Abstract
Background
A risk factor assessment that reliably predicts whether patients are predisposed to intracranial aneurysm (IA) rupture has yet to be formulated. As such, the clinical management of unruptured IA remains unclear. Our aim was to determine whether impaired arterial distensibility and hypertrophic remodeling might be indicators of risk for IA rupture.
Material/Methods
The study population (n=49) was selected from consecutive admissions for either unruptured IA (n=23) or ruptured IA (n=26) from January to December 2010. Hemodynamic measures were taken from every patient, including systolic and diastolic blood pressure using a sphygmomanometer. Unruptured IA and ruptured IA characteristics, including aneurysmal shape, size, angle, aspect ratio, and bottleneck factor, were measured and calculated from transverse brain CT angiography images. With ultrasound, the right common carotid artery intima-media thickness was measured, as well as the lumen diameter during systole and diastole. Arterial wall strain, distensibility, stiffness index, and elastic modulus were calculated and compared between patients with unruptured IAs and ruptured IAs. A p-value less than 0.05 was considered statistically significant.
Results
General demographic data did not differ between patients with unruptured IAs and ruptured IAs. Greater mean intima-media thickness (p=0.013), mean stiffness index (p=0.044), and mean elastic modulus (p=0.026) were observed for patients with ruptured IAs. Moreover, mean strain (p=0.013) and mean distensibility (p=0.024) were decreased in patients with ruptured IAs.
Conclusions
Patients with ruptured IAs demonstrated decreased arterial distensibility and increased intima-media thickness at the level of the carotid arteries. By measuring these parameters via ultrasound, it may be possible to predict whether patients with existing IAs might rupture and hemorrhage into the subarachnoid space.
Keywords: ruptured, intracranial aneurysm, predisposing factors, elasticity, carotid intima-media thickness, ultrasound
Background
Population-based and autopsy studies report that the incidence of intracranial aneurysms (IAs) may be as high as 10% and peaks in the sixth decade of life. Females with a family history of IA are especially at risk [1–3]. Subarachnoid hemorrhages (SAHs) due to ruptured IAs account for 10% and 40% of deaths before hospitalization and after a 1-month hospital stay, respectively [1,4]. More than 35% of patients with SAH develop major neurological deficits preceding hospital discharge, even if they demonstrated favorable Glasgow Coma Scores [5–8]. Although it is easier to detect unruptured IAs with various imaging modalities, it is difficult to predict if or when they will rupture, which poses a dilemma for both patients and physicians [9–11].
Acquired and hereditary risk factors contribute to the multifactorial etiology of unruptured IA, including sex, hypertension, atherosclerosis, alcohol consumption, and smoking [12]. Parameters utilized for rupture assessment include aneurysm size, shape, and location, in addition to angle and flow hemodynamics [13–18]. Cumulative arterial wall deterioration as a result of constant remodeling characterized by degeneration and inflammatory cell infiltration lead to IA rupture and subsequent SAH [19,20]. Altered arterial wall elastic properties and hypertrophic remodeling might predispose IAs to rupture [21–23]. Arterial wall elasticity and intima-media thickness can be estimated non-invasively via ultrasound to indirectly evaluate arterial wall strength [24–26]. We hypothesized that hypertrophic intimal remodeling and impaired elastic properties detected in the right carotid artery might co-occur with IA rupture and thus may predict an impending IA rupture.
Material and Methods
A total of 49 patients consecutively admitted with a diagnosis of IA (n=23) or ruptured IA (n=26) between January and December 2010 were included in this study. Study subjects were screened and removed from the study if they qualified for the following exclusion criteria: history of stroke, heart failure, severe heart valve disease, renal dysfunction, diabetes mellitus (DM), and local or systemic acute infection. The study protocol was designed in accordance with the Helsinki Declaration and was approved by the institutional ethics committee. Written informed consent was obtained from all participants.
Blood pressure measurement
Maximum blood pressure (BPmax) was the systolic BP and minimum blood pressure (BPmin) was the diastolic BP. Blood pressure was measured from the right brachial artery with a sphygmomanometer (Omron HEM 705CP, Colson) after a 10-minute resting period. Heart rate and BP were measured just before ultrasound examination, and within the first 3 days following IA rupture to avoid falsely elevated BPs from SAH-induced vasospasm.
Ultrasound examination
Ultrasound examinations were performed for patients with ruptured IAs within the first 3 days of rupture to avoid SAH induced vasospasm and following DSA. Specifically, the Aplio XG scanner equipped with a 10 MHz linear array transducer (Toshiba Medical Systems, Tokyo, Japan) was used. A pulse repetition frequency of 3 kHz with an automatic cutoff filter ranging from 1 to 3 kHz was utilized. M-mode ultrasound was performed at a speed of 50 mm/sec.
The right common carotid artery (CCA) was examined while the patient assumed the supine position with slight head elevation. The transducer was positioned parallel to the CCA such that the lumen’s diameter was maximized in the longitudinal plane. Maximum (Dmax) and minimum (Dmin) internal lumen diameters were measured at 1 to 2 cm proximal to the CCA bifurcation in magnified M-mode during systole and diastole. Intima-media thickness measurements were taken at this same location, but were derived in B-mode.
Elastic properties
Arterial elastic properties, including distensibility, strain, stiffness index, and elastic modulus, were measured to determine the stress on the right CCA wall during diastole and systole [27,28]. Strain was defined as the percent change in CCA artery lumen diameter during systole and diastole. The following calculations were performed to determine the aforementioned measures:
Statistics
Statistical Package for the Social Sciences for Windows (SPSS ver. 18, Chicago, IL, USA) software was used to analyze all data. Descriptive parameters were expressed as the mean ± the standard deviation or via percentages. Variations between groups were compared with the Mann-Whitney U test. Correlation analyses were performed with the Spearman test. A p-value less than 0.05 was considered significant. The right CCA internal diameter was measured by an expert, blinded observer (A.D.). To evaluate inter-observer reliability, minimum and maximum CCA internal diameter and distensibility were also calculated by a second observer (M.I.). An inter-class correlation coefficient (ICC) above 0.72 was considered sufficient.
Results
A comparison between unruptured IA and ruptured IA groups in terms of demographics and aneurysm characteristics is provided in Table 1. There were no differences in age, sex, and heart rate between groups. Patients with unruptured IA had a mean age of 47.1±12.0 years, with ages that ranged from 26 to 68 years. Patients with ruptured IA had a mean age of 48.6±11.5 years, ranging from 31 to 67 years. Unruptured IA and ruptured IA characteristics included shape, size, angle, aspect ratio, and bottleneck factor. For the entire study population, unruptured IA and ruptured IA locations included the internal carotid artery (ICA) in 14% of cases, the anterior communicating artery (ACoA) in 20% of cases, the middle cerebral artery (MCA) in 41% of cases, and the vertebral and basilar arteries (V-B) in 25% of of cases (Figures 1 and 2).
Table 1.
Demographic data and aneurysm characteristic comparisons.
| Parameter | Unruptured IAs (n=23) | Ruptured IAs (n=26) | p-value | |
|---|---|---|---|---|
| Age (years) | 47.1±12.0 | 48.6±11.5 | 0.42 | |
|
| ||||
| Gender (male/female) | 9/14 | 10/16 | 0.19 | |
|
| ||||
| BMI (kg/m2) | 25.1±2.7 | 27.5±2.3 | 0.35 | |
|
| ||||
| Heart rate (/min) | 69±7 | 74±8 | 0.23 | |
|
| ||||
| IAs Location | ICA n (%) | 4 (57%) | 3 (43%) | 0.21 |
| ACoA n (%) | 3 (30%) | 7 (70%) | 0.01 | |
| MCA n (%) | 9 (45%) | 11 (55%) | 0.07 | |
| V-B n (%) | 7 (58%) | 5 (42%) | 0.02 | |
|
| ||||
| Shape | Sphere n (%) | 9 (69%) | 4 (31%) | 0.01 |
| Oval n (%) | 9 (43%) | 12 (57%) | 0.07 | |
| Lobulated n (%) | 6 (40%) | 9 (60%) | 0.04 | |
|
| ||||
| Size (mm) | Height | 4.1±2.1 | 5.2±2.3 | 0.22 |
| Width | 3.6±2.1 | 4.4±1.8 | 0.14 | |
| Neck | 2.7±1.2 | 2.4±1.1 | 0.11 | |
|
| ||||
| Angle(°) | 121±18 | 85±21 | 0.04 | |
|
| ||||
| Aspect ratio (height/Neck) | 1.2±0.2 | 1.7±0.3 | 0.02 | |
|
| ||||
| Bottleneck factor (width/Neck) | 1.1±0.2 | 1.4±0.2 | 0.03 | |
Mean ±SD; BMI – body mass index; BPmax – systolic blood pressure; BPmin – diastolic blood pressure; ΔP – pulse pressure; Dmax – systolic diameter; Dmin – diastolic diameter; IAs – intracranial aneurysms.
Figure 1.
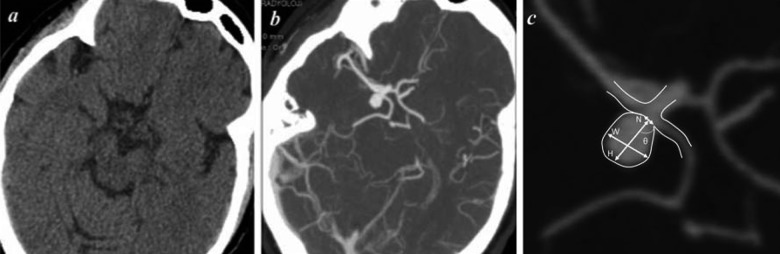
Brain CT of a 79 year-old woman with an unruptured intracranial aneurysm. (A) Transverse CT without contrast, (B) CT-angiography, (C) magnified image of aneurysm where H is size, W is dome width, N is neck width, and θ is angle. Calculations are H/N (aspect ratio) and W/N (bottleneck factor).
Figure 2.
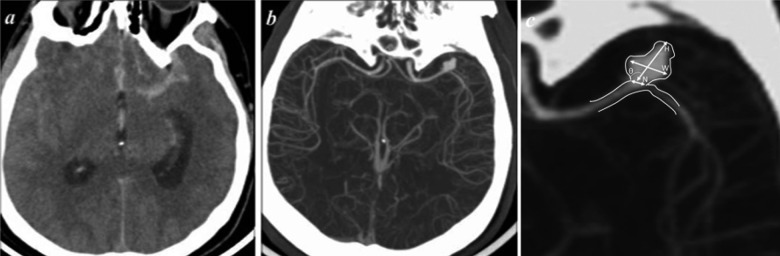
Brain CT of a 41 year-old male with a ruptured intracranial aneurysm with associated SAH. (A) Transverse CT image without contrast, (B) CT-angiography, and (C) aneurysm morphology assessment. Where H is size, W is dome width, N is neck width, and è is angle.
The unruptured IA group exhibited a decreased mean CCA intima-media thickness at 0.52±0.12 cm vs. 0.61±0.13 cm for the ruptured IA group (p=0.013). However, the unruptured IA group exhibited increased mean CCA lumen diameter change (ΔD) at 0.7±0.1 cm as compared to 0.5±0.1 cm for the ruptured IA group (p=0.04) (Figures 3 and 4). Mean CCA stiffness index was greater for the ruptured IA group at 6.0±0.5 vs. 6.7±0.5 for the IA group (p=0.044). This was also true for the mean CCA elastic modulus, as this measure was higher for the ruptured IA group at 0.9±0.3 compared to the IA group at 0.7±0.2 (p=0.026). Mean CCA strain was decreased for the ruptured IA group at 6.1±1.7 vs. 8.1±1.9 for the IA group (p=0.013). This same trend was observed for mean CCA distensibility, as the ruptured IA group mean value was lower at 1.8±0.4 than the IA group at 2.3±0.5 (p=0.024). Table 2 illustrates a comparison of all these calculated mean values between groups.
Figure 3.
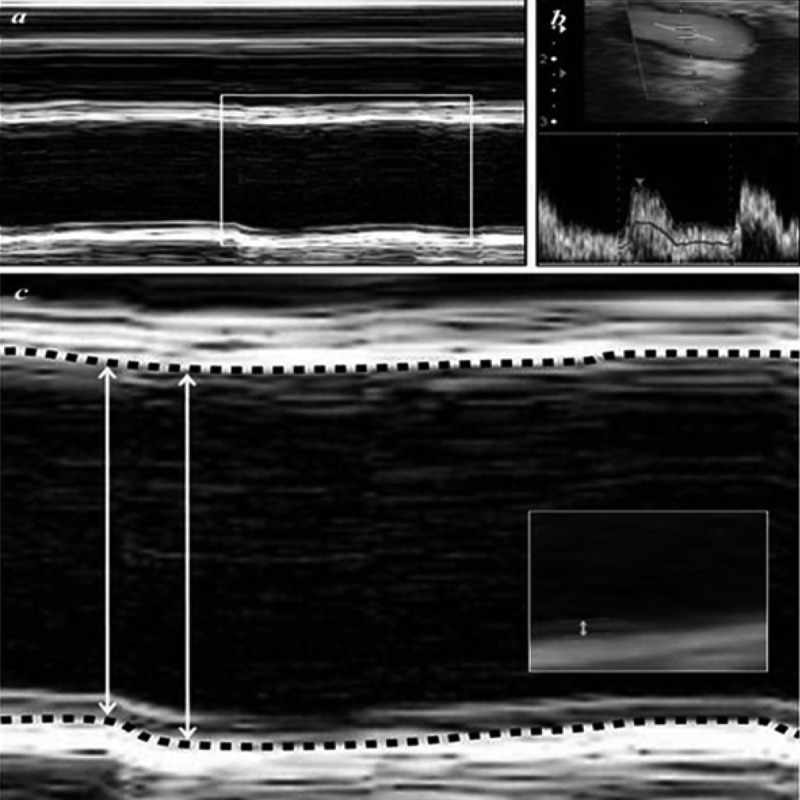
Ultrasound imaging of the right CCA for same patient as in Figure 1. (A) M-mode, (B) Doppler, and (C) ultrasound images of the CCA. The large parallel white lines are the CCA wall, which moves as the heart beats. The black area between the large parallel lines represents the CCA lumen. Lumen diameter is indicated with the double-headed arrow. The dashed lines highlight the intima-media junction, and the small double-headed arrow (see inset) indicates the intima-media thickness.
Figure 4.
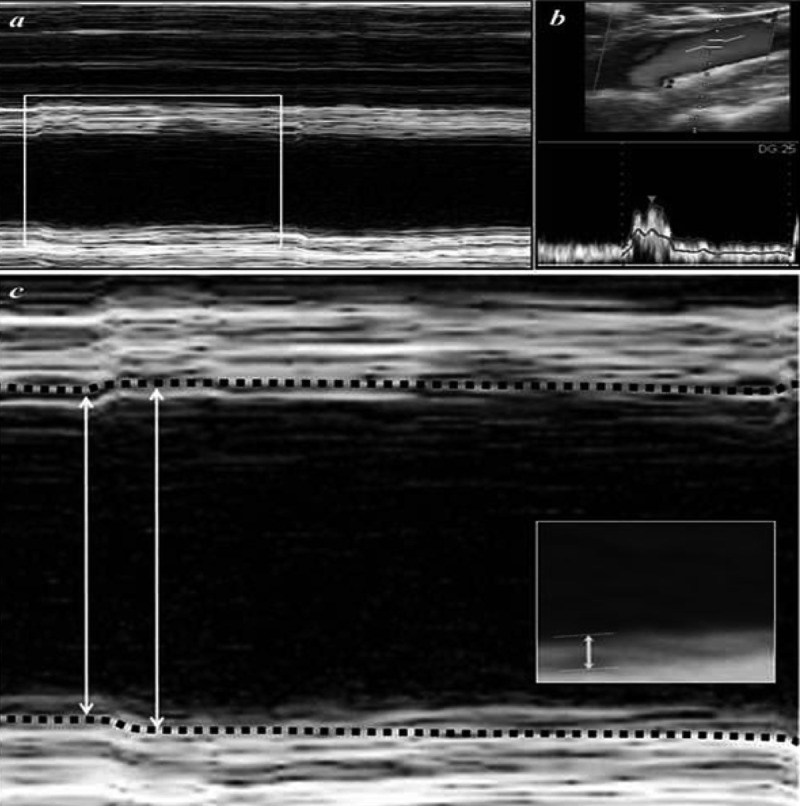
Ultrasound imaging of the right CCA from the same patient in Figure 3. (A) M-mode, (B) Doppler, and (C) ultrasound images of the CCA. Lumen diameter is indicated with the double-headed arrows. The dashed lines highlight the intima-media junction, and the small double-headed arrow (inset) indicates the intima-media thickness.
Table 2.
Right CCA elastic properties comparison.
| Unruptured IAs (n=23) | Ruptured IAs (n=26) | p-value | |
|---|---|---|---|
| IMT (mm) | 0.52±0.12 | 0.61±0.13 | 0.013 |
| Dmax (mm) | 6.9±0.4 | 7.1±0.3 | 0.14 |
| Dmin (mm) | 6.2±0.3 | 6.6±0.2 | 0.08 |
| ΔD (mm) | 0.7±0.1 | 0.5±0.1 | 0.04 |
| BPmax (mmHg) | 117±6 | 134±8 | 0.08 |
| BPmin (mmHg) | 72±5 | 83±8 | 0.09 |
| ΔP (mmHg) | 45±4 | 51±5 | 0.07 |
| Strain (%) | 8.1±1.9 | 6.1±1.7 | 0.013 |
| Distension (×10−6 cm2/dyne) | 2.3±0.5 | 1.8±0.4 | 0.024 |
| Stiffness index | 6.0±0.5 | 6.7±0.4 | 0.044 |
| Elastic modulus (×10−6 cm2/dyne) | 0.7±0.2 | 0.9±0.3 | 0.026 |
IAs – Intracranial aneurysms.
Discussion
The risk of rupture per year for patients with asymptomatic IAs has been reported at 0.05% [8]. In population-based studies, the prevalence of IAs ranged widely, from 0.2% to 10% [2,10]. As such, much research has been devoted to studying IAs and the factors that facilitate rupture. Reduced arterial elasticity was observed in patients with ruptured IAs in a recent ex vivo study [11]. The relationship between arterial wall distensibility and stress is still a matter of debate [29,30]. Patients with IA rupture exhibit decreased arterial wall distensibility compared to those with unruptured IAs [11,25]. This phenomenon is widely accepted, even though one ex vivo study asserted the opposite upon measuring arterial wall distensibility in the anterior cerebral, radial, and dorsalis pedis arteries. Moreover, arterial distensibility measurements have been commonly performed on the CCA [29,31]. In agreement with most studies, it is preferred to perform investigations on arterial wall distensibility in vivo. Therefore, we designed an in vivo study using measurements from the right CCA, such that our outcomes might aid in advancing the clinical management of patients with unruptured IA.
Numerous studies have been performed that focused on parameters predicting rupture, such as aneurysm location, size, shape, and angle [14–18]. IAs with an irregular shape and multilobular contours are associated with increased focal stress and distensibility [32,33]. Elliptical IAs are more prone to rupture than spherical IAs [34,35]. Decreased bottleneck and aspect ratios are also correlated with increased rupture risk [14,17]. However, once rupture has occurred, spherical aneurysms become more oval-shaped; thus, an aneurysm’s elliptical quality may not be distinctive of increased rupture risk [4]. A recent study based on biomechanical models of rupture reported that rupture risk is associated with increased aneurysm size [11]. The cutoff value for increased rupture risk was determined to be 10 mm; however, the vast majority of IAs reported in other studies range between 5 to 9 mm [8,36]. Because of the high prevalence of IAs less than 10 mm in diameter, it is important to use criteria other than size to determine rupture risk [33]. It has been postulated that rupture occurs in small-sized IAs if they rapidly expand [4]. However, surgical interventions are not indicated for small IAs, since the rupture risk is relatively low [10,37].
Recent studies have emphasized that the pathophysiology of IA rupture could be evaluated biophysically [7,28]. An experimental study focusing on biomechanical alterations between unruptured IAs and ruptured IAs showed that patients with greater arterial wall rigidity were more prone to IA rupture [11]. Rigidity can be determined by the elastic properties of the arterial wall tissue, and a reduction in elasticity significantly contributes to the pathobiology of IA rupture [19,25]. Rupture risk for abdominal aortic aneurysms has also been evaluated in several studies [28,38]. It has been widely reported that arterial distensibility with comorbid vasculopathy predisposes patients to abdominal aortic aneurysm formation, dissection, and rupture [39]. These studies asserted that aneurysmal rupture occurred when wall tension exceeded the strength limit of the artery wall [40]. Another study evaluating the aneurysmal expansion limit demonstrated a correlation between the extent of distensibility and rupture [41,42]. Increased arterial wall stiffness, loss of elasticity, and decreased perivascular support might be associated with vasculopathies resulting from aging, essential hypertension, diabetes mellitus, and vasculitis [23,31,43–45].
Intimal remodeling and arterial stiffness might be associated with defects in elastin, collagen, and extracellular matrix [41,46]. Specifically, defective collagen contributes to arterial wall weakness due to decreased wall distensibility, which facilitates IA rupture [11,40]. Deficiencies in elastin are also correlated to intimal hypertrophy [26,27,47]. Aneurysmal growth and rupture has been associated with TNF-mediated inflammation [19]. In fact, patients with IA rupture exhibit exaggerated intimal hypertrophy [25,46,48]. Hemodynamic studies also highlight parameters that modulate aneurysmal wall rigidity, growth, and rupture [35,36,40]. Diminished arterial wall elasticity has been associated with a large discrepancy between diastolic and systolic BP (ΔP), which contributes to increased arterial resistance [38,40,43,49]. This phenomenon of continuous, increased mechanical loading augments IA wall stress, which facilitates IA fatigue and rupture [5,45]. Our results were in accordance with these data, as a high ΔP was associated with IA rupture.
Overall, our results are in parallel with most in vivo studies performed on the extracranial arteries. We found that patients with IA rupture exhibit impaired CCA elasticity, which is associated with hypertrophic remodeling not observed in unruptured IAs. Impaired distensibility might not be completely attributed to IA rupture, as vasculopathy may also contribute to IA rupture [12,20]. Our data suggest that decreased CCA elasticity and increased intima-media measurements might be associated with an increased likelihood of IA rupture, which may be helpful in decision-making for IA clinical management.
Limitations
The study sample size was small, lacked a healthy control group, and no long-term follow-up was performed. However, all study subjects ultimately received surgical or interventional treatment.
Conclusions and Future Perspectives
Our findings demonstrate that patients with ruptured IAs exhibit impaired CCA wall distensibility and increased intima-media thickness, which suggests hypertrophic remodeling. Thus, determining CCA distensibility and intima-media thickness might be useful in determining whether an IA may rupture in the future. To better determine whether CCA elastic properties predispose patients to IA rupture, a larger prospective study design might be more informative, as the development of rupture may be correlated with changes in these factors over time. It is our hope that developing a set of predictive parameters for IA rupture will guide clinical management so to prevent complications such as subarachnoid hemorrhage.
Acknowledgements
We thank Dr Ercan Tuncel, Professor of Radiology, Uludag University for his dedicated contributions.
Footnotes
Conflict of interest
We declare that we have no conflict of interest.
Source of support: Departmental sources
References
- 1.Rinkel GJ, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke. 1998;29:251–56. doi: 10.1161/01.str.29.1.251. [DOI] [PubMed] [Google Scholar]
- 2.Loewenstein JE, Gayle SC, Duffis EJ, et al. The natural history and treatment options for unruptured intracranial aneurysms. Int J Vasc Med. 2012;2012:898052. doi: 10.1155/2012/898052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh SY, Kwon JT, Park YS, et al. Clinical features of acute subdural hematomas caused by ruptured intracranial aneurysms. J Korean Neurosurg Soc. 2011;50:6–10. doi: 10.3340/jkns.2011.50.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.You SH, Kong DS, Kim JS, et al. Characteristic features of unruptured intracranial aneurysms: predictive risk factors for aneurysm rupture. J Neurol Neurosurg Psychiatry. 2010;81:479–84. doi: 10.1136/jnnp.2008.169573. [DOI] [PubMed] [Google Scholar]
- 5.Beck J, Rohde S, Berkefeld J, et al. Size and location of ruptured and unruptured intracranial aneurysms measured by 3-dimensional rotational angiography. Surg Neurol. 2006;65:18–25. doi: 10.1016/j.surneu.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Terzidou C, Dalianis G, Zacharaki F. Ocular symptomatology, management, and clinical outcome of a giant intracranial aneurysm. Case Report Med. 2012;2012:643965. doi: 10.1155/2012/643965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White PM. The detection and management of unruptured intracranial aneurysms. Brain. 2000;123:205–21. doi: 10.1093/brain/123.2.205. [DOI] [PubMed] [Google Scholar]
- 8.Beck J, Rohde S, el Beltagy M, et al. Difference in configuration of ruptured and unruptured intracranial aneurysms determined by biplanar digital subtraction angiography. Acta Neurochir. 2003;145:861–65. doi: 10.1007/s00701-003-0124-0. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimoto Y, Wakai S. Cost-effectiveness analysis of screening for asymptomatic, unruptured intracranial aneurysms. A mathematical model. Stroke. 1999;30:1621–27. doi: 10.1161/01.str.30.8.1621. [DOI] [PubMed] [Google Scholar]
- 10.Wanke I, Doerfler A, Dietrich U, et al. Endovascular treatment of unruptured intracranial aneurysms. AJNR Am J Neuroradiol. 2002;23:756–61. [PMC free article] [PubMed] [Google Scholar]
- 11.Costalat V, Sanchez M, Ambard D, et al. Biomechanical wall properties of human intracranial aneurysms resected following surgical clipping. J Biomech. 2011;44:2685–91. doi: 10.1016/j.jbiomech.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 12.Luukkonen TM, Pöyhönen M, Palotie A, et al. A balanced translocation truncates Neurotrimin in a family with intracranial and thoracic aortic aneurysm. J Med Genet. 2012;49:621–29. doi: 10.1136/jmedgenet-2012-100977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amenta PS, Yadla S, Campbell PG, et al. Analysis of nonmodifiable risk factors for intracranial aneurysm rupture in a large, retrospective cohort. Neurosurgery. 2012;70:693–99. doi: 10.1227/NEU.0b013e3182354d68. [DOI] [PubMed] [Google Scholar]
- 14.Nader-Sepahi A, Casimiro M, Sen J, Kitchen ND. Is aspect ratio a reliable predictor of intracranial aneurysm rupture? Neurosurgery. 2004;54:1343–47. doi: 10.1227/01.neu.0000124482.03676.8b. [DOI] [PubMed] [Google Scholar]
- 15.Clarke G, Mendelow AD, Mitchell P. Predicting the risk of rupture of intracranial aneurysms based on anatomical location. Acta Neurochir. 2005;147:259–63. doi: 10.1007/s00701-004-0473-3. [DOI] [PubMed] [Google Scholar]
- 16.Lall RR, Eddleman CS, Bendok BR, Batjer HH. Unruptured intracranial aneurysms and the assessment of rupture risk based on anatomical and morphological factors: sifting through the sands of data. Neurosurg Focus. 2009;26:E2. doi: 10.3171/2009.2.FOCUS0921. [DOI] [PubMed] [Google Scholar]
- 17.Hoh BL, Sistrom CL, Firment CS, et al. Bottleneck factor and height-width ratio: association with ruptured aneurysms in patients with multiple cerebral aneurysms. Neurosurgery. 2007;61:716–22. doi: 10.1227/01.NEU.0000298899.77097.BF. [DOI] [PubMed] [Google Scholar]
- 18.Raij L, Gonzalez-Ochoa AM. Vascular compliance in blood pressure. Curr Opin Nephrol Hypertens. 2011;20:457–64. doi: 10.1097/MNH.0b013e3283499d7b. [DOI] [PubMed] [Google Scholar]
- 19.Jayaraman T, Paget A, Shin YS, et al. TNF-alpha-mediated inflammation in cerebral aneurysms: a potential link to growth and rupture. Vasc Health Risk Manag. 2008;4:805–17. doi: 10.2147/vhrm.s2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tulamo R, Frösen J, Junnikkala S, et al. Complement activation associates with saccular cerebral artery aneurysm wall degeneration and rupture. Neurosurgery. 2006;59:1069–76. doi: 10.1227/01.NEU.0000245598.84698.26. [DOI] [PubMed] [Google Scholar]
- 21.Sakata N, Takebayashi S, Shimizu K, et al. A case of segmental mediolytic arteriopathy involving both intracranial and intraabdominal arteries. Pathol Res Pract. 2002;198:493–97. doi: 10.1078/0344-0338-00290. [DOI] [PubMed] [Google Scholar]
- 22.Giannarelli C, Bianchini E, Bruno RM, et al. Local carotid stiffness and intimamedia thickness assessment by a novel ultrasound-based system in essential hypertension. Atherosclerosis. 2012;223:372–77. doi: 10.1016/j.atherosclerosis.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 23.Rogowicz-Frontczak A, Araszkiewicz A, Pilacinski S, et al. Carotid intima-media thickness and arterial stiffness in type 1 diabetic patients with and without microangiopathy. Arch Med Sci. 2012;8:484–90. doi: 10.5114/aoms.2012.29404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjällmark A, Lind B, Peolsson M, et al. Ultrasonographic strain imaging is superior to conventional non-invasive measures of vascular stiffness in the detection of age-dependent differences in the mechanical properties of the common carotid artery. Eur J Echocardiogr. 2010;11:630–36. doi: 10.1093/ejechocard/jeq033. [DOI] [PubMed] [Google Scholar]
- 25.Maltete D, Bellien J, Cabrejo L, et al. Hypertrophic remodeling and increased arterial stiffness in patients with intracranial aneurysms. Atherosclerosis. 2010;211:486–91. doi: 10.1016/j.atherosclerosis.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Karwowski W, Naumnik B, Szczepański M, Myśliwiec M. The mechanism of vascular calcification – a systematic review. Med Sci Monit. 2012;18(1):RA1–11. doi: 10.12659/MSM.882181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selzer RH, Mack WJ, Lee PL, et al. Improved common carotid elasticity and intima-media thickness measurements from computer analysis of sequential ultrasound frames. Atherosclerosis. 2001;154:185–93. doi: 10.1016/s0021-9150(00)00461-5. [DOI] [PubMed] [Google Scholar]
- 28.van‘t Veer M, Buth J, Merkx M, et al. Biomechanical properties of abdominal aortic aneurysms assessed by simultaneously measured pressure and volume changes in humans. J Vasc Surg. 2008;48:1401–7. doi: 10.1016/j.jvs.2008.06.060. [DOI] [PubMed] [Google Scholar]
- 29.Godia EC, Madhok R, Pittman J, et al. Carotid artery distensibility: a reliability study. J Ultrasound Med. 2007;26:1157–65. doi: 10.7863/jum.2007.26.9.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tóth M, Nádasy GL, Nyár I, et al. Are there systemic changes in the arterial biomechanics of intracranial aneurysm patients? Pflugers Arch. 2000;439:573–78. doi: 10.1007/s004249900154. [DOI] [PubMed] [Google Scholar]
- 31.Greene ER, Lanphere KR, Sharrar J, Roldan CA. Arterial distensibility in systemic lupus erythematosus. Med Biol Soc. 2009;2009:1109–12. doi: 10.1109/IEMBS.2009.5334459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boutouyrie P, Germain DP, Fiessinger JN, et al. Increased carotid wall stress in vascular Ehlers-Danlos syndrome. Circulation. 2004;109:1530–35. doi: 10.1161/01.CIR.0000121741.50315.C2. [DOI] [PubMed] [Google Scholar]
- 33.Groth M, Forkert ND, Buhk JH, et al. Comparison of 3D computer-aided with manual cerebral aneurysm measurements in different imaging modalities. Neuroradiology. 2013;55:171–78. doi: 10.1007/s00234-012-1095-8. [DOI] [PubMed] [Google Scholar]
- 34.Rohde S, Lahmann K, Beck J, et al. Fourier analysis of intracranial aneurysms: towards an objective and quantitative evaluation of the shape of aneurysms. Neuroradiology. 2005;47:121–26. doi: 10.1007/s00234-004-1324-x. [DOI] [PubMed] [Google Scholar]
- 35.Foutrakis GN, Yonas H, Sclabassi RJ. Saccular aneurysm formation in curved and bifurcating arteries. AJNR Am J Neuroradiol. 1999;20:1309–17. [PMC free article] [PubMed] [Google Scholar]
- 36.Vlak MH, Rinkel GJ, Greebe P, et al. Trigger factors for rupture of intracranial aneurysms in relation to patient and aneurysm characteristics. J Neurol. 2012;259:1298–302. doi: 10.1007/s00415-011-6341-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pierot L, Barbe C, Spelle L, et al. Endovascular treatment of very small unruptured aneurysms: rate of procedural complications, clinical outcome, and anatomical results. Stroke. 2010;41:2855–59. doi: 10.1161/STROKEAHA.110.588830. [DOI] [PubMed] [Google Scholar]
- 38.Evrengul H, Tanriverdi H, Kilic ID, et al. Aortic stiffness and flow-mediated dilatation in normotensive offspring of parents with hypertension. Cardiol Young. 2012;22:451–56. doi: 10.1017/S104795111200008X. [DOI] [PubMed] [Google Scholar]
- 39.van Laake LW, Vainas T, Dammers R, et al. Systemic dilation diathesis in patients with abdominal aortic aneurysms: a role for matrix metalloproteinase-9? Eur J Vasc Endovasc Surg. 2005;29:371–77. doi: 10.1016/j.ejvs.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Wilson KA, Lee AJ, Lee AJ, et al. The relationship between aortic wall distensibility and rupture of infrarenal abdominal aortic aneurysm. J Vasc Surg. 2003;37:112–17. doi: 10.1067/mva.2003.40. [DOI] [PubMed] [Google Scholar]
- 41.Wilson KA, Lindholt JS, Hoskins PR, et al. The relationship between abdominal aortic aneurysm distensibility and serum markers of elastin and collagen metabolism. Eur J Vasc Endovasc Surg. 2001;21:175–78. doi: 10.1053/ejvs.2001.1303. [DOI] [PubMed] [Google Scholar]
- 42.Gaál EI, Salo P, Kristiansson K, et al. Intracranial aneurysm risk locus 5q23.2 is associated with elevated systolic blood pressure. PLoS Genet. 2012;8:e1002563. doi: 10.1371/journal.pgen.1002563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zapolski T, Wysokiński A. Left atrium volume index is influenced by aortic stiffness and central pulse pressure in type 2 diabetes mellitus patients: a hemodynamic and echocardiographic study. Med Sci Monit. 2013;19:153–64. doi: 10.12659/MSM.883818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orlova IA, Nuraliev EY, Yarovaya EB, Ageev FT. Prognostic value of changes in arterial stiffness in men with coronary artery disease. Vasc Health Risk Manag. 2010;6:1015–21. doi: 10.2147/VHRM.S13591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van de Putte EM, Uiterwaal CS, Bots ML, et al. Is chronic fatigue syndrome a connective tissue disorder? A cross-sectional study in adolescents. Pediatrics. 2005;115:415–22. doi: 10.1542/peds.2004-1515. [DOI] [PubMed] [Google Scholar]
- 46.Mizuguchi Y, Oishi Y, Miyoshi H, et al. Impact of statin therapy on left ventricular function and carotid arterial stiffness in patients with hypercholesterolemia. Circ J. 2008;72:538–44. doi: 10.1253/circj.72.538. [DOI] [PubMed] [Google Scholar]
- 47.Celik T, Iyisoy A, Kursaklioglu H, et al. Impaired aortic elastic properties in young patients with prehypertension. Blood Press Monit. 2006;11:251–55. doi: 10.1097/01.mbp.0000209084.55364.3a. [DOI] [PubMed] [Google Scholar]
- 48.Seth S, Goyal NK, Jagia P, et al. Carotid intima-medial thickness as a marker of disease activity in Takayasu’s arteritis. Int J Cardiol. 2006;108:385–90. doi: 10.1016/j.ijcard.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 49.Mattace-Raso FU, van den Meiracker AH, Bos WJ, et al. Arterial stiffness, cardiovagal baroreflex sensitivity and postural blood pressure changes in older adults: the Rotterdam Study. J Hypertens. 2007;25:1421–26. doi: 10.1097/HJH.0b013e32811d6a07. [DOI] [PubMed] [Google Scholar]


