SUMMARY
Immature double-positive (CD4+CD8+) thymocytes respond to negatively selecting peptide-MHC ligands by forming an immune synapse that sustains contact with the antigen-presenting cell (APC). Using fluorescently labeled peptides, we showed that as few as two agonist ligands could promote APC contact and subsequent apoptosis in reactive thymocytes. Furthermore, we showed that productive signaling for positive selection, as gauged by nuclear translocation of a green fluorescent protein (GFP)-labeled NFATc construct, did not involve formation of a synapse between thymocytes and selecting epithelial cells in reaggregate thymus cultures. Antibody blockade of endogenous positively selecting ligands prevented NFAT nuclear accumulation in such cultures and reversed NFAT accumulation in previously stimulated thymocytes. Together, these data suggest a “gauntlet” model in which thymocytes mature by continually acquiring and reacquiring positively selecting signals without sustained contact with epithelial cells, thereby allowing them to sample many cell surfaces for potentially negatively selecting ligands.
INTRODUCTION
The mature T cell repertoire arises as the result of interactions between immature T cell precursors (double-positive [DP] thymocytes) and antigen-presenting cells (APCs) within the thymus. A strong interaction between the thymocyte’s T cell receptor molecules (TCRs) and self-peptide-self-MHC on any of the APCs it encounters is likely to induce programmed cell death (termed negative selection, or clonal deletion), whereas failure to achieve a sufficient threshold of TCR signaling results eventually in death by neglect (von Boehmer et al., 2003). Juxtaposed between these two outcomes is an intermediate mode of engagement with self-peptide-MHC that can induce sweeping transcriptional changes that result eventually in the thymocyte’s maturation to the single positive (SP [CD4+ or CD8+]) stage (Huang et al., 2004). Various studies have shown that this last process of positive selection requires prolonged interactions with appropriate epithelial cells (Germain, 2002; Kisielow and Miazek, 1995; Liu and Bosselut, 2004; Yasutomo et al., 2000), but the nature of those contacts and of the TCR signaling that accompanies them is not well understood. It is known, however, that thymocytes are extremely sensitive to their cognate peptide-MHC ligands, even more so than their mature T cell counterparts (Daniels et al., 2006; Davey et al., 1998). Negative selection of TCR transgenic thymocytes has been shown to occur in response to far fewer peptides per APC (on average) than mature T cells bearing the same transgenic TCRs (Peterson et al., 1999).
With respect to mature T cells, it has been known for some time that their interactions with agonist ligands are characterized by the formation of an immune synapse (Grakoui et al., 1999; Huppa and Davis, 2003), a tight interface between T cells and APCs (Davis et al., 2007) that can sustain contact and productive signaling for hours in the case of CD4+ T cells (Huppa et al., 2003). This led to the suggestion that immature T cells might arrest their migration and form a similar structure with selecting thymic epithelial cells (TECs) (Bousso et al., 2002; Hogquist et al., 2003; Starr et al., 2003). Indeed, we and others have shown previously that DP thymocytes respond to negatively selecting ligands, presented either by thymic APCs (Richie et al., 2002) or supported lipid bilayers (Hailman et al., 2002), by adhering to those APCs and polarizing CD3ζ, Lck, and CD4 into an immune synapse, albeit with differences in localization of TCR and other signaling molecules when compared to mature effector T cells. However, although thymocytes appear to alter their pattern of motility under conditions of positive selection (Bhakta et al., 2005; Bousso et al., 2002), whether immune synapses are formed could not be assessed.
In this study, we first sought to define the signaling threshold for negative selection. Using a biotinylated version of a peptide derived from moth cytochrome C (MCC, residues 88–103) (Irvine et al., 2002), we estimated the number of peptides bound to APCs in bulk cultures and also precisely determined the number of peptides bound to I-Ek on an APC by video fluorescence microscopy. We then observed the interaction of thymocytes derived from 5c.c7 TCR transgenic mice with these APCs and found that as few as two MCC peptides within a thymocyte:APC interface promoted apoptosis of the thymocyte within 1.5–4 hr. Strikingly, however, in cultures of whole thymi in which an estimated ~5%–10% of APCs bear enough peptides to promote apoptosis, negative selection was still >95% efficient. This observation suggests that thymocytes must sample large numbers of APCs before committing to either positive or negative selection, rather than sustain contact with individual positively selecting APCs.
In order to address the question of whether immune-synapse formation operates during positive selection, we expressed the transcription factor NFATc1 as a green fluorescent protein (GFP) fusion protein in immature DP thymocytes. This provided us with an indicator that permits the observation of signaling in these cells under conditions that promote positive selection of 5c.c7 TCR transgenic thymocytes in a reaggregate thymus organ culture (RTOC) system (Hare et al., 1999; Jenkinson and Anderson, 1994). We found that a large percentage of thymocytes (~20%–30%) mobilized NFATc1 into their nuclei in the presence of stromal cells expressing positively selecting I-Ek ligands. This response could be blocked by an antibody against I-Ek or one which binds a small subset of endogenous I-Ek molecules and has previously been shown to block positive selection of 5c.c7 T cells in vivo (Baldwin et al., 1999). Mature T cells did not mobilize NFATc1 under these conditions, indicating that this is a stage-specific phenomenon. These results suggest that positive selection involves the continuous low-level stimulation of immature thymocytes from multiple contacts with endogenous pMHC ligands on thymic epithelial cells. In contrast to mature T cells, this type of stimulation promotes the translocation of NFAT into the nucleus for prolonged periods of time, presumably to induce genes needed for differentiation to the single-positive stage.
RESULTS
Quantifying the Signal Threshold for Negative Selection in Thymus Cultures
T cells bearing the 5c.c7 TCR recognize MCC-I-Ek as an agonist ligand, and immature 5c.c7 thymocytes respond to this same ligand by undergoing negative selection. Modification of MCC with a biotin label does not disturb the interaction with TCR and peptide-MHC (Irvine et al., 2002), and by labeling this peptide with streptavidin (SA)-phycoerythrin (PE) (Irvine et al., 2002), we could quantify the number of MCC-I-Ek complexes presented by APCs in fetal thymic organ cultures (FTOCs) derived from 5c.c7, invariant chain deficient (Cd74−/−) 16-day-old embryos. Invariant chain deficiency drastically restricts the repertoire of self-peptides bound to class II MHC and in particular prevents presentation of those peptides that promote positive selection of I-Ek-restricted MCC-reactive T cells (Tourne et al., 1995). Under normal conditions, these cultures contain less than 2% CD4+ SP cells and >90% DP cells after 3 days’ incubation.
We first determined the relationship between the concentration of MCC-biotin added to these cultures and MCC-biotin loaded onto the I-Ek molecules of thymic stromal cells. 5c.c7 Cd74−/− e16 FTOC treated with varying amounts of MCC-biotin were trypsinized after 3 days’ culture, and the resulting cell suspension was labeled with SA-PE and anti-I-Ek. Neither procedure disturbs cell-surface MCC-I-Ek (data not shown). The median fluorescent intensity of I-Ek+ cells was determined by flow cytometry and was related to the absolute number of PE molecules with PE Quantibrite beads (Figure 1A). By determining peptide numbers on CH27 cells by flow cytometry at high doses and by microscopy at low doses, we showed that this relation remains approximately linear, down to a regime of one peptide per APC (Figure S1 available online). We then used flow cytometry to determine the proportion of living DP cells remaining in 5c.c7 Cd74−/− e16 FTOC after 3 days’ culture with varying doses of MCC-biotin (Figure 1B). Negative selection of MCC-reactive thymocytes still occurred at doses of MCC-biotin that equate to an average of less than one peptide per APC. This estimate was surprisingly low, and so we sought to refine it further.
Figure 1. Number of Peptides Required for Negative Selection in FTOC.

(A) Thymi from day 16 5c.c7 Cd74−/− embryos were cultured for 3 days in the presence of varying concentrations of biotin-MCC. Thymi were then dispersed into single-cell suspensions with trypsin and EDTA, and cells were stained with SA-PE and then anti-I-Ek and analyzed by flow cytometry. I-Ek+ cells were gated and we converted their median PE fluorescence intensity to number of SA-PE molecules using a standard curve generated with QuantiBRITE PE beads (BD Biosciences, r2 > 0.99). We used this number to generate a relation between peptide concentration and peptides loaded per APC (number of biotin-MCC per APC = 115 × [biotin-MCC], in μM, r2 = 0.979).
(B) Thymi from day 16 5c.c7 Cd74−/− embryos were cultured for 3 days in the presence of varying concentrations of biotin-MCC. Thymocytes were then recovered and stained for CD4 and CD8, and the percentage of surviving DP cells was determined by FACS analysis. The percentage of surviving DP cells is plotted against both concentration of biotin-MCC and the number of biotin-MCC per I-Ek+ cell according to the correspondence shown in part (A). The depicted results represent three independent experiments.
Enumerating Peptides Required for Negative Selection in Thymocyte:APC Couples
FTOC studies gave us an estimate for the number of peptides that provoke negative selection, but this estimate was based on extrapolation from large peptide numbers in bulk cultures that do not possess the full repertoire of endogenous peptides, some of which may be important for facilitating T cell signaling. This estimate is thus unlikely to be precise. We therefore sought to determine the number of peptides required to induce thymocyte deletion on a single-cell level.
We chose CH27 cells as APCs for these experiments because their intrinsic fluorescence is low, and therefore single PE molecules can be reliably and precisely detected on their surface by video fluorescence microscopy (Irvine et al., 2002; Li et al., 2004; Purbhoo et al., 2004). CH27 cells were pulsed with MCC-biotin, washed, and labeled with SA-PE. We carried out both steps at 4°C in the presence of azide to prevent internalization and representation of unlabeled peptide-MHC complexes. Labeled CH27 cells were then allowed to interact with 5c.c7 Cd74−/− thymocytes. Because these thymocytes failed to receive TCR signals in vivo, they were >95% preselection DP cells. We could then observe their interaction by fluorescence video microscopy in medium doped with 2 mM Ca2+ (which does not increase thymocytes’ dose response to MCC, see Figure S2) and fluorescein-labeled Annexin V. Annexin V binds to phophatidylserine, whose exposure on the cell surface is a hallmark of programmed cell death. This allowed us to identify thymocytes that had committed to apoptosis by their accumulation of the fluorescein label.
Figure 2 shows the response of 5c.c7 Cd74−/− thymocytes to one (Figures 2A-2C) or two (Figures 2D-2F) biotin-MCC presented in the interface between the thymocyte and a CH27 APC. A single peptide was unable to provoke apoptosis even after 6 hr in any of these experiments, whereas two or more peptides were sufficient to induce thymocyte apoptosis within 2 hours in a large majority of cell couples observed (Figure 2G). Negative selection occurred in response to two peptides in the thymocyte-APC interface even when no other peptides were present on the APC (e.g., as depicted in Figure 2E). In light of these results, it is striking that deletion was so efficient in FTOC. Under conditions in which APCs possess an estimated average of 0.2 peptides, APCs presenting two or more peptides would be very rare (less than one in 20 APCs under a normal distribution, or less than one in ten APCs in a distribution in which an APC either presents zero or two peptides), and yet 90% of thymocytes still undergo negative selection in such cultures (Figure 1B). When less than ~1% of APCs were expected to possess two or more MCC-I-Ek, over 50% of reactive thymocytes were deleted from our thymus cultures. It seems likely then that thymocytes sample a large number of thymic APCs before committing to their fate, an idea that is incompatible with the notion of positive selection via any long-lasting synapse formation with one epithelial cell. To explore this issue, we set out to observe thymocyte:epithelial cell interactions that lead to positive selection using NFAT as an indicator for productive signaling.
Figure 2. Thymocyte Apoptosis in Response to a Defined Number of Peptide-MHC Ligands.

CH27 cells were loaded with biotin-MCC, extensively washed and labeled with SA-PE, and imaged together with 5c.c7 Cd74−/− thymocytes in the presence of 2 mM Ca2+ and Annexin V-FITC. SA-PE signal was acquired within 5 min of interaction in 1 micron sections and then reconstructed with Metamorph software so that the number of SA-PE molecules present could be determined. FITC fluorescence was assessed every 15 min for 6.5 hr.
(A and B) A DIC image (A) and PE fluorescence image (B) of a thymocyte-CH27 interaction. PE fluorescence images are rotated 90° so that the thymocyte-APC interface faces the viewer. This interface contains a single peak of PE fluorescence corresponding in fluorescence intensity to a single SA-PE molecule.
(C) DIC (top) and Annexin V-FITC fluorescence images (bottom) of this cell couple over time. Frames are taken 30 min apart; the last frame represents 6 hr after interaction.
(D and E) A DIC image (D) and PE fluorescence image (E) of a thymocyte-CH27 interaction in which the interface contains a single peak of PE fluorescence with double the intensity of that in (B), corresponding to 2 SA-PE molecules.
(F) A time-lapse of DIC and Annexin V-FITC shows that the thymocyte has committed to apoptosis after 1.5–2 hr of interaction (frames are 30 min).
(G) Summary of data represented in (A)–(F), n = 12–18 cell couples for each group.
NFAT Activity as a Hallmark of Positive Selection
Prior work had indicated that calcineurin activity is necessary for positive but not for negative selection (Neilson et al., 2004), and this suggested that NFAT mobilization might be a useful indicator for positively selecting signals in live thymocytes. Although there are four NFAT family members expressed in T cells, NFATc1 expression was easily detected (Figures 3A and 3B) and is sufficient to allow T cell maturation in the absence of the other thymic NFAT family members (Ranger et al., 1998). Constitutively active NFAT family members have also been shown to promote positive selection (Amasaki et al., 2002; Hayden-Martinez et al., 2000).
Figure 3. Nuclear Accumulation of Thymocytes’ Endogenous NFATc1 in Positively Selecting Thymi.
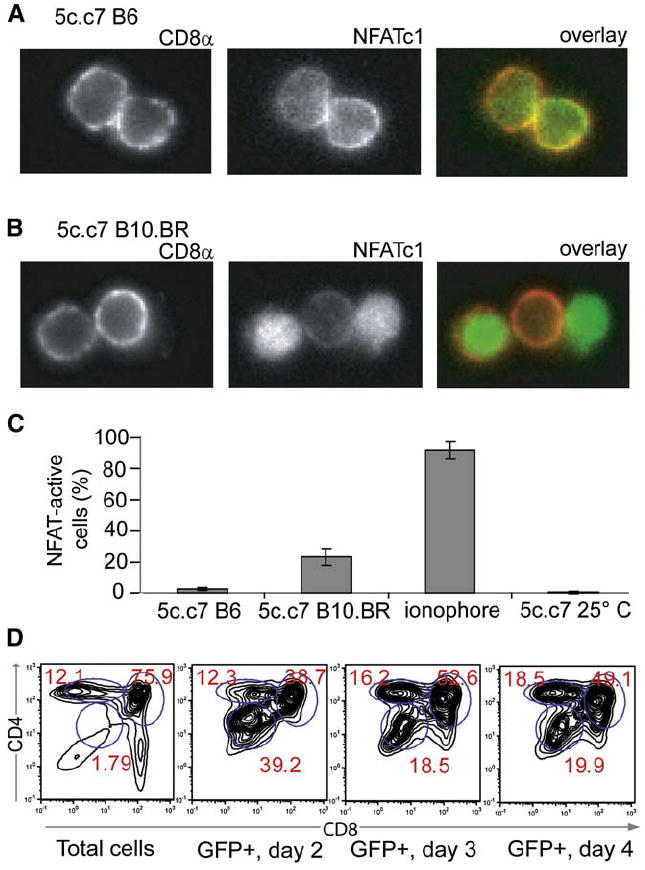
(A) Thymi were dissected from 5c.c7 B6 mice and thymocytes were liberated directly into 4% paraformaldehyde. Fixed cells were then permeabilized and stained with anti-CD8α PE and mouse anti-NFATc1 and then goat anti-mouse Alexa 488. The left panel shows CD8α staining, the middle panel shows NFATc1 staining, and the right panel shows an overlay with CD8α staining in red and NFATc1 staining in green.
(B) 5c.c7 B10.BR thymocytes were isolated and stained as in (A).
(C) Summary of data from (A) and (B), and for 5c.c7 B6 thymocytes treated with the ionophore A23187 for 10 min prior to fixation, or thymi allowed to rest in RT PBS for 5 min prior to thymocyte liberation and fixation. Data are expressed as the proportion (mean ± standard deviation) of CD8+ cells with nuclear NFAT accumulation (>50% nuclear Alexa 488 staining, n > 300) from two mice for each condition.
(D) NFAT transcriptional activity correlates with thymocyte maturation in vivo. 5c.c7 DP thymocytes transduced with NFAT-promoter-driven GFP were injected intrathymically into B10.BR recipients; 5 × 107 thymocytes were prepared from recipient mice at the indicated days after injection, stained for surface expression of CD4 and CD8, and analyzed by flow cytometry. Shown are the CD4-CD8 profiles of total ungated cells (first panel) or cells gated for expression of NFAT-driven GFP on day 2 (second panel), day 3 (third panel), and day 4 (fourth panel) after intrathymic injection. Each profile is representative of two to three mice.
To correlate endogenous NFAT activity with positive selection in vivo in our 5c.c7 transgenic TCR system, we fixed freshly isolated 5c.c7 thymocytes from selecting and nonselecting backgrounds for immunofluorescent staining and analysis. More than 20% of DP cells from positively selecting 5c.c7 B10.BR thymi had predominantly nuclear NFATc1 (Figures 3A-3C). Only a tiny fraction of DP thymocytes from the nonselecting 5c.c7 B6 background showed such NFAT activation, suggesting that NFATc1 nuclear localization might be a useful indicator for positively selecting signals.
To further establish NFAT as a faithful indicator of positive-selection events, we generated a self-inactivating retrovirus (Kripke-Skillern and Nolan, 2002) that directed the expression of GFP under the control of an NFAT-binding cassette (Figure S3A). A small but substantial population of mature T cells (8%–10%) infected with this construct upregulated GFP in response to antigen stimulation or ionomycin-induced calcium flux, and this upregulation could be blocked by the calcineurin inhibitor FK506, demonstrating the reporter’s fidelity (Figure S4). We then intrathymically injected reporter-infected 5c.c7 DP cells into B10.BR hosts. By gating on the small proportion of GFP-positive cells on subsequent days after injection, we could show that thymocytes displaying NFAT-DNA-binding activity were enriched for those subsets of DP cells that were undergoing positive selection in vivo (Correia-Neves et al., 2001; Sant’Angelo et al., 1998) (CD4intCD8int and CD4hiCD8int subsets; Figure 3D, and see also Figure S5).
Expression and Localization of NFATc1-GFP
In order to observe NFATc1 mobilization during thymocyte selection in RTOC, we made a retroviral construct of NFATc1, tethered by a 21 amino acid linker to GFP (Figure S3B). As expected, NFATc1-GFP was found predominantly in the cytoplasm of resting cells (see the first panels of Figures 4A and 4B). To verify that overexpression of the altered NFAT had no deleterious effects on thymic selection, we generated RTOC containing DP thymocytes transduced with NFATc1-GFP and analyzed them by flow cytometry after 3 days. DP thymocytes were selected into the CD4+ SP lineage in similar proportions regardless of whether they expressed NFATc1-GFP, and a negatively selecting concentration of agonist MCC peptide resulted in a complete absence of DP thymocytes that was also independent of NFATc1-GFP expression (Figures S6A–S6D).
Figure 4. Characterization of the NFATc1-GFP Retrovirus.

(A) Video time-lapse microscopy of a mature 5c.c7 T cell expressing NFATc1-GFP encountering a CH27 B cell pulsed with 1 μM MCC. The top row shows DIC, the middle row shows Fura 340/380 ratio in a false-color scale, and the bottom row shows NFATc1-GFP. Times in seconds are given relative to the onset of Ca2+ flux above the DIC images, and the ratio of nuclear to cytosolic gfp signal is indicated below the fluorescence images.
(B) Video time-lapse microscopy of 5c.c7 DP thymocytes expressing NFATc1-GFP in RTOC with B10.BR thymic stromal cells with no peptide added. The top row shows DIC, and the bottom row shows NFATc1-GFP. Times in minutes are given relative to the onset of imaging, and the ratio of nuclear to cytosolic GFP signal is indicated below the fluorescence images.
(C) Summary of video microscopy data, presented as the average number of NFATc1-GFP+ cells displaying nuclear accumulation (>50% of total GFP signal confined to the nuclear region in consecutive time points) of NFAT, among >100 cells in each case. For mature T cells, only cells displaying Ca2+ flux (fura 2am 340/380 ratio > 1.5) were considered for analysis. RTOC were composed of B10.BR thymic stromal cells unless otherwise indicated.
NFATc1-GFP Nuclear Translocation as a Sensor for TCR Signaling
We first observed the dynamics of NFATc1-GFP localization in mature T cells interacting with APCs. Not surprisingly, mature 5c.c7 T cells exhibited rapid nuclear import of NFATc1-GFP upon engagement with CH27 B cells presenting agonist MCC-I-Ek complexes (Figure 4A). This NFAT response followed quickly upon initiation of calcium flux and was accompanied by tight apposition of T cell and B cell membranes. NFAT was retained in the nuclei of APC-engaged T cells for the duration of the experiment (>30 min in many cases). When CH27 B cells presented only their endogenous peptide-MHC ligands, no NFAT mobilization occurred (Figure 4C).
To examine NFAT mobilization in immature thymocytes, we generated RTOC composed of NFATc1-GFP-transduced 5c.c7 DP thymocytes together with B10.BR thymic stromal cells for imaging by live-cell video microscopy (Richie et al., 2002). As expected, the vast majority of DP thymocytes displayed a nuclear localization of NFATc1-GFP when thymic APCs presented the negatively selecting ligand MCC-I-Ek (Figure 4C). Although the initiation of cell-cell contact could not accurately be determined in RTOC, NFAT nuclear import was rapid because NFAT was present in the nuclei of nearly all thymocytes, even when MCC was added only 5 min prior to the onset of imaging. Consistent with previous work, DP thymocytes under these conditions form stable interactions with thymic APCs, complete with the formation of an immune synapse, as gauged by the accumulation of TCR, Lck (Richie et al., 2002), and CD4 (data not shown). Given the stability of cell-cell interactions, it is not surprising that responding DP thymocytes retained NFAT in their nuclei for upward of 30 min.
However, we also found that a smaller but substantial proportion of DP thymocytes mobilized NFAT in a stable fashion in response to thymic APCs presenting only endogenous peptide-MHC ligands (Figures 4B and 4C). This observation was not just a reflection of the difference in microenvironment or APCs because mature T cells did not translocate NFAT when placed in these cultures (Figure 4C). It is also not an artifact of the NFATc1-GFP construct because treatment with the calcineurin inhibitor Cyclosporin caused this construct to be exported from thymocyte nuclei within 5–15 min (Figure S6E). These observations suggest that sustained thymocyte signaling occurs in these positively selecting RTOC cultures.
TCR Signaling Despite the Lack of Stable Thymocyte-Stromal Cell Interactions in RTOC
We previously showed that thymocytes form immune synapses in positively selecting RTOC at a much lower frequency and with different cell-surface-molecule dynamics than in negatively selecting RTOC (Richie et al., 2002). To determine whether these rare synapses represented positively selecting interactions, we generated RTOC containing CD3ζ-GFP-transduced 5c.c7 DP thymocytes together with thymic stromal cells and a small population of PKH26-labeled B10.BR thymic dendritic cells (DCs) or TECs. We could then enumerate thymocytes that formed a stable interface with APCs presenting either negatively selecting MCC-I-Ek or endogenous peptide-MHC ligands and find out whether the thymocytes accumulated their TCRs in that interface (Figure 5). Dendritic cells presenting MCC-I-Ek adhered to and promoted TCR accumulation in nearly half of the DP thymocytes available to them (Figure 5B). In contrast, DCs presenting only endogenous ligands bound fewer than 5% of available thymocytes (Figure 5A). Similarly, thymic epithelial cells presenting positively selecting ligands did not promote stable contacts with CD3ζ accumulation, unlike TECs presenting negatively selecting MCC-I-Ek (Figures 5C and 5D, and enumerated in Figure 5E), although the lower efficiency with which TECs provoke synapses relative to DCs may also contribute to this observation. The small number of stable contacts formed in RTOC containing B10.BR epithelial cells was formed toward I-E-deficient APCs and was thus unlikely to represent positively selecting interactions (Figure 5E, white bar). Further analyses of synapse formation are presented in Figure S7.
Figure 5. Paucity of Stable Thymocyte-Thymic APC Contacts in Positively Selecting RTOC.
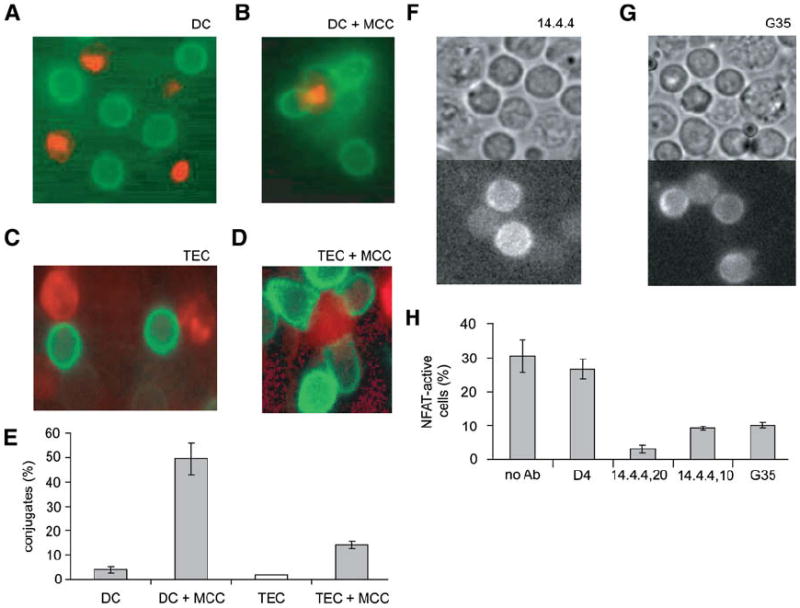
(A) A representative image of an RTOC composed of purified 5c.c7 DP thymocytes expressing CD3ζ-GFP (in green), with unlabeled B10.BR TECs, and PKH26-labeled B10.BR thymic DCs (in red).
(B) RTOC generated as in (A), but DCs additionally present MCC-I-Ek.
(C) A representative image of an RTOC composed of purified 5c.c7 DP thymocytes expressing CD3ζ-GFP (in green), with unlabeled B6 TECs, and PKH26-labeled B10.BR TECs (in red).
(D) RTOC generated as in (C), but TECs additionally present MCC-I-Ek.
(E) Summary of data from (A)–(D). Data are expressed as the average percentage of thymocytes with >1.3-fold polarized accumulation of CD3ζ-GFP toward an unlabeled B6 APC (white bars), or >1.3-fold polarized accumulation of CD3ζ-GFP toward a contact with a labeled B10.BR APC (gray bars) for more than 3 min; data are averages (± SD) over three experiments, with >50 cells per experiment.
(F–H) Positively selecting ligands are responsible for NFAT mobilization in 5c.c7 thymocytes in selecting RTOC. (F) and (G) are representative images of RTOC composed of 5c.c7 DP thymocytes expressing NFATc1-GFP and B10.BR stromal cells, treated with (F) 20 μg/ml 14.4.4 or (G) 500 μg/ml G35 antibody 4 hr prior to imaging. (H) shows a summary of data from RTOC containing 5c.c7 DP thymocytes expressing NFATc1-GFP and treated with the indicated antibodies. Data are expressed as the average percentage (± SD) of GFP+ thymocytes displaying a sustained (>5 min) nuclear accumulation (>50% nuclear GFP signal) of NFATc1-GFP over three experiments, with n > 50 for each experiment.
Despite this lack of synapse formation, thymic epithelial cells elicited an NFAT response from DP thymocytes. To directly verify that this NFAT mobilization occurred in response to endogenous MHC ligands, we used monoclonal antibodies to block either I-Ek (14.4.4) generally or a subset of self-peptide-I-Ek complexes (G35) that includes those necessary for the positive selection of 5c.c7 TCR-bearing thymocytes (Baldwin et al., 1999). Both antibodies inhibit the progression of 5c.c7 thymocytes to the CD4+ SP lineage in B10.BR mice (Baldwin et al., 1999) and in FTOC (data not shown), and both antibodies also inhibited the NFAT response of 5c.c7 thymocytes in B10.BR RTOC when added 4 hr prior to imaging (shown in Figures 5F and 5G, and quantified in Figure 5H). An isotype control antibody, D4 (which binds MCC-I-Ek complexes but does not interfere with thymic selection in mice or FTOC [Baldwin et al., 1999]), did not have a marked effect on NFAT mobilization. 5c.c7 thymocytes in nonselecting B6 RTOC also did not mobilize NFAT into their nuclei (2.8% thymocytes with nuclear NFAT, Figure 4C). Thus, a fairly large proportion of DP 5c.c7 thymocytes (20%–30%) experience self-peptide-MHC-dependent signaling, whereas few if any of them form an immune synapse. As additional evidence, we find that an antibody that blocks LFA-1 in FTOC did not prevent positive selection despite inhibiting immune-synapse formation (Figure S8).
Transient and Serial Encounters Lead to Sustained NFAT Nuclear Translocation
Direct visualization also supported the idea that thymocytes sustain productive signaling without sustaining cell-cell contact. Figure 6 shows the progression over time of 5c.c7 DP thymocytes through reaggregate cultures composed of B10.BR thymic stromal cells. In these representative time-lapse images, we see NFATc1-GFP translocated to the thymocyte nucleus upon encounter with a thymic APC. However, thymocytes did not alter their morphology to increase their contact area with that APC (i.e., no flattened or cupped interface as in Figure 5D). In fact, the thymocyte, although rounded in morphology, was not tethered to any particular epithelial cell and could immediately move away from the putatively stimulating APC (marked with a red asterisk in the panel in which NFAT translocation occurs, and again in the last panel of Figure 6A). We identified the stimulating APC on the basis of its initiating contact with the thymocyte 30–60 s prior to NFAT translocation (because it is difficult to identify the stimulating APC precisely under these conditions, real-time imaging of thymocytes leaving contact with labeled stimulating epithelial cells is shown further in Movies S1 and S2). Nonetheless, NFAT remains in the nucleus of these cells. Note that the percentage of responding thymocytes increased strictly with time (Figure 6C) with only one exception (n > 50) because once confined to the nucleus, NFAT was not re-exported to the cytoplasm in the course of these experiments (>30 min). However, if we blocked calcineurin activity after ionophore treatment, NFAT returned to the thymocyte cytoplasm in well under 30 min (~10 min, Figure S6E).
Figure 6. Sustained Nuclear NFAT in DP Thymocytes Results from Intermittent, Transient Contact with Selecting Ligands.

(A) Time-lapse video microscopy of 5c.c7 DP thymocytes expressing NFATc1-GFP in RTOC with B10.BR stromal cells. RTOCs were allowed to cool to room temperature to quench cell signaling prior to imaging at 37°C. The putatively stimulating epithelial cell is marked by a red asterisk.
(B) Time-lapse video microscopy of 5c.c7 DP thymocytes expressing NFATc1-GFP in RTOC with B10.BR stromal cells. We added 20 μg/ml anti-I-Ek antibody 14.4.4 immediately prior to imaging.
(C) Percentage of DP thymocytes with nuclear NFATc1-GFP (>50% nuclear GFP signal) over time in selecting RTOC.
(D) Percentage of DP thymocytes with nuclear NFATc1-GFP (>50% nuclear GFP signal) over time in RTOC with 14.4.4 added at 0 min. Individual traces in (C) and (D) are separate experiments tracking 12–20 cells each.
To test whether continued contact with MHC ligands is required for NFAT retention in the nucleus (as opposed to some thymocyte-specific mechanism), we used the 14.4.4 antibody to block endogenous peptide-I-Ek immediately prior to video microscopic visualization. We found a reduction in the number of responding thymocytes after 5 min of antibody treatment, and those thymocytes that had nuclear NFAT at the onset of imaging released it into their cytosol over the course of several minutes (Figures 6B and 6D). Thus, transient but repeated engagements with MHC ligands presented by thymic APCs seem to be required for sustained NFAT activity in immature T cells.
DISCUSSION
We have shown here that immature thymocytes initiate programmed cell death in response to contact with as few as two agonist peptide-MHC ligands on an APC. Importantly, this is fewer peptides than are required to elicit an effector response from mature T cells of the same antigen specificity (Irvine et al., 2002; Peterson et al., 1999) and also agrees very closely with earlier estimates of the range of peptides required (Peterson et al., 1999). Although CH27 B cells possess the costimulatory and adhesion molecules thought to be important for negative selection (Graham et al., 2006), thymic APCs may of course be different in terms of the number of peptides they require to delete self-reactive DP T cells, although it is hard to imagine that the threshold could be placed much lower. Negative selection can also occur at the semimature SP stage (Kishimoto and Sprent, 1997), although we and others (Villunger et al., 2003) find that 5c.c7 DP and semimature SP thymocytes are similarly sensitive to agonist stimuli. This property of heightened sensitivity at the DP and semimature SP stage (Davey et al., 1998; Li et al., 2007) presumably enforces the stringency of central tolerance. If the densities of particular determinants in the thymus and periphery are similar (Marrack et al., 1993), and immature T cells delete themselves in response to fewer peptides than an effector T cell can respond to, it becomes unlikely that an autoreactive T cell clone could both fail to find negatively selecting ligands in the thymus and yet find enough ligands in the periphery to provoke an effector response.
This result is also interesting in light of our results in whole-thymus cultures. Even under conditions in which only a few APCs present sufficiently many ligands to induce negative selection, negative selection was still remarkably efficient. This suggests that the efficiency of deletion must stem not only from the sensitivity of thymocytes for their ligands but also from some ability of thymocytes to seek out those rare APCs within the thymus that are competent to delete them. Such an interpretation would be at odds with a single-niche model of positive selection (Merkenschlager et al., 1994) or with models of stable contacts during the course of positive selection (Starr et al., 2003). If a thymocyte could complete its maturation (either to the fully mature SP stage or to the medulla-bound semimature SP stage) by contacting the first epithelial cell it encountered, it would fail to experience a large portion of the thymic self-peptide repertoire (Anderson et al., 2002; Barton and Rudensky, 1999a). Instead, we show here that transient but repeated encounters with selecting ligands are necessary and sufficient to promote sustained NFAT signaling in immature DP thymocytes and that these encounters did not involve immune-synapse formation in a reaggregate culture system that supports positive selection. When we also saw that sustained NFAT activity correlates with progression toward the mature T cell stage, we inferred from these observations that maturing thymocytes run a gauntlet of repeated engagement with many different APCs as they move through the thymus. This mode of signal acquisition would also help to explain the elegant data of Merkenschlager et al. (1994), who showed that negative selection could remain efficient with as few as one in 10–20 thymic APCs presenting appropriate ligands—in fact, we arrive at a very similar estimate from our peptide-counting studies.
NFAT is a particularly informative marker for TCR signaling (Hooijberg et al., 2000) because its nuclear import rapidly indicates the initiation of TCR signaling, whereas its nuclear residence indicates the duration of that signaling, and because its nuclear translocation closely correlates with its transcriptional activity (Neal and Clipstone, 2001), which includes the regulation of thousands of genes in mature T cells (Diehn et al., 2002). The ability to determine whether downstream effectors such as NFAT sustain their activity is of particular interest given the role that duration of signaling appears to have in governing positive selection and also in the lineage decision of selected T cells (Kisielow and Miazek, 1995; Liu and Bosselut, 2004; Yasutomo et al., 2000). Here, we have shown that NFAT activity was found preferentially in those thymocytes undergoing positive selection in vivo, whereas in RTOCs a large fraction of DP thymocytes translocate NFAT to their nuclei in a manner that can be inhibited by blocking selecting ligands (Baldwin et al., 1999). This suggests that the thymocytes that mobilize NFAT into the nucleus are likely to progress toward full maturation.
Although effector T cells seem to rely on the tight T cell-APC contact mediated by immune-synapse formation to sustain signaling, and DP T cells display a similar behavior when responding to negatively selecting signals, DP thymocytes displayed neither behavior in response to positively selecting ligands. Indeed they did not even adhere tightly enough to thymic epithelium presenting such ligands to remain in contact with those cells over the course of minutes in a RTOC. Nonetheless, thymocytes can maintain their downstream NFAT response through re-exposure to selecting ligands on nearby epithelial cells. It is likely then that thymocytes achieve the duration of signaling required for maturation through many transient encounters, potentially with many different epithelial cells, rather than the prolonged contact with a single epithelial cell that immune-synapse formation might promote. As long as a thymocyte re-encountered selecting ligands within the 5–15 min required for NFAT nuclear export, NFAT transcriptional activity could be maintained by many separate thymocyte:stromal cell liaisons, similar to how mature T cells appear to acquire and accumulate signals from T cell:DC interactions in 3D collagen matrices (Gunzer et al., 2000) and lymph nodes (Miller et al., 2004; Miller et al., 2002).
Recent work has shown that calcium signaling promotes an arrest of thymocyte motility in thymus tissue slices (Bhakta et al., 2005). These pauses in motility were generally brief (10–20 min) and were followed by subsequent cycles of motility and arrest. The duration of thymocyte-epithelial cell contact we observed is even shorter (<5 min), possibly because of the less compact RTOC structure. Nonetheless, the reacquisition of motility in thymus slices (Bhakta et al., 2005) and intact thymus lobes (Witt et al., 2005) imply that thymocytes are not forming stable synapse-mediated contacts (which can last for hours in mature T helper cells [Faroudi et al., 2003; Huppa et al., 2003]). Because NFAT is re-exported from the nucleus slowly after the cessation of signaling, NFAT could act to “remember” recent encounters with selecting ligands (Tomida et al., 2003). This would explain why we observed continuous nuclear NFAT accumulation, whereas Bhakta et al. observed punctuated calcium signaling.
The efficiency with which TCR transgenic thymocytes are positively selected correlates with the number of stromal cells expressing selecting MHC in chimeric reaggregate thymus cultures (Merkenschlager et al., 1994). This might indicate that thymocytes compete for cellular niches within the thymus in which they can continually engage their selecting ligands (Merkenschlager, 1996). However, the efficiency of positive selection has also been shown to correlate with the expression of selecting MHC in a genetically homogeneous thymus (Berg et al., 1990; Wong et al., 2000). Thus, the density of MHC determines the efficiency of positive selection even under conditions of equal cellular space. The requirement that thymocytes continually re-encounter selecting ligands in order to maintain productive signaling might explain both observations. A thymocyte’s chance of reencountering ligands compatible with their TCR would decline in both cases, whether because the requisite MHC was limited to fewer cells, or because it was expressed at a lower density on all cells.
These findings bear particularly on how thymocytes access the self-antigenic information presented by the thymus. Although peripheral mechanisms can help to ameliorate autoimmune responses, immune tolerance is critically reliant on the thymus’ ability to remove immature T cells carrying self-reactive TCRs (Anderson et al., 2002; Daniels et al., 2006; Sprent and Kishimoto, 2001; Venanzi et al., 2004). For this purpose, it seems likely that thymic APCs are most effective as a group. Because the number of potential self-determinants outnumbers the MHC complexes that can be expressed by any one APC, with a large variation in the abundance of any particular determinant (Barton and Rudensky, 1999a, b; Chmielowski et al., 2000), no one APC can present the entire array of self-peptides. A self-reactive thymocyte might therefore have to scan many such cells before finding one that can promote negative selection or TCR editing.
A lack of synapse-sustained contact might also enhance the efficacy of positive selection. If thymocytes need to continually scan (Miller et al., 2004; Wu et al., 2002) epithelial cells for peptide-MHCs that promote positively selecting signals, this would ensure a lower error rate in this critical transition, preventing the maturation of thymocytes that are “restricted” only to very rare peptide-MHC. This also raises the possibility that T cells might preferentially recognize the ligands that selected them once they reach the periphery. Prior work has shown that mature CD4+ T cells need to interact with endogenous peptide-MHC in the periphery in order to maintain optimal responsiveness (Stefanova et al., 2002) and that particular endogenous peptide-MHC can synergize with agonist ligands to activate T cells, whereas other self peptides can’t (Krogsgaard et al., 2005). Positive selection might therefore select T cells not only for their ability to recognize self-MHC but also more specifically for their ability to utilize particular synergistic self-peptide-MHC complexes (Davis et al., 2007).
EXPERIMENTAL PROCEDURES
Mice
5c.c7 mice on the B10.BR background were obtained from Taconic Farms. We obtained 5c.c7 B6 mice by crossing 5c.c7 onto the C57Bl/6 background for more than seven generations (Richie et al., 2002). We obtained 5c.c7 Cd74−/− mice by crossing 5c.c7 onto Cd74−/− B10.BR mice (a kind gift of C. Benoist and D. Mathis). All mice were bred and maintained at the Stanford University Department of Comparative Medicine Animal Facility (protocol 3540) in accordance with National Institutes of Health guidelines.
Antibodies
Anti-I-Ek (14.4.4 s) and anti-LFA-1 (anti-CD11a, M17/4) were obtained from BD Biosciences. Anti-NFATc1 (7A6) was a kind gift of G. Crabtree. The generation of anti-MCC/I-Ek antibodies G35 and D4 is described in (Baldwin et al., 1999).
Retroviral Constructs and Virus Preparation
CD3ζ-GFP in a modified form of the pIB vector was as previously described (Ehrlich et al., 2002). The NFATc1-GFP fusion was kindly provided by M. Krummel (UCSF) and cloned into a modified pMSCV vector (see Figure S1B). The minimal NFAT promoter (Graef et al., 1999) (see also http://crablab.stanford.edu) driving eGFP was cloned into the CKHGppSin vector (a gift of G. Nolan and K. Kripke-Skillern) to generate the Npro construct (see Figure S1A). Constructs were transfected into Phoenix-ecotropic cells (a gift of Dr. Nolan) and selected for construct integration by treatment with Blasticidin, Zeocin, or Puromycin (Invitrogen).
Infection and Isolation of Thymocytes and T Cells
For reaggregate cultures, e16 thymic lobes were dissociated and thymocytes were resuspended in virus-containing supernatant, then centrifuged at 32°C at 750 G for 1 hr. Cells were then transferred to 37°C overnight. For intrathymic injection, thymi from 4- to 6-week-old 5c.c7 mice were dissociated, then DP and SP cells were depleted with anti-CD4 dynalbeads (Dynal Biotech). DN cells were then transduced as above. In both cases, cells were allowed to mature to the DP stage 12–16 hr at 37°C. For mature cells, lymph nodes from 4- to 6-week-old 5c.c7 mice were dissociated and T cells were stimulated by addition of 5 μM MCC peptide (day 0). Lymph node preparations were additionally treated with 250 U/ml IL-2 after 12–16 hr and were transduced by 1.5 hr centrifugation in viral supernatant 6 hr later. T cells were used for in vitro and microscopy studies on day 5 of culture.
Flow Cytometry
Cell-surface molecules were stained with anti-CD4 (GK1.5 or RM4-5), anti-CD8α (53-6.7), anti-I-Ek (14.4.4 s), streptavidin-PE, and/or anti-Vβ3 (KJ25), all from BD Biosciences. FITC-Z-VAD-FMK (CaspACE) was from Promega. Live cells were identified by their forward- and side-scatter profiles and exclusion of propidium iodide. Samples were run on a FACStar (Becton Dickinson) and analyzed with Flowjo software (Treestar).
Immunofluorescent Staining of Fresh Ex Vivo Thymocytes
Thymi were dissected from 4- to 6-week-old 5c.c7 B10.BR or 5c.c7 B6 mice and were either immediately dissociated in 4% paraformaldehyde (Sigma) in PBS or treated 10 min with 0.5 μM A23187 and then fixed for 30 min. Cells were then plated onto poly-L-lysine treated chambers (Lab-Tek), washed 3× PBS, permeabilized with 0.1% Triton X-100 for 10 min, blocked with TNB 1 hr, and stained with anti-NFATc1 (7A6), with subsequent staining with biotinylated goat anti-mouse IgG and finally streptavidin Alexa 488 and anti-CD8 PE (BD Biosciences).
Intrathymic Injection
Npro-transduced DP cells were injected into 4- to 6-week-old B10.BR recipients as previously described (Jerabek and Weissman, 2001). A total of 107 cells were extensively washed and resuspended in 10 μl PBS for injection with a 10 μl Hamilton syringe after anesthetization with ketamine and xylazine or with Avertin. Mice were euthanized and 5 × 106 thymocytes were analyzed by flow cytometry after 2–7 days.
Generation of Reaggregate Cultures
e15 thymi were cultured for 6–8 days on 0.8 micron filters (Millipore) floated on culture medium containing 0.36 mg/ml 2-deoxyguanosine (Sigma) for removal of thymocytes and dendritic cells, and thymic stromal cells (TSC) were prepared by dissociation of thymic lobes with trypsin and EDTA. Residual bone marrow-derived cells were removed with streptavidin Dynalbeads coated with biotinylated anti-CD45 (BD Biosciences). Thymic dendritic cells were isolated by collagenase D (Boeringer) treatment of 4- to 6-week-old B10.BR thymi, followed by density separation on a gradient of 14.5% metrizamide (Sigma) and purification with anti-CD11c miltenyi beads (Miltenyi Biotech). Stromal cells or dendritic cells were labeled with a PKH26 linker kit (Sigma). Transduced thymocyte preparations were enriched to >97% DP cells with streptavidin Dynalbeads coated with biotinylated anti-CD8 (53-6.7, BD Biosciences), and cells were released from the beads by treatment with trypsin and EDTA (Sigma). A total of 1.5 to 3 × 105 TSCs were mixed with 1 to 2 × 105 DP cells and centrifuged 750 G in nontissue-culture-treated V-bottom 96-well plates. Plates were then transferred to a sealed chamber containing 70% 02, 25% N2, and 5% CO2 (Praxair Bioblend), and cells were allowed to reaggregate 4–12 hr prior to imaging or transfer to 0.8 micron filters for culture at 37°C.
In Vitro Responses
A total of 5 × 105 day 5 poststimulation 5c.c7 T cells were mixed with 2.5 × 105 CH27 cells with or without 2 μM MCC peptide. Cells were harvested and analyzed by flow cytometry at indicated times, gating on live CD4+ cells. Cells were treated with 0.5 μM A23187, 1 nM FK506, and/or 50 ng/ml Cyclosporin A (Sigma).
Microscopy
RTOC cultures were transferred with a cut 200 μl pipette tip to an FCS2 chamber (Bioptechs) and sandwiched between a slide and coverslip separated by a 0.25 mm gasket. Mature T cells and thymocyte suspensions were loaded with fura 2am (Molecular Probes) for 20 min and washed prior to imaging in eight-well borosilicate chambers (Lab-Tek), and CH27 B lymphoma cells were used as APCs. Cells were imaged at 37°C and 5% CO2 with a Zeiss Axiovert-100TV microscope as described (Richie et al., 2002). NFATc1-GFP was considered nuclear when the ratio of nuclear GFP signal to cytosolic GFP signal was greater than one in consecutive frames (nuclear accumulated thymocytes: 1.68 ± 0.543; cytosolic accumulated thymocytes: 0.543 ± 0.212).
Peptide Counting
For FTOC, e16 thymi were dissected and cultured on 0.8 micron filters (Millipore) in standard culture medium supplemented with biotinylated MCC (Irvine et al., 2002). Cells were dispersed after 3 days with trypsin and EDTA, then stained with 14.4.4-Fitc and streptavidin-PE. A standard curve of PE fluorescence was generated with QuantiBRITE PE beads (BD Biosciences). For microscopy studies, CH27 cells expressing H-2Kb (Purbhoo et al., 2004) were incubated with biotinylated MCC at 4°C for 1 hr, then washed extensively in ice-cold PBS/5% FCS/0.1% NaN3 before staining with 10 μg/ml SA-PE at 4°C in PBS/5% FCS/0.1% NaN3 for 40 min. CH27 cells were then washed extensively in PBS/5% FCS for removal of free SA-PE. Thymocytes and CH27 cells were then incubated for 5 min at 37°C to allow cell couples to form, then spun gently onto a Lab-Tek chambered coverglass (Nalge Nunc) precoated with anti-H-2Kb to immobilize CH27 cells. SA-PE was visualized across the whole cell at 1 micron intervals with 100 ms exposure times, and the images were reconstructed into a 3D image with Metamorph software (Universal Imaging Corp). Individual PE molecules under these conditions yielded a single peak of fluorescence intensity of ~70 ± 12 units; peaks of fluorescence intensity at the CH27 cell membrane with intensities of 60–80 units over background were thus identified as single peptides. Multiple peptides could occur as multiple peaks of 60–80 unit fluorescence, or as single peaks of 60–80, 120– 160, or 180–240 units. Peaks of greater than 250 units of fluorescence were counted as four or greater peptides. For apoptosis determination, the medium was spiked with Annexin V (1:20, BD Biosciences) and 2 mM CaCl2.
Supplementary Material
Acknowledgments
We thank M.F. Krummel for the original NFATc1-GFP construct; G. Nolan, K. Kripke-Skillern, and R. Wolkowitz for the CKHGppSin vector; and M. Kuhns for the modified pMSCV vector. We also thank J.J.T. Owen for his guidance, Q.-J. Li and F. Tynan for critical reading and J.R. Neilson, M. Winslow, and E. Gallo for productive discussions. This work was supported by Howard Hughes Medical Institute predoctoral fellowships (P.J.R.E. and L.I.R.E.) and by the US National Institutes of Health (R01 AIO22511) and Howard Hughes Medical Institute (M.M.D.).
Footnotes
SUPPLEMENTAL DATA
Supplemental Data include nine figures and two movies and can be found with this article online at http://www.immunity.com/supplemental/S1074-7613(08)00460-3.
References
- Amasaki Y, Adachi S, Ishida Y, Iwata M, Arai N, Arai K, Miyatake S. A constitutively nuclear form of NFATx shows efficient transactivation activity and induces differentiation of CD4(+)CD8(+) T cells. J Biol Chem. 2002;277:25640–25648. doi: 10.1074/jbc.M201860200. [DOI] [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- Baldwin KK, Reay PA, Wu L, Farr A, Davis MM. A T cell receptor-specific blockade of positive selection. J Exp Med. 1999;189:13–24. doi: 10.1084/jem.189.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton GM, Rudensky AY. Evaluating peptide repertoires within the context of thymocyte development. Semin Immunol. 1999a;11:417–422. doi: 10.1006/smim.1999.0199. [DOI] [PubMed] [Google Scholar]
- Barton GM, Rudensky AY. Requirement for diverse, low-abundance peptides in positive selection of T cells. Science. 1999b;283:67–70. doi: 10.1126/science.283.5398.67. [DOI] [PubMed] [Google Scholar]
- Berg LJ, Frank GD, Davis MM. The effects of MHC gene dosage and allelic variation on T cell receptor selection. Cell. 1990;60:1043–1053. doi: 10.1016/0092-8674(90)90352-f. [DOI] [PubMed] [Google Scholar]
- Bhakta NR, Oh DY, Lewis RS. Calcium oscillations regulate thymocyte motility during positive selection in the three-dimensional thymic environment. Nat Immunol. 2005;6:143–151. doi: 10.1038/ni1161. [DOI] [PubMed] [Google Scholar]
- Bousso P, Bhakta NR, Lewis RS, Robey E. Dynamics of thymocyte-stromal cell interactions visualized by two-photon microscopy. Science. 2002;296:1876–1880. doi: 10.1126/science.1070945. [DOI] [PubMed] [Google Scholar]
- Chmielowski B, Muranski P, Kisielow P, Ignatowicz L. On the role of high- and low-abundance class II MHC-peptide complexes in the thymic positive selection of CD4(+) T cells. Int Immunol. 2000;12:67–72. doi: 10.1093/intimm/12.1.67. [DOI] [PubMed] [Google Scholar]
- Correia-Neves M, Mathis D, Benoist C. A molecular chart of thymocyte positive selection. Eur J Immunol. 2001;31:2583–2592. doi: 10.1002/1521-4141(200109)31:9<2583::aid-immu2583>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Daniels MA, Teixeiro E, Gill J, Hausmann B, Roubaty D, Holmberg K, Werlen G, Hollander GA, Gascoigne NR, Palmer E. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- Davey GM, Schober SL, Endrizzi BT, Dutcher AK, Jameson SC, Hogquist KA. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J Exp Med. 1998;188:1867–1874. doi: 10.1084/jem.188.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MM, Krogsgaard M, Huse M, Huppa JB, Lillemeier BF, Li QJ. T cells as a self-referential, sensory organ. Annu Rev Immunol. 2007;25:681–695. doi: 10.1146/annurev.immunol.24.021605.090600. [DOI] [PubMed] [Google Scholar]
- Diehn M, Alizadeh AA, Rando OJ, Liu CL, Stankunas K, Botstein D, Crabtree GR, Brown PO. Genomic expression programs and the integration of the CD28 costimulatory signal in T cell activation. Proc Natl Acad Sci USA. 2002;99:11796–11801. doi: 10.1073/pnas.092284399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich LI, Ebert PJ, Krummel MF, Weiss A, Davis MM. Dynamics of p56lck translocation to the T cell immunological synapse following agonist and antagonist stimulation. Immunity. 2002;17:809–822. doi: 10.1016/s1074-7613(02)00481-8. [DOI] [PubMed] [Google Scholar]
- Faroudi M, Zaru R, Paulet P, Muller S, Valitutti S. Cutting edge: T lymphocyte activation by repeated immunological synapse formation and intermittent signaling. J Immunol. 2003;171:1128–1132. doi: 10.4049/jimmunol.171.3.1128. [DOI] [PubMed] [Google Scholar]
- Germain RN. T-cell development and the CD4–CD8 lineage decision. Nat Rev Immunol. 2002;2:309–322. doi: 10.1038/nri798. [DOI] [PubMed] [Google Scholar]
- Graef IA, Mermelstein PG, Stankunas K, Neilson JR, Deisseroth K, Tsien RW, Crabtree GR. L-type calcium channels and GSK-3 regulate the activity of NF-ATc4 in hippocampal neurons. Nature. 1999;401:703–708. doi: 10.1038/44378. [DOI] [PubMed] [Google Scholar]
- Graham DB, Bell MP, Huntoon CJ, Griffin MD, Tai X, Singer A, McKean DJ. CD28 ligation costimulates cell death but not maturation of double-positive thymocytes due to defective ERK MAPK signaling. J Immunol. 2006;177:6098–6107. doi: 10.4049/jimmunol.177.9.6098. [DOI] [PubMed] [Google Scholar]
- Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: A molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- Gunzer M, Schafer A, Borgmann S, Grabbe S, Zanker KS, Brocker EB, Kampgen E, Friedl P. Antigen presentation in extracellular matrix: Interactions of T cells with dendritic cells are dynamic, short lived, and sequential. Immunity. 2000;13:323–332. doi: 10.1016/s1074-7613(00)00032-7. [DOI] [PubMed] [Google Scholar]
- Hailman E, Burack WR, Shaw AS, Dustin ML, Allen PM. Immature CD4(+)CD8(+) thymocytes form a multifocal immunological synapse with sustained tyrosine phosphorylation. Immunity. 2002;16:839–848. doi: 10.1016/s1074-7613(02)00326-6. [DOI] [PubMed] [Google Scholar]
- Hare KJ, Jenkinson EJ, Anderson G. In vitro models of T cell development. Semin Immunol. 1999;11:3–12. doi: 10.1006/smim.1998.0151. [DOI] [PubMed] [Google Scholar]
- Hayden-Martinez K, Kane LP, Hedrick SM. Effects of a constitutively active form of calcineurin on T cell activation and thymic selection. J Immunol. 2000;165:3713–3721. doi: 10.4049/jimmunol.165.7.3713. [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Starr TK, Jameson SC. Receptor sensitivity: When T cells lose their sense of self. Curr Biol. 2003;13:R239–R241. doi: 10.1016/s0960-9822(03)00161-1. [DOI] [PubMed] [Google Scholar]
- Hooijberg E, Bakker AQ, Ruizendaal JJ, Spits H. NFAT-controlled expression of GFP permits visualization and isolation of antigen-stimulated primary human T cells. Blood. 2000;96:459–466. [PubMed] [Google Scholar]
- Huang YH, Li D, Winoto A, Robey EA. Distinct transcriptional programs in thymocytes responding to T cell receptor, Notch, and positive selection signals. Proc Natl Acad Sci USA. 2004;101:4936–4941. doi: 10.1073/pnas.0401133101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppa JB, Davis MM. T-cell-antigen recognition and the immunological synapse. Nat Rev Immunol. 2003;3:973–983. doi: 10.1038/nri1245. [DOI] [PubMed] [Google Scholar]
- Huppa JB, Gleimer M, Sumen C, Davis MM. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat Immunol. 2003;4:749–755. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–849. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- Jenkinson EJ, Anderson G. Fetal thymic organ cultures. Curr Opin Immunol. 1994;6:293–297. doi: 10.1016/0952-7915(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Jerabek L, Weissman IL. Intrathymic Injection for Analysis of T-Cell Progenitor Activity. In: K CA, Jordan CT, editors. Methods in Molecular Medicine. Totowa, NJ: Humana Press; 2001. pp. 161–165. [DOI] [PubMed] [Google Scholar]
- Kishimoto H, Sprent J. Negative selection in the thymus includes semimature T cells. J Exp Med. 1997;185:263–271. doi: 10.1084/jem.185.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisielow P, Miazek A. Positive selection of T cells: Rescue from programmed cell death and differentiation require continual engagement of the T cell receptor. J Exp Med. 1995;181:1975–1984. doi: 10.1084/jem.181.6.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke-Skillern K, Nolan GP. PhD thesis. Stanford University, Department of Immunology; Stanford, CA: 2002. HIV-1 Promoter Genetics. [Google Scholar]
- Krogsgaard M, Li QJ, Sumen C, Huppa JB, Huse M, Davis MM. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature. 2005;434:238–243. doi: 10.1038/nature03391. [DOI] [PubMed] [Google Scholar]
- Li QJ, Chau J, Ebert PJ, Sylvester G, Min H, Liu G, Braich R, Manoharan M, Soutschek J, Skare P, et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Li QJ, Dinner AR, Qi S, Irvine DJ, Huppa JB, Davis MM, Chakraborty AK. CD4 enhances T cell sensitivity to antigen by coordinating Lck accumulation at the immunological synapse. Nat Immunol. 2004;5:791–799. doi: 10.1038/ni1095. [DOI] [PubMed] [Google Scholar]
- Liu X, Bosselut R. Duration of TCR signaling controls CD4–CD8 lineage differentiation in vivo. Nat Immunol. 2004;5:280–288. doi: 10.1038/ni1040. [DOI] [PubMed] [Google Scholar]
- Marrack P, Ignatowicz L, Kappler JW, Boymel J, Freed JH. Comparison of peptides bound to spleen and thymus class II. J Exp Med. 1993;178:2173–2183. doi: 10.1084/jem.178.6.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkenschlager M. Tracing interactions of thymocytes with individual stromal cell partners. Eur J Immunol. 1996;26:892–896. doi: 10.1002/eji.1830260426. [DOI] [PubMed] [Google Scholar]
- Merkenschlager M, Benoist C, Mathis D. Evidence for a single-niche model of positive selection. Proc Natl Acad Sci USA. 1994;91:11694–11698. doi: 10.1073/pnas.91.24.11694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MJ, Hejazi AS, Wei SH, Cahalan MD, Parker I. T cell repertoire scanning is promoted by dynamic dendritic cell behavior and random T cell motility in the lymph node. Proc Natl Acad Sci USA. 2004;101:998–1003. doi: 10.1073/pnas.0306407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- Neal JW, Clipstone NA. Glycogen synthase kinase-3 inhibits the DNA binding activity of NFATc. J Biol Chem. 2001;276:3666–3673. doi: 10.1074/jbc.M004888200. [DOI] [PubMed] [Google Scholar]
- Neilson JR, Winslow MM, Hur EM, Crabtree GR. Calcineurin B1 is essential for positive but not negative selection during thymocyte development. Immunity. 2004;20:255–266. doi: 10.1016/s1074-7613(04)00052-4. [DOI] [PubMed] [Google Scholar]
- Peterson DA, DiPaolo RJ, Kanagawa O, Unanue ER. Cutting edge: Negative selection of immature thymocytes by a few peptide-MHC complexes: Differential sensitivity of immature and mature T cells. J Immunol. 1999;162:3117–3120. [PubMed] [Google Scholar]
- Purbhoo MA, Irvine DJ, Huppa JB, Davis MM. T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol. 2004;5:524–530. doi: 10.1038/ni1058. [DOI] [PubMed] [Google Scholar]
- Ranger AM, Oukka M, Rengarajan J, Glimcher LH. Inhibitory function of two NFAT family members in lymphoid homeostasis and Th2 development. Immunity. 1998;9:627–635. doi: 10.1016/s1074-7613(00)80660-3. [DOI] [PubMed] [Google Scholar]
- Richie LI, Ebert PJ, Wu LC, Krummel MF, Owen JJ, Davis MM. Imaging synapse formation during thymocyte selection: Inability of CD3zeta to form a stable central accumulation during negative selection. Immunity. 2002;16:595–606. doi: 10.1016/s1074-7613(02)00299-6. [DOI] [PubMed] [Google Scholar]
- Sant’Angelo DB, Lucas B, Waterbury PG, Cohen B, Brabb T, Goverman J, Germain RN, Janeway CA., Jr A molecular map of T cell development. Immunity. 1998;9:179–186. doi: 10.1016/s1074-7613(00)80600-7. [DOI] [PubMed] [Google Scholar]
- Sprent J, Kishimoto H. The thymus and central tolerance. Philos Trans R Soc Lond B Biol Sci. 2001;356:609–616. doi: 10.1098/rstb.2001.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr TK, Daniels MA, Lucido MM, Jameson SC, Hogquist KA. Thymocyte sensitivity and supramolecular activation cluster formation are developmentally regulated: A partial role for sialylation. J Immunol. 2003;171:4512–4520. doi: 10.4049/jimmunol.171.9.4512. [DOI] [PubMed] [Google Scholar]
- Stefanova I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420:429–434. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- Tomida T, Hirose K, Takizawa A, Shibasaki F, Iino M. NFAT functions as a working memory of Ca2+ signals in decoding Ca2+ oscillation. EMBO J. 2003;22:3825–3832. doi: 10.1093/emboj/cdg381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourne S, Nakano N, Viville S, Benoist C, Mathis D. The influence of invariant chain on the positive selection of single T cell receptor specificities. Eur J Immunol. 1995;25:1851–1856. doi: 10.1002/eji.1830250709. [DOI] [PubMed] [Google Scholar]
- Venanzi ES, Benoist C, Mathis D. Good riddance: Thymocyte clonal deletion prevents autoimmunity. Curr Opin Immunol. 2004;16:197–202. doi: 10.1016/j.coi.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Villunger A, Marsden VS, Strasser A. Efficient T cell receptor-mediated apoptosis in nonobese diabetic mouse thymocytes. Nat Immunol. 2003;4:717. doi: 10.1038/ni0803-717. [DOI] [PubMed] [Google Scholar]
- von Boehmer H, Aifantis I, Gounari F, Azogui O, Haughn L, Apostolou I, Jaeckel E, Grassi F, Klein L. Thymic selection revisited: How essential is it? Immunol Rev. 2003;191:62–78. doi: 10.1034/j.1600-065x.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- Witt CM, Raychaudhuri S, Schaefer B, Chakraborty AK, Robey EA. Directed migration of positively selected thymocytes visualized in real time. PLoS Biol. 2005;3:e160. doi: 10.1371/journal.pbio.0030160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P, Goldrath AW, Rudensky AY. Competition for specific intrathymic ligands limits positive selection in a TCR transgenic model of CD4+ T cell development. J Immunol. 2000;164:6252–6259. doi: 10.4049/jimmunol.164.12.6252. [DOI] [PubMed] [Google Scholar]
- Wu LC, Tuot DS, Lyons DS, Garcia KC, Davis MM. Two-step binding mechanism for T-cell receptor recognition of peptide MHC. Nature. 2002;418:552–556. doi: 10.1038/nature00920. [DOI] [PubMed] [Google Scholar]
- Yasutomo K, Doyle C, Miele L, Fuchs C, Germain RN. The duration of antigen receptor signalling determines CD4+ versus CD8+ T-cell lineage fate. Nature. 2000;404:506–510. doi: 10.1038/35006664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


