Abstract
Background
Plumbagin, a quinonoid constituent isolated from the root of Plumbago zeylanica L., has been proven to possess anti-tumor activity both in vitro and in vivo. However, its anti-tumor properties for human tongue carcinoma have not been reported. This study aimed to investigate the inhibitory effect and the underlying mechanism of plumbagin on the growth of human tongue carcinoma cells.
Material/Methods
Cell proliferation ability was detected by EdU incorporation assay and colony formation assay. Cell-cycle distribution was determined by flow cytometric analysis using propidium iodide (PI) staining. Cellular apoptosis was then evaluated by flow cytometry and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. Western blotting was applied to assay the expression of Bax and Bcl-2.
Results
Plumbagin inhibited the growth and proliferation of Tca8113 cells in vitro in a concentration- and time-dependent manner. The cell cycles of plumbagin-treated Tca8113 cells were arrested at the G2/M phase. Cells treated with plumbagin presented the characteristic morphological changes of apoptosis. The ratio of Bax/Bcl-2 was raised by plumbagin in a concentration-dependent manner.
Conclusions
These results indicate that plumbagin induces the apoptosis of Tca8113 cells through mitochondria-mediated pathway.
Keywords: plumbagin, human tongue carcinoma, proliferation, cell cycle arrest, apoptosis
Background
Oral cancer is a common tumor around the world, particularly in developing countries [1]. In the past decade, the incidence and mortality rates of oral cancer have been increasing in many regions of the world such as Taiwan and the United Kingdom [2,3]. More than 90% of oral cancers are histopathologically squamous cell carcinomas (SCCs) where the most common site of involvement is the tongue [4,5]. Despite advances in surgery, radiotherapy and chemotherapy, the 5-year survival rate of oral cancer patients have not greatly improved over the past several decades and remains at ~50% [6,7]. Moreover, advanced oral squamous cell carcinoma (OSCC) has a high morbidity and treatment frequently results in significant mutilation and compromised functions [8]. Therefore, there is an urgent need for the development of a more effective therapy to treat early and advanced OSCC.
Over 2,500 years, a large number of traditional Chinese medicines have been widely used to treat and prevent various diseases including cancer, diabetes, hypertension and Alzheimer disease [9–12]. In recent years, many herbal components have been purified and identified as effective anticancer agents. Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) is a natural naphthoquinone isolated from the root of Plumbago zeylanica L [13,14]. In traditional Chinese medicine, P. zeylanica L. has been extensively used for the treatment of rheumatoid arthritis, dysmenorrhea, bruising, and cancer [15,16]. Like daunorubicin, mitoxanthrone and adriamycin, plumbagin is structurally derived from naphthoquinone [16,17]. Plumbagin has been proven to possess anti-tumor activity both in a variety of cell lines and animal models [13,18–21]. The previous study showed that plumbagin has the ability to suppress azoxymethane-induced intestinal carcinogenesis in rats, suggesting its chemopreventive activity [22]. Besides anticancer effects, plumbagin also exhibited radiosensitizing properties in experimental mouse tumors as well as in tumor cells in vitro[23–25].
Apoptosis, a form of programmed cell death, plays a fundamental role in the elimination of damaged or unwanted cells [26]. Failure of tumor cells to undergo apoptosis translates into malignant potential and chemotherapeutic resistance [27]. Similar to other malignancies, apoptosis of tumor cells significantly influences the trend of progression and remission in tongue cancers. Reduced apoptotic cancer cell number was closely related with poor prognosis of tongue carcinomas [5]. A pallet of genes are involved in the control of apoptosis such as the Bcl-2 family whose oncogenic potential has been demonstrated in oral tumorigenesis [28]. Bcl-2, a potent suppressor of apoptosis, can form a heterodimer with the apoptotic protein Bax and thereby neutralize its apoptotic effects. Therefore, alteration in the ratio of Bax/Bcl2 is a decisive factor which plays an important role in determining whether cells will undergo apoptosis. Recent studies have shown that a large number of anticancer drugs exert their therapeutic effects by inducing apoptosis in malignant cells [27].
In this study, we investigated the cell growth inhibition activity of plumbagin using a cellular model and examined its effects on cell cycle and apoptosis in human oral tongue squamous cell carcinoma (OTSCC) Tca8113 cells. Furthermore, we assayed the levels of Bcl-2 and Bax in Tca8113 cells treated with plumbagin.
Material and Methods
Reagents
Plumbagin was purchased from Sigma-Aldrich (St Louis, MO). This compound was dissolved in dimethyl sulfoxide (DMSO) where the final DMSO concentration in all cell cultures was kept at below 0.1% (v/v) with no detectable cytotoxic effects on cell growth.
Propidium iodide (PI) was obtained from BD Biosciences (San Jose, CA). 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), acridine orange (AO), ethidium bromide (EB), Hoechst 33258 and Hoechst 33342 were purchased from Sigma-Aldrich (St. Louis, MO). The protease inhibitor cocktail was obtained from Roche (Mannheim, Germany). Finally, the antibodies against B-cell lymphoma-2 (Bcl-2), Bcl-2–associated X protein (Bax), and β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell lines and cell culture
Tca8113 cell line, a poorly differentiated oral tongue squamous cell carcinoma cell line, was kindly provided by Key Laboratory of Oral Biomedicine Ministry of Education, School & Hospital of Stomatology, Wuhan University, Wuhan, China. Tca8113 cells were grown in 25-cm2 culture flasks and incubated in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum, 100 units/liter penicillin, and 10 mg/liter streptomycin. Cells were maintained at 37°C in an atmosphere containing 5% CO2.
Cell viability assay
Cell viability was quantitatively determined by the MTT assay. Briefly, Tca8113 cells were plated in 96-well culture plates (1×104 cells/well) and allowed to attach overnight at 37°C in a humidified 5% CO2 atmosphere. Cells were treated with the plumbagin at 3.125–50 μM for 12, 24, 48 or 72 hr, and 20 μl 5.0 mg/ml MTT reagent was added to each well and incubated in dark for an additional 4 hr at 37°C in a humidified environment with 5% CO2. The plates were then centrifuged at 1,500 g for 5 min and the supernatant was removed. The cell pellets were dissolved in 100 μl DMSO. The absorbance was determined using the microplate reader (Bio-Rad, Hercules, CA) at a wavelength of 570 nm, with background subtraction at a wavelength of 630 nm. The 50% inhibitory concentration (IC50) was calculated from survival curves using the Bliss method. All experiments were performed with 6 wells for each concentration, and repeated at least three times.
EdU incorporation assay
Cell proliferation or DNA synthesis was assessed by 5-ethynyl-2’-deoxyuridine (EdU) fluorescence staining and completed according to the manufacturer’s instructions (Cell-Light™ EdU DNA Cell Proliferation Kit, Ruibo Biotech, Guangzhou, China). The procedure was as follows: Tca8113 cells were plated in 96-well culture plates (1×104 cells/well), treated with plumbagin for 24 hr, washed with phosphate-buffered saline (PBS) and then incubated with 50 μM EdU for 2 hr. Subsequently, the cells were fixed with 4% paraformaldehyde in PBS for 30 min at room temperature followed by washing twice with PBS and treated with 0.5% Triton X-100 for 10 min at room temperature for permeabilization. The cells were then washed with PBS and incubated with the 1× Apollo® reaction cocktail for 30 min at room temperature in dark. After removing the cocktail, the cells were washed twice with 0.5% Triton X-100 in PBS, and then treated with 1× Hoechst 33342 solution for another 30 min at room temperature with light. Finally, after washing with PBS for five times, the cells were examined with fluorescence microscopy and photographed (Olympus DP 71, Tokyo, Japan). Photographs of the cells were processed and analyzed.
Colony formation assay
The cells were seeded at a density of 300/mL into 6-well culture plates, treated with plumbagin for 24 hr, then washed with PBS and fresh medium was added. Colonies were allowed to grow for 14 days. After removing the medium, each well was carefully washed twice with PBS. The cells were fixed in methanol for 15 min and then stained with crystal violet for 20 min. Finally, positive colony formations (more than 50 cells per colony) were counted. The survival cell fraction was expressed as the ratio of plating efficiency of treated cells to that of untreated control cells.
Flow cytometry
The effect of plumbagin treatment on cell cycle was determined by flow cytometric analysis using PI staining as described [29]. Briefly, the cells were exposed to plumbagin at 2.5, 5.0 or 10.0 μM for 24 hr. After plumbagin treatment, both floating and attached cells were collected, washed, and fixed in 70% ethanol overnight at −20°C. Then, the cells were washed twice with ice-cold PBS, resuspended in PBS, and stained with PI solution that contained 50 μg/ml PI and 25 μg/ml RNase. Stained cells were analyzed on a BD FACS Caliber Cell flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Data was then analyzed using CellQuest Pro software (Becton Dickinson, Franklin Lakes, NJ).
To quantify drug-induced apoptosis, annexin V/PI staining was performed using flow cytometry. Briefly after plumbagin treatment, both floating and attached cells were collected and stained with annexin V and PI using the annexin V-FITC apoptosis detection kit (Nanjing KeyGen Biotech Co., Nanjing, China) according to the protocol provided by the manufacturer. The cells were then exposed to plumbagin at different concentrations for 24 hr. Double staining was used to distinguish between viable, apoptotic (early or late) and necrotic cells. The resulting fluorescence was measured by flow cytometry using a BD FACS Caliber cell flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Resulting data were analyzed using the CellQuest Pro software (Becton Dickinson, Franklin Lakes, NJ).
Cellular morphological observations
Tca8113 in DMEM containing 10% FBS were seeded into 6-well culture plates and incubated overnight. Plumbagin (2.5, 5 or 10 μM) was then added to the cell culture and the cellular morphology was observed using phase-contrast microscopy (Olympus CKX31, Tokyo, Japan) at the indicated times.
Terminal deoxynucleotidyl transferase-mediated biotin dUTP nick end labeling (TUNEL) assay
To detect apoptotic cells, in situ end labeling of the 3’-OH end of the DNA fragments generated by apoptosis-associated endonucleases was performed with the Colorimetric TUNEL Apoptosis Assay kit (Beyotime Institute of Biotechnology, Haimen, China). Briefly, cells were seeded on cover slips and treated with the indicated concentration of plumbagin for 24 hr and cells were fixed in 4% paraformaldehyde. Then, the cells were permeabilized with 0.1% Triton X-100 in PBS while blocking endogenous peroxidase activity with 0.3% H2O2 in methanol. Subsequently, the TdT reaction mix was added to the sections on the slide and then the cells were incubated at 37°C for 60 min inside a humidified chamber for the end labeling reaction to occur. For negative control the TdT enzyme was replaced with PBS. After the incubation of cells with Streptavidin-HRP and diaminobenzidine, apoptotic DNA fragmentation was detected by visualizing labeled DNA using a light microscope. The percentage of apoptotic cells were calculated by counting the stained cells in 12 fields, each containing a total of 50 cells.
Western blotting assay
Tca8113 cells were treated with plumbagin. Total cell lysates were extracted and subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) electrophoresis, and then transferred to high-quality polyvinylidene difluoride (PVDF) membranes. After blocking with 5% nonfat milk for 1 hr, membranes were incubated with primary antibodies specific to Bax, Bcl-2, and β-actin for 1 hr. After washing three times with TBS-T, the membranes were incubated with the corresponding secondary antibody. The signals were detected by the enhanced chemiluminescence blotting detection system (The ECL substrate kit, Amersham, Piscataway, NJ).
Statistical analysis
All data are expressed as the mean ± standard deviation (SD). Differences among groups were analyzed by the Student’s t-test or analysis of variance (ANOVA) when multiple groups were involved. The P value reported was two-sided and a value of P<0.05 was considered statistically significant. All analyses were performed using the SPSS software (Version 12.0, SPSS Inc., Chicago, IL).
Results
Plumbagin treatment decreased the viability of Tca8113 cells
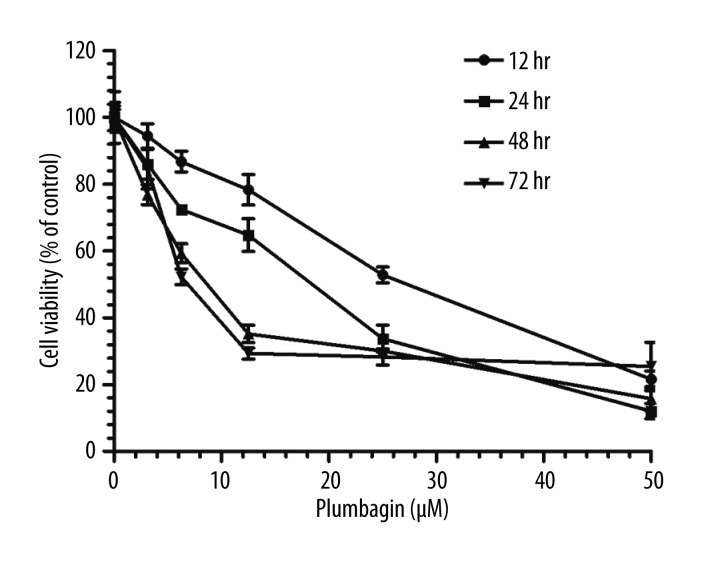
Earlier studies have indicated that plumbagin exerted extensive anticancer activities on cancer cells [18,20,21]. To explore the effects of plumbagin on Tca8113 cell viability, we treated the cells with plumbagin at various concentrations. As shown in Figure 1, the MTT colorimetric assay indicated that plumbagin inhibited the viability of Tca8113 cells in a concentration- and time-dependent manner. The IC50 values of plumbagin in Tca8113 cells for 12, 24, 48 and 72 hr incubation times were 37.53, 22.98, 3.89 and 5.62 μM, respectively.
Figure 1.
Concentration- and time-dependent inhibitory effect of plumbagin on Tca8113 cells. Data are the mean ±SD of six independent experiments.
Plumbagin suppressed the proliferation of Tca8113 cells
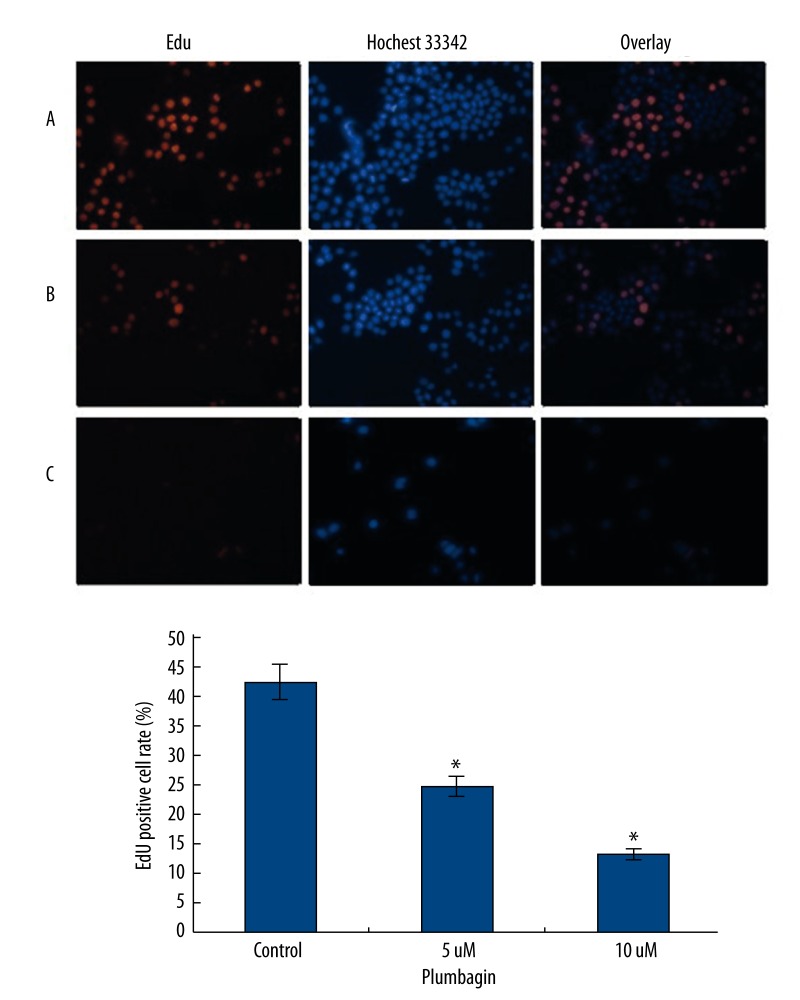
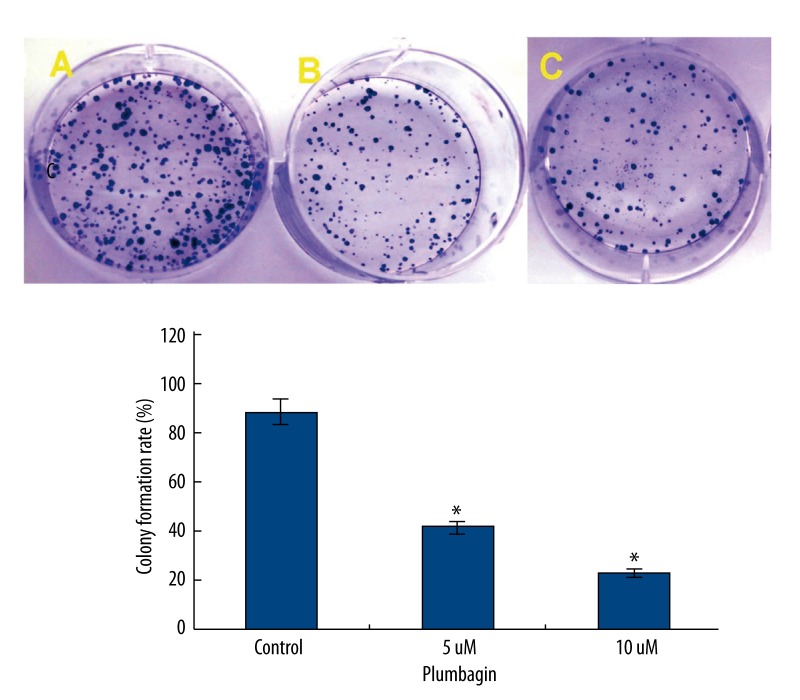
To determine whether plumbagin had inhibitory effects on the growth of Tca8113 cells, we first tested the rates of cell proliferation by EdU fluorescence staining, which directly measured active DNA synthesis or S-phase synthesis of the cell cycle [33,34]. An EdU incorporation assay indicated that the mean percentage of positive cells in the treated groups were significantly lower than that in the control group (P<0.05) (Figure 2). The use of a colony formation assay further demonstrated that the treatment of plumbagin resulted in decreased cell proliferation of Tca8113 cells (Figure 3). These results clearly indicated that plumbagin suppressed the in vitro growth of cultured Tca8113 cells.
Figure 2.
Measurement of anti-proliferation effects of plumbagin by EdU incorporation assay. Tca8113cells were incubated in the medium alone or with the medium containing plumbagin for 24 hr. Before stopping the cell culture, the cells were exposed to EdU for 2 hr and stained as described in the Materials and Methods. Red fluorescence represents the EdU-positive cells; and blue fluorescence from the Hoechst stain represents the total cells (magnification: ×400). A) the medium alone, the mean percentage of positive cells is 42.18%; B) treatment with 5 μM plumbagin, the mean percentage of positive cells is 24.63%; and C) treatment with 10 μM plumbagin, the mean percentage of positive cells is 13.38%. The positive cell rate showed a significant decrease compared to the control cells (* P<0.05). Triplicate experiments were performed.
Figure 3.
Plumbagin suppresses the colony formation of Tca8113 cells. Tca8113cells were incubated in the medium alone or the medium containing plumbagin for 24 hr, and the medium was replaced with fresh medium and incubated for 14 days. (A) the medium alone; (B) 5 μM plumbagin; and (C) 10 μM plumbagin. Colony formation rate was significantly decreased compared to the control cells (* P<0.05). Triplicate experiments were performed.
Plumbagin induced G2/M arrest in Tca8113 cells
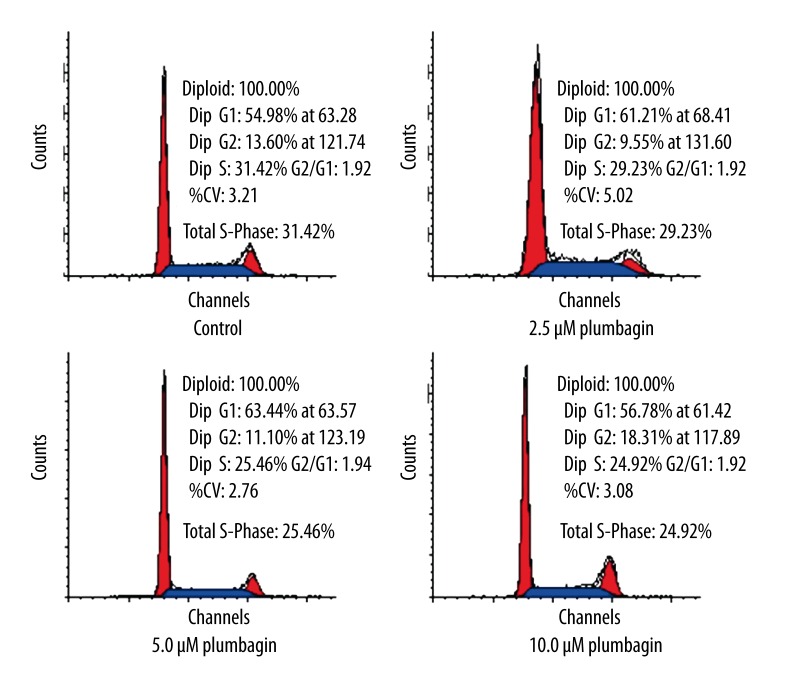
To investigate whether the growth inhibitory effect of plumbagin was related to cell cycle arrest or an apoptotic process in Tca8113 cells, we analyzed the cell cycle using flow cytometric analysis. The results showed that plumbagin induced G2/M arrest at a concentration of 10 μM for 24 hr in addition to a significant decrease in S phase populations. However, the percentage of G2/G1 fraction did not differ significantly between the control and plumbagin-treated Tca8113 cells (Figure 4).
Figure 4.
Cell cycle distribution in Tca8113 cells treated with plumbagin for 24 hr. Plumbagin dose-dependently induced G2/M arrest and a significant decrease in S phase populations. The percentages of G2- and S-phases cells were notably different between the control and plumbagin-treated Tca8113cells (P<0.05). Triplicate experiments were performed.
Plumbagin induced apoptosis of Tca8113 cells
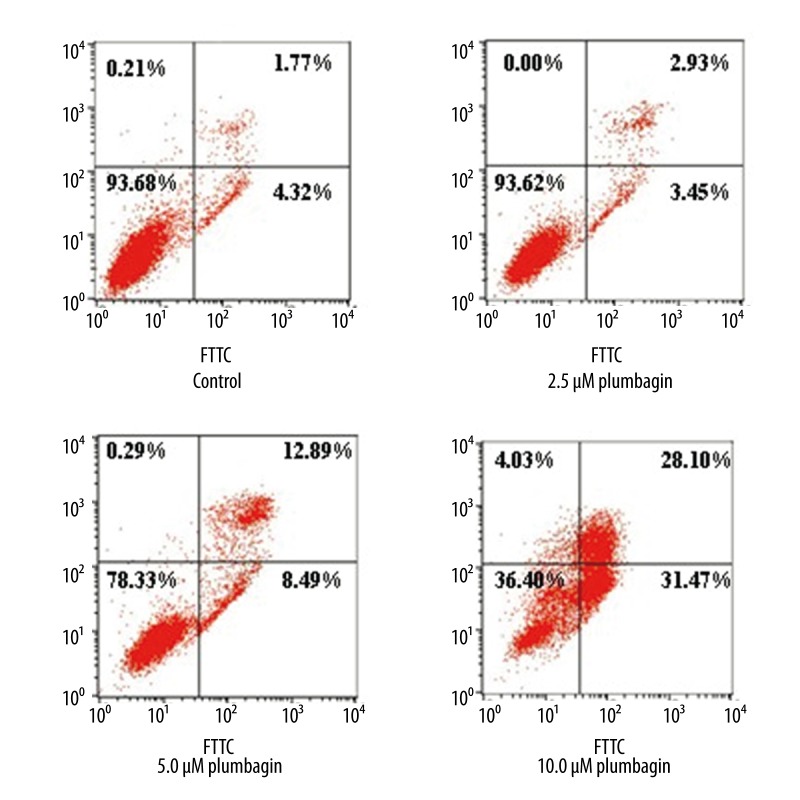
Previous studies demonstrated that inducing apoptosis was one of the mechanisms of antitumor effects of plumbagin [14,20,35]. Consistent with previous studies, we also observed that plumbagin induced apoptosis in cultured human tongue carcinoma cells. The number of apoptotic cells induced by plumbagin was measured by staining with annexin V-FITC/PI and flow cytometry. Plumbagin exerted strong apoptosis-inducing effects on Tca8113 cells at 5.0 and 10 μM when incubated for 24 hr, with an apoptotic rate of 8.49% and 31.47%, respectively. The cellular necrotic rates of Tca8113 cells induced by plumbagin were 12.89% and 28.10%, respectively. The cellular apoptotic rates and necrotic rates did not differ significantly between the control and the 2.5 μM plumbagin-treated Tca8113cells (Figure 5). These findings indicated that apoptosis was a part of the plumbagin-mediated anticancer effects on cancer cells.
Figure 5.
Cell apoptosis in Tca8113 cells treated with plumbagin for 24 hr. Plumbagin induced apoptosis in cultured Tca8113 cells in a concentration-dependent manner. Apoptosis was significantly increased in plumbagin-treated Tca8113 cells compared to the control cells (P<0.05). The cellular apoptotic rates and necrotic rates did not differ significantly between the control and cells treated with 2.5 μM plumbagin (P>0.05). Triplicate experiments were performed.
To determine the characteristics of plumbagin-induced Tca8113 cell death, morphologic changes were examined using phase-contrast microscopy. The Tca8113 cells exposure to plumbagin (5 and 10 μM) for 24 hr resulted in morphologic alterations characteristic of apoptosis, including membrane blebbing, nuclear condensation, and fragmentation (Figure 6).
Figure 6.
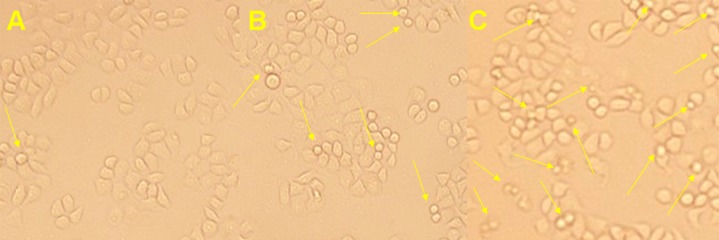
Plumbagin-induced apoptotic morphological changes in Tca8113 cells. Tca8113 cells were incubated in the medium alone or with the medium containing plumbagin for 24 hr. (A) the medium alone; (B) 5 μM plumbagin; and (C) 10 μM plumbagin. Arrows indicate multiblebbing cells and apoptotic bodies (Magnification: × 400).
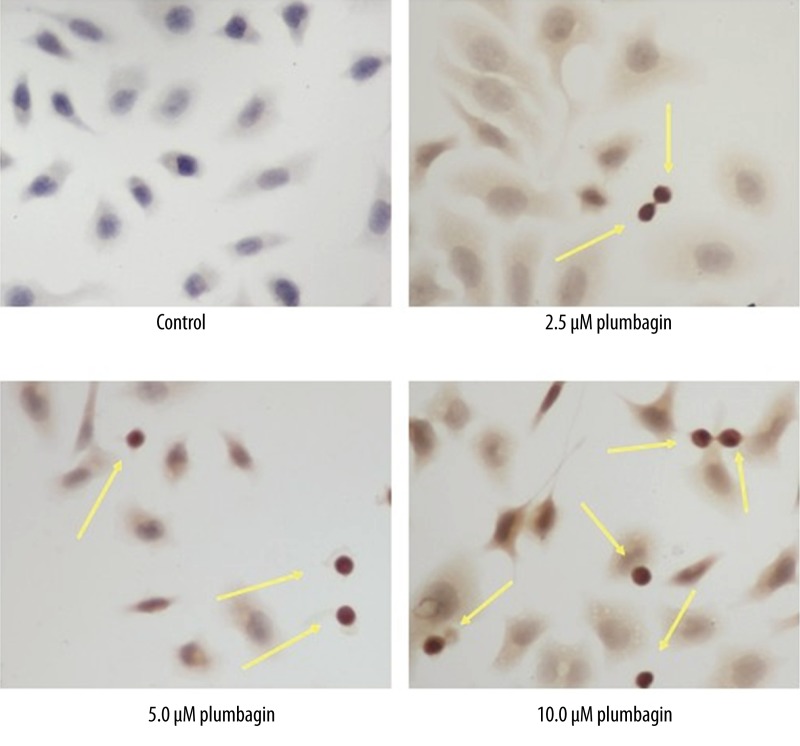
Plumbagin-induced apoptosis was further confirmed by conducting a TUNEL assay. The TUNEL assay is a simple and sensitive technique that requires a smaller number of cells for detecting DNA damage while simultaneously allowing in situ detection of DNA fragmentation. The cells treated with the plumbagin showed clear nuclear condensation and also incorporated the labeled nucleotide into the DNA. The control cells without plumbagin did not show a positive TUNEL reaction. This result indicated that plumbagin was effective in inducing DNA fragmentation in Tca8113 cells (Figure 7).
Figure 7.
Plumbagin-induced apoptotic morphological changes in Tca8113 cells. Tca8113 cells were incubated in the medium alone or medium containing plumbagin for 24 hr. Arrows indicate fragmented nuclei and nuclei-shrunk characteristic changes of apoptosis (magnification: ×400). The cellular apoptotic rates of four groups were 4.05%, 4.68%, 8.74% and 34.24%, respectively. Apoptosis in Tca8113 cells treated with 5.0 μM or 10 μM plumbagin was significant increased compared to the control cells (P<0.05). Triplicate experiments were performed.
Plumbagin increased Bax/Bcl-2 ratio in Tca8113 cells
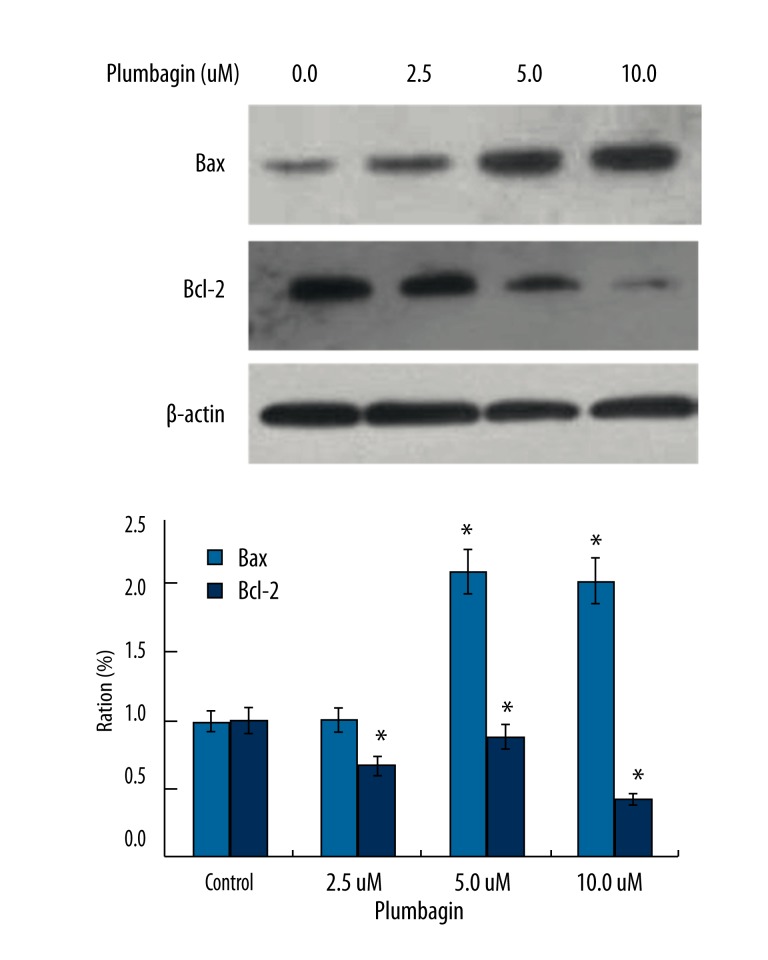
To investigate the anticancer mechanism of plumbagin, we examined the expression of the proapoptotic protein Bax and anti-apoptotic protein Bcl-2, two typical proteins involved in the cell apoptosis process. Western blotting indicated that plumbagin treatment resulted in a significant increase of Bax expression and a dramatical decrease of Bcl-2 expression in the Tca8113 cells was found which led to an increase in the Bax/Bcl-2 ratio (Figure 8). The results further confirmed that the anticancer property of plumbagin on human tongue carcinoma might be due to not only cell cycle arrest but also induction of cellular apoptosis.
Figure 8.
Effect of plumbagin on the expression of Bax and Bcl-2 proteins in Tca8113 cells. Tca8113 cells were incubated in the medium alone or the medium containing plumbagin for 24 hr. A) the medium alone; B) 2.5 μM plumbagin; C) 5 μM plumbagin; and D) 10 μM plumbagin. Bax was dramatically increased while Bcl-2 was significant decreased compared to the control cells (* P<0.05). Triplicate experiments were performed.
Discussion
In this study, we investigated the anticancer effect of plumbagin in human tongue carcinoma cells and we demonstrated that plumbagin directly inhibited the proliferation of Tca8113 cells by inducing the cancer cells to undergo S-G2/M phase arrest and apoptosis. It is possible that plumbagin may trigger the mitochondrial apoptotic pathway indicated by a change in Bax/Bcl-2 ratios. OSCC is the sixth most frequent solid cancer worldwide and tongue carcinoma is the most common type of OSCC [5]. Because of unsatisfactory outcomes associated with the treatment of advanced tongue carcinoma, there is an urgent need to intensify our efforts to identify novel agents that could delay or prevent the development of tongue carcinoma.
Some natural naphthoquinones have been reported to possess apoptosis-inducing activities in a variety of human cancer cell lines and animal models [17,36,37]. Plumbagin is a major active component of the herb P. zeylanica L, which has been safely used for centuries in China as well as other Asian countries to treat a variety of ailments [15,16,38]. This natural naphthoquinone is a potent pharmaceutical substance that exerts multiple biological and pharmacological activities such as anti-leishmanial, antimicrobial, antiviral, antiatherosclerotic, and antitumor properties [18,20,39–42]. However, no study has been reported about the effects of plumbagin in the prevention and/or treatment of human tongue carcinoma. This is the first report that suggests that plumbagin could suppress the proliferation and induce apoptosis of human tongue carcinoma Tca8113 cells. Data presented here indicated that 5 and 10 μM plumbagin were able to inhibit cell proliferation and induce apoptosis in cancer cells. The range of concentrations used was consistent with many other studies that investigated the antitumour effect of plumbagin in cultured cancer cells [13,35,43].
Detection of DNA synthesis in proliferating cells relies on the incorporation of labeled DNA precursors into cellular DNA during the S phase of the cell cycle [33]. EdU is a thymidine analogue in which a terminal alkyne group replaces the methyl group in the 5 position that is subsequently incorporated into cellular DNA during DNA replication. The terminal alkyne group is then detected through its reaction with fluorescent azides, in a Cu(I)-catalyzed [3 + 2] cycloaddition (“click” chemistry) [33,34,44]. The EdU incorporation assay and the clonogenic assay showed that plumbagin induced a concentration-dependent inhibitory effect on Tca8113 cells. This effect was confirmed by Hochest 33258 and AO/EB fluorescent staining. In agreement with other studies, we demonstrated that plumbagin inhibited cell growth and induced apoptosis in Tca8113 cells in a concentration- and time-dependent manner.
To better understand the mechanism of plumbagin-mediated cell proliferation inhibition, we investigated cell cycle distribution using flow cytometric analysis. Treatment with plumbagin resulted in a significant growth arrest in the G2/M phase of Tca8113 cells. The G2/M arrest observed in this report is similar to that observed in response to plumbagin treatment in breast cancer, lung cancer, and melanoma cells [35,38]. In contrast, Powolny et al. [14] reported that the percentage of G2/M fraction did not differ significantly between the DMSO-treated control and the plumbagin-treated prostate cancer cells. The percentage of G2/G1 fraction did not differ significantly between control and plumbagin-treated Tca8113cells.
To confirm whether the cytotoxic effects induced by plumbagin in Tca8113 cells involved apoptotic changes, cells were examined for characteristic apoptotic patterns using light microscopy and TUNEL assay. Apoptosis is characterized by a set of morphologic changes including chromatin condensation, nuclear fragmentation, membrane blebbing and cell shrinkage [27]. The induction of the apoptotic cell death by plumbagin was supported by typical morphological and molecular hallmarks including chromatin fragmentation and phosphatidylserine exposure. Apoptotic cells that were labeled with annexin V-FITC and excluding PI were then quantified by flow cytometry. The results involving the induction of apoptosis in Tca8113 cells by plumbagin are consistent with previous findings using other cancer cell lines such as human melanoma cells, cervical cancer cells, and breast cancer cells [45,46]. Bcl-2 family members are apoptosis regulatory proteins which are strongly correlated with the oncogenesis and progression of OSCC [28,47,48]. The Bcl-2 family proteins are known to regulate apoptosis by changing its relative levels. Bcl-2, a major anti-apoptotic protein, can form a heterodimer complex with the proapoptotic member Bax, thereby neutralizing its proapoptotic effects. Therefore, the ratio of Bax/Bcl-2 is a decisive factor and plays an important role in determining whether cells will undergo death or survival [25,43]. The present study demonstrated that plumbagin treatment could lead to an increase in the Bax/Bcl-2 ratio. This change in proapoptotic/antiapoptotic protein ratio might contribute to the apoptosis-promoting activity of plumbagin.
Conclusions
In summary, our data suggest that plumbagin is a potential anticancer agent against human tongue carcinoma with anti-proliferative potential and proapoptotic effects. Plumbagin-induced apoptosis is associated with change in the Bax/Bcl-2 ratio. Further mechanistic studies in vitro and in vivo will be required to assess the potential of plumbagin in prevention and treatment of tongue carcinoma.
Footnotes
Source of support: This work was funded by the Natural Science Foundation of Jiangxi Province, Nanchang, China (2010GZY0302)
References
- 1.Zhou ZT, Yang Y, Ge JP. The preventive effect of salvianolic acid B on malignant transformation of DMBA-induced oral premalignant lesion in hamsters. Carcinogenesis. 2006;27(4):826–32. doi: 10.1093/carcin/bgi271. [DOI] [PubMed] [Google Scholar]
- 2.Mackenzie J, Ah-See K, Thakker N, et al. Increasing incidence of oral cancer amongst young persons: what is the aetiology? Oral Oncol. 2000;36(4):387–89. doi: 10.1016/s1368-8375(00)00009-9. [DOI] [PubMed] [Google Scholar]
- 3.Su C-C, Lin Y-Y, Chang T-K, et al. Incidence of oral cancer in relation to nickel and arsenic concentrations in farm soils of patients’ residential areas in Taiwan. BMC Public Health. 2010;10(1):67. doi: 10.1186/1471-2458-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyamoto S, Yasui Y, Kim M, et al. A novel rasH2 mouse carcinogenesis model that is highly susceptible to 4-NQO-induced tongue and esophageal carcinogenesis is useful for preclinical chemoprevention studies. Carcinogenesis. 2008;29(2):418–26. doi: 10.1093/carcin/bgm225. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Huang H, Sun L, et al. MiR-21 indicates poor prognosis in tongue squamous cell carcinomas as an apoptosis inhibitor. Clin Cancer Res. 2009;15(12):3998–4008. doi: 10.1158/1078-0432.CCR-08-3053. [DOI] [PubMed] [Google Scholar]
- 6.Sano D, Choi S, Milas ZL, et al. The effect of combination anti-endothelial growth factor receptor and anti-vascular endothelial growth factor receptor 2 targeted therapy on lymph node metastasis: a study in an orthotopic nude mouse model of squamous cell carcinoma of the oral tongue. Arch Otolaryngol Head Neck Surg. 2009;135(4):411–20. doi: 10.1001/archoto.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khandelwal S, Solomon MC. Cytomorphological analysis of keratinocytes in oral smears from tobacco users and oral squamous cell carcinoma lesions – a histochemical approach. Int J Oral Sci. 2010;2(1):45–52. doi: 10.4248/IJOS10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun L, Diamond ME, Ottaviano AJ, et al. Transforming growth factor-β 1 promotes matrix metalloproteinase-9-mediated oral cancer invasion through snail expression. Mol Cancer Res. 2008;6(1):10–20. doi: 10.1158/1541-7786.MCR-07-0208. [DOI] [PubMed] [Google Scholar]
- 9.Nagler R, Bahar G, Shpitzer T, Feinmesser R. Concomitant analysis of salivary tumor markers − a new diagnostic tool for oral cancer. Clin Cancer Res. 2006;12(13):3979–84. doi: 10.1158/1078-0432.CCR-05-2412. [DOI] [PubMed] [Google Scholar]
- 10.Xia M, Wang M, Tashiro S, et al. Dracorhodin perchlorate induces A375-S2 cell apoptosis via accumulation of p53 and activation of caspases. Biol Pharm Bull. 2005;28(2):226–32. doi: 10.1248/bpb.28.226. [DOI] [PubMed] [Google Scholar]
- 11.Ma Z, Otsuyama K-i, Liu S, et al. Baicalein, a component of Scutellaria radix from Huang-Lian-Jie-Du-Tang (HLJDT), leads to suppression of proliferation and induction of apoptosis in human myeloma cells. Blood. 2005;105(8):3312–18. doi: 10.1182/blood-2004-10-3915. [DOI] [PubMed] [Google Scholar]
- 12.Lee CS, Kim YJ, Lee MS, et al. 18β-Glycyrrhetinic acid induces apoptotic cell death in SiHa cells and exhibits a synergistic effect against antibiotic anti-cancer drug toxicity. Life Sci. 2008;83(13–14):481–89. doi: 10.1016/j.lfs.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Aziz MH, Dreckschmidt NE, Verma AK. Plumbagin, a medicinal plant-derived naphthoquinone, is a novel inhibitor of the growth and invasion of hormone-refractory prostate cancer. Cancer Res. 2008;68(21):9024–32. doi: 10.1158/0008-5472.CAN-08-2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powolny AA, Singh SV. Plumbagin-induced apoptosis in human prostate cancer cells is associated with modulation of cellular redox status and generation of reactive oxygen species. Pharm Res. 2008;25(9):2171–80. doi: 10.1007/s11095-008-9533-3. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh YJ, Lin LC, Tsai TH. Measurement and pharmacokinetic study of plumbagin in a conscious freely moving rat using liquid chromatography/tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;844(1):1–5. doi: 10.1016/j.jchromb.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 16.Chen ZF, Tan MX, Liu LM, et al. Cytotoxicity of the traditional Chinese medicine (TCM) plumbagin in its copper chemistry. Dalton Trans. 2009;(48):10824–33. doi: 10.1039/b910133k. [DOI] [PubMed] [Google Scholar]
- 17.Kanaan YM, Das JR, Bakare O, et al. Biological evaluation of 2,3-dichloro-5,8-dimethoxy-1,4-naphthoquinone as an anti-breast cancer agent. Anticancer Res. 2009;29(1):191–99. [PubMed] [Google Scholar]
- 18.Shieh JM, Chiang TA, Chang WT, et al. Plumbagin inhibits TPA-induced MMP-2 and u-PA expressions by reducing binding activities of NF-κB and AP-1 via ERK signaling pathway in A549 human lung cancer cells. Mol Cell Biochem. 2010;335(1–2):181–93. doi: 10.1007/s11010-009-0254-7. [DOI] [PubMed] [Google Scholar]
- 19.Chen CA, Chang HH, Kao CY, et al. Plumbagin, isolated from Plumbago zeylanica, induces cell death through apoptosis in human pancreatic cancer cells. Pancreatology. 2010;9(6):797–809. doi: 10.1159/000210028. [DOI] [PubMed] [Google Scholar]
- 20.Xu KH, Lu DP. Plumbagin induces ROS-mediated apoptosis in human promyelocytic leukemia cells in vivo. Leuk Res. 2010;34(5):658–65. doi: 10.1016/j.leukres.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 21.Shih YW, Lee YC, Wu PF, et al. Plumbagin inhibits invasion and migration of liver cancer HepG2 cells by decreasing productions of matrix metalloproteinase-2 and urokinase- plasminogen activator. Hepatol Res. 2009;39(10):998–1009. doi: 10.1111/j.1872-034X.2009.00540.x. [DOI] [PubMed] [Google Scholar]
- 22.Sugie S, Okamoto K, Rahman KM, et al. Inhibitory effects of plumbagin and juglone on azoxymethane-induced intestinal carcinogenesis in rats. Cancer Lett. 1998;127(1–2):177–83. doi: 10.1016/s0304-3835(98)00035-4. [DOI] [PubMed] [Google Scholar]
- 23.Prasad VS, Devi PU, Rao BS, Kamath R. Radiosensitizing effect of plumbagin on mouse melanoma cells grown in vitro. Indian J Exp Biol. 1996;34(9):857–58. [PubMed] [Google Scholar]
- 24.Devi PU, Rao BS, Solomon FE. Effect of plumbagin on the radiation induced cytogenetic and cell cycle changes in mouse Ehrlich ascites carcinoma in vivo. Indian J Exp Biol. 1998;36(9):891–95. [PubMed] [Google Scholar]
- 25.Nair S, Nair RR, Srinivas P, et al. Radiosensitizing effects of plumbagin in cervical cancer cells is through modulation of apoptotic pathway. Mol Carcinog. 2008;47(1):22–33. doi: 10.1002/mc.20359. [DOI] [PubMed] [Google Scholar]
- 26.Gopalakrishnan A, Tony Kong AN. Anticarcinogenesis by dietary phytochemicals: cytoprotection by Nrf2 in normal cells and cytotoxicity by modulation of transcription factors NF-κB and AP-1 in abnormal cancer cells. Food Chem Toxicol. 2008;46(4):1257–70. doi: 10.1016/j.fct.2007.09.082. [DOI] [PubMed] [Google Scholar]
- 27.Khan N, Afaq F, Mukhtar H. Apoptosis by dietary factors: the suicide solution for delaying cancer growth. Carcinogenesis. 2007;28(2):233–39. doi: 10.1093/carcin/bgl243. [DOI] [PubMed] [Google Scholar]
- 28.Popovic B, Jekic B, Novakovic I, et al. Bcl-2 expression in oral squamous cell carcinoma. Ann N Y Acad Sci. 2007;1095:19–25. doi: 10.1196/annals.1397.003. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Hao H, He S, et al. Lipoxin A4 and its analogue suppress the tumor growth of transplanted H22 in mice: the role of antiangiogenesis. Mol Cancer Ther. 2010;9(8):2164–74. doi: 10.1158/1535-7163.MCT-10-0173. [DOI] [PubMed] [Google Scholar]
- 30.Wang R, Fang X, Lu Y, Wang S. The PDBbind database: collection of binding affinities for protein-ligand complexes with known three-dimensional structures. J Med Chem. 2004;47(12):2977–80. doi: 10.1021/jm030580l. [DOI] [PubMed] [Google Scholar]
- 31.Yang L, Luo H, Chen J, et al. SePreSA: a server for the prediction of populations susceptible to serious adverse drug reactions implementing the methodology of a chemical-protein interactome. Nucleic Acids Res. 2009;37(Web Server issue):W406–12. doi: 10.1093/nar/gkp312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L, Chen J, Shi L, et al. Identifying unexpected therapeutic targets via chemical-protein interactome. PLoS One. 2010;5(3):e9568. doi: 10.1371/journal.pone.0009568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci USA. 2008;105(7):2415–20. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu Y, Arora A, Min W, et al. EdU incorporation is an alternative non-radioactive assay to 3H-thymidine uptake for in vitro measurement of mice T-cell proliferations. J Immunol Methods. 2009;350(1–2):29–35. doi: 10.1016/j.jim.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Wang CC, Chiang YM, Sung SC, et al. Plumbagin induces cell cycle arrest and apoptosis through reactive oxygen species/c-Jun N-terminal kinase pathways in human melanoma A375.S2 cells. Cancer Lett. 2008;259(1):82–98. doi: 10.1016/j.canlet.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Hernandez-Munoz LS, Gomez M, Gonzalez FJ, et al. Towards a molecular-level understanding of the reactivity differences for radical anions of juglone and plumbagin: an electrochemical and spectroelectrochemical approach. Org Biomol Chem. 2009;7(9):1896–903. doi: 10.1039/b822684a. [DOI] [PubMed] [Google Scholar]
- 37.Sethi G, Ahn KS, Aggarwal BB. Targeting nuclear factor-κB activation pathway by thymoquinone: role in suppression of antiapoptotic gene products and enhancement of apoptosis. Mol Cancer Res. 2008;6(6):1059–70. doi: 10.1158/1541-7786.MCR-07-2088. [DOI] [PubMed] [Google Scholar]
- 38.Kuo PL, Hsu YL, Cho CY. Plumbagin induces G2-M arrest and autophagy by inhibiting the AKT/mammalian target of rapamycin pathway in breast cancer cells. Mol Cancer Ther. 2006;5(12):3209–21. doi: 10.1158/1535-7163.MCT-06-0478. [DOI] [PubMed] [Google Scholar]
- 39.Dzoyem JP, Tangmouo JG, Lontsi D, et al. In vitro antifungal activity of extract and plumbagin from the stem bark of Diospyros crassiflora Hiern (Ebenaceae) Phytother Res. 2007;21(7):671–74. doi: 10.1002/ptr.2140. [DOI] [PubMed] [Google Scholar]
- 40.Kuete V, Tangmouo JG, Meyer JJ, Lall N. Diospyrone, crassiflorone and plumbagin: three antimycobacterial and antigonorrhoeal naphthoquinones from two Diospyros spp. Int J Antimicrob Agents. 2009;34(4):322–25. doi: 10.1016/j.ijantimicag.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Chan-Bacab MJ, Pena-Rodriguez LM. Plant natural products with leishmanicidal activity. Nat Prod Rep. 2001;18(6):674–88. doi: 10.1039/b100455g. [DOI] [PubMed] [Google Scholar]
- 42.Sharma I, Gusain D, Dixit VP. Hypolipidaemic and antiatherosclerotic effects of plumbagin in rabbits. Indian J Physiol Pharmacol. 1991;35(1):10–14. [PubMed] [Google Scholar]
- 43.Ahmad A, Banerjee S, Wang Z, et al. Plumbagin-induced apoptosis of human breast cancer cells is mediated by inactivation of NF-κB and Bcl-2. J Cell Biochem. 2008;105(6):1461–71. doi: 10.1002/jcb.21966. [DOI] [PubMed] [Google Scholar]
- 44.Limsirichaikul S, Niimi A, Fawcett H, et al. A rapid non-radioactive technique for measurement of repair synthesis in primary human fibroblasts by incorporation of ethynyl deoxyuridine (EdU) Nucleic Acids Res. 2009;37(4):e31. doi: 10.1093/nar/gkp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia M, Wang D, Wang M, et al. Dracorhodin perchlorate induces apoptosis via activation of caspases and generation of reactive oxygen species. J Pharmacol Sci. 2004;95(2):273–83. doi: 10.1254/jphs.fpj03102x. [DOI] [PubMed] [Google Scholar]
- 46.Xia MY, Wang MW, Cui Z, et al. Dracorhodin perchlorate induces apoptosis in HL-60 cells. J Asian Nat Prod Res. 2006;8(4):335–43. doi: 10.1080/10286020500035300. [DOI] [PubMed] [Google Scholar]
- 47.Camisasca DR, Honorato J, Bernardo V, et al. Expression of Bcl-2 family proteins and associated clinicopathologic factors predict survival outcome in patients with oral squamous cell carcinoma. Oral Oncol. 2009;45(3):225–33. doi: 10.1016/j.oraloncology.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 48.Ribeiro DA, Salvadori DM, Marques ME. Abnormal expression of bcl-2 and bax in rat tongue mucosa during the development of squamous cell carcinoma induced by 4-nitroquinoline 1-oxide. Int J Exp Pathol. 2005;86(6):375–81. doi: 10.1111/j.0959-9673.2005.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]