–restricted TCRs have been identified, a role for specific peptides in TCR signal amplification in the periphery is not clear for CD8+ T cells. MHC class I molecules seem uniformly able to boost agonist signaling, perhaps as a result of the higher affinity of CD8 for MHC class I molecules (relative to the affinity of CD4 for MHC class II molecules)6. To test the connection between selecting pMHC and peripheral T cell activation, we therefore needed to identify peptides that positively select an MHC class II–restricted TCR.
Such peptides would also be useful for examining the mechanisms that control the signaling threshold between positive and negative selection. CD4+CD8+ double positive (DP) thymocytes enact a genetic program that arrests their development and renders them more mortal than any other T cell population. For example, DP cells have higher expression of the ‘gatekeeper’ transcription factors E2A and HEB13 and lower expression of prosurvival molecules such as NF-κB14 and Bcl-2 (ref. 15) than do mature T cells. Another such regulator is probably the microRNA miR-181a16,17. MicroRNAs are a class of small, noncoding RNA molecules of 20–22 base pairs that specifically target and either degrade or repress the translation of cognate mRNA18. Specifically, miR-181a intrinsically modulates TCR sensitivity by suppressing the expression of several phosphatases that act on multiple nodes of the TCR signaling network17.
DP thymocytes are more sensitive to pMHC ligands than are their mature counterparts19. DP thymocytes will commit to apoptosis after encountering as few as two agonist pMHC ligands20, whereas mature T cells require many more for productive signaling to occur21-23. Immature DP thymocytes have roughly six- to tenfold higher expression of miR-181a than do mature T cells (depending on the genetic background), and miR-181a is at least partially responsible for this thymocyte hypersensitivity; inhibition of miR-181a leads to diminished TCR signaling and impaired positive and negative selection in thymocytes with a fixed TCR specificity17. It seems natural to suppose, then, that in a thymus with a diverse TCR repertoire, miR-181a might enforce central tolerance by facilitating negative selection in response to moderate-affinity interactions between TCRs and self peptide–MHC complexes.
Here we report the identification of six endogenous peptides (of more than 90 peptides examined) able to mediate positive selection of the MHC class II–restricted 5C.C7 TCR, which recognizes a fragment of moth cytochrome c (MCC) in the context of I-Ek. The strongest positively selecting peptide was also the most able to enhance activation of naive 5C.C7 T cells, whereas none of the nonselecting peptides could do so. This result underscores the peptide-specific nature of the involvement of self peptide–MHC in antigen recognition by CD4+ T cells and suggests a reason for the peptide-specific nature of thymic self-restriction. In addition, we demonstrate that inhibiting miR-181a in thymus cultures did indeed lead to the production of mature autoreactive CD4+ single-positive (SP) T cells specific for particular self peptides of moderate affinity. Thus, the sensitivity provided by the higher miR-181a expression in thymocytes relative to that in mature T cells enforces central tolerance to particular moderate-affinity self peptide–MHC complexes.
RESULTS
Self peptides that positively select the 5C.C7 TCR
A large number of peptides have been reported to be presented in the context of I-Ek by a B lymphoma cell line24. A coarse analysis has suggested that MHC class II–bound peptides are not very different in thymic and peripheral antigen-presenting cells (APCs)25. Therefore, we hypothesized that these peptides might be suitable candidates for positively selecting ligands.
To narrow our search, we first tested the ability of these peptides to elicit expression of the activation marker CD69 (ref. 8) on DP thymocytes from 5C.C7 mice deficient in the invariant chain (Ii), which chaperones I-Ek molecules to the endosomes, where they can be loaded with peptides. Accordingly, Ii-deficient mice have lower cell surface expression of I-Ek, and these I-Ek complexes present a ‘redacted’ repertoire of self peptides. Among the absent epitopes are those required for positive selection of MCC–I-Ek–reactive T cells; thus, over 95% of 5C.C7 Ii-deficient thymocytes are preselection DP cells11. Several peptides induced appreciable CD69 upregulation in this assay (Table 1).
Table 1.
Positively selecting peptides for the 5C.C7 TCR
| Peptide | P1 | P5 | P9 | CD69+ (%) | Selection | [Min] | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MCC | A | N | E | R | A | D | L | I | A | Y | L | K | Q | A | T | K | 69 | − | |||||
| ATPase 11C | S | S | P | D | E | I | A | L | V | K | G | A | K | R | F | G | F | 31 | ++ & − | 100 μM | |||
| MSA | T | P | T | L | V | E | A | A | R | N | L | G | R | V | G | 26 | None | NA | |||||
| Fetuin | I | P | G | G | P | V | R | L | C | P | G | R | I | R | 22 | + | 200 μM | ||||||
| GP | A | Q | R | A | E | L | I | A | L | T | Q | A | L | K | M | 20 | +++ | 50 μM | |||||
| αTryp | Y | D | R | N | T | K | S | P | L | F | V | G | K | V | 13 | ++ | 1 mM | ||||||
| ER60 | G | F | P | T | I | Y | F | S | P | A | N | K | K | L | 12 | ++ | 150 μM | ||||||
| WDFY1 | H | S | V | I | M | W | D | I | G | G | R | K | G | 8 | None | NA | |||||||
| MCT1 | F | I | S | I | G | F | S | Y | A | F | P | K | S | L | 7 | None | NA | ||||||
| cCyt | V | N | K | E | I | Q | N | A | V | Q | G | V | K | 4 | None | NA | |||||||
| UK1 | I | P | L | I | M | L | I | N | K | A | R | N | K | A | E | 3 | + | 250 μM | |||||
| β2m | H | P | P | H | I | E | I | Q | M | L | K | N | G | K | K | I | P | 2 | + | 500 μM | |||
| gp250 | S | A | P | G | L | I | I | A | T | G | S | V | G | K | N | L | 2 | None | ND | ||||
| No peptide | 2 | None | NA |
Peptide induction of CD69 expression and selection strength. For analysis of CD69 expression, 5C.C7 Ii-deficient thymocytes and CH27 APCs were cultured 4 h with peptides identified before24 (concentration, 20–100 μM; peptides presented in their putative I-Ek-binding registers with P1 and P9 anchors bolded); not all peptides elicited a detectable CD69 response (most of those are not presented here). For analysis of the ability of each peptide to positively select 5C.C7-bearing thymocytes, FTOC were established from E16 5C.C7 Ii-deficient embryos and peptides were added 1 d later; after 5 d of culture, thymocytes were liberated, stained for CD4, CD8, CD69 and CD62L and analyzed by flow cytometry (Fig. 1). Selection strength is presented as the percentage of CD4+ SP T cells generated (−, negative selection; ++ & −, 3–6% CD4+ SP cells at 100–200 μM, and negative selection at 200 μM and above; None, <1% CD4+ SP cells; +, 1–2% CD4+ SP cells; ++, 3–6% CD4+ SP cells; +++, 7–12% CD4+ SP cells) and the minimum concentration ([Min]) of peptide required to observe selection events, relative to the background selection of a paired FTOC with no peptide added (typically 0.5–1%). Data are from three independent experiments (CD69 expression; average) or are representative of at least three experiments per peptide (selection strength).
We next tested whether the addition of any of these 5C.C7-reactive peptides allowed the developmental progression of 5C.C7 Ii-deficient DP thymocytes to the mature CD69−CD62L+CD25− CD4+ SP stage in fetal thymic organ culture (FTOC). Contrary to our expectations, the most potent CD69-inducing peptide was not the most potent at fomenting the maturation of 5C.C7 T cells (Fig. 1 and Table 1; >25% of gated CD4+ cells were also CD69−CD62L+ (data not shown)). This ATPase 11c–derived peptide negatively selected 5C.C7 thymocytes at doses of 200 μM or higher and could only moderately positively select 5C.C7 thymocytes at lower doses (100–200 μM; Fig. 1 and Table 1).
Figure 1.
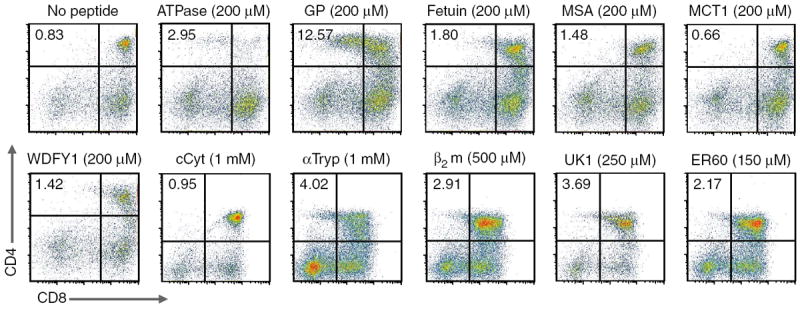
Positive selection of 5C.C7 T cells by endogenous peptides. Expression of CD4 and CD8 in 5C.C7 Ii-deficient FTOC cultured for 4 d with peptides in Table 1 (peptide concentrations, above plots); cultures were also analyzed for expression of CD25, CD69 and CD62L (data not shown). The cCyt, αTryp, β2m, UK1 and ER60 results are from separate experiments and are gated according to their respective no-peptide controls. MSA, mouse serum albumin; MCT1, monocarboxylate transporter 1; WDFY1, WD repeat and FYVE domain–containing 1; cCyt, complement cytolysis inhibitor; αTryp, α-anti-trypsin; β2m, β2-microglobulin; UK1, unknown 1. Data are representative of at least three independent experiments for each peptide.
Instead, the peptide that most efficiently induced positive selection was derived from the group-associated antigen–protease–polymerase (Gag-Pro-Pol) polypeptide (called ‘GP peptide’ here). A ‘blast’ search of the GP peptide sequence showed that it could be generated from four independent chromosomal loci. Mela (encoding melanoma antigen; GenBank accession numbers 6715563 and DQ366147) is on chromosome 8, E1, and apparently belongs to the protein-coding sequence of an endogenous retrovirus26. Rmcf2 (encoding the protein ‘resistance to MCF virus 2’; GeneID accession number 619210 and GenBank accession number AY999005) is on chromosome 18, 56.0 cM; this peptide sequence also belongs to a truncated gag protein27. The gene encoding the mKIAA1466 protein (GenBank accession number BAC65796.1), a mouse ortholog of the human gene encoding mKIAA1466, can be mapped onto 17 chromosomes of the mouse genome, but only two regions—Gm3168 (predicted gene 3168; GenBank accession number XM_001476722.1) on chromosome 8, C5 and Gm3579 (predicted gene 3579; GenBank accession number XM_001477842.1) on chromosome 4, D2.3—are predicted to express protein28. Transcripts corresponding to all four genes were abundantly expressed in stromal cells from the B10.A and B10.BR mouse strains, and two of these transcripts were expressed in particularly large amounts in purified 6C3+I-Ek+ cortical thymic epithelial cells (Supplementary Fig. 1). As GP peptide can be processed and presented by I-Ek in cells24, this suggests that GP–I-Ek should be available to developing DP thymocytes at the anatomical site of positive selection.
GP peptide induced the highest percentage of CD4+ SP cells in FTOC (>10% at 200 μM; Fig. 1). The next most efficient peptide was derived from the cysteine protease of the endoplasmic reticulum ER60, which was among the weakest in terms of CD69 upregulation. No other peptide yielded more than 5% CD4+ SP cells in FTOC (although a β2-microglobulin-derived peptide produced very small numbers of CD4+ SP cells that were functionally mature T cells; Supplementary Fig. 2). Finally, the glycoprotein gp250–derived peptide did not elicit CD69 upregulation in 5C.C7 thymocytes (Table 1), which is notable because Lo et al. show in this issue that gp250 can positively select the MCC–I-Ek–reactive AND TCR29.
Selecting peptides in mature T cell activation
ER60’s ability to positively select 5C.C7 T cells is notable because ER60, when present with agonist peptide in an engineered I-Ek ‘pseudo-dimer’30, has been reported to enhance recall responses of primed 5C.C7 T cells. However, this has not been reported in the context of supported lipid bilayers31. As the peptides involved in both positive selection and coactivation are among the weakest ligands known to have any effect on TCR signaling, it seemed natural to suppose that these groups of peptides might overlap to some degree.
To investigate that possibility, we used the antibody G35, which binds to a small subset of endogenous peptide–I-Ek complexes that are required for positive selection of 5C.C7 T cells32. We prepared splenic B10.BR APCs, treated them with G35, washed them extensively before loading them with MCC, and finally incubated them with naive 5C.C7 T cells. The CD69 response of 5C.C7 T cells was attenuated by pretreatment with G35 (Fig. 2a). As G35 also weakly binds to MCC–I-Ek itself, a possible complication is that residual-free G35 might have simply bound to and blocked MCC–I-Ek. To control for this, we used antibody D4, which, like G35, was raised against MCC–I-Ek. But unlike G35, D4 does not cross-react with positively selecting peptide–I-Ek complexes32. Pretreatment with D4 did not impede CD69 upregulation on 5C.C7 T cells. In an analogous experiment, Ii-deficient APCs that lack 5C.C7-selecting peptides were less stimulatory than their wild-type counterparts, even when they presented equal amounts of MCC (Supplementary Fig. 3).
Figure 2.
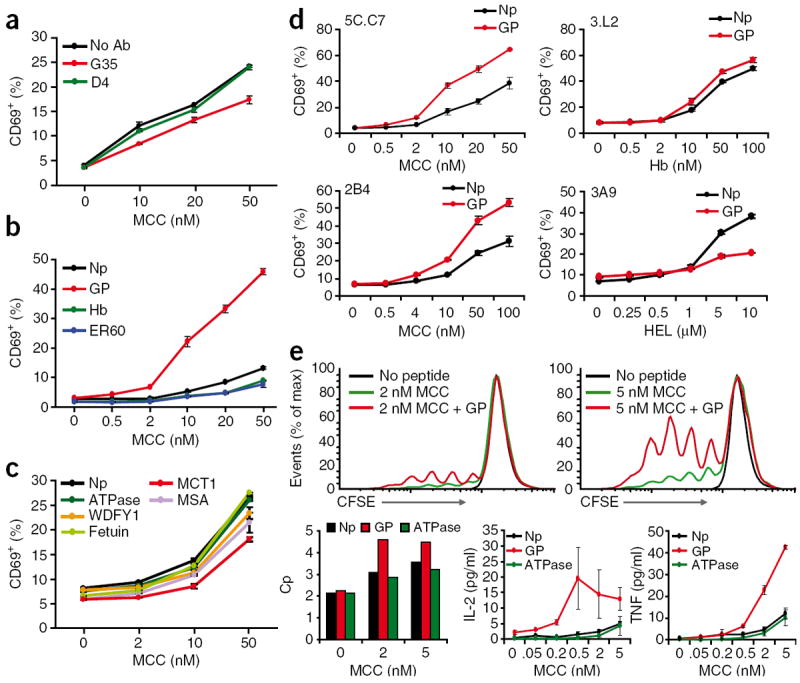
An endogenous selecting peptide contributes to mature T cell activation. (a) Flow cytometry analysis of CD69 expression by naive 5C.C7 CD4+ T cells stimulated for 4 h by B10.BR splenocytes; before use as APCs, the B10.BR splenocytes were depleted of T cells, then treated with nothing (No Ab) or with antibody G35 or D4 (500 μg/ml each), washed extensively and loaded with MCC (concentration, horizontal axis). (b,c) Flow cytometry analysis of the CD69 response of naive 5C.C7 T cells stimulated for 4 h by B10.BR Ii-deficient splenocytes; before use as APCs, the B10.BR splenocytes were depleted of T cells and loaded with MCC alone (Np) or MCC plus 5 μM peptide (key; MCC concentration, horizontal axis). (d) Flow cytometry analysis of CD69 expression by 5C.C7, 3.L2, 2B4 or 3A9 TCR-transgenic T cells incubated for 5 h with B10.BR APCs plus their nominal antigenic peptides (concentration, horizontal axis) with (GP) or without (Np) 5 μM GP peptide. Hb, hemoglobin; HEL, hen egg lysozyme. (e) CFSE profiles (top row) of 5C.C7 lymph node suspensions loaded with the cytosolic dye CFSE for 10 min and washed, followed by the addition of no peptide or MCC (final concentration, 2 nM or 5 nM) with (+ GP) or without GP peptide (final concentration, 5 μM); after 4 d, cells were stained for CD4 and CD8 and assessed by flow cytometry. Bottom row, T cell–proliferative capacity (Cp; left), and supernatant concentrations of IL-2 and tumor necrosis factor (TNF) assessed by fluorescence-based cytometric bead array (bottom middle and right) of the 5C.C7 lymph node cells described above cultured with MCC (concentration, horizontal axes) plus no peptide, GP peptide or ATPase peptide. Data are representative of three (a,c,e) or five (b,d) experiments (mean and s.d.).
G35 also bound to GP–I-Ek and ER60–I-Ek and blocked the positive selection of 5C.C7 thymocytes exposed to these peptides (Supplementary Fig. 4). Nonetheless, it is entirely possible that there exists a subset of G35-bound determinants that have no role in thymocyte selection but might nonetheless influence mature T cell activation. To more directly assess the role of positively selecting pMHC in mature T cell activation, we determined if any of the positively selecting peptides we had found could boost the responses of naive 5C.C7 T cells to MCC.
For this, we used Ii-deficient B10.BR splenic APCs. As these cells lack positively selecting pMHC, we reconstituted them with various peptides along with MCC and assessed their ability to stimulate naive 5C.C7 recombination-activating gene 2–deficient T cells. At low concentrations of MCC, GP peptide enhanced CD69 expression (Fig. 2b), but neither hemoglobin (another I-Ek-binding peptide and a null ligand for 5C.C7) nor ER60 could do so. The slightly lower MCC dose response seen in the presence of these two peptides may be due to their competition with MCC for MHC loading. Somewhat unexpectedly, ATPase 11c peptide did not enhance MCC-induced CD69 expression, and neither did any other peptide that we tested (Fig. 2c).
Notably, the enhanced response to APCs loaded with both MCC and GP peptide relative to the response to MCC alone was not due to an artifact of peptide loading. By measuring the peptide loaded using a biotinylated version of MCC21, we found that at the concentrations used here, GP peptide did not alter the amount of MCC loaded onto MHC (Supplementary Fig. 5a). We also observed enhanced CD69 expression when we loaded and washed away excess MCC before incubation with GP peptide; in this case, the two peptides were never in competition with each other (Supplementary Fig. 5b).
GP peptide–mediated enhancement of signaling was also TCR specific, as neither 3.L2 nor 3A9 TCR-transgenic T cells responded any more vigorously to their cognate agonist peptides (hemoglobin amino acids 64–76 in the context of I-Ek, and hen egg lysozyme amino acids 46–61 in the context of I-Ak, respectively) when presented by B10.BR splenic APCs in the presence of GP peptide (Fig. 2d). GP peptide actually seemed to inhibit the 3A9 response, but this inhibition was due to competition for peptide loading, as it was abolished when hen egg lysozyme and GP peptide were loaded sequentially (Supplementary Fig. 6). GP peptide did boost the response of 2B4 TCR–transgenic T cells, but this might reasonably have been due to some shared specificity, as 2B4 also recognizes MCC–I-Ek as its cognate antigen. Finally, GP also enhanced 5C.C7 T cells’ functional response to MCC–I-Ek, in terms of proliferation and cytokine production (Fig. 2e). Thus, among 95 peptides, GP was the most capable in terms of both positive selection and the ability to assist the naive T cell response.
Central tolerance requires miR-181a
Published work has suggested that miR-181a enhances TCR signaling sensitivity in thymocytes relative to mature T cells17. To examine the role of miR-181a in thymic selection, we made use of an antagomir33 specific for miR-181a (antagomir 181a)17. Antagomirs are 5′-modified and methylated antisense RNA molecules that target and specifically inhibit their cognate miRNA molecules33 (Supplementary Fig. 7). As a control, we used an otherwise identical antagomir with its seed region ‘scrambled’. The seed region is composed of nucleotides 2–7 of the mature miRNA and critically determines its specificity for target mRNA.
It has been suggested that the greater TCR sensitivity of DP thymocytes is important for deletion of self-specific thymocytes19. To investigate whether dampening the sensitivity of DP thymocytes to TCR signaling would result in the production of mature ‘self-avid’ and self-reactive T cells, we generated FTOCs from wild-type B10.BR mice at embryonic day 16 and treated them with antagomirs beginning 1 d after the establishment of the culture (the equivalent of embryonic day 17, when DP cells have just begun to appear; Fig. 3a). After 6 more days we liberated thymocytes from these cultures, washed away any leftover antagomir and added freshly isolated T cell–depleted irradiated B10.BR splenocytes as APCs. After another 4 d we analyzed the activation status of the responding T cells. Indeed, a small fraction of the CD4+ SP T cells that had matured in the antagomir 181a–treated FTOC upregulated CD69 and released the proinflammatory cytokines interferon-γ, tumor necrosis factor and IL-17 in response to irradiated syngeneic splenocytes (Fig. 3b,c). T cells that had matured in the control antagomir–treated FTOCs did not show this self-reactivity. Thus, thymic miR-181a expression is important for the elimination of self-reactive thymocytes.
Figure 3.
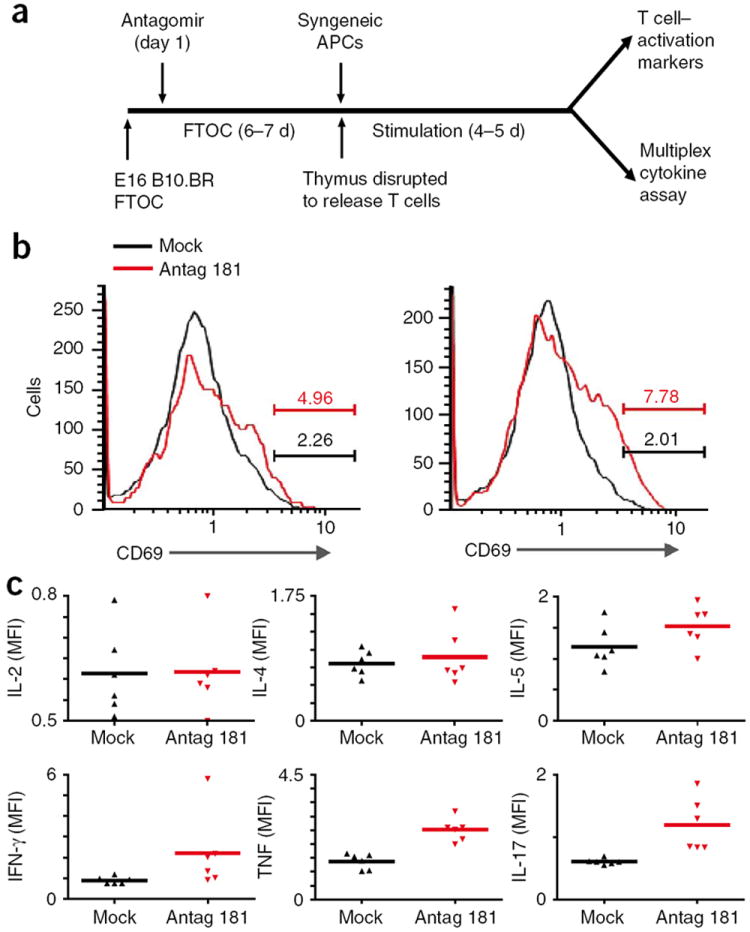
Importance of miR-181a for T cell central tolerance. (a) Experimental setup: E16 B10.BR FTOC were established and 1 d later were treated with antagomir 181a (35 μg/ml) or antagomir with a ‘scrambled’ seed region (mock treatment); after 6–7 total days of culture, T cells were dissociated and washed and then incubated with B10.BR (syngeneic) splenic APCs for an additional 4–5 d, then cells were stained for CD4, CD8 and CD69 and analyzed by flow cytometry (top right) and supernatants were collected for assessment of cytokine concentrations (bottom right). (b) CD69 profiles of CD4+ T cells from FTOCs treated with antagomir with a ‘scrambled’ seed region (Mock) or with miR-181a-specific antagomir (Antag 181). Numbers above bracketed lines indicate percent CD69+ cells. Data are representative of two independent experiments. (c) Fluorescence–based cytometric bead array of cytokines in supernatants from the cultures in b. Each symbol represents an individual sample; small horizontal lines indicate the mean. Data are representative of two independent experiments, each in triplicate.
Together with published results17, such autoreactivity suggested that miR-181a might be crucial for setting a low threshold for signaling in DP thymocytes. However, the induction of autoimmunity involves a complex interaction of conventional and regulatory T cells34. As the development of natural regulatory T cells also relies on TCR signaling in the thymus, one explanation for our observations could be the ablation or alteration of regulatory T cells by miR-181a inhibition. Therefore, we sought more direct evidence of a miR-181a-dependent shift in the conventional TCR repertoire. Specifically, we hypothesized that self peptide–MHC complexes with moderate affinity for TCRs would generate signals strong enough to prompt deletion of normal thymocytes; however, in the absence of miR-181a, thymocytes bearing these TCRs would not be eliminated and would be allowed to mature into autoreactive T cells.
We reasoned that the GP and ATPase 11c peptides should represent moderate-affinity self ligands for the 5C.C7 TCR because ATPase 11c is strong enough to induce negative selection in 5C.C7 transgenic thymocytes at sufficiently high doses and GP peptide is strong enough to influence mature 5C.C7 T cell activation. By using thymi in which the 5C.C7 TCRβ chain is fixed but the TCRα chain is allowed to vary, we hoped to generate a semi-diverse TCR repertoire that would still be enriched for TCRs that recognize GP and ATPase as positively selecting peptides. Diversity of the TCRα chain should allow the production of some TCRs that bind GP peptide and/or ATPase11c with affinity high enough to induce negative selection in an otherwise normal thymus. To find out if these TCRs are spared from negative selection in the absence of miR-181a, we treated 5C.C7 TCR β-chain-transgenic FTOCs with antagomir 181a or mismatched control antagomir and then liberated the resulting thymocytes after 7 d of culture. We allowed these cells to interact with T cell–depleted splenic B10. BR APCs for another 4 d and analyzed the resulting T cell populations with tetramers of GP–I-Ek or ATPase 11c–I-Ek (Fig. 4). Consistent with what has been seen in healthy mice35 and human subjects36, control mock-treated thymi produced few GP peptide– or ATPase 11c–reactive T cells (Fig. 4b,d). However, thymi treated with antagomir 181a produced considerably more T cells specific for GP–I-Ek or ATPase 11c–I-Ek in terms of the total number of tetramer-positive cells (Fig. 4a,c) and as a percentage of total T cells (Fig. 4b,d). Furthermore, the tetramer-specific T cell populations expanded in the presence of additional GP or ATPase 11c peptide (Fig. 4a,c), which shows that miR-181a is necessary to prevent the development of T cells that are specific for and reactive to self peptide–MHC complexes of moderate affinity.
Figure 4.
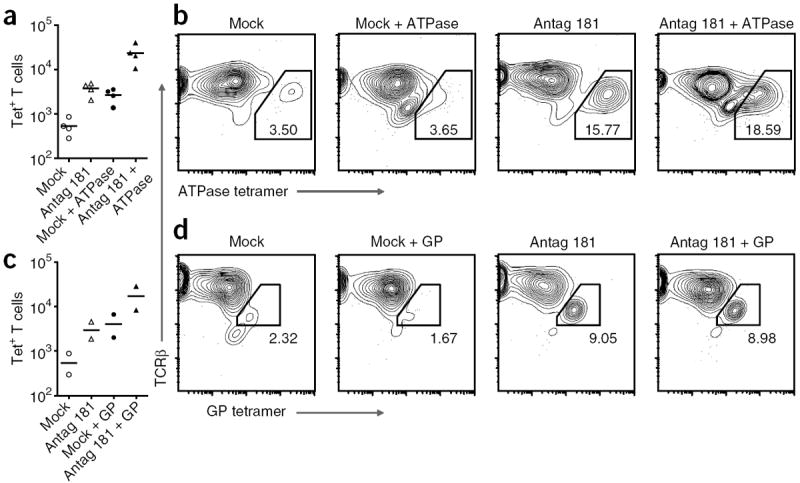
Negative selection threshold set by miR-181a. Flow cytometry of cells from E16 5C.C7 TCRβ-transgenic FTOC (established as described in Fig. 3a) cultured for 7 d with antagomir 181a (35 μg/ml) or antagomir with a ‘scrambled’ seed region (Mock), then dissociated and washed and incubated for 4 d with B10.BR splenic APCs with or without 5 μM ATPase 11c peptide (a,b) or GP peptide (c,d) and then stained for CD4, CD8 and TCRβ and with ATPase–I-Ek tetramer (a,b) or GP–I-Ek tetramer (c,d). (a,c) Tetramer-positive (Tet+) T cells. Each symbol represents an independent experiment; small horizontal lines indicate the mean. (b,d) TCRβ and tetramer profiles of CD4+ T cells. Numbers in or adjacent to outlined areas indicate percent tetramer-positive cells among total CD4+ T cells. Data are representative of four experiments (ATPase 11c tetramer) or two experiments (GP tetramer).
Feedback regulation of miR-181a by TCR signaling
Individual T cells can vary substantially in their expression of different signaling molecules and, consequently, T cells bearing the same TCR can respond differently to a particular peptide-MHC ligand37. Therefore, effective central tolerance might involve not only the selection of an appropriate TCR repertoire but also setting of the appropriate signaling sensitivity in individual T cells. The miR-181a-dependent transition from higher to lower TCR sensitivity as cells progress from the DP stage to the SP stage might simply be part of the developmental program of T cell maturation. Alternatively, it might represent ‘tuning’ of TCR sensitivity by alteration of the amount of miR-181a expression according to the strength of TCR interactions with thymic self peptide–MHC complexes.
We sought to determine whether miR-181a downregulation is one of the many long-term changes in gene expression that result from positive selection, or whether it is more acutely and directly related to TCR signaling. First we stimulated 5C.C7 Ii-deficient preselection DP thymocytes with plate-bound I-Ek complexes and measured their expression of miR-181a relative to that of unstimulated thymocytes after 1 h by quantitative PCR. As this time frame is too short for DP cells to commit to positive selection38 (and in any case, DP cells do not progress to the SP stage in such cultures), any changes we observed should have represented acute regulation by TCR signaling. DP thymocytes downregulated miR-181a to 25–33% of their resting amounts in response to acute stimulation with MCC–I-Ek or GP–I-Ek (Fig. 5a).
Figure 5.
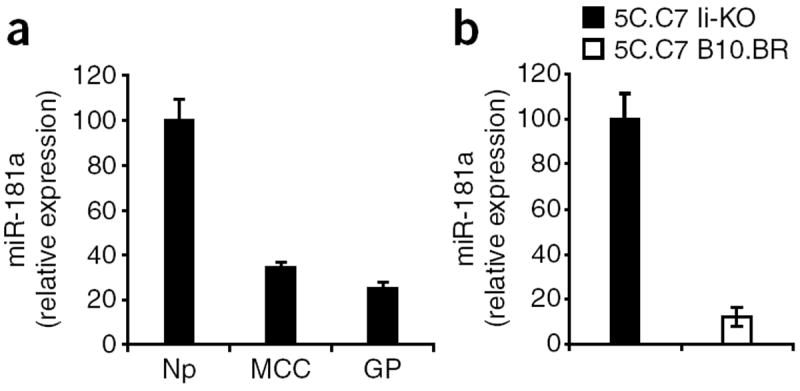
Thymocytes downregulate miR-181a in response to selection signals. (a) Quantitative real-time PCR analysis of mature miR-181a in lysates of 5C.C7 Ii-deficient thymocytes allowed to interact for 1 h with plate-bound MCC–I-Ek or GP–I-Ek complexes or with no protein. Results are presented relative to expression in response to no protein, set as 100. Data represent two independent experiments (error bars, s.d.). (b) Quantitative real-time PCR analysis of miR-181a in DP 5C.C7 TCR–transgenic Ii-deficient thymocytes (5C.C7 Ii-KO) and 5C.C7 TCR–transgenic B10.BR thymocytes (5C.C7 B10.BR). Results are presented relative to expression in 5C.C7 Ii-KO thymocytes, set as 100. Data represent two independent experiments (error bars, s.d.).
Next we purified DP thymocytes from nonselecting 5C.C7 Ii-deficient thymi and from positively selecting 5C.C7 B10.BR thymi. In DP cells that have not yet begun their progression toward the SP stage, any differences in miR-181a expression should reflect a response to acute TCR stimulation rather than differentiation. We noted considerable downmodulation of miR-181a in DP cells from the positively selecting environment relative to that in DP cells from nonselecting thymi (Fig. 5b). Together these data suggest that TCR signals directly feed back to modulate miR-181a expression, and thus TCR sensitivity, according to the strength of the selection signal in the thymus.
DISCUSSION
Here we have shown that of 95 endogenous I-Ek-bound peptides, 6 could positively select CD4+ T cells bearing the 5C.C7 TCR in Ii-deficient FTOCs. This report and the accompanying report of Lo et al.29 are the first to our knowledge to identify endogenous peptides able to positively select T cells bearing MHC class II–restricted TCRs. Like peptides that positively select the MHC class I–restricted OT-I TCR8, the strongest of these 5C.C7 positively selecting peptides, GP, structurally resembles the 5C.C7 nominal antigen MCC; the two peptides share four identical residues and a chemically similar fifth residue. The conserved residues in MCC and GP peptide are those that provide the MHC anchors39. Thus, the underlying ‘shapes’ of MCC– I-Ek and GP–I-Ek might be similar, although the particular side chains that underlie the fine specificity of the 5C.C7 TCR for each pMHC complex are very different. This finding agrees with the observation that a peptide-dependent MHC conformation is important for selection40 and is also consistent with published reports of degeneracy or polyspecificity in the TCR-contacting residues of peptides as a result of positive selection41. In addition, GP peptide is structurally compatible with the published positive selection–inhibiting G35 antibody32, and G35 bound to GP–I-Ek and blocked GP–I-Ek–mediated positive selection. These features of GP peptide are thus consistent with what is known about the peptides that positively select 5C.C7 in particular, and TCRs in general, in vivo. The concentration of GP peptide required for its activity in FTOC was somewhat higher than what is required for the best positively selecting peptides for OT-I TCR. This may have been due in part to differences in MHC expression and/or peptide loading in the FTOCs deficient in β2-microglobulin and transporter associated with antigen processing used in MHC class I–restricted systems and the Ii-deficient FTOC used here, or it may have reflected the relatively large amount of GP peptide available in the thymus.
However, the sequence similarity between GP peptide and MCC may not be a ‘hard and fast’ rule governing selection. The other self peptides that—albeit more weakly—drove positive selection of 5C.C7 T cells were less similar in sequence to MCC. Also, gp250, a peptide able to positively select the MCC–I-Ek–responsive AND TCR, does not seem to share extensive homology with the AND nominal antigenic peptide29. Furthermore, GP–I-Ek failed to stimulate a response from AND thymocytes, and gp250–I-Ek did not elicit a response from 5C.C7 thymocytes. Thus, although these two TCRs recognize the same antigen, their fine specificity for positively selecting ligands is very different.
GP peptide was also notable because it acted in synergy with MCC to activate mature 5C.C7 T cells. This contrasts with what has been reported for CD8+ OT-I T cells, in which endogenous pMHC complexes are either neutral42 or uniformly synergistic regardless of peptide sequence43. In the latter case, this was attributed to the relatively high-affinity interaction between CD8 and the invariant portion of MHC class I (ref. 44); strong binding of CD8 to MHC class I may overwhelm and obviate the need for the binding of TCR to any particular endogenous pMHC. As the affinity of CD4 for MHC class II is much lower, weak interactions between TCRs and particular endogenous pMHC complexes may contribute in a biologically meaningful way to the overall functional outcome of interactions between CD4+ T cells and MHC class II–expressing cells. Alternatively, there may yet be an unidentified peptide-specific role for self MHC in enhancing CD8+ T cell responses.
The identity of these positively selecting peptides was also useful in answering questions related to central tolerance. It is known that miR-181a controls the TCR signaling sensitivity threshold of T lymphocytes. Here we have provided direct evidence that this miR-181a-dependent threshold crucially determines the affinity of TCRs that are deleted in the thymus versus those that escape to the periphery. DP thymocytes are normally more sensitive to TCR stimuli than are their peripheral naive counterparts, due at least in part to higher miR-181a expression17. When we reduced miR-181a expression in DPs undergoing thymic selection, the resulting mature T cells recognized and were activated by particular high-affinity positively selecting self peptides. Thus, if DPs were allowed to undergo thymic selection with the TCR sensitivity of peripheral mature T cells, the result would probably be autoreactivity. For this reason, stage-specific miR-181a-mediated ‘tuning’ of TCR sensitivity seems to be a necessary feature of successful immunity. It is notable that mature T cells, already very sensitive to even single agonist ligands21, must be made less sensitive than they otherwise could be to function properly. Nonetheless, positively selecting peptides retain some measure of activity that can influence peripheral mature T cell function. It has been shown that gp250 facilitates homeostatic maintenance and proliferation of AND T cells29, and GP peptide acted to enhance the response of 5C.C7 naive T cells. These observations raise two important points.
First, there exist weak epitopes that influence peripheral T cell function but that cannot be detected by assaying mature T cells. Stage-specific alterations in TCR sensitivity can be taken advantage of to discover these epitopes, as we have done here for GP peptide. Second, the ability to alter responsiveness toward particular peptides by modulating miR-181a expression might be a way to ‘tune’ responsiveness in the periphery. Expression of miR-181a is quite low in naive T cells and lower still in activated T cells, but in those populations, miR-181a expression may be heterogeneous. We have shown here that DP thymocytes modulated miR-181a expression in response to positively selecting self peptide–MHC complexes in the thymus. This TCR-mediated feedback regulation of TCR sensitivity may serve to ‘tune’ T cells according to the affinity of their TCRs so that, for example, a T cell with a particularly ‘self-avid’ TCR could still become a productive member of the mature repertoire, as long as miR-181a was expressed in amounts low enough to prevent overt autoreactivity. As self peptide–MHC is the major driver of the maintenance of naive T cells, stronger TCR signaling in response to self peptide–MHC in the periphery might more strongly suppress miR-181a expression than would weaker signaling. Thus, miR-181a might represent a way to normalize T cell responsiveness despite differing TCR affinity for self ligands.
In the context of dynamic miR-181a expression, the ability of endogenous self peptides to influence mature T cell function seems to be a natural and beneficial outcome, as miR-181a downregulation can lower TCR sensitivity to self ligands but cannot abolish the TCR’s affinity for those ligands. By the same token, it might be expected that particularly avid positively selecting self peptides would retain some more overt measure of reactivity even when miR-181a is downregulated to only a few dozen copies per cell, as it is in peripheral T cells. In fact, some self peptides can enhance naive T cell responses to their nominal antigens by maintaining a state of phosphorylative readiness5 or perhaps by providing unique ‘coagonistic’ signals45. Here we have shown that at least in one case for the 5C.C7 TCR, the positively selecting peptide and the mature T cell response–enhancing peptide are one and the same.
This raises the question of what precisely positive selection is selecting for. It has long been thought that selecting for TCRs that have a weak affinity for self peptide–MHC would enrich the TCR repertoire for the ability to recognize foreign antigen presented by self MHC46. However, evolutionary pressure on variable-region gene segments has already conferred substantial MHC specificity41,47 and self MHC restriction48,49. Furthermore, as shown here and by Lo et al.29 for MHC class II-restricted T cells, positive selection of a given TCR is extremely specific for self peptides and not just for self MHC, in that 6 of 95 peptides could select 5C.C7 T cells and 1 of 95 could select AND T cells29. This peptide specificity is further underscored by the observation that 5C.C7 and AND29, two TCRs that are restricted to I-Ek and recognize the same foreign I-Ek-bound peptide, are positively selected by distinct self peptides. Similarly, only 2 among the 27 self peptides tested drove maturation of the MHC class I–restricted OT-I TCR8. These results suggest that positive selection is testing for a more stringent interaction than binding to self MHC alone. One possibility for this parameter might be the ability of a particular self peptide–MHC complex to participate in TCR signaling in mature T cells, as suggested before50. In support of this, T cells that bind to an antigen–I-Ab tetramer are positively selected in both b-haplotype and d-haplotype mice but are better able to respond to that antigen if they are selected by I-Ab (ref. 49). However, this response-enhancing ability does not seem to be common, as we and Lo et al. have found only one such self peptide for 5C.C7 and one for AND29, respectively, among 95 candidates. Given this high degree of specificity, we suggest that at least some of the TCRs generated in the thymus cannot use any particular self peptide–MHC complex in this way, and so it is advantageous to select those clones that can. Thus, we believe that positive selection selects for TCRs that can interact with those self peptide–MHC complexes that are useful in the periphery for maintaining homeostasis4,29 and/or enhancing T cell responses to foreign antigens5,29,50.
ONLINE METHODS
Mice
The 5C.C7 mice on the B10.BR background were from Taconic Farms. The 5C.C7 Ii-deficient mice were obtained by crossing of 5C.C7 mice with Ii-deficient (Cd74−/−) B10.BR mice (a gift from C. Benoist and D. Mathis). The 5C.C7 B6 mice were obtained by crossing of 5C.C7 onto the C57BL/6 background for more than seven generations51. The 2B4 mice were generated as described10. The 3A9 and N3.L2 mice were from Jackson Laboratories. All mice were bred and maintained at the Stanford University Department of Comparative Medicine Animal Facility (protocol 3540) in accordance with guidelines of the US National Institutes of Health.
Antibodies, soluble I-Ek complexes, tetramers and antagomirs
Generation of the MCC–I-Ek–specific antibodies G35 and D4 has been described32. ATPase–I-Ek and GP–I-Ek monomers were prepared, biotinylated and made into tetramers as described52. Antagomirs (from Alnylam) were synthesized as described33 with the following sequences: antagomir 181a, 5′-Cy3-soAsoCoUoCoAoCoCoGoAoCoAoGoCoGoUoUoGoAoAoUsoGso UsoUs-Chol-3′, and mm-antagomir 181a (‘scrambled’ antagomir), 5′-Cy3-soA-soCoUoCoAoCoCoGoAoCoAoGoCoGoUoUoUoUoUoAsoUsoAsoUs-Chol-3′ (where ‘Cy3’ is indocarbocyanine; ‘s’ indicates phosphorothioate linkage; ‘o’ indicates 2′-O-methyl-modified nucleotides; and ‘Chol’ indicates cholesterol linked through a hydroxyprolinol linkage).
Peptides
Crude peptides representing those described before24 were from Sigma Genosys and were used for the initial screening of CD69 upregulation (Table 1). Peptide candidates (from Biosynthesis, Elim Biopharmaceuticals or Anaspec) were purified by high-performance liquid chromatography before use in other experiments. Biotinylated peptides were generated as described21.
Quantitative RT-PCR
A ‘blast’ search of the GP peptide sequence showed that it could be generated from four independent chromosomal loci of the mouse genome. Transcripts for these genes were detected by quantitative PCR with the following primers: GP-DQ366147 (PCR product length, 207 base pairs) sense, 5′-CCCCTCTGAGTCTGACCCTAGAA-3′, and antisense, 5′-GTCCGTGGGGACCAGGAGAG-3′; GP-AY999005 (PCR product length 202 base pairs) sense, 5′-CCCCTCTGAGTCTAACTTTGCAG-3′, and antisense, 5′-TGGGGACCAGGACAAAACACTC-3′; GP-XM001477842 and GP-6XM001476722 (PCR product length, 189 base pairs) sense, 5′-GTTCGAATCAGGACAAGGGTG-3′, and antisense, 5′-GTCAAAACCA GGGCCTGCAC-3′; ATPase 11c (amplify both splicing isoforms; PCR product length, 186 base pairs) sense, 5′-ACCGCAGACCCTCGATTGTATA-3′, and antisense, 5′-GGTTCCAAATGTCCAATTTCCA-3′; and internal control SDHA (encoding the flavoprotein succinate dehydrogenase complex, subunit A; PCR product length, 160 base pairs) sense, 5′-TTATTGCTACTGGGGGCTACG GG-3′, and antisense, 5′-AGGCAGCCAGCACCGTATATACC-3′. An Eppendorf Realplex2 and an SYBR fast quantitative PCR kit (KAPA biosystems) were used for quantitative PCR. Reaction conditions were 95 °C for 2 min, followed by 40 cycles of 95 °C for 15 s, 55 °C for 25 s and 68 °C for 20 s.
Flow cytometry
Cell surface molecules were stained with antibody to CD4 (anti-CD4; GK1.5 or RM4-5), anti-CD8α (53-6.7), anti–I-Ek (14.4.4s), strepta-vidin-PE, anti-CD69 (H1.2F3), anti-TCRβ (H57), anti-CD25 (PC61), anti-BP-1 (6C3), and/or anti-CD62L (MEL-14; all from BD Biosciences). Live cells were identified by their forward-scatter and side-scatter profiles. Samples were run on a Cytomics FC500 (Beckman Coulter) or FACStar (Becton-Dickinson) and data were analyzed with FlowJo software. Cells were sorted on a FACSAria (Becton-Dickinson).
Thymocyte culture
For these cultures, 5C.C7 Ii-deficient or 5C.C7 B6 thymocytes (2 × 105 to 3 × 105 cells) were incubated for 4 h with CH27 B lymphoma cells (1.5 × 105 to 2 × 105) and crude peptide at concentrations ranging from 20 μM to 100 μM. Expression of CD4, CD8 and CD69 was then analyzed by flow cytometry.
FTOC
Fetal thymi at embryonic days 16–17 were dissected and placed on a 0.8-μm filter supported by a membrane support pad (both from Millipore) in 1.5 ml culture medium and were cultured for 4–7 d at 37 °C. Purified peptides, antagomirs and/or antibodies were added on day 1 of culture.
Peripheral T cell culture
Lymph nodes were dissected and enriched for T cells by depletion of B220+ cells with anti-B220 Dynal beads (Invitrogen) and depletion of I-Ek+ cells with biotin–anti–I-Ek (BD Biosciences) and streptavidin Dynal beads (Invitrogen). APCs were prepared from spleen; red blood cells were removed with NH4Cl buffer and T cells were removed with anti-Thy-1 Dynal beads (Invitrogen). T cells (1 × 105 to 2 × 105) were incubated for various times at 37 °C with APCs (1.5 × 105 to 3 × 105). For analysis of cell proliferation, lymph node cells were labeled for 10 min with CFSE (1.3 μM per 1 × 106 cells) in Hank’s balanced-salt solution plus 0.2% (vol/vol) FCS, then were washed in medium containing 10% (vol/vol) FCS. Proliferative capacity was calculated as described53. Cytokine secretion into culture supernatants was analyzed by cytometric bead array (BD Biosciences).
Statistics
Significance was assessed by Student’s t-test, and P values of less than 0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank P. Allen (Washington University) for peptide sequence data and critical discussions; C.-Z. Chen for comments on the manuscript; D. Mathis and C. Benoist (Harvard University) for Ii-deficient mice; E.W. Newell and Y. Wong for help with cell sorting; and T. Mao for help with RNA blot analysis. Supported by the Howard Hughes Medical Institute (P.J.R.E. and M.M.D.), Duke University (Q.-J. L.) and the US National Institutes of Health (RO1 AIO22511).
Footnotes
AUTHOR CONTRIBUTIONS
P.J.R.E., Q.-J.L. and M.M.D. conceived of the project, designed experiments and interpreted results; P.J.R.E. and Q.-J.L. did experiments and analyzed data; S.J. quantified miR-181a in terms of TCR signaling feedback and expression analysis for GP peptides; J.X. contributed critical reagents and technical support; and P.J.R.E., Q.-J.L. and M.M.D. prepared the manuscript.
Note: Supplementary information is available on the Nature Immunology website.
COMPETING INTERESTS STATEMENT
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at http://www.nature.com/natureimmunology/.
References
- 1.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 2.Hogquist KA, Gavin MA, Bevan MJ. Positive selection of CD8+ T cells induced by major histocompatibility complex binding peptides in fetal thymic organ culture. J Exp Med. 1993;177:1469–1473. doi: 10.1084/jem.177.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldrath AW, Bevan MJ. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 1999;11:183–190. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 5.Stefanova I, Dorfman JR, Tsukamoto M, Germain RN. On the role of self-recognition in T cell responses to foreign antigen. Immunol Rev. 2003;191:97–106. doi: 10.1034/j.1600-065x.2003.00006.x. [DOI] [PubMed] [Google Scholar]
- 6.Yachi PP, Ampudia J, Gascoigne NR, Zal T. Nonstimulatory peptides contribute to antigen-induced CD8–T cell receptor interaction at the immunological synapse. Nat Immunol. 2005;6:785–792. doi: 10.1038/ni1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krogsgaard M, Davis MM. How T cells ‘see’ antigen. Nat Immunol. 2005;6:239–245. doi: 10.1038/ni1173. [DOI] [PubMed] [Google Scholar]
- 8.Santori FR, et al. Rare, structurally homologous self-peptides promote thymocyte positive selection. Immunity. 2002;17:131–142. doi: 10.1016/s1074-7613(02)00361-8. [DOI] [PubMed] [Google Scholar]
- 9.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 10.Berg LJ, et al. Antigen/MHC-specific T cells are preferentially exported from the thymus in the presence of their MHC ligand. Cell. 1989;58:1035–1046. doi: 10.1016/0092-8674(89)90502-3. [DOI] [PubMed] [Google Scholar]
- 11.Tourne S, Nakano N, Viville S, Benoist C, Mathis D. The influence of invariant chain on the positive selection of single T cell receptor specificities. Eur J Immunol. 1995;25:1851–1856. doi: 10.1002/eji.1830250709. [DOI] [PubMed] [Google Scholar]
- 12.Barton GM, Rudensky AY. Requirement for diverse, low-abundance peptides in positive selection of T cells. Science. 1999;283:67–70. doi: 10.1126/science.283.5398.67. [DOI] [PubMed] [Google Scholar]
- 13.Jones ME, Zhuang Y. Acquisition of a functional T cell receptor during T lymphocyte development is enforced by HEB and E2A transcription factors. Immunity. 2007;27:860–870. doi: 10.1016/j.immuni.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voll RE, et al. NF-kappa B activation by the pre-T cell receptor serves as a selective survival signal in T lymphocyte development. Immunity. 2000;13:677–689. doi: 10.1016/s1074-7613(00)00067-4. [DOI] [PubMed] [Google Scholar]
- 15.Linette GP, et al. Bcl-2 is upregulated at the CD4+CD8+ stage during positive selection and promotes thymocyte differentiation at several control points. Immunity. 1994;1:197–205. doi: 10.1016/1074-7613(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 16.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 17.Li QJ, et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Leung AK, Sharp PA. Function and localization of microRNAs in mammalian cells. Cold Spring Harb Symp Quant Biol. 2006;71:29–38. doi: 10.1101/sqb.2006.71.049. [DOI] [PubMed] [Google Scholar]
- 19.Davey GM, et al. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J Exp Med. 1998;188:1867–1874. doi: 10.1084/jem.188.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebert PJ, Ehrlich LI, Davis MM. Low ligand requirement for deletion and lack of synapses in positive selection enforce the gauntlet of thymic T cell maturation. Immunity. 2008;29:734–745. doi: 10.1016/j.immuni.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–849. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- 22.Li QJ, et al. CD4 enhances T cell sensitivity to antigen by coordinating Lck accumulation at the immunological synapse. Nat Immunol. 2004;5:791–799. doi: 10.1038/ni1095. [DOI] [PubMed] [Google Scholar]
- 23.Purbhoo MA, Irvine DJ, Huppa JB, Davis MM. T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol. 2004;5:524–530. doi: 10.1038/ni1058. [DOI] [PubMed] [Google Scholar]
- 24.Felix NJ, et al. Alloreactive T cells respond specifically to multiple distinct peptide-MHC complexes. Nat Immunol. 2007;8:388–397. doi: 10.1038/ni1446. [DOI] [PubMed] [Google Scholar]
- 25.Marrack P, Ignatowicz L, Kappler JW, Boymel J, Freed JH. Comparison of peptides bound to spleen and thymus class II. J Exp Med. 1993;178:2173–2183. doi: 10.1084/jem.178.6.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayashi H, et al. Molecular cloning and characterization of the gene encoding mouse melanoma antigen by cDNA library transfection. J Immunol. 1992;149:1223–1229. [PubMed] [Google Scholar]
- 27.Wu T, Yan Y, Kozak CA. Rmcf2, a xenotropic provirus in the Asian mouse species Mus castaneus, blocks infection by polytropic mouse gammaretroviruses. J Virol. 2005;79:9677–9684. doi: 10.1128/JVI.79.15.9677-9684.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okazaki N, et al. Prediction of the coding sequences of mouse homologues of KIAA gene: II. The complete nucleotide sequences of 400 mouse KIAA-homologous cDNAs identified by screening of terminal sequences of cDNA clones randomly sampled from size-fractionated libraries. DNA Res. 2003;10:35–48. doi: 10.1093/dnares/10.1.35. [DOI] [PubMed] [Google Scholar]
- 29.Lo W-L, Felix NJ, Walters JJ, Rohrs H, Gross ML, Allen PM. An endogenous peptide positively selects and augments the activation and survival of peripheral CD4+ T cells. Nat Immunol. 2009 Oct 4; doi: 10.1038/ni.1796. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krogsgaard M, et al. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature. 2005;434:238–243. doi: 10.1038/nature03391. [DOI] [PubMed] [Google Scholar]
- 31.Ma Z, Sharp KA, Janmey PA, Finkel TH. Surface-anchored monomeric agonist pMHCs alone trigger TCR with high sensitivity. PLoS Biol. 2008;6:e43. doi: 10.1371/journal.pbio.0060043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baldwin KK, Reay PA, Wu L, Farr A, Davis MMA. T cell receptor-specific blockade of positive selection. J Exp Med. 1999;189:13–24. doi: 10.1084/jem.189.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krutzfeldt J, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 34.Goodnow CC. Multistep pathogenesis of autoimmune disease. Cell. 2007;130:25–35. doi: 10.1016/j.cell.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 35.Reddy J, et al. Detection of autoreactive myelin proteolipid protein 139–151-specific T cells by using MHC II (IAs) tetramers. J Immunol. 2003;170:870–877. doi: 10.4049/jimmunol.170.2.870. [DOI] [PubMed] [Google Scholar]
- 36.Danke NA, Koelle DM, Yee C, Beheray S, Kwok WW. Autoreactive T cells in healthy individuals. J Immunol. 2004;172:5967–5972. doi: 10.4049/jimmunol.172.10.5967. [DOI] [PubMed] [Google Scholar]
- 37.Feinerman O, Veiga J, Dorfman JR, Germain RN, Altan-Bonnet G. Variability and robustness in T cell activation from regulated heterogeneity in protein levels. Science. 2008;321:1081–1084. doi: 10.1126/science.1158013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yasutomo K, Doyle C, Miele L, Fuchs C, Germain RN. The duration of antigen receptor signalling determines CD4+ versus CD8+ T-cell lineage fate. Nature. 2000;404:506–510. doi: 10.1038/35006664. [DOI] [PubMed] [Google Scholar]
- 39.Reay PA, Kantor RM, Davis MM. Use of global amino acid replacements to define the requirements for MHC binding and T cell recognition of moth cytochrome c (93–103) J Immunol. 1994;152:3946–3957. [PubMed] [Google Scholar]
- 40.Stefanski HE, Jameson SC, Hogquist KA. Positive selection is limited by available peptide-dependent MHC conformations. J Immunol. 2000;164:3519–3526. doi: 10.4049/jimmunol.164.7.3519. [DOI] [PubMed] [Google Scholar]
- 41.Huseby ES, et al. How the T cell repertoire becomes peptide and MHC specific. Cell. 2005;122:247–260. doi: 10.1016/j.cell.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 42.Sporri R, Reis e Sousa C. Self peptide/MHC class I complexes have a negligible effect on the response of some CD8+ T cells to foreign antigen. Eur J Immunol. 2002;32:3161–3170. doi: 10.1002/1521-4141(200211)32:11<3161::AID-IMMU3161>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 43.Yachi PP, Lotz C, Ampudia J, Gascoigne NR. T cell activation enhancement by endogenous pMHC acts for both weak and strong agonists but varies with differentiation state. J Exp Med. 2007;204:2747–2757. doi: 10.1084/jem.20062610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia KC, et al. CD8 enhances formation of stable -cel T-cell receptor/MHC class I molecule complexes. Nature. 1996;384:577–581. doi: 10.1038/384577a0. [DOI] [PubMed] [Google Scholar]
- 45.Davis MM, et al. T cells as a self-referential, sensory organ. Annu Rev Immunol. 2007;25:681–695. doi: 10.1146/annurev.immunol.24.021605.090600. [DOI] [PubMed] [Google Scholar]
- 46.Fink PJ, Bevan MJ. H-2 antigens of the thymus determine lymphocyte specificity. J Exp Med. 1978;148:766–775. doi: 10.1084/jem.148.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scott-Browne JP, White J, Kappler JW, Gapin L, Marrack P. Germline-encoded amino acids in the αβ T-cell receptor control thymic selection. Nature. 2009;458:1043–1046. doi: 10.1038/nature07812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zerrahn J, Held W, Raulet DH. The MHC reactivity of the T cell repertoire prior to positive and negative selection. Cell. 1997;88:627–636. doi: 10.1016/s0092-8674(00)81905-4. [DOI] [PubMed] [Google Scholar]
- 49.Chu HH, et al. Positive selection optimizes the number and function of MHCII-restricted CD4+ T cell clones in the naive polyclonal repertoire. Proc Natl Acad Sci USA. 2009;106:11241–11245. doi: 10.1073/pnas.0902015106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krogsgaard M, Juang J, Davis MM. A role for “self” in T-cell activation. Semin Immunol. 2007;19:236–244. doi: 10.1016/j.smim.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richie LI, et al. Imaging synapse formation during thymocyte selection: inability of CD3ζ to form a stable central accumulation during negative selection. Immunity. 2002;16:595–606. doi: 10.1016/s1074-7613(02)00299-6. [DOI] [PubMed] [Google Scholar]
- 52.Altman JD, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 53.Gudmundsdottir H, Wells AD, Turka LA. Dynamics and requirements of T cell clonal expansion in vivo at the single-cell level: effector function is linked to proliferative capacity. J Immunol. 1999;162:5212–5223. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


