Abstract
Acetaldehyde, the first metabolite of ethanol, reacts with DNA to form adducts, including N2-ethyl-2′-deoxyguanosine (N2-Et-dG). Although the effects of N2-Et-dG on DNA polymerases have been well studied, nothing is known about possible effects of this lesion on transcription by RNA polymerases (RNAPs). Using primer extension assays in vitro, we found that a single N2-Et-dG lesion is a strong block to both mammalian RNAPII and two other multisubunit RNAPs, (yeast RNAPII and Escherichia coli RNAP), as well as to T7 RNAP. However, the mechanism of transcription blockage appears to differ between the multisubunit RNAPs and T7 RNAP. Specifically, all three of the multisubunit RNAPs can incorporate a single rNTP residue opposite the lesion, whereas T7 RNAP is essentially unable to do so. Using the mammalian RNAPII, we found that CMP is exclusively incorporated opposite the N2-Et-dG lesion. In addition, we also show that the accessory transcription factor TFIIS does not act as a lesion bypass factor, as it does for other nonbulky DNA lesions; instead, it stimulates the polymerase to remove the CMP incorporated opposite the lesion by mammalian RNAPII. We also include models of the N2-Et-dG within the active site of yeast RNAPII, which are compatible with our observations.
Acetaldehyde (ACD)2 is a genotoxin, known animal carcinogen, and suspected human carcinogen (1, 2). Although small amounts of ACD are produced endogenously during threonine catabolism (3), the most significant source of human exposure to ACD is via the metabolism of ethanol. In the human body, ethanol is first converted to ACD via the enzyme alcohol dehydrogenase, and ACD is further converted to acetate via aldehyde dehydrogenase (ALDH), primarily by the hepatic enzyme ALDH2. Approximately 50% of East Asian individuals are deficient in ALDH2 activity because of an amino acid substitution resulting in an inactive enzyme (4). ALDH2-deficient individuals are at a substantially elevated risk of esophageal cancer when they drink heavily, and other mechanistic evidence indicates that ACD is responsible for the increased cancer risk (2).
Several studies have shown that ACD can react with DNA to form adducts (5–12). One of the first identified and most well studied ACD-derived lesions is N2-ethyl-2′-deoxyguanosine (N2-Et-dG) (Fig. 1). N2-Et-dG is the stable form of N2-Eti-dG, the immediate product of the ACD reaction with dG. In the presence of basic compounds such as histones and polyamines, ACD can also give rise to other DNA adducts (13, 14). Elevated levels of these ACD-related DNA adducts, including N2-Et-dG, have been observed in white blood cell DNA in humans following alcohol consumption, with significantly higher levels observed in ALDH2-deficient individuals (15). Thus, the biological effects of these DNA lesions are of potential clinical relevance.
FIGURE 1.

Formation of N2-Et-dG by the reaction of acetaldehyde and guanosine. The initial reaction product is N2-EtI-dG, which undergoes a reduction reaction to yield the stable DNA lesion N2-Et-dG, which was used in the experiments reported here.
In view of the relationship between alcohol and cancer, the effect of N2-Et-dG on DNA replication and mutagenesis have been well studied. N2-Et-dG is a strong block to DNA polymerase α (16) but is efficiently bypassed by the replicative polymerase δ (17) (see also Ref. 18). No published data are available for this lesion with the replicative polymerase ∊. In addition, cells have specialized DNA polymerases that can bypass N2-Et-dG in an error-free manner (16, 19, 20). In human cells in vivo, the major genotoxic effects of the lesion appear to be the result of replication blockage, with some capacity for generating mutations including single base deletions and transversions (21).
In contrast to the effect on DNA polymerases, no studies have been done to examine the effects of this lesion on transcription by RNA polymerases (RNAPs). Therefore, in the present work we investigated the effects of N2-Et-dG on transcription by mammalian RNAPII, as well as two other multisubunit RNAPs, yeast RNAPII and Escherichia coli RNAP. To address the possibility of bypass factors, we also tested the effect of the transcription factor TFIIS, which has been recently shown to stimulate lesion bypass during transcription past the oxidized guanosine lesion 8-oxo-dG (22, 23), to allow mammalian RNA-PII to bypass N2-Et-dG.
As noted above, ALDH2 plays a key role on the metabolism of ACD. Because ALDH2 is localized to the mitochondria (4), it follows that ACD formed from ethanol metabolism must be able to enter mitochondria to be a substrate for the enzyme. As a result, it is likely that, especially in ALDH2-deficient individuals, ACD adduction of hepatic mitochondrial DNA could be significant. Therefore, in addition to investigating the effect of N2-Et-dG on transcription by RNAPII, we also examined the effect of the lesion on transcription by T7RNAP, as a model for mitochondrial RNAP. Eukaryotic mitochondrial RNAPs are single subunit polymerases that are highly homologous to T7 and other single subunit phage polymerases, especially in the residues and overall structure of the active site (24).
We found that a single N2-Et-dG lesion is a strong block to both mammalian and other multisubunit RNAPII, as well as to T7 RNAP. Interestingly, however, all three of the multisubunit RNAPs can incorporate a single rNTP residue opposite the lesion, whereas T7 RNAP is unable to do so. Using the mammalian RNAPII, we found that CMP is exclusively incorporated opposite the N2-Et-dG lesion. We also show that the accessory transcription factor TFIIS does not act as a lesion bypass factor, as it does for other nonbulky DNA lesions; instead, it stimulates the polymerase to remove the CMP incorporated opposite the lesion.
MATERIALS AND METHODS
Oligonucleotide Synthesis—A phospohoramidite containing N2-Et-dG was synthesized by Glen Research (Sterling, VA). Oligonucleotides containing the lesion were synthesized on an ABI DNA synthesizer using standard chemistry and deprotection and purified by denaturing PAGE.
The sequence of the DNA template strand used in the in vitro transcription was: 5′-CATGCTGATGAATTCCTTCNCTA-CTTTCCTCTCCATTT-3′. The underlined N indicates the position of either deoxyguanosine or the N2-Et-dG.
High pressure liquid chromatography-purified RNA primers were obtained from IDT (Coralville, IA). The sequence of the RNA oligonucleotide used for running start transcription was 5′-AGAGGAAAGU-3′, and that for standing start transcription was 5′-AGGAAAGUAG-3′. The sequence of the 30-mer RNA marker was 5′-UAGGUUCCACCUUACCAGCCUU-UUACAGAU-3′
RNA Primer Labeling with [γ-32P]ATP by T4 Polynucleotide Kinase—RNA primers were labeled with 30 μCi of [γ-32P]ATP (PerkinElmer Life Sciences) at 5′ end by T4 polynucleotide kinase (New England Biolabs). The reaction was carried out at 37 °C for 30 min and heat-inactivated at 65 °C for 15 min. The unincorporated nucleotides were removed by NucAway spin column (Ambion, TX) according to the manufacturer’s instructions.
Proteins—Mammalian RNAPII (from calf thymus) and yeast RNA-PIIs were purified as described (25). Full-length N-terminal histidine-tagged human TFIIS was expressed in the bacterial expression vector pET21 in BL-21 cells (Invitrogen). TFIIS was purified by nickel-nitrilotriacetic acid affinity chromatography as per company recommendations (Qiagen), followed by a final column employing MonoS (Bio-Rad) (supplemental Fig. S1). E. coli RNAP and T7 RNAP were purchased from Epicenter (Madison, WI).
In Vitro Transcription—The methodology for analyzing the effects of DNA lesions on transcription was based on the direct assembly of transcription elongation complexes introduced by Kashlev and co-workers (26). The single-stranded DNA template was used at 2 μm, and mixed with the RNA primer (2 μm) in the reaction buffer specific for each polymerase. The amount of RNA primer used assumed complete recovery after the spin column purification step. Mammalian RNAPII buffer was: 50 mm Tris-HCl (pH 7.6), 10 mm dithiothreitol, 6 mm MgCl2 and 40 mm (NH4)2SO4. The same buffer was also used for yeast RNAPII. E. coli RNAP reaction buffer was 20 mm HEPES (pH 7.9), 40 mm KCl, and 5 mm MgCl2. T7 RNAP reaction buffer was 20 mm HEPES (pH 7.9), 10 mm magnesium acetate, 20 mm sodium acetate, 1 mm dithiothreitol, and 0.1 mm EDTA.
To set up the reactions, 1 μl of RNA primer (2 pmol) was annealed to the template in a volume of 8 μl by first heating the mixture to 45 °C for 5 min and then cooled to room temperature (approximately 24 °C) 2 °C every 2 min (26). Then 1 μl of a 1.13 μm solution of mammalian RNAPII or yeast RNAPII was added to the reaction and incubated for 10 min at room temperature. These conditions give a nominal molar ratio of primer:template to polymerase of ≈2. For the prokaryotic enzymes, the reactions contained 10 units (1 μl) of E. coli RNAP or 50 units (1 μl) of T7 RNAP.
In vitro transcription was started by adding the indicated ribonucleotide(s) (final concentration, 100 μm) and stopped by removing samples and mixing with 2× formamide gel loading dye (95% (v/v) formamide, 025% (w/v) bromphenol blue, 0.025% (w/v) xylene cyanol, and 5 mm EDTA, pH 8.0) at the specific time points. The transcription products were separated on 20% acrylamide, 7 m urea gels and detected by autoradiography.
Quantitative Analysis of Apparent Km Values—The apparent Km is the concentration of NTP at which the rate of incorporation opposite dG or N2-Et-dG is half-maximal during a fixed reaction time (27). To determine the apparent Km values for CTP incorporation opposite dG or N2-Et-dG, standing start experiments were performed with varying concentrations of CTP as shown in Fig. 3, with incubation for 20 min at 25 °C. The resulting gels were dried, and the results were quantified using a Typhoon Imager (Molecular Dynamics), with subtraction of the background values at time 0 (27). Apparent Km values were obtained by nonlinear curve fitting using GraphPad Prism. The values given are the means ± S.E., based on two independent determinations.
FIGURE 3.
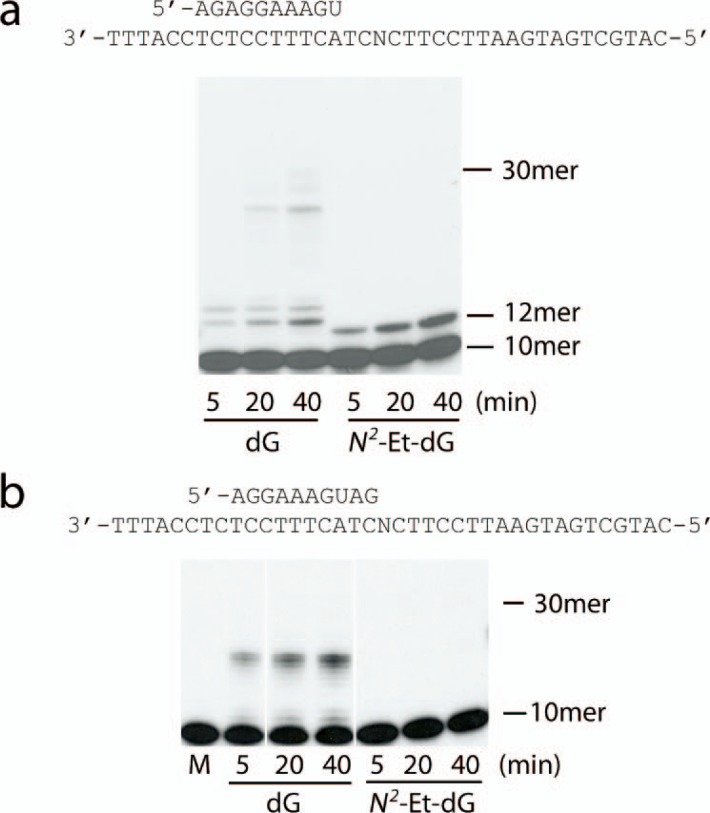
N2-Et-dG is a strong block to transcription in the standing start reaction. a, a 10-mer RNA oligonucleotide (top strand) annealed to the same DNA template (bottom strand). N indicates either guanine or N2-Et-dG. b, mammalian RNAPII transcription on either control or lesion template. Only one nucleotide insertion was detected on the lesion template (11-mer). c, individual nucleotides (100 μm each) were added to the standing start reactions, and samples collected at the indicated times. CMP was incorporated by mammalian RNAPII opposite the lesion. d, mammalian RNAPII was incubated with increasing concentrations of CTP for 20 min, and the amount of extended primer on either the control or N2-Et-dG containing template was determined. Representative results are shown; the values from duplicate experiments were quantified, and the values are reported in the text. e, quantitative analysis of additional incorporation experiments using restricted ranges of CTP concentration. The curves were generated using nonlinear regression analysis (GraphPad Prism), and the resulting apparent Km values are indicated. The data points are the means ± S.E. of duplicate determinations.
RESULTS
N2-Et-dG Is a Strong Block to Transcription Elongation by Mammalian RNAPII—To study the effects of N2-Et-dG on transcription, we utilized a primer extension assay, in which an RNA primer is first annealed to a lesion-containing or control (lesion-free) oligonucleotide DNA template. RNAP is then added, and transcription is initiated by the addition of rNTPs. This strategy is adapted from the methodology of Kashlev and co-workers (26) and is analogous to the methodology used to study the effects of DNA lesions on DNA polymerases (16, 19, 20). During transcription by RNAPs under these conditions, the nontemplate DNA strand is not required for transcription (26, 29, 30), and the absence of the nontemplate strand does not affect the stability of eukaryotic RNAPII transcription elongation complexes (31).
In our initial experiments, we used a “running start” set-up in which a 10-mer primer was annealed to a 38-mer DNA template two nucleotides 3′ to the lesion (Fig. 2a). As shown in Fig. 2b, the N2-Et-dG lesion poses as a strong blockage for transcription by calf RNAPII. Over time, a slow incorporation of nucleotides opposite the lesion base is observed.
FIGURE 2.
N2-Et-dG is a strong block to transcription by multisubunit RNAPs. a, diagram of the 10-mer RNA primer (top strand) annealed to a 38-mer DNA template (bottom strand). The N represents either guanine or N2-Et-dG lesion. b, a running start study using mammalian RNAP II. Transcription was initiated by the addition of rNTPs (100 μm each), and samples were analyzed at the indicated times. Transcription on the N2-Et-dG lesion-containing template was blocked after inserting one nucleotide (13-mer). c, running start studies with either yeast RNAPII or E. coli RNAP. Both enzymes are able to incorporate a nucleotide opposite the N2-Et-dG, as indicated by the accumulation of the 13-mer.
We repeated these experiments using two other purified multisubunit RNAPs. As shown in Fig. 2c, similar to the results using mammalian RNAPII, both yeast RNAPII and E. coli RNAP were also able to incorporate a single nucleotide opposite the N2-Et-dG lesion before stalling. Also, in contrast to mammalian RNAPII, with these enzymes, a minimal amount of bypass product was observable.
Inspection of the results shown in Fig. 2c indicated that the three polymerases differ in their ability to incorporate a nucleotide opposite the lesion. These differences are illustrated by comparing the relative amount of the 13-mer band (which represents nucleotide incorporation opposite the lesion) to the 12-mer, which represents transcription up to the site of the lesion. Because the 12-mer band is by definition the result of active polymerization, the relative amount of the 13-mer band to the 12-mer band is a measure of the fraction of active polymerases that were able to incorporate nucleotide opposite the lesion.
From the results shown in Fig. 2 as well as other analyses (not shown) the 13-mer/12-mer ratio at the 40-min time point for the yeast polymerase is 2.7–4.5, compared with 0.34–0.83 for the calf polymerase and 0.6–0.85 for the E. coli polymerase. On the basis of these differences, as well as the faster time course of nucleotide incorporation opposite the lesion for the E. coli polymerase compared with the calf (Fig. 2), our results indicate that the ability to incorporate nucleotide opposite the N2-Et-dG lesion is highest for yeast RNAPII, followed by the E. coli RNAP and then the calf RNAPII.
It should be noted that using the 13-mer/12-mer ratio as a basis for comparison normalizes for any variation in amount of transcription in different samples. Such variation, which is seen in Figs. 2 and 5, may be due to differences in the number of active polymerase molecules in a given enzyme preparation and/or to the efficiency of formation of the primer-template-enzyme complexes.
FIGURE 5.
The single subunit T7 RNAP cannot insert a nucleotide opposite the N2-Et-dG, under running start (a) or standing start (b) conditions. In b, the order of the lanes as shown was electronically modified (white lines) to compensate for a sample loading error. All of the lanes in b were from the same film exposure of the same gel processed identically.
Together, our results show that under running start conditions, the N2-Et-dG lesion is a very strong block to transcription elongation by mammalian RNAP II, as well as yeast RNAPII and E. coli RNAP. All three multisubunit polymerases are able to incorporate nucleotide opposite the lesion, prior to stalling, although the ability to incorporate nucleotide opposite the lesion varies between the three enzymes.
Mammalian RNAP II Incorporates CMP Opposite N2-Et-dG during Transcription—We next carried out a standing start RNA extension study using mammalian RNAPII with template containing either dG or N2-Et-dG as the first template base to be transcribed. Fig. 3b shows that under standing start conditions, mammalian RNAPII was also able to slowly incorporate a single nucleotide opposite the lesion before stalling, consistent with the running start results.
In Fig. 3b, we note that a limited degree of read-through product beyond the lesion site is clearly observable, in contrast to the results with the running start set-up (Fig. 2b). Because the template is identical in both cases, but the primer is annealed to different locations in the running start versus the standing start experiments, the difference between the two is most likely due in part to different initial RNAPII/nucleic acid structures. Such an interpretation is fully compatible with previous findings (32). The difference may also reflect differences in the number of possible configurations that the ethyl group can assume in the running versus standing start modes, an issue we will return to under “Discussion.” The important point here is that the results from the running and standing start experiments are in agreement in that in both conditions, the polymerase is able to slowly incorporate 1 nucleotide opposite the lesion.
To determine which nucleotide was incorporated opposite the N2-Et-dG lesion, the standing start RNA extension study was conducted as in Fig. 3b, but with each of the four ribonucleotides added separately at a concentration of 100 μm. As expected, under these conditions, mammalian RNAPII prefers to incorporate CMP opposite the unmodified template dG (Fig. 3c). With N2-Et-dG lesion as the template, only cytosine incorporation was observed, although clearly more slowly than with a template dG (Fig. 3d). These results suggest that Watson-Crick base pairing specificity was still preserved during nucleotide incorporation opposite N2-Et-dG.
To quantify the magnitude of the effect of the lesion on nucleotide incorporation, we determined the concentration of NTP at which the rate of incorporation opposite dG or N2-Et-dG is half-maximal during a fixed reaction time (27). This value, the apparent Km, has been used by other workers to compare the relative rates of nucleotide incorporation by RNAPs under various experimental conditions (27, 33). Representative data from these initial experiments, which used a wide range of CTP concentrations, are shown in Fig. 3d. Based on these analyses, the apparent Km for CTP incorporation opposite template dG was in the low nanomolar range, in good agreement with the apparent Km values obtained by Kornberg and co-workers (27) for yeast RNA-PII using a similar analysis. In contrast, with the N2-Et-dG template, the apparent Km for CTP incorporation was in the low micromolar range.
To increase the accuracy of our apparent Km determinations, we carried out additional experiments using a smaller range of CTP concentrations around the apparent Km values predicted for the dG and N2-Et-dG templates based on the initial analysis. As shown in Fig. 3e, these experiments gave an apparent Km for CTP incorporation opposite template dG of 3.95 nm, compared with 5.76 μm for the N2-Et-dG template, indicating that the N2-Et-dG reduces CTP incorporation by ≈1500-fold.
TFIIS Does Not Stimulate Transcription Past the N2-Et-dG Lesion—The general transcription elongation factor TFIIS is known for its role in stimulating the arrested RNAPII to cleave the nascent mRNA during transcription (34, 35). This generates a new 3′ end that allows transcription to resume. On helix-distorting DNA lesions such as a cyclobutane pyrimidine dimer, the addition of TFIIS stimulates the polymerase stalled at the lesion to backtrack (36, 37). In contrast, other data indicate that TFIIS can act as a bypass factor by stimulating transcription past nonbulky DNA lesions such as 8-oxo-dG (22, 23).
To investigate whether TFIIS can affect the ability of mammalian RNAPII to bypass N2-Et-dG, we first carried out a running start experiment similar to that described above (Fig. 2), including increasing amounts of TFIIS (Fig. 4a). This condition is most analogous to the in vivo situation, in which transcription takes place in the presence of rNTPs and TFIIS is present. When the template with N2-Et-dG lesion was transcribed in the absence of TFIIS, mammalian RNAPII alone was able to incorporate some nucleotide opposite the lesion, consistent with the results shown in Fig. 2b. However, with increasing TFIIS concentrations, the band corresponding to nucleotide incorporation opposite the lesion was reduced and completely abolished at high TFIIS concentrations. Importantly, TFIIS alone in the absence of RNAPII did not produce any transcript cleavage (Fig. 4a, IIS lanes).
FIGURE 4.
TFIIS is not a transcription bypass factor for mammalian RNAPII at the N2-Et-dG lesion. a, TFIIS was added to the running start reaction at increasing concentrations. The nucleotide opposite the lesion is removed when TFIIS is presented at the higher concentration (1.0- and 10-fold). Samples in lanes marked IIS were incubated with the amount of TFIIS used in the 10-fold ratio lanes but no RNAPII. b, a 11-mer RNA primer is annealed to the template, where a cytosine on the 3′ end is opposite either a guanine or a N2-Et-dG lesion base. c, increasing concentrations of TFIIS stimulate transcript cleavage by 2 nucleotides with either the dG or N2-Et-dG template. All of the incubations were for 40 min.
The effect of TFIIS observed in Fig. 4a could be due to either TFIIS preventing incorporation of nucleotide opposite the lesion or the stimulation of transcript cleavage following nucleotide incorporation. To address this issue, we carried out another experiment using an RNA primer with a cytosine opposite the N2-Et-dG or dG and incubation in the presence of rNTPs. As shown in Fig. 4b, in the absence of TFIIS, small amounts of extension products are visible with the N2-Et-dG template. However, with increasing TFIIS concentrations, the amount of extension is reduced, with the concomitant appearance of a product 1 nucleotide shorter than the original primer (Fig. 4b). This result indicates that even in the presence of rNTPs, TFIIS stimulates the removal of CMP incorporated opposite the lesion.
To determine the extent of TFIIS-stimulated transcript cleavage, we repeated the experiment shown in Fig. 4b in the absence of rNTPs (Fig. 4c). Under these conditions, we observed the appearance of an additional TFIIS-dependent band 2 nucleotides shorter than the starting RNA primer, with both the control and lesion-containing templates. This observation is consistent with others showing that TFIIS stimulated cleavage of transcription complexes stalled because of the absence of rNTPs occurs in dinucleotide increments (38).
Taken together, these results indicate that TFIIS does not act as a bypass factor for transcription past N2-Et-dG by mammalian RNAPII. Rather, the TFIIS-induced cleavage activity reduced the steady-state level of nucleoside incorporation opposite the lesion.
The N2-Et-dG Lesion Blocks Nucleotide Incorporation by T7 RNAP—As mentioned earlier, the mitochondrial localization of ALDH2 indicates that ACD can enter the mitochondrion and therefore might adduct mitochondrial DNA, especially in ALDH2-deficient individuals. To evaluate the blockage effect of the lesion on mitochondrial DNA transcription, we used T7 RNAP as a model enzyme, based on its homology to mitochondrial RNAP. The T7 reactions were conducted in a similar fashion as reactions employing the multisubunit RNAPs.
Under running start conditions, transcription of the N2-Et-dG template by T7 RNAP results in a 12-mer. However, in contrast to the results using multisubunit RNAPs (Fig. 2), no 13-mer product resulting from nucleotide incorporation opposite the lesion is detectable under these conditions (Fig. 5a). Consistent with this result, we were also unable to detect nucleotide incorporation opposite the lesion in the standing start set-up (Fig. 5b), although with very long film exposures, some bypass transcripts can be detected (data not shown).
DISCUSSION
We provide herein data that show that the ACD-derived DNA lesion N2-Et-dG is a strong block to both multisubunit and single-subunit RNAPs. Mammalian RNAPII, as well two other multisubunit RNAPs, are able to slowly incorporate a nucleoside opposite the N2-Et-dG lesion, whereas further transcription is strongly inhibited. Thus, the lesion interferes with transcription at both the incorporation and extension steps. Experiments using single rNTPs showed that mammalian RNAPII exclusively incorporates CMP opposite a N2-Et-dG lesion, which is a non-mutagenic event, but the incorporation of CMP opposite N2-Et-dG is reduced by a factor of ≈1500 compared with incorporation opposite dG. In contrast to the multisubunit RNAPs, the single-subunit T7 RNAP is unable to incorporate any rNTP opposite the lesion. Together, these results indicate that the N2-Et-dG lesion is a strong block to transcription by both multisubunit and single-subunit RNAPs.
Our results can be compared with published studies of the effects of N2-Et-dG on DNA polymerases. With regard to the replicative DNA polymerase α, for which N2-Et-dG was shown to be a strong blocking lesion, the only nucleotide that was incorporated opposite the lesion was dCMP, a nonmutagenic event (16). However, during DNA replication, the cells have available a variety of specialized DNA polymerases that can bypass replication blocking DNA lesions (39). With regard to N2-Et-dG, the translesion polymerase η as well as other polymerases can bypass N2-Et-dG, incorporating dCMP in the process (16, 19, 20). Thus, during replication, polymerase switching is likely to mitigate arrested replication resulting from N2-Et-dG in vivo.
In contrast, RNAPs are exclusively processive enzymes; specialized translesion RNAPs analogous to DNA polymerase η do not exist. When encountering obstacles to transcription, RNAPs do have accessory elongation factors available that can stimulate bypass in some cases (22, 23). However, our results indicate that the association of TFIIS with RNAPII stalled at an N2-Et-dG will not stimulate transcription past the lesion, but in fact accentuates the transcription blocking effect by stimulating transcript cleavage. Thus, although the initial effect of N2-Et-dG on transcription and replication is similar (i.e. polymerase blocking in both cases), the effects of the subsequent cellular responses to the blocked polymerases are likely to be very different.
Biological Considerations—Our results using a minimal system demonstrate that N2-Et-dG is a strong block to transcription. However, in vivo, the ultimate effect of the lesion will depend upon the efficiency of DNA repair, as well as accessory factors that modulate the effect of the lesion on transcription. With regard to repair, we have been unable to detect cleavage of oligo-nucleotides containing of N2-Et-dG by whole cell extracts from rat liver, under conditions in which glycosylase activity toward uracil, 8-oxo-dG, and ethenoadenine were readily observed.3 These negative results argue against the possibility that N2-Et-dG is a substrate for glycosylase initiated BER. Likewise, we found that N2-Et-dG is not a substrate for the E. coli direct repair enzyme AlkB (40). Although the eukaryotic AlkB homologs were not tested, they show only weak activity against ethylated bases (40). Finally, the absence of mismatch repair had no detectable effect on the genotoxicity of N2-Et-dG replicated in human cells (41). Thus, at present, the repair pathway responsible for the repair of N2-Et-dG, if any, remains to be elucidated. Also, it is important to bear in mind that our experiments are done with N2-Et-dG, whereas the initial lesion formed in vivo is N2-Eti-dG. N2-Et-dG itself is, however, detectable in the human body after ethanol consumption (15).
Turning to the question of transcription bypass factors, we initially tested the ability of TFIIS to stimulate transcription bypass past N2-Et-dG in part because it has been shown that TFIIS can stimulate transcription past another guanosine lesion, 8-oxo-dG (22, 23). Although we found that TFIIS does not stimulate transcriptional bypass past N2-Et-dG, we note that TFIIS is also not able to stimulate transcription past a different DNA lesion, thymine glycol. However, the Cockayne syndrome B protein, Elongin, and TFIIF are all able to stimulate transcription past thymine glycol (22). Thus, it remains possible that one of these factors can stimulate transcription past N2-Et-dG in vivo. Additional studies will be necessary to test this possibility.
The mitochondrial localization of ALDH2 indicates that ACD can enter the mitochondrion and therefore might accumulate in mitochondrial DNA, especially in ALDH2-deficient individuals. We used T7 RNAP as a model for mitochondrial RNAP, in an effort to address the possible effects of the N2-Et-dG in mitochondria. Our observation that N2-Et-dG is an essentially complete block to T7 RNAP in vitro suggests that, to the extent that this lesion is formed in mitochondrial DNA in vivo, it could impede mitochondrial gene expression and therefore interfere with mitochondrial function. In fact, in preliminary results we have found that N2-Et-dG also blocks transcription by purified histidine-tagged yeast mitochondrial RNAP.4 Because the liver is the primary site of alcohol metabolism to acetaldehyde (by alcohol dehydrogenase) and ALDH2-deficient individuals are known to have higher levels of N2-Et-dG in cellular DNA after alcohol consumption (15), the inhibition of mitochondrial RNA transcription might be especially significant in the liver of ALDH2-deficient individuals after alcohol consumption.
The Function of TFIIS during Transcription at an N2-Et-dG Lesion—On nondamaged DNA templates, the two major roles for TFIIS that have been identified are stimulation of transcription elongation and as a transcript cleavage stimulatory factor (34). Likewise, at sites of DNA damage, TFIIS can either stimulate transcript cleavage (36, 37) or act as a bypass factor (22, 23). Our results clearly demonstrate that at N2-Et-dG, TFIIS does not act as a bypass factor; rather, it stimulates transcript cleavage and in doing so contributes to the transcription blocking effect of the lesion.
Hawley and co-workers (42) proposed a kinetic portioning model to explain the proofreading function of TFIIS in which the slow extension of elongation from a mismatched terminus allows time for TFIIS to stimulate cleavage. During the resynthesis step after transcription cleavage, correct nucleotide incorporation allows transcription to continue. Crystal structures of yeast TFIIS complexed with RNAPII are consistent with this model (43).
Our observations on the effect of N2-Et-dG can be understood in the context of an analogous model. In essence, the slow incorporation of CMP opposite the lesion, and slow extension of the rC: N2-Et-dG base pair allows time for TFIIS to stimulate transcript cleavage analogous to the proofreading model. However, in contrast to the proofreading model, during the resynthesis step following transcript cleavage, the polymerase reincorporates CMP opposite the lesion, again triggering TFIIS-stimulated transcript cleavage. The net result in the presence of TFIIS is a futile cycle of CMP incorporation, transcript cleavage, and resynthesis, resulting in a steady-state condition of polymerase stalling 1 nucleotide before the lesion (Fig. 4a).
Structural Considerations—Dimitri et al. (44) recently tested the ability of 1,N2-ethnoguanine to affect transcription of human RNAPII (in a HeLa nuclear extract) and T7 RNAP. These authors found that 1,N2-ethnoguanine was a complete block to human RNAPII, whereas T7 RNAP could bypass the lesion. These results are somewhat the opposite of what we found with N2-Et-dG. However, the differences can likely be explained by the different structural effects of the lesion. In 1,N2-ethnoguanine, the modification is an etheno group with covalent bonds between both N2 and C-1 of the purine ring. This results in an additional ring to the purine, which blocks access to the active site of RNAPII. In contrast, in N2-Et-dG the ethyl group is attached to the N2 via a single bond. As such, the ethyl group hangs below the guanine base and can therefore potentially adapt a number of different configurations within this space.
Modeling the effect of N2-Et-dG on transcription by T7RNAP is complicated by the large structural difference between the open and closed configurations of this enzyme (45, 46). Our current results do not allow us to determine whether the blocking effect of the lesion on transcription takes place in the open or closed states, and therefore we have not attempted to model these possibilities until additional data are available.
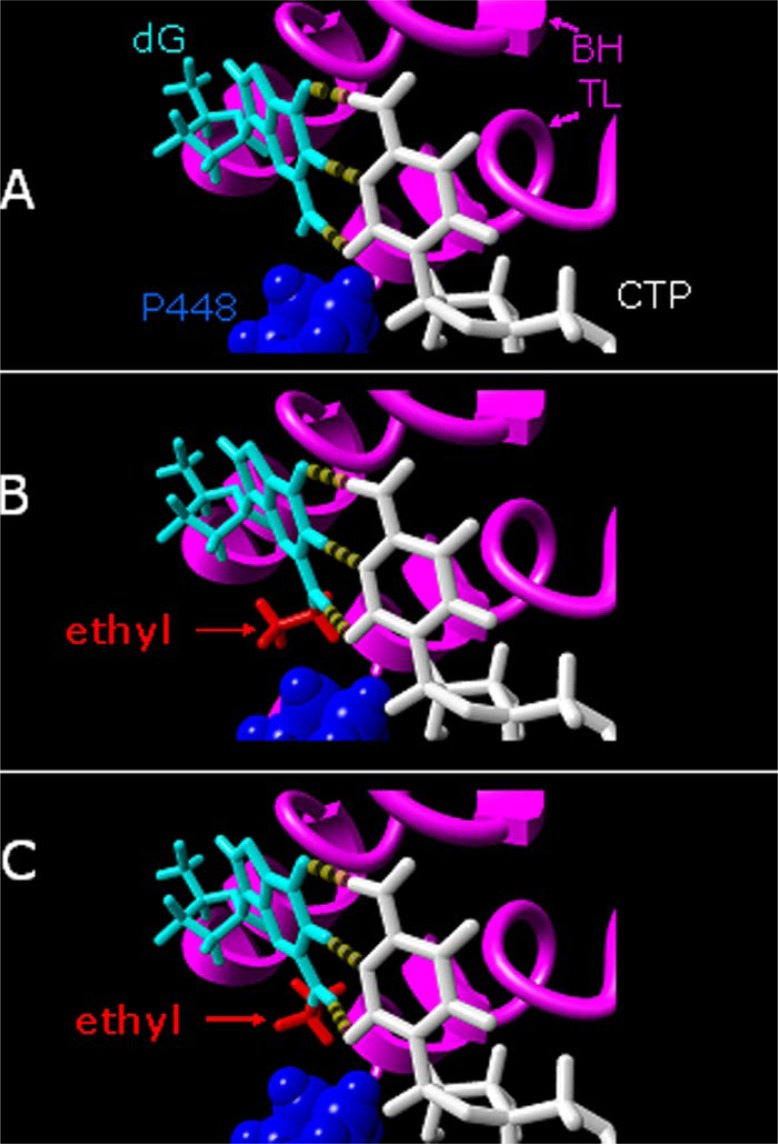
Regarding RNAPII, our observations indicate that within the active site the ethyl group in N2-Et-dG can adopt one or more configurations that are compatible with the incorporation of CMP opposite the lesion. Using molecular modeling, we have been able to identify multiple configurations of the ethyl group within the polymerase that are compatible with hydrogen bonding between the guanosine base and the cytosine of an incoming CTP. Two examples are shown in Fig. 6. The multiple distinct possible configurations of N2-Et-dG in the active site may also provide insight into why we see some bypass of the lesion in the standing start experiments but not in the running start.
FIGURE 6.
Energy-minimized models of the active site yeast RNAPII with a template dG (A) or different configurations of N2-Et-dG (B and C) base paired to an incoming CTP. The amino acid residue most likely to clash with some configurations of the ethyl group N2-Et-dG is Rpb1 Pro448. The two different configurations of the ethyl group within the active site shown in B and C avoid a clash between the lesion and Pro448 or any other amino acid within the active site, while allowing three H-bonds (dashed lines) between the template G and incoming CTP. Additional abbreviations: BH, bridge helix; TL, trigger loop. Energy minimization and modeling were done using YASARA-WHAT-IF (28). For additional information about the modeling, see supplementary text.
Specifically, it may be the case that some configurations of the ethyl group in the active site are compatible with both CMP incorporation and subsequent extension, whereas other configurations of the ethyl group allow CMP incorporation but inhibit subsequent extension. If so, it is possible that configurations that are compatible with subsequent extension might be disfavored under running start conditions compared with the standing start, because of either occupancy of the active site or other locations of the polymerase by rNTPs (47), allosteric effects of rNTPs (48–51), or different configurations of the trigger loop (27, 52). Ultimately, determination of the location(s) of the ethyl group with the active site of the enzyme will require direct structural analysis using x-ray crystallography.
Acknowledgments
We thank Cheryl Marietta for helpful comments on the text.
Footnotes
The abbreviations used are: ACD, acetaldehyde; N2-Et-dG, N2-ethyl-2′-deoxy-guanosine; RNAP, RNA polymerase; ALDH, aldehyde dehydrogenase.
P. J. Brooks, unpublished observations.
T.-F. Cheng, J. Abraham, and P. J. Brooks, unpublished observations.
This work was supported by the Division of Clinical and Intramural Research, National Institute on Alcohol Abuse and Alcoholism. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1 and supplemental text.
REFERENCES
- 1.Dellarco VL. Mutat Res. 1988;195:1–20. doi: 10.1016/0165-1110(88)90013-9. [DOI] [PubMed] [Google Scholar]
- 2.Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Altieri A, Cogliano V. Lancet Oncol. 2007;8:292–293. doi: 10.1016/s1470-2045(07)70099-2. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa H, Gomi T, Fujioka M. Int J Biochem Cell Biol. 2000;32:289–301. doi: 10.1016/s1357-2725(99)00113-2. [DOI] [PubMed] [Google Scholar]
- 4.Crabb DW, Matsumoto M, Chang D, You M. Proc Nutr Soc. 2004;63:49–63. doi: 10.1079/pns2003327. [DOI] [PubMed] [Google Scholar]
- 5.Fraenkel-Conrat H, Singer B. Proc Natl Acad Sci U S A. 1988;85:3758–3761. doi: 10.1073/pnas.85.11.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaca CE, Fang JL, Schweda EK. Chem Biol Interact. 1995;98:51–67. doi: 10.1016/0009-2797(95)03632-v. [DOI] [PubMed] [Google Scholar]
- 7.Fang JL, Vaca CE. Carcinogenesis. 1997;18:627–632. doi: 10.1093/carcin/18.4.627. [DOI] [PubMed] [Google Scholar]
- 8.Hecht SS, McIntee EJ, Wang M. Toxicology. 2001;166:31–36. doi: 10.1016/s0300-483x(01)00436-x. [DOI] [PubMed] [Google Scholar]
- 9.Wang M, McIntee EJ, Cheng G, Shi Y, Villalta PW, Hecht SS. Chem Res Toxicol. 2000;13:1149–1157. doi: 10.1021/tx000118t. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Wang M, Villalta PW, Luo X, Feuer R, Jensen J, Hatsukami DK, Hecht SS. Chem Res Toxicol. 2007;20:108–113. doi: 10.1021/tx060232x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang M, Yu N, Chen L, Villalta PW, Hochalter JB, Hecht SS. Chem Res Toxicol. 2006;19:319–324. doi: 10.1021/tx0502948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooks PJ, Theruvathu JA. Alcohol. 2005;35:187–193. doi: 10.1016/j.alcohol.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Inagaki S, Esaka Y, Goto M, Deyashiki Y, Sako M. Biol Pharm Bull. 2004;27:273–276. doi: 10.1248/bpb.27.273. [DOI] [PubMed] [Google Scholar]
- 14.Theruvathu JA, Jaruga P, Nath RG, Dizdaroglu M, Brooks PJ. Nucleic Acids Res. 2005;33:3513–3520. doi: 10.1093/nar/gki661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuda T, Yabushita H, Kanaly RA, Shibutani S, Yokoyama A. Chem Res Toxicol. 2006;19:1374–1378. doi: 10.1021/tx060113h. [DOI] [PubMed] [Google Scholar]
- 16.Perrino FW, Blans P, Harvey S, Gelhaus SL, McGrath C, Akman SA, Jenkins GS, LaCourse WR, Fishbein JC. Chem Res Toxicol. 2003;16:1616–1623. doi: 10.1021/tx034164f. [DOI] [PubMed] [Google Scholar]
- 17.Choi JY, Guengerich FP. J Mol Biol. 2005;352:72–90. doi: 10.1016/j.jmb.2005.06.079. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda T, Terashima I, Matsumoto Y, Yabushita H, Matsui S, Shibutani S. Biochemistry. 1999;38:929–935. doi: 10.1021/bi982134j. [DOI] [PubMed] [Google Scholar]
- 19.Choi JY, Angel KC, Guengerich FP. J Biol Chem. 2006;281:21062–21072. doi: 10.1074/jbc.M602246200. [DOI] [PubMed] [Google Scholar]
- 20.Choi JY, Guengerich FP. J Biol Chem. 2006;281:12315–12324. doi: 10.1074/jbc.M600112200. [DOI] [PubMed] [Google Scholar]
- 21.Upton DC, Wang X, Blans P, Perrino FW, Fishbein JC, Akman SA. Mutat Res. 2006;599:1–10. doi: 10.1016/j.mrfmmm.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Charlet-Berguerand N, Feuerhahn S, Kong SE, Ziserman H, Conaway JW, Conaway R, Egly JM. EMBO J. 2006;25:5481–5491. doi: 10.1038/sj.emboj.7601403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuraoka I, Suzuki K, Ito S, Hayashida M, Kwei JS, Ikegami T, Handa H, Nakabeppu Y, Tanaka K. DNA Repair (Amst) 2007;6:841–851. doi: 10.1016/j.dnarep.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Masters BS, Stohl LL, Clayton DA. Cell. 1987;51:89–99. doi: 10.1016/0092-8674(87)90013-4. [DOI] [PubMed] [Google Scholar]
- 25.Hu X, Malik S, Negroiu CC, Hubbard K, Velalar CN, Hampton B, Grosu D, Catalano J, Roeder RG, Gnatt A. Proc Natl Acad Sci U S A. 2006;103:9506–9511. doi: 10.1073/pnas.0603702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komissarova N, Kireeva ML, Becker J, Sidorenkov I, Kashlev M. Methods Enzymol. 2003;371:233–251. doi: 10.1016/S0076-6879(03)71017-9. [DOI] [PubMed] [Google Scholar]
- 27.Wang D, Bushnell DA, Westover KD, Kaplan CD, Kornberg RD. Cell. 2006;127:941–954. doi: 10.1016/j.cell.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vriend G. J Mol Graph. 1990;8:52–56. doi: 10.1016/0263-7855(90)80070-v. [DOI] [PubMed] [Google Scholar]
- 29.Kashkina E, Anikin M, Brueckner F, Pomerantz RT, McAllister WT, Cramer P, Temiakov D. Mol Cell. 2006;24:257–266. doi: 10.1016/j.molcel.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Pomerantz RT, Temiakov D, Anikin M, Vassylyev DG, McAllister WT. Mol Cell. 2006;24:245–255. doi: 10.1016/j.molcel.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kireeva ML, Komissarova N, Waugh DS, Kashlev M. J Biol Chem. 2000;275:6530–6536. doi: 10.1074/jbc.275.9.6530. [DOI] [PubMed] [Google Scholar]
- 32.Gnatt A, Fu J, Kornberg RD. J Biol Chem. 1997;272:30799–30805. doi: 10.1074/jbc.272.49.30799. [DOI] [PubMed] [Google Scholar]
- 33.Svetlov V, Vassylyev DG, Artsimovitch I. J Biol Chem. 2004;279:38087–38090. doi: 10.1074/jbc.C400316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fish RN, Kane CM. Biochim Biophys Acta. 2002;1577:287–307. doi: 10.1016/s0167-4781(02)00459-1. [DOI] [PubMed] [Google Scholar]
- 35.Wind M, Reines D. Bioessays. 2000;22:327–336. doi: 10.1002/(SICI)1521-1878(200004)22:4<327::AID-BIES3>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donahue BA, Yin S, Taylor JS, Reines D, Hanawalt PC. Proc Natl Acad Sci U S A. 1994;91:8502–8506. doi: 10.1073/pnas.91.18.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tornaletti S. Mutat Res. 2005;577:131–145. doi: 10.1016/j.mrfmmm.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 38.Izban MG, Luse DS. J Biol Chem. 1993;268:12874–12885. [PubMed] [Google Scholar]
- 39.Prakash S, Johnson RE, Prakash L. Annu Rev Biochem. 2005;74:317–353. doi: 10.1146/annurev.biochem.74.082803.133250. [DOI] [PubMed] [Google Scholar]
- 40.Koivisto P, Robins P, Lindahl T, Sedgwick B. J Biol Chem. 2004;279:40470–40474. doi: 10.1074/jbc.M407960200. [DOI] [PubMed] [Google Scholar]
- 41.Upton DC, Wang X, Blans P, Perrino FW, Fishbein JC, Akman SA. Chem Res Toxicol. 2006;19:960–967. doi: 10.1021/tx060084a. [DOI] [PubMed] [Google Scholar]
- 42.Thomas MJ, Platas AA, Hawley DK. Cell. 1998;93:627–637. doi: 10.1016/s0092-8674(00)81191-5. [DOI] [PubMed] [Google Scholar]
- 43.Kettenberger H, Armache KJ, Cramer P. Cell. 2003;114:347–357. doi: 10.1016/s0092-8674(03)00598-1. [DOI] [PubMed] [Google Scholar]
- 44.Dimitri A, Goodenough AK, Guengerich FP, Broyde S, Scicchitano DA. J Mol Biol. 2008;375:353–366. doi: 10.1016/j.jmb.2007.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yin YW, Steitz TA. Cell. 2004;116:393–404. doi: 10.1016/s0092-8674(04)00120-5. [DOI] [PubMed] [Google Scholar]
- 46.Temiakov D, Patlan V, Anikin M, McAllister WT, Yokoyama S, Vassylyev DG. Cell. 2004;116:381–391. doi: 10.1016/s0092-8674(04)00059-5. [DOI] [PubMed] [Google Scholar]
- 47.Westover KD, Bushnell DA, Kornberg RD. Cell. 2004;119:481–489. doi: 10.1016/j.cell.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 48.Foster JE, Holmes SF, Erie DA. Cell. 2001;106:243–252. doi: 10.1016/s0092-8674(01)00420-2. [DOI] [PubMed] [Google Scholar]
- 49.Holmes SF, Erie DA. J Biol Chem. 2003;278:35597–35608. doi: 10.1074/jbc.M304496200. [DOI] [PubMed] [Google Scholar]
- 50.Nedialkov YA, Gong XQ, Hovde SL, Yamaguchi Y, Handa H, Geiger JH, Yan H, Burton ZF. J Biol Chem. 2003;278:18303–18312. doi: 10.1074/jbc.M301103200. [DOI] [PubMed] [Google Scholar]
- 51.Zhang C, Burton ZF. J Mol Biol. 2004;342:1085–1099. doi: 10.1016/j.jmb.2004.07.070. [DOI] [PubMed] [Google Scholar]
- 52.Vassylyev DG, Vassylyeva MN, Zhang J, Palangat M, Artsimovitch I, Landick R. Nature. 2007;448:163–168. doi: 10.1038/nature05931. [DOI] [PubMed] [Google Scholar]