Abstract
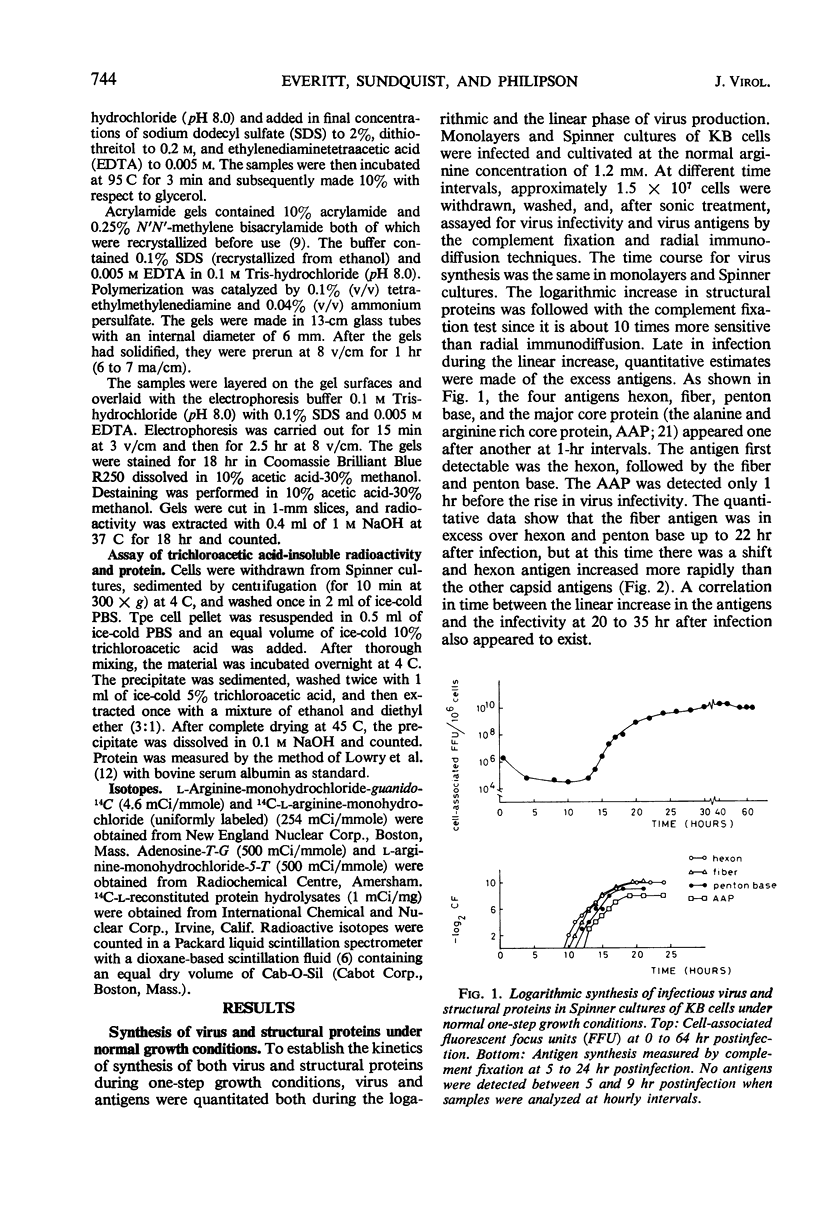
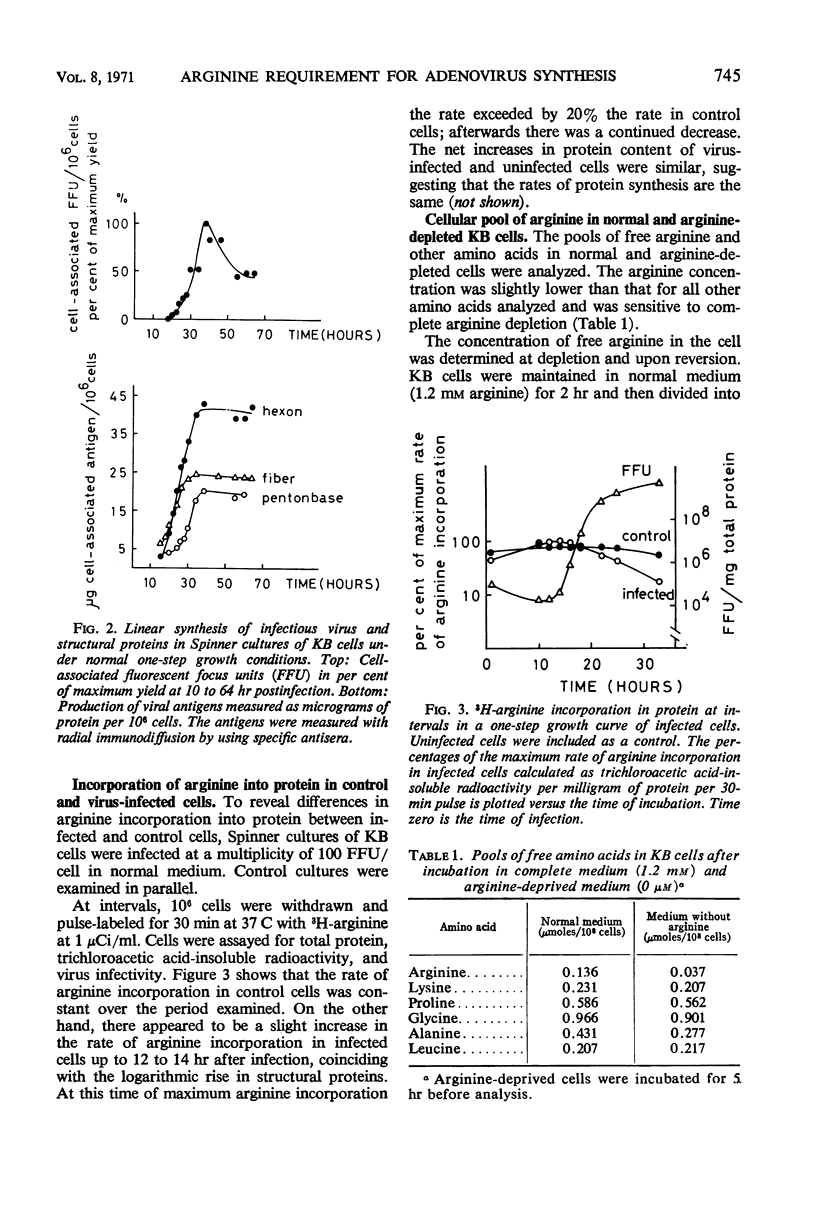
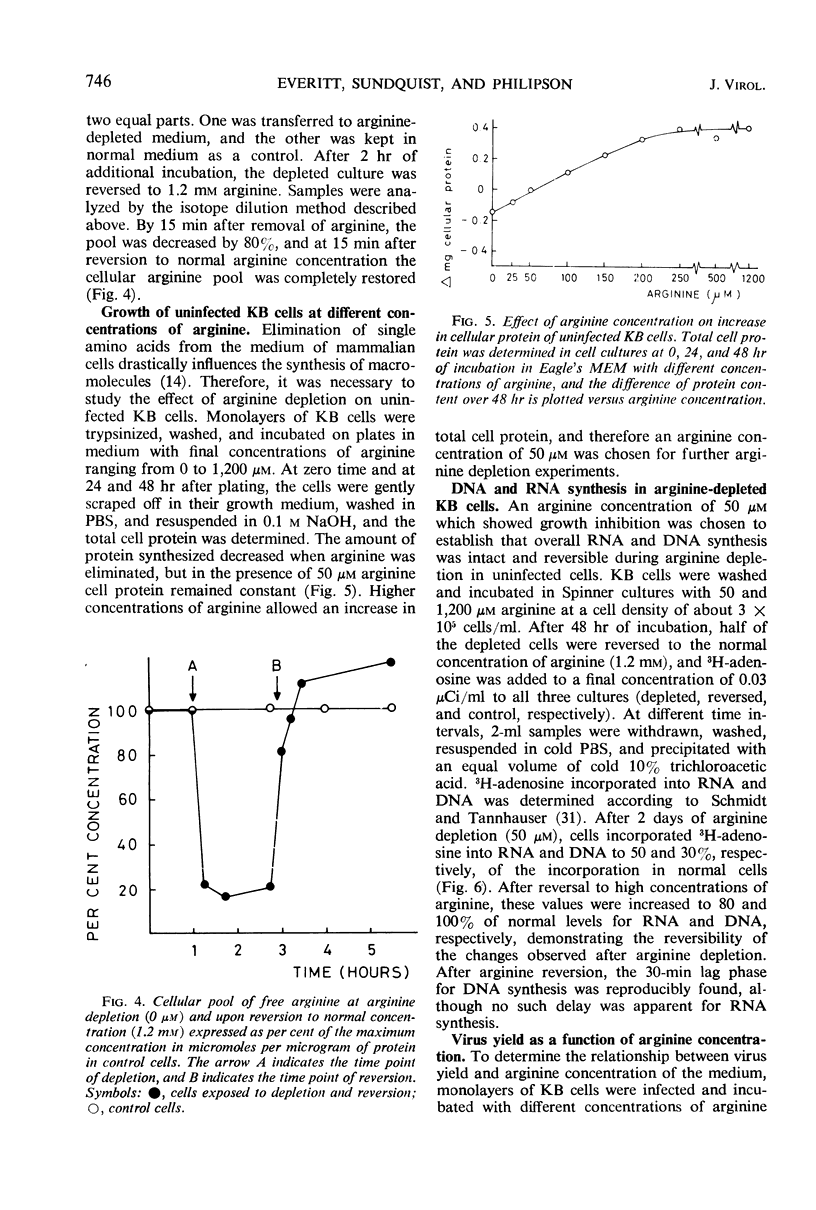
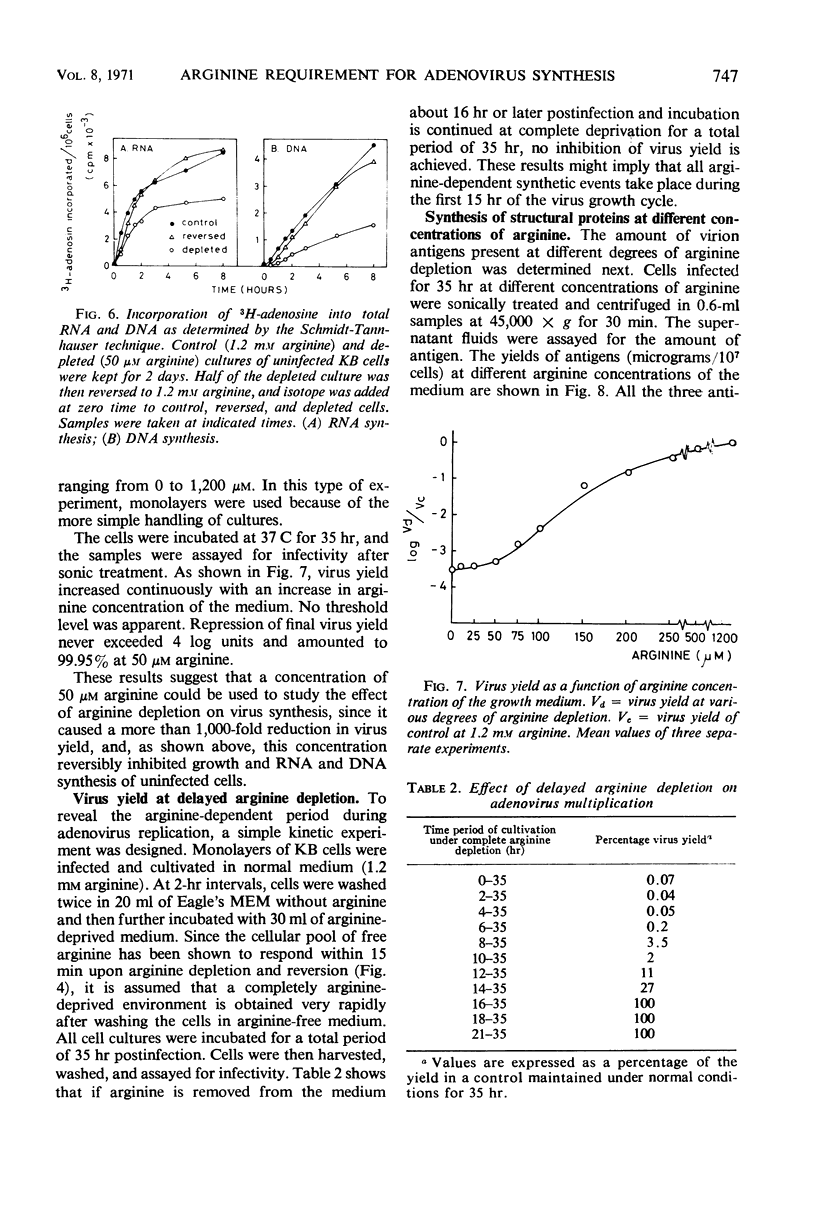
The mechanism of the arginine requirement for adenovirus was studied in cultures of KB cells infected with adenovirus type 2. Macromolecular synthesis was found to be severely impaired in uninfected cells under complete arginine deprivation, whereas an arginine concentration of 50 μm yielded a moderate and reversible inhibition of growth and nucleic acid synthesis. At this concentration, viral structural proteins were accumulated in excess although the virus yield was reduced more than 1,000-fold. The arginine-sensitive step appeared to occur early during the first 15 hr postinfection in the virus growth cycle. Virus-infected cells deprived of arginine to 50 μm showed, when reversed, a 4- to 5-hr lag period before the increase in virus growth was observed. Analysis of the radioactive pattern of labeled virions synthesized after reversion showed that all polypeptides were synthesized after addition of arginine to the medium, and none of the virion-polypeptides which are revealed by gel electrophoresis appeared to be preferentially synthesized after arginine reversion. The excess pool of structural proteins formed during depletion appeared to a large extent to be unavailable for virus assembly.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker Y., Olshevsky U., Levitt J. The role of arginine in the replication of herpes simplex virus. J Gen Virol. 1967 Oct;1(4):471–478. doi: 10.1099/0022-1317-1-4-471. [DOI] [PubMed] [Google Scholar]
- Brunschede H., Bremer H. Synthesis and breakdown of proteins in Escherichia coli during amino-acid starvation. J Mol Biol. 1971 Apr 14;57(1):35–57. doi: 10.1016/0022-2836(71)90118-5. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- GREEN M., PINA M. Biochemical studies on adenovirus multiplication. IV. Isolation, purification, and chemical analysis of adenovirus. Virology. 1963 May;20:199–207. doi: 10.1016/0042-6822(63)90157-0. [DOI] [PubMed] [Google Scholar]
- Goldblum N., Ravid Z., Becker Y. Effect of withdrawal of arginine and other amino acids on the synthesis of tumour and viral antigens of SV 40 virus. J Gen Virol. 1968 Jul;3(1):143–146. doi: 10.1099/0022-1317-3-1-143. [DOI] [PubMed] [Google Scholar]
- Inglis V. B. Requirement of arginine for the replication of herpes virus. J Gen Virol. 1968 Jul;3(1):9–17. doi: 10.1099/0022-1317-3-1-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laver W. G. Isolation of an arginine-rich protein from particles of adenovirus type 2. Virology. 1970 Jul;41(3):488–500. doi: 10.1016/0042-6822(70)90170-4. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh P. C., Oie H. K. Role of lysine in the replication of reovirus: I. Synthesis of complete and empty virions. J Virol. 1969 Dec;4(6):890–895. doi: 10.1128/jvi.4.6.890-895.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early events of virus-cell interaction in an adenovirus system. J Virol. 1969 Oct;4(4):323–338. doi: 10.1128/jvi.4.4.323-338.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden B. E., Vaughan M. H. Synthesis of ribosomal proteins in the absence of ribosome maturation in methionine-deficient HeLa cells. J Mol Biol. 1968 Dec;38(3):431–435. doi: 10.1016/0022-2836(68)90398-7. [DOI] [PubMed] [Google Scholar]
- Maden B. E., Vaughan M. H., Warner J. R., Darnell J. E. Effects of valine deprivation on ribosome formation in HeLa cells. J Mol Biol. 1969 Oct 28;45(2):265–275. doi: 10.1016/0022-2836(69)90104-1. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. II. Soluble proteins, cores, top components and the structure of the virion. Virology. 1968 Sep;36(1):126–136. doi: 10.1016/0042-6822(68)90122-0. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- POLASA H., GREEN M. BIOCHEMICAL STUDIES ON ADENOVIRUS MULTIPLICATION. 8. ANALYSIS OF PROTEIN SYNTHESIS. Virology. 1965 Jan;25:68–79. doi: 10.1016/0042-6822(65)90253-9. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Höglund S. Sructural proteins of adenoviruses. 3. Purification and characterization of the adenovirus type 2 penton antigen. Virology. 1969 Sep;39(1):90–106. doi: 10.1016/0042-6822(69)90351-1. [DOI] [PubMed] [Google Scholar]
- Philipson L. Attachment and eclipse of adenovirus. J Virol. 1967 Oct;1(5):868–875. doi: 10.1128/jvi.1.5.868-875.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L., Lonberg-Holm K., Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968 Oct;2(10):1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prage L., Pettersson U., Höglund S., Lonberg-Holm K., Philipson L. Structural proteins of adenoviruses. IV. Sequential degradation of the adenovirus type 2 virion. Virology. 1970 Oct;42(2):341–358. doi: 10.1016/0042-6822(70)90278-3. [DOI] [PubMed] [Google Scholar]
- Prage L., Pettersson U. Structural proteins of adenoviruses. VII. Purification and properties of an arginine-rich core protein from adenovirus type 2 and type 3. Virology. 1971 Aug;45(2):364–373. doi: 10.1016/0042-6822(71)90337-0. [DOI] [PubMed] [Google Scholar]
- Rouse H. C., Schlesinger R. W. An arginine-dependent step in the maturation of type 2 adenovirus. Virology. 1967 Nov;33(3):513–522. doi: 10.1016/0042-6822(67)90128-6. [DOI] [PubMed] [Google Scholar]
- Rowe W. C., Huggins A. K., Baldwin E. A radioisotopic assay system for enzymes of the ornithine-urea cycle. Anal Biochem. 1970 May;35(1):167–176. doi: 10.1016/0003-2697(70)90022-9. [DOI] [PubMed] [Google Scholar]
- Rubin I. B., Goldstein G. An ultrasensitive isotope dilution method for the determination of L-amino acids. Anal Biochem. 1970 Feb;33(2):244–254. doi: 10.1016/0003-2697(70)90293-9. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Becker Y. A maturation factor for adenovirus. Virology. 1968 May;35(1):18–27. doi: 10.1016/0042-6822(68)90301-2. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Hayashi K., Sanderson P. J., Pereira H. G. Adenovirus antigens--a study of their properties and sequential development in infection. J Gen Virol. 1967 Oct;1(4):495–507. doi: 10.1099/0022-1317-1-4-495. [DOI] [PubMed] [Google Scholar]
- Russell W. C., McIntosh K., Skehel J. J. The preparation and properties of adenovirus cores. J Gen Virol. 1971 Apr;11(1):35–46. doi: 10.1099/0022-1317-11-1-35. [DOI] [PubMed] [Google Scholar]
- SCHARFF M. D., SHATKIN A. J., LEVINTOW L. ASSOCIATION OF NEWLY FORMED VIRAL PROTEIN WITH SPECIFIC POLYRIBOSOMES. Proc Natl Acad Sci U S A. 1963 Oct;50:686–694. doi: 10.1073/pnas.50.4.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Schlesinger R. W. Adenoviruses: the nature of the virion and of controlling factors in productive or abortive infection and tumorigenesis. Adv Virus Res. 1969;14:1–61. doi: 10.1016/s0065-3527(08)60556-4. [DOI] [PubMed] [Google Scholar]
- White D. O., Scharff M. D., Maizel J. V., Jr The polypeptides of adenovirus. 3. Synthesis in infected cells. Virology. 1969 Jul;38(3):395–406. doi: 10.1016/0042-6822(69)90152-4. [DOI] [PubMed] [Google Scholar]
- Willems M., Penman M., Penman S. The regulation of RNA synthesis and processing in the nucleolus during inhibition of protein synthesis. J Cell Biol. 1969 Apr;41(1):177–187. doi: 10.1083/jcb.41.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters A. L., Consigli R. A. Effects of arginine deprivation on polyoma virus infection of mouse embryo cultures. J Gen Virol. 1971 Jan;10(1):53–63. doi: 10.1099/0022-1317-10-1-53. [DOI] [PubMed] [Google Scholar]
- Winters W. D., Russell W. C. Studies on the assembly of adenovirus in vitro. J Gen Virol. 1971 Feb;10(2):181–194. doi: 10.1099/0022-1317-10-2-181. [DOI] [PubMed] [Google Scholar]



