Abstract
Dentin sialophosphoprotein (DSPP) is critical for proper mineralization of tooth dentin, and mutations in DSPP cause inherited dentin defects. Dentin phosphoprotein (DPP) is the C-terminal cleavage product of DSPP that binds collagen and induces intrafibrillar mineralization. We isolated DPP from individual pigs and determined that its N-terminal and C-terminal domains are glycosylated and that DPP averages 155 phosphates per molecule. Porcine DPP is unstable at low pH and high temperatures, and complexing with collagen improves its stability. Surprisingly, we observed DPP size variations on SDS-PAGE for DPP isolated from individual pigs. These variations are not caused by differences in proteolytic processing or degrees of phosphorylation or glycosylation, but rather to allelic variations in Dspp. Characterization of the DPP coding region identified 4 allelic variants. Among the 4 alleles, 27 sequence variations were identified, including 16 length polymorphisms ranging from 3 to 63 nucleotides. None of the length variations shifted the reading frame, and all localized to the highly redundant region of the DPP code. The 4 alleles encode DPP domains having 551, 575, 589, or 594 amino acids and completely explain the DPP size variations. DPP length variations are polymorphic and are not associated with dentin defects.
About 85% of the organic material in tooth dentin is type I collagen. The non-collagenous proteins in dentin are predominantly cleavage products of dentin sialophosphoprotein (DSPP).2 DSPP is processed by proteases into numerous parts. In pig, the three major cleavage products are dentin sialoprotein (DSP) (1), dentin glycoprotein (DGP) (2), and dentin phosphoprotein (DPP). The linear order of the structural domains in porcine DSPP is DSP-DGP-DPP. In the rat, the designation DSP is used to include all of the non-DPP region of DSPP (DSP-DPP). The initial proteolytic cleavage of DSPP is in a conserved context and releases DPP so that DPP proteins isolated from various mammals share the same N-terminal sequence: Asp-Asp-Pro-Asn (DDPN) (3, 4). The early work on rat DSP and DPP was performed without knowing that DSP and DPP are initially part of the same protein and must be secreted in equal amounts. Based upon the results of these early studies, it became generally accepted that DPP is ten times more abundant than DSP in dentin extracts (5–7). As DPP and DSP are expressed in a 1:1 ratio (8), to get to a 10:1 ratio DSP must be rapidly degraded, and/or there must be alternative forms of DSP that were not previously recognized, which, when counted, bring the ratio back to 1:1. In fact, high molecular weight forms of DSP have been discovered in rat dentin (9). Porcine DSP is a highly glycosylated proteoglycan that forms covalent dimers (1). These glycosylations protect some parts of the protein from detection by DSP polyclonal antibodies, so it seems likely that DSP cleavage products may be more abundant than was originally thought.
Defects in human DSPP cause various types of inherited dentin defects, including dentin dysplasia type II and dentinogenesis imperfecta types II and III (10, 11). Dspp knock-out mice exhibit dentin defects reminiscent of those observed in human dentinogenesis imperfecta (12). The DSPP mutations reported so far are primarily along intron-exon borders near the 5′-end of the gene (13–19). This region encodes DSP, the N-terminal DSPP cleavage (20). The DPP coding region in the last exon of DSPP (exon 5) has a highly redundant region that is prone to artifacts during reverse transcription and polymerase chain reactions (21). Technical difficulties have so far thwarted mutational analyses of the DPP code in kindreds with inherited dentin defects. The only sequence variation reported to be disease-causing in the DPP region was a compound insertion (6 codons) and a deletion (12 codons) in the repetitive region (22). These insertions and deletions, however, are apparently normal sequence variations (polymorphisms) that do not cause dental disease in kindred. Alignment of 4 human DSPP sequences in GenBank™ identified 10 length variations in the DPP redundant region, ranging in length from 3 to 153 nucleotides and all maintaining the reading frame (10). An independent analysis of the kindred with the compound insertion/deletion found similar (but not identical) deletions and insertions in the DPP redundant region in normal controls and identified a missense mutation (p.P17S) in the DSP code that functional studies showed caused retention of the DSPP protein in the endoplasmic reticulum (11). Separately, a p.P17S missense alteration was found in the affected members of another family with inherited dentin defects and was thought to be causing the disease (19). These findings suggest that there may be extensive sequence length variations in the human DPP redundant region and that these length variations are compatible with normal function.
DPP protein has never been extensively characterized, and it is still not known if DPP is glycosylated (23). In this study, we isolated porcine DPP, determined its abundance relative to DSP, characterized its post-translational modifications, and determined the molecular basis for variability in the size of DPP protein in dentin extracts.
EXPERIMENTAL PROCEDURES
All experimental procedures involving the use of animals were reviewed and approved by the Institutional Animal Care and Use Program at the University of Michigan.
Preparation of Dentin Powder—Tooth germs of permanent second molars were surgically extracted with a hammer and chisel from the maxillae and mandibles of six-month-old pigs, within minutes of each animal’s termination at the Michigan State University Meat Laboratory (East Lansing, MI). Typically two maxillary and two mandibular second molars were obtained from each animal. The developmental stage of the molars was advanced in crown formation, although prior to the onset of root formation. The soft tissue was removed with forceps, and secretory and maturation stage enamel was scraped off with a curette. The remaining hard tissue was reduced to powder using a jaw crusher (Retsch, Newtown, PA).
Extraction of Proteins from Dentin Powder—Dentin powder (1.7–4.4 gm each) obtained from 22 pigs, individually, or combined from ∼8 pigs (40 gm) for large scale preparations, was suspended with 50 mm Tris-HCl, 4 m guanidine buffer, pH 7.4 containing Protease Inhibitor Mixture Set III (1 mm AEBSF, 0.8 μm aprotinin, 50 μm bestatin, 15 μm E-64, 20 μm leupeptin, and 10 μm pepstatin) (Calbiochem, San Diego, CA) and 1 mm 1,10-phenanthroline (Sigma). Each sample was intensively agitated with an orbital shaker for 24 h at 4 °C. The insoluble material was pelleted by centrifugation (15,900 × g), and the guanidine extraction repeated for two more days. The insoluble guanidine extracts were dialyzed against 16 liters of 0.5 m acetic acid (HAc) containing 5 mm benzamidine (Sigma), 1 mm phenyl-methylsulfonyl fluoride (Sigma), and 1 mm 1,10-phenanthroline. Each day the calcium concentration in the reservoir was measured using the Calcium Reagent Set (Pointe Scientific, Canton, MI), and the HAc/protease inhibitor solution was replaced. After 5 days, the calcium ion concentration of the HAc reservoir fell below 0.2 mm, indicating that the tooth mineral had fully dissolved. The dialysis bag contents were centrifuged, and the pellet was extracted with 0.5 m acetic acid, 2 m NaCl (AN), which dissolved dentin phosphoprotein (DPP) and DSP proteoglycan products. The AN supernatant was fractionated to purify DPP and high molecular weight DSP-containing proteoglycan.
Purification of Porcine DPP—AN extracts (∼10–28 mg each) were dissolved in 0.05% trifluoroacetic acid and fractionated by reversed phase-high performance liquid chromatography (RP-HPLC) using a Discovery C-18 column (1.0 cm × 25 cm, Sigma-Aldrich/Supelco) run at a flow rate of 1.0 ml/min and monitored at 220 nm (Buffer A: 0.05% trifluoroacetic acid; Buffer B: 80% acetonitrile, Buffer A).
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)—SDS-PAGE was performed using Novex 4–20% Tris-glycine or NuPAGE 3–8% Tris-acetate gels (Invitrogen). Samples were dissolved in Laemmli sample buffer (Bio-Rad), and electrophoresis was carried out using a current of 20 mA for 65 min or 150 V for 1 h, respectively. The gels were stained with Simply Blue Safe Stain (Invitrogen) or Stains-all (Sigma). The apparent molecular weights of protein bands were estimated by comparison with SeeBlue® Plus2 Pre-Stained Standard (Invitrogen).
Release of N- and O-Linked Glycan Chains by Glycopeptidase A and O-Glycanase Digestion—Acid phosphatase-treated DPP (0.2 mg each) in 0.1 m citrate phosphate buffer (pH 5.0) or in 0.25 m sodium phosphate buffer (pH 5.0) was incubated with 1 milliunit of glycopeptidase A (Seikagaku America, East Fal-mouth, MA) or a mixture of 2.5 milliunits of O-glycosidase and 5 milliunits of sialidase Au (QA-Bio, LLC, Palm Desert, CA) containing the Protease Inhibitor Mixture Set II for 48 h or 2 h at 37 °C, respectively. At the end of the incubation period, aliquots were analyzed by SDS-PAGE. The six purified pronase-digested DPP peptides were digested with glycopeptidase A, precipitated with three volumes of ice-cold ethanol, and pelleted by centrifugation for 10 min at 10,000 × g. Glycans in the supernatant were evaporated and stored at −80 °C.
Quantitative Determination of Phosphoserine in DPP—DPP from 22 individual pigs (0.5–0.7 mg each) was partially hydrolyzed with 0.2 ml of 6 n HCl for 3 h at 110 °C. After the reaction, the sample was evaporated and dissolved in 1 ml of deionized (DI) water, and 0.25 ml was fractionated on a TSK-gel SAX column (4.6 mm × 15 cm, TOSOH, Tokyo, JPN). The column was equilibrated with 40 mm potassium phosphate buffer (pH 4.0), and eluted with the same buffer at a flow rate of 1.5 ml/min at room temperature. Phosphoserine was detected by monitoring absorbance at 210 nm, and authentic phosphoserine, phosphothreonine, and phosphotyrosine (Sigma) were used as references. The mol phosphates released was divided by the starting weight × 100 g/mol (100-kDa molecular mass) to calculate phosphate per molecule.
Quantitative Determination of DPP Phosphorylation by Acid Phosphatase Digestion—DPP (150 μg each) in 150 ml of 10 mm sodium acetate, 50 mm EDTA buffer (pH 5.8) from individual pigs was incubated with 0.2 unit of acid phosphatase (white potato) (Sigma) containing the Protease Inhibitor Mixture Set II for 48 h at 37 °C. Free phosphorus in aliquots (20 ml) of acid phosphatase digests was measured by colorimetric assay using the Inorganic Phosphorus Reagent Set (Pointe Scientific, Canton, MI). The number of phosphates per molecule was calculated assuming a molecular mass of 100 kDa (Table 1).
TABLE 1.
Moles of phosphate per mole of DPP
All values based upon 100 kDa/molecule.
| Pig | IP | P-Ser | Ave | Pig | IP | P-Ser | Ave |
|---|---|---|---|---|---|---|---|
| A | 217 | 199 | 208 | L | 254 | 211 | 233 |
| B | 227 | 184 | 206 | M | 217 | 180 | 199 |
| C | 218 | 198 | 208 | N | 229 | 184 | 207 |
| D | 202 | 189 | 196 | O | 215 | 193 | 204 |
| E | 234 | 192 | 213 | P | 235 | 183 | 209 |
| F | 246 | 203 | 225 | Q | 206 | 175 | 191 |
| G | 255 | 196 | 226 | R | 262 | 202 | 232 |
| H | 203 | 181 | 192 | S | 252 | 228 | 240 |
| I | 272 | 177 | 225 | T | 251 | 237 | 244 |
| J | 231 | 156 | 194 | U | 216 | 185 | 201 |
| K | 249 | 167 | 208 | V | 221 | 194 | 208 |
|
| |||||||
| Total | 2554 | 2042 | 2298 | Total | 2558 | 2172 | 2365 |
| Ave | 232 | 186 | 209 | Ave | 233 | 197 | 215 |
Extraction of DPP from Gels—Three DPP protein bands from large scale dentin preparations were excised from a polyacrylamide gel after Stains-all staining. Each gel slice was transferred to a D-Tube Dialyzer (midi size) (Novagen/EMD Chemicals, Inc., Gibbstown, NJ) and electroeluted with 25 mm Tris, 250 mm Tricine, 0.025% SDS buffer (pH 8.5) at 150 V for 3 h. The eluate (DPP) was precipitated with 20% trichloroacetic acid. The pellet was incubated in acetone overnight at −20 °C and centrifuged at 4 °C for 30 min at 14,000 × g. The supernatant was decanted, the pellet dried under a hood, and characterized by Edman sequencing.
Purification of DPP Glycopeptide by Pronase Digestion—DPP (25 mg) was digested with acid phosphatase, dialyzed against water for 2 days, and lyophilized. Acid phosphatase-treated DPP (18 mg) was dissolved with 1 ml of 50 mm Tris-HCl buffer, pH 8.0. This solution was incubated with 0.1 mg of pronase (Calbiochem) for 15 h at 37 °C. The digest was fractionated by size exclusion chromatography using a Sephadex G-15 column (1.6 cm × 100 cm, GE Healthcare Life Sciences) equilibrated with 50 mm Tris-HCl buffer, pH 8.0. DPP peptides were eluted with the same buffer at a flow rate of 0.2 ml/min at 4 °C with absorbance monitored at 220 nm, and analyzed for glycosylation using a phenol-sulfuric acid assay at 490 nm. The fraction of peptides containing DPP glycopeptide was eluted in the first peak, and this fraction was separated by hydrophilic interaction liquid chromatography (HILIC) using a polyhydroxyethyl A column (4.6-mm inner diameter × 20 cm, The Nest Group, Inc., Southborough, MA) equilibrated with 15 mm triethylamine phosphate (TEAP) in 95% acetonitrile (pH 3.0). Peptides were eluted with a linear acetonitrile gradient containing 15 mm TEAP in 5% acetonitrile (pH 3.0) at a flow rate of 0.5 ml/min at room temperature, while monitoring the absorbance at 220 nm. Six peaks were collected and evaporated. Aliquots were used for both amino acid sequence and glycosylation analyses.
Preparation and Isolation of 2-AA-labeled Glycans—Glycans released by glycopeptidase A were labeled with the 2-aminobenzoic acid (2-AA) labeling kit (QA-Bio, Palm Desert, CA), and labeled glycans were purified by LudgerClean S cartridge (QA-Bio). The 2-AA glycans were fractionated by normal phase (NP) HPLC using a SUPELCOSIL LC-NH2 column (4.6 mm × 25 cm, Sigma-Aldrich/Supelco). The column was equilibrated with 2% acetic acid and 1% tetrahydrofuran in acetonitrile. Glycans were eluted with a linear gradient using 5% acetic acid, 1% tetrahydrofuran, 3% triethylamine in water at a flow rate of 0.7 ml/min at room temperature. For the detection of 2-AA-glycans, an excitation wavelength of 230 nm and an emission wavelength of 420 nm were used.
Effect of pH and Temperature for Stability of DPP Structure—DPP (1 mg) was dissolved with 0.2 ml of DI water. A 20-μl aliquot was mixed with 180 μl of 50 mm sodium acetate or 50 mm Tris-HCl or 50 mm carbonate buffers to achieve a final pH of 4, 5, 6, 7, 8, 9, 10, or 11 (50 mm sodium acetate for pH 4 and 5, 50 mm Tris-HCl for pH 6–8, or 50 mm carbonate buffer for pH 9–11), respectively. Each sample was divided into five tubes (40 μl each). One tube was immediately stored at −80 °C, another tube was incubated for 5 min at 95 °C, and the other three tubes were incubated for 20 h at 4, 20, or 37 °C, respectively. Aliquots (20 μl) of samples were separated by SDS-PAGE and visualized with Stains-all.
Effect of Collagen on the Stability of DPP—DPP (0.2 mg) was dissolved with 0.2 ml of 0.5 m acetic acid. An aliquot of sample (50 μl) was then mixed with 0.2 ml of 0.5 m acetic acid containing 0.2 mg of acid-soluble human placenta type I collagen (Abcam, Cambridge, MA). Another aliquot (50 μl) was mixed with 0.2 ml of 0.5 m acetic acid only. Two samples with and without the collagen were divided into five tubes (50 μl each). Each sample was incubated and analyzed by SDS-PAGE.
Amino Acid Analysis—The purified peptide samples (0.02–0.03 mg) were hydrolyzed with 6 n HCl at 115 °C for 16 h. The amino acid analyses were performed using a Beckman Model 7300 automatic amino acid analyzer.
Automated Edman Degradation—Automated Edman degradation used the Applied Biosystems Procise 494 cLC protein sequencer at the W. M. Keck Facility at Yale University.
Characterization of DPP Genomic Sequences—Genomic DNA was isolated from the ear auricles of the 22 pigs investigated using the DNeasy Tissue kit (Qiagen, Valencia, CA). The DNA from eight pigs (B, J, H, M, N, P, R, V) was used as template to clone and characterize the DPP coding region. A segment from Dspp exon 5 containing the entire DPP coding region was amplified by polymerase chain reaction (PCR) using the primer pair: 5′-TGGACCCAGC-AAAACACATA and 5′-AATCGT-AGCCAAGCTGGAGA). The reactions ran for 35 cycles, with denaturing at 94 °C for 30 s, annealing at 56 °C for 30 s, and extension at 72 °C for 3 min using the Expand High Fidelity plus PCR system (Roche Applied Sciences). The amplification products for each pig were separated on 1% agarose gels containing ethidium bromide. The DNA bands were visualized with long wavelength UV light, excised with a razor blade, purified using a QIAquick gel extraction kit (Qiagen), ligated into pCR2.1-topo vector (Invitrogen), and used to transform Top10 competent cells (Invitrogen). Individual colonies were grown in LB broth and DNA isolated using the SV Mini-preps DNA Purification System (Promega). Sequencing was carried out at the University of Michigan DNA Sequencing Core using 4 separate primers; two used for original amplification and two internal primers (5′-AGTGATGGCAATGGTG-ACAA and 5′-GATTGCTGTCACTGCCTTCA-3′). Because of the cloning and characterization of some PCR artifacts, a second set of PCR/cloning reactions was conducted, and only clones isolated from multiple independent PCR reactions were accepted as being the true DPP alleles.
RESULTS
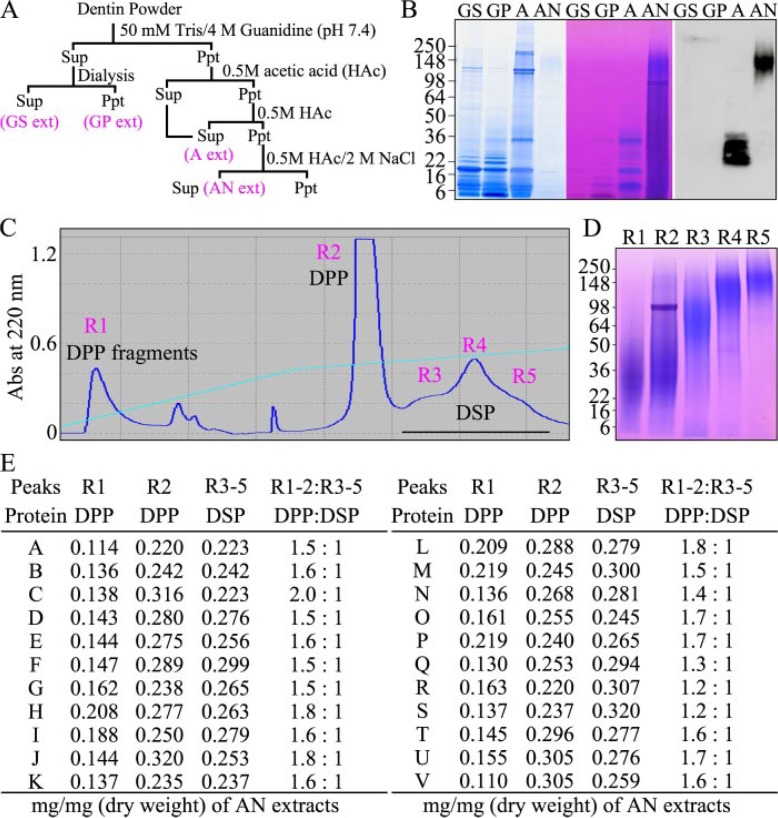
Relative Abundance of DSP and DPP in Porcine Dentin—Previously, we developed a strategy for sequentially extracting proteins from porcine dentin and used DSP and DGP antibodies to identify where DSPP-derived cleavage products fractionated during the procedure (1, 2, 24). This extraction scheme is shown in Fig. 1A. Dentin powder is first homogenized in guanidine. Dialysis of the guanidine extract causes some precipitation, separating it into guanidine soluble “GS” and guanidine pellet “GP” extracts. The original guanidine pellet is demineralized with acetic acid yielding an acid or “A” extract. The acid pellet is extracted with acetic acid/NaCl, generating the “AN” extract. The GS and GP extracts contain primarily enamel protein cleavage products and proteases (2, 24). The A extract contains soluble collagen, 32-kDa enamelin (from enamel), osteocalcin, and relatively small cleavage products from the non-DPP portion of DSP (the N-terminal fragments of DSP, DGP, and extended DGP). Very little protein in the AN extract stains with CBB, but this extract is rich in Stains-all-positive DSPP-derived dentin proteins (Fig. 1B). Lower molecular weight protein was removed from the AN extract by size exclusion chromatography, and the high molecular weight DSPP-derived proteins are separated into DPP and DSP components by RP-HPLC. Five RP fractions are evident in the chromatogram (Fig. 1C). R1 contains DPP fragments, R2 contains intact DPP and DPP fragments, R3 contains the central proteoglycan core of DSP, R4 contains DSP, and R5 contains DSP-DGP, or the entire non-DPP region of DSPP (Fig. 1D) (24).
FIGURE 1.
Relative abundance of DSP and DPP in developing porcine molars. A, strategy for isolating dentin proteins from dentin powder that generates 4 extracts: the guanidine supernatant (GS extract), guanidine pellet (GP extract), acid extract (A extract), and acid/NaCl (AN extract). B, SDS-PAGE (4–20% gradient gel) stained with CBB (left), Stains-all (center), and a Western blot immunostained with an anti-DSP Ab (right). High molecular weight DSPP-derived proteins in the AN extract do not stain with CBB, but stain deeply with Stains-all. High molecular weight DSPP-derived proteins are in the AN extract are purified by size exclusion chromatography (not shown) followed by RP-HPLC. C, RP-HPLC chromatogram exhibits five peaks labeled R1 through R5. D, SDS-PAGE (4–20% gradient gel) stained with Stains-all shows DPP fragments (R1), intact DPP with DPP fragments (R2), DSP proteoglycan core (R3), DSP proteoglycan (R4), and DSP-DGP proteoglycan (R5). The weight of the AN extract and fractions R1 through R5 were measured for dentin obtained from each of 22 pigs (labeled A through V). E, table showing the dry weights of fractions R1, R2, and R3 through R5 divided by the total dry weight of the AN extract, and the dry weight of DPP (R1+R2) relative to DSP (R3–R5) in the AN extracts.
To quantify the relative abundance of DPP and high molecular weight DSP, we isolated and weighed the AN extract from the second molars of 22 individual pigs and isolated the five RP-HPLC fractions containing DSPP-derived proteins. We normalized for variations in protein quantity among the pig extracts by dividing the quantity of protein in each RP fraction by the total amount of protein in the AN extract and calculated the total amount of DPP (R1+R2) relative to the total amount of high molecular weight DSP in fractions (R3+R4+R5). On a weight basis, porcine dentin in the second molars of six-month-old pigs contains between 1.2 and 2.0 times more DPP than high molecular weight DSP (Fig. 1E). There are, however, low molecular weight DSP cleavage products outside of the AN extract (lane A, Fig. 1B). These cannot be efficiently isolated and their quantity was not determined for individual pigs, but including the small DSP cleavage products in the A extract would have brought the ratio of DSP to DPP (on a weight basis) very close to 1:1, which is consistent with the fact that DPP and DSP are synthesized from the same mRNA transcript in 1:1 molar ratios (8, 21).
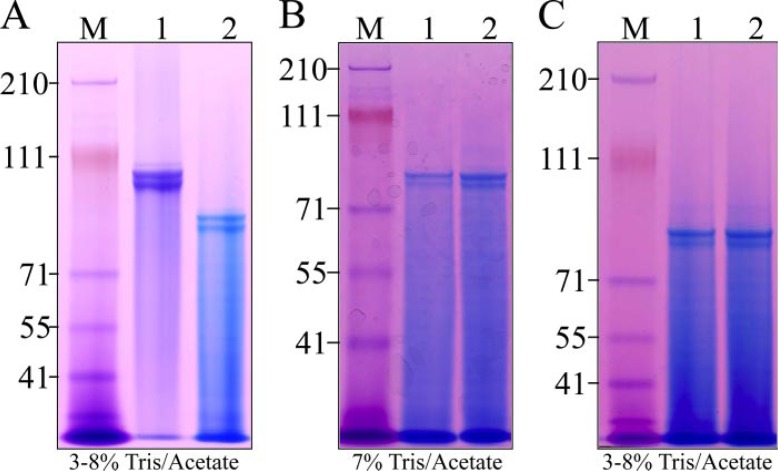
DPP Post-translational Modifications—An estimate of the gross levels of post-translational modifications in porcine dentin phosphoprotein was made by examining DPP before and after dephosphorylation and deglycosylation (Fig. 2). Porcine DPP in our large scale preparations (that combine dentin powder from 8 pigs) typically appears as two prominent bands, often accompanied by one or two distinct but weaker DPP bands. We originally assumed that the multiple DPP bands, all migrating near 100 kDa on SDS-PAGE, to be due to variations in the amount of DPP post-translational modification. Dephosphorylation of DPP with acid phosphatase, however, causes these DPP bands to shift to a lower apparent molecular weight on SDS-PAGE (Fig. 2A), but does not alter the pattern of multiple bands. N-deglycosylation (Fig. 2B) and O-deglycosylation (Fig. 2C) of the dephosphorylated DPP does not appreciably alter the mobility of DPP on SDS-PAGE and also does not alter the pattern of multiple bands. Each DPP band was excised from the gel and characterized by N-terminal sequencing. All three DPP bands gave the same N terminus: DDPNXXIE. These results demonstrate that the various DPP bands extracted from porcine dentin do not differ from each other because of variations in the levels of phosphorylation, glycosylation, or through the use of multiple cleavage sites to release DPP from the parent DSPP protein. We then characterized the number of phosphates on DPP and identified DPP glycosylation sites.
FIGURE 2.
Multiple DPP bands are not due to variable levels of phosphorylation or glycosylation. A, DPP in large scale preparations (from 8 pigs) typically shows 2 prominent bands and 1 or 2 additional faint bands (lane 1). Dephosphorylation with acid phosphatase shifts the multiple bands down the gel, but the pattern of multiple bands is preserved. B, N-deglycosylation of the dephosphorylated DPP with glycopeptidase A (lane 2) does not significantly alter the mobility of DPP or its pattern of multiple bands (lane 1). C, O-deglycosylation of dephosphorylated and N-deglycosylated DPP (lane 2) does not significantly alter the mobility of DPP or its pattern of multiple bands (lane 1). The 3 DPP bands from lane 1 in panel A were excised from the gel and characterized by N-terminal sequencing. All gave the same result: DDPNS, which is the conserved DPP N-terminal sequence.
We isolated DPP from 22 individual pigs and determined the numbers of phosphate per molecule using two independent methods. One method measures the amount of phosphoserine released after acid hydrolysis, the other measures inorganic phosphate released by acid phosphatase. Using the apparent molecular mass of DPP (100 kDa) on SDS-PAGE to convert from grams to moles, the number of phosphates per molecule of DPP came to an average of 212 per molecule. This number of phosphates would contribute 17 kDa to the mass of the protein.
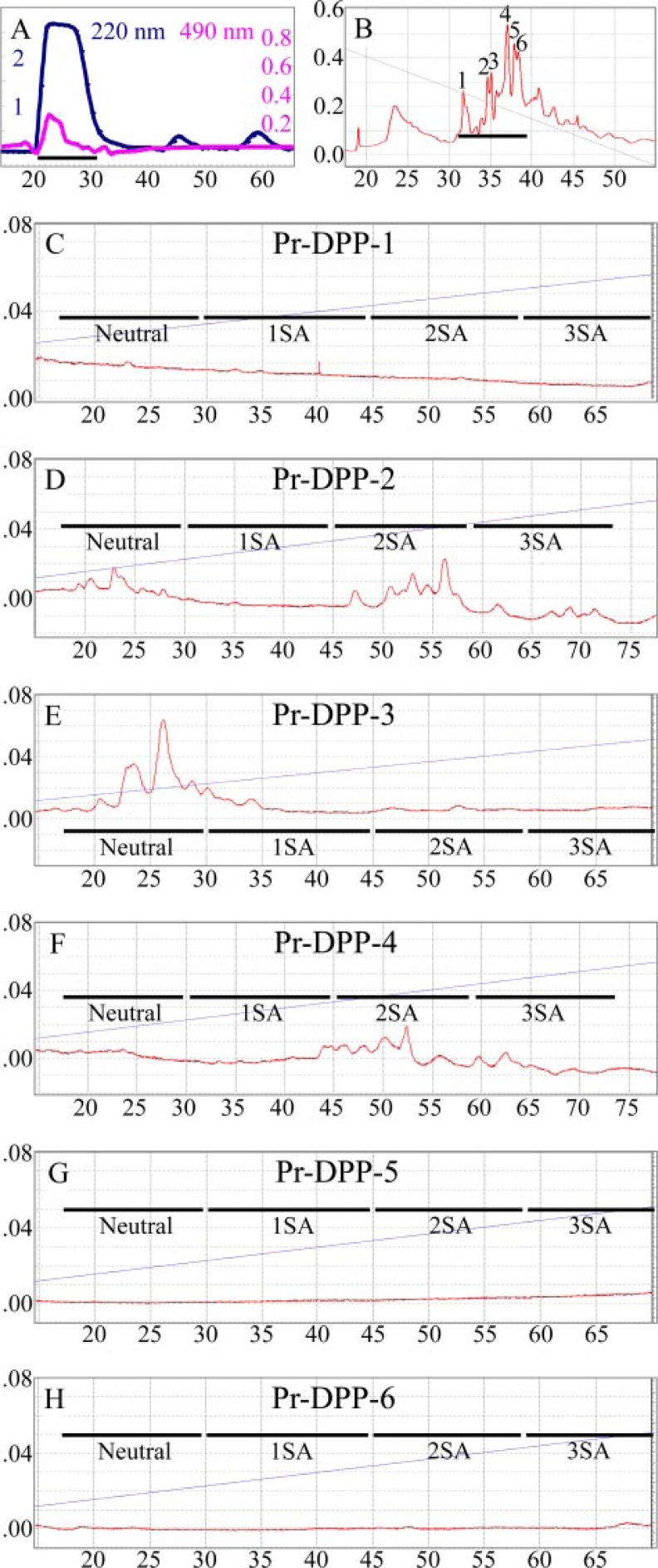
To identify specific glycosylation sites, DPP was digested with pronase, a mixture of proteolytic enzymes that digests glycoproteins down to short glycopeptides that are protected from further degradation by their bulky carbohydrate chains. The pronase digestion products were fractionated by size exclusion chromatography and assayed for the presence of glycosylation (Fig. 3A). The glycosylation-positive peak was separated into six fractions (labeled Pr-DPP-1 through Pr-DPP-6) by HILIC (Fig. 3B). The protein in each fraction was deglycosylated and characterized by N-terminal sequencing (Table 2). The glycosylations were fluorescently labeled and analyzed by NP-HPLC (Fig. 3, C–H). No labeled glycosylations were observed for Pr-DPP-1, Pr-DPP-5, and Pr-DPP-6. Two N-linked glycosylation sites were identified in the other fractions: at Asn525 (Pr-DPP-2 and Pr-DPP-3) and Asn937 (numbered for the smallest DSPP allele; Pr-DPP-4). Based upon its retention time on the NP-HPLC column, the glycosylation at Asn525 can have either no sialic acid (Pr-DPP3) or two sialic acids (Pr-DPP2). The glycosylation at Asn937 has two sialic acids (Pr-DPP4). These results demonstrate that porcine DPP is glycosylated at two positions, and that variations in glycosylation cannot account for the multiple bands of DPP observed on SDS-PAGE.
FIGURE 3.
Identification of two N-glycosylation sites in DPP. A, size exclusion chromatogram of pronase digestion products of DPP with absorbance monitored at 220 nm (blue line). Aliquots were analyzed for glycosylation by measuring the absorbance at 490 nm (magenta) after performing the phenol-sulfuric acid assay. The fraction applied to the next column is underlined. B, chromatogram of glycosylation-positive fraction (peak 1 in A) divided into 6 parts by hydrophilic interaction-HPLC. The 6 Pr-DPPs were digested with glycopeptidase A, and the released N-glycosylations were fluorescently labeled. C–H, chromatograms of labeled N-glycans on NP-HPLC and monitored with an excitation wavelength of 230 nm and an emission wavelength of 420 nm. No N-glycans were released from the Pr-DPP fraction 1 (C), 5 (G), or 6 (H). N-glycans were eluted in order of the number of sialic acids (SA) indicated as Neutral, 1SA, 2SA, and 3SA (34). The N-glycan for Pr-DPP-3 (E) has no SA, while those for Pr-DPP-2 (D) and Pr-DPP-4 (F) have two sialic acids.
TABLE 2.
N-terminal sequences of 6 pronase-generated DPP peptides
| Pr-DPP | Sequence | Positionsa | N-glycan |
|---|---|---|---|
| 1 | DDPNXXE | 458–464 | − |
| 2 | SKEEAE | 502–508 | + |
| 3 | AEEDXTSD | 506–513 | + |
| 4 | SDDSXS | 918–924 | + |
| 5 | SDES | 490–494 | − |
| 6 | DSASD | 965–969 | − |
Numbering for DSPP allele pDSPP1023.
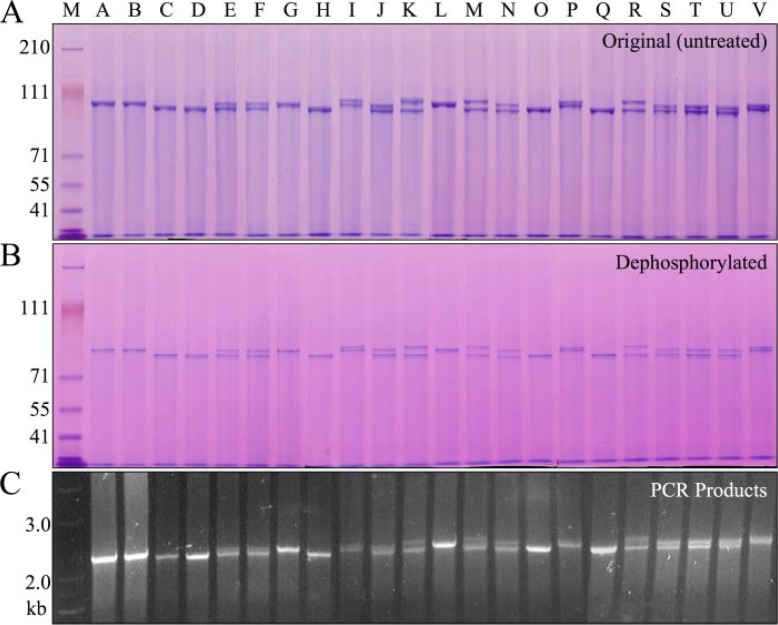
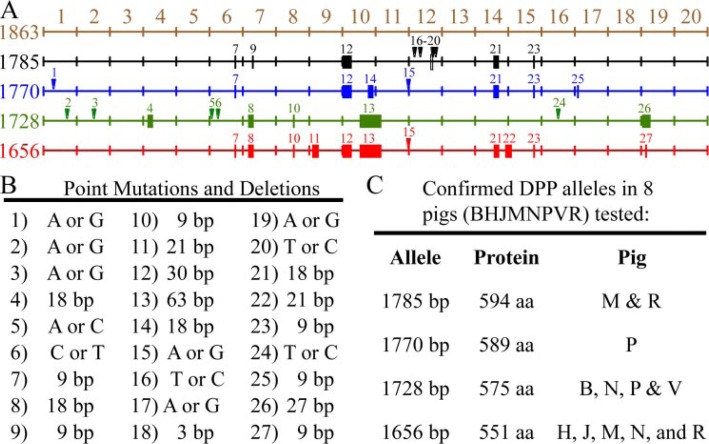
Allelic Polymorphisms as the Source of DPP Length Variations—DPP protein was separately isolated from 22 pigs and analyzed by SDS-PAGE (Fig. 4A). Each pig showed either one or two DPP bands of equal intensity, migrating near 100 kDa. DPP bands from six pigs representing the full range of DPP size variations were characterized by Edman sequencing and all displayed the same N-terminal sequence: DDPNXXE. Dephosphorylation caused the DPP bands to shift to a lower position on the gel, but did not alter the pattern of bands (Fig. 4B). We amplified the DPP coding region (which is not interrupted by any introns) using genomic DNA isolated from the same 22 pigs used to obtain the DPP protein and found length variations in the DPP code that closely correlated with the length variations observed at the protein level (Fig. 4C).
FIGURE 4.
Length polymorphisms of DPP protein and coding region. A, SDS-PAGE showing variations in the mobility of DPP protein isolated from 22 individual pigs. 9 of 22 pigs showed only a single band of DPP (A, B, C, D, G, H, L, O, Q). Thirteen pigs showed 2 DPP bands (E, F, I, J, K, M, N, P, R, S, T, U, V). Although no individual pig showed more than 2 DPP bands, 6 bands of non-identical sizes could be discerned among the 22 pigs. B, dephosphorylation of DPP from the 22 pigs shifted the bands lower on the gel but did not alter the pattern of bands. C, DPP PCR products using genomic DNA as template generated an identical pattern of 1 or 2 bands.
The DPP PCR products from 8 of the pigs were cloned and characterized. Some length variations in the DPP amplification products were identified as PCR artifacts (data not shown), as they could not be reproduced by repeating the experiment. PCR products that represented singular events were consistently ignored in favor of only reproducible outcomes. We characterized 4 groups of DPP cDNAs that were generated from multiple independent PCR cloning experiments, and these clones can account for all of the DPP size variability observed at the protein level. The DPP clones correspond to 4 allelic variations of the DPP code in the Dspp genes of this group of pigs. The 4 clones varied in length because of the presence of multiple in-frame deletions or insertions. All of the length variations localized to the highly redundant region of the DPP code (Fig. 5A). The DPP coding region has 27 sequence variations among the 4 alleles (Fig. 5B). Of these, 11 are point mutations (changing 5 amino acids) and 16 are length variations ranging from 3 to 63 bp that maintain the open reading frame (Table 1 and supplemental data).
FIGURE 5.
DPP allelic variations. A, mapping of sequence variations in the porcine DPP coding region. Point mutations are indicated by numbered arrowheads; deletions are indicated by solid rectangles. On the left is the number of base pairs in each DPP allele. The first map (1863 bp, brown) is not an allele, but a merged reference sequence that contains all of the DPP code found on the 4 DPP alleles characterized. The 20 divisions in the coding region correspond to rows of 93 nucleotides each in the DNA alignment of the DPP alleles (Table 1 and supplemental data). The 4 DPP alleles had 1785 (black), 1770 (blue), 1728 (green), and 1656 (red) bp. B, key to the 27 sequence variations observed in the 4 DPP alleles characterized. C, listing of the pigs analyzed for sequence variations, the number of coding bp in each allele, the number of amino acids (aa) in the DPP protein, and the pigs shown to host each allele. The predominant alleles in the 22 pigs were 1656 and 1728 bp. Based upon the mobility of the proteins (Fig. 4A), and the cloning results, these alleles were likely present in 15 or 14 of the 22 pigs, respectively (1656: C2, D2, E, F, H2, J, K, M, N, O2, Q2, R, S, T, U; 1728: A2, B2, E, F, G2, I, J, L2, N, P, S, T, U, V). Only 6 of the 22 pigs hosted other alleles (I, K, M, P, R, V). The 1770 allele in pig P also appeared to be present in pigs I and V. The 1785 allele in pigs M and R also appeared to be in pig K. The frequency of the 4 characterized alleles in the 22 pigs (44 alleles) were 1656: 20 alleles; 1728: 18 alleles; 1770: 3 alleles; 1785: 3 alleles.
The DPP coding regions in the 4 alleles are 1656 (EU419998), 1728 (EU419999), 1770 (EU420000), and 1785 bp (EU419997) in length (Fig. 5C). Because the lengths of the DPP coding region closely correlate with the apparent sizes of the protein bands on SDS-PAGE (Fig. 3, A and B), it was possible to deduce the distribution of the 4 DPP alleles in the 22 pigs investigated. In these pigs, there are 44 DPP alleles in total: 20 (1656 bp), 18 (1728 bp), 3 (1770 bp), and 3 (1785 bp). Thus, the two smallest DPP alleles (1656 bp and 1728 bp) predominate among the pigs in this group. These commercial pigs have no apparent dental phenotype, and the observed DPP sequence variations are not associated with any loss of function.
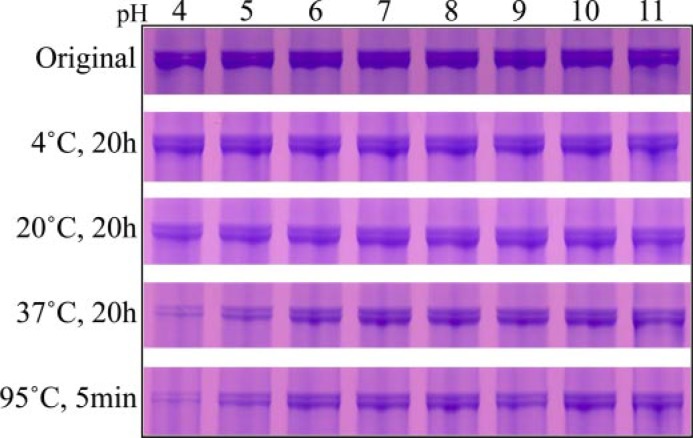
DPP Stability—The effects of pH and temperature on the stability of DPP were examined (Fig. 6). Dentin phosphoprotein is stable for at least 20 h at 4 °C and at 20 °C within the entire range of pH values tested (pH 4–11). At 37 °C, DPP is sensitive to hydrogen ion concentration, and a significant reduction in the amount of DPP is observed after 20 h at pH 5 and below. The degradation of DPP at low pH 5 occurs more rapidly if the protein is heated at 95 °C for 5 min. The instability of DPP observed at low pH and high temperature is reduced in the presence of type I collagen (Fig. 7, A–C).
FIGURE 6.
Instability of DPP at low pH and high temperature. SDS-PAGE of DPP bands stained with Stains-all that have been incubated at the pH shown at the top and the temperature and length of time shown on the left. DPP is particularly unstable if heated at low pH.
FIGURE 7.
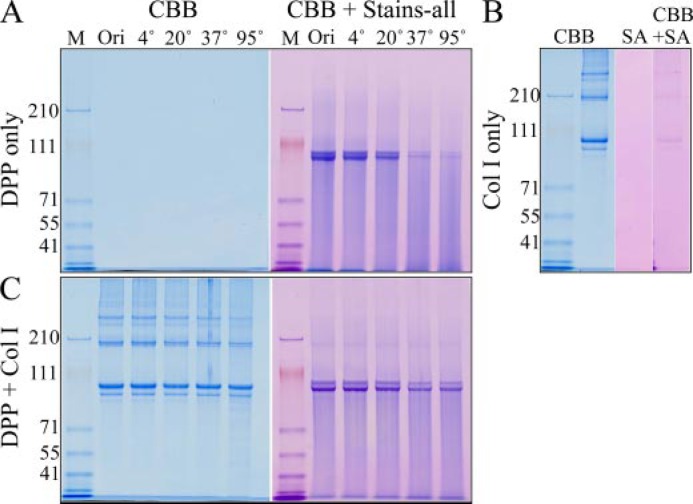
Collagen-DPP interactions increase the stability of DPP and induce collagen fibril assembly. A, SDS-PAGE stained with CBB or CBB plus Stains-all. DPP does not stain with CBB but can be visualized with Stains-all. DPP is not stable when incubated below pH 6 at higher temperature. B, SDS-PAGE showing type I collagen (Col I). Col I stains with CBB but does not stain with Stains-all (SA). C, SDS-PAGE of Col I and DPP after being mixed together and incubated at 4, 20, 37, or 95 °C. The presence of collagen reduces the instability of DPP at 37 and 95 °C.
DISCUSSION
Relative Abundance of DSP and DPP—Using the porcine animal model, which provides abundant dentin proteins and having previously surveyed dentin extracts for the presence of DSPP-derived proteins, we were able to quantify levels of DSPP-derived proteins from developing pig molars and determined that there were approximately equal weights of DSPP cleavage products from the DSP-DGP and DPP regions in our dentin extracts. There are differences, however in the way these proteins are degraded. After the release of DPP, DSP-DGP is processed into numerous relatively small cleavage products (∼17–35 kDa) that can be found in the A extract. Larger DSP-DGP pieces that contain the two long chondroitin sulfate attachments are in the AN extract. DPP degradation products do not form discrete bands, but rather show a continuum of all sizes that make a Stains-all-positive smear on SDS-PAGE (Fig. 1D, R1 and R2). This pattern might reflect an inherent chemical instability rather than processing by proteases (25).
DPP Post-translational Modifications—DPP is the most acidic protein known and has an isoelectric point of 1.1 (26). The low electric point is due to its large number of acidic amino acids and phosphorylated serines. Porcine DPP migrates at about 100 kDa on SDS-PAGE. When DPP is isolated from individual pigs, either one or two DPP bands are observed. We have demonstrated that the presence of multiple bands is due to allelic variation. Porcine DPP is relatively homogeneous with respect to its post-translational modifications. This contrasts with rat DPP where multiple forms have been proposed that vary according to their degree of phosphorylation (3).
We determined that the average number of phosphates per DPP molecule is 212 when using DPPs apparent molecular mass (100 kDa) on SDS-PAGE to calculate moles of DPP in a weighed sample. The results of the cloning and post-translational modification studies, however, suggest that 73 kDa might be a better estimate for the molecular mass of porcine DPP. The predominant forms of DPP in our population of pigs contain 551 or 575 amino acids. Without post-translational modifications, the common DPP forms have calculated molecular masses of 54.6 and 57.1 kDa. If DPP were 73 kDa, the average number of phosphates per molecule would be 155 (73 kDa/mol × 212 P/100 kDa/mol = 155 P). This number of phosphates (155/molecule) has a mass of 12.4 kDa (155 P × 80 Da/p = 12.4 kDa). The breakdown for the 73-kDa native protein is: 56-kDa protein + 12.4-kDa p + 4.6-kDa N-glycos = 73 kDa. As there are 310 serines per molecule, half of the serines in DPP are phosphorylated.
The DPP deduced amino acid sequences of the 4 DPP alleles are aligned in Fig. 8. There are eight potential N-linked glycosylation sites (NXS, where x≠P) in the N-terminal region of DPP and five in the C-terminal region. Pronase digests of DPP generated short glycopeptides derived from one site in each of these regions. It seems unlikely that additional sites are glycosylated as the mobility of DPP on SDS-PAGE does not change appreciably following deglycosylation.
FIGURE 8.
Alignment of the DPP deduced amino acid sequences of 4 porcine DSPP alleles pDSPP1066/DPP594 (black), pDSPP1061/DPP589 (blue), pDSPP1047/DPP575 (green), and pDSPP1023/DPP551, (red). Key: five amino acid substitutions are in bold. Asparagines (N) in the appropriate context for glycosylation are underlined. Asparagines determined to be glycosylated following pronase digestions of DPP are in bold and underlined.
DPP Length Polymorphisms—The size of DPP varies among species. Rat, bovine, and porcine DPPs have an apparent molecular mass just under 100 kDa (3, 27), while human DPP migrates at about 140 kDa on SDS-PAGE (28). It is becoming increasingly apparent that the size of DPP also varies within species. These observations suggest that DPP function is not affected by length variations in its repeat region. The functional significance (or insignificance) of DPP length variations is important when considering whether a particular sequence variation is disease-causing or simply a polymorphism. It is also important when considering DPP function.
Our studies of 22 commercial pigs demonstrate that DPP allelic variations at the DNA level translate into size variations at the protein level. The 4 Dspp alleles showed 16 DPP length variations ranging from 1 to 21 amino acids and 5 single amino acid substitutions. There is no apparent loss of function due to this variability. Previously we reported the cloning of two porcine DSPP (pDSPP) cDNA clones encoding a total of 600 and 593 amino acids, respectively: pDSPP600/DPP128 (Acc. AY161863) and pDSPP593/pDPP121 (Acc. AY161862). The DPP part of these clones was short and apparently had most of the DPP region deleted during the cloning procedure, possibly during the reverse transcription reaction (21). Only the DPP region was affected by artifacts, and the early DSPP clones demonstrated that porcine DPP begins at Asp473 of DSPP. Therefore, the 4 DPP allelic variants identified in the 22 pigs can be designated pDSPP1023/DPP551, pDSPP1047/DPP575, pDSPP1061/DPP589, and pDSPP1066/DPP594. The pDSPP1023/DPP551 and pDSPP1047/DPP575 alleles predominate (38/44 alleles) in our group of pigs and express the two major DPP bands observed in protein preparations from the same animals. By comparison, rat DSPP protein has 970 amino acids, 523 of which are in the DPP portion of the protein (rDSPP970/DPP523, Acc. AJ403971) (29), while human DSPP is larger and may also show allelic length variations (hDSPP1301/DPP839, Acc. NM_014208; hDSPP1253/DPP791 Acc. AAF42472) (30).
DPP Instability—The DPP domain is inherently unstable and degrades when heated in vitro (25). Increasing concentrations of SDS helped protect against thermal degradation. We confirmed these findings and add that DPP is unstable at low pH and that type I collagen helps protect DPP from thermal degradation, presumably by forming Col I-DPP complexes.
DPP Is an Intrinsically Disordered Protein—Proteins that do not appear to adopt a well-defined conformation under native conditions are increasingly being categorized as intrinsically disordered proteins (IDP) (31). The tendency for proteins to be intrinsically disordered can be predicted from their amino acid sequences. DPP is 29% aspartic acid and 54% serine, with half of the serines phosphorylated. The PONDR computational software for predicting naturally disordered structures gives DPP its highest possible score (1.0) over a continuous region extending for more than 350 amino acids (32). NMR analysis showed that bovine DPP is a molecule of uniformly high mobility, which is consistent with an intrinsically disordered protein (33).
In summary, our results demonstrate that the amounts of protein in porcine dentin extracts that are derived from the DSP-DGP and DPP regions of DSPP are about equal. Porcine DPP is extensively phosphorylated, averaging 155 phosphates per molecule, and is glycosylated at its N-terminal and C-terminal domains. Although porcine DPP shows an apparent molecular mass of 100 kDa on SDS-PAGE, we estimate its actual size to be 73 kDa, based upon the deduced masses of its amino acid chain and post-translational modifications. Allelic length variations in the DPP coding region translate into size variations at the protein level. Such length variations are exceedingly rare in proteins. They occur, however, within the highly redundant DPP region and represent normal variations that do not interfere with protein function.
Acknowledgments
We thank Tom Forton, manager of the Michigan State University Meat Laboratory and members of the Michigan State University Department of Animal Science for their kind assistance in obtaining fresh developing molars from pigs. We thank Dr. Myron Crawford, director of W. M. Keck Foundation Biotechnology Resource Laboratory at Yale University and Nancy Williams for the amino acid analysis and protein sequencing.
Footnotes
This work was supported, in whole or in part, by Grants DE15846 and DE11301 (NIDCR). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The abbreviations used are: DSPP, dentin sialophosphoprotein; 2-AA, 2-aminobenzoic acid; AEBSF, 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride; CBB, Coomassie Brilliant Blue; Col I, type I collagen; DGP, dentin glycoprotein; DPP, dentin phosphoprotein; DSP, dentin sialoprotein; HILIC, hydrophilic interaction liquid chromatography; HPLC, high performance liquid chromatography; IDP, intrinsically disordered protein; NMR, nuclear magnetic resonance; NP, normal phase; Pr, pronase; RP, reversed phase; RP-HPLC, reverse phase high performance liquid chromatography; Tricine, N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine.
The on-line version of this article (available at http://www.jbc.org) contains supplemental sequence data.
REFERENCES
- 1.Yamakoshi Y, Hu JC, Fukae M, Iwata T, Kim JW, Zhang H, Simmer JP. J Biol Chem. 2005;280:1552–1560. doi: 10.1074/jbc.M409606200. [DOI] [PubMed] [Google Scholar]
- 2.Yamakoshi Y, Hu JC, Fukae M, Zhang H, Simmer JP. J Biol Chem. 2005;280:17472–17479. doi: 10.1074/jbc.M413220200. [DOI] [PubMed] [Google Scholar]
- 3.Butler WT, Bhown M, DiMuzio MT, Cothran WC, Linde A. Arch Biochem Biophys. 1983;225:178–186. doi: 10.1016/0003-9861(83)90021-8. [DOI] [PubMed] [Google Scholar]
- 4.Huq NL, Cross KJ, Talbo GH, Riley PF, Loganathan A, Crossley MA, Perich JW, Reynolds EC. J Dent Res. 2000;79:1914–1919. doi: 10.1177/00220345000790111701. [DOI] [PubMed] [Google Scholar]
- 5.Butler WT, Bhown M, Dimuzio MT, Linde A. Coll Relat Res. 1981;1:187–199. doi: 10.1016/s0174-173x(81)80019-2. [DOI] [PubMed] [Google Scholar]
- 6.Butler WT. Eur J Oral Sci. 1998;106:204–210. doi: 10.1111/j.1600-0722.1998.tb02177.x. [DOI] [PubMed] [Google Scholar]
- 7.Patel P. Nat Genet. 2001;27:129–130. doi: 10.1038/84728. [DOI] [PubMed] [Google Scholar]
- 8.MacDougall M, Simmons D, Luan X, Nydegger J, Feng J, Gu TT. J Biol Chem. 1997;272:835–842. doi: 10.1074/jbc.272.2.835. [DOI] [PubMed] [Google Scholar]
- 9.Qin C, Brunn JC, Baba O, Wygant JN, McIntyre BW, Butler WT. Eur J Oral Sci. 2003;111:235–242. doi: 10.1034/j.1600-0722.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim JW, Simmer JP. J Dent Res. 2007;86:392–399. doi: 10.1177/154405910708600502. [DOI] [PubMed] [Google Scholar]
- 11.Hart PS, Hart TC. Cells Tissues Organs. 2007;186:70–77. doi: 10.1159/000102682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sreenath T, Thyagarajan T, Hall B, Longenecker G, D’Souza R, Hong S, Wright JT, MacDougall M, Sauk J, Kulkarni AB. J Biol Chem. 2003;278:24874–24880. doi: 10.1074/jbc.M303908200. [DOI] [PubMed] [Google Scholar]
- 13.Xiao S, Yu C, Chou X, Yuan W, Wang Y, Bu L, Fu G, Qian M, Yang J, Shi Y, Hu L, Han B, Wang Z, Huang W, Liu J, Chen Z, Zhao G, Kong X. Nat Genet. 2001;27:201–204. doi: 10.1038/84848. [DOI] [PubMed] [Google Scholar]
- 14.Malmgren B, Lindskog S, Elgadi A, Norgren S. Hum Genet. 2004;114:491–498. doi: 10.1007/s00439-004-1084-z. [DOI] [PubMed] [Google Scholar]
- 15.Kim JW, Nam SH, Jang KT, Lee SH, Kim CC, Hahn SH, Hu JC, Simmer JP. Hum. Genet. 2004;115:248–254. doi: 10.1007/s00439-004-1143-5. [DOI] [PubMed] [Google Scholar]
- 16.Kim JW, Hu JC, Lee JI, Moon SK, Kim YJ, Jang KT, Lee SH, Kim CC, Hahn SH, Simmer JP. Hum Genet. 2005;116:186–191. doi: 10.1007/s00439-004-1223-6. [DOI] [PubMed] [Google Scholar]
- 17.Holappa H, Nieminen P, Tolva L, Lukinmaa PL, Alaluusua S. Eur J Oral Sci. 2006;114:381–384. doi: 10.1111/j.1600-0722.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- 18.Song Y, Wang C, Peng B, Ye X, Zhao G, Fan M, Fu Q, Bian Z. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:360–374. doi: 10.1016/j.tripleo.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Chen L, Liu J, Zhao Z, Qu E, Wang X, Chang W, Xu C, Wang QK, Liu M. BMC Med Genet. 2007;8:52. doi: 10.1186/1471-2350-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ritchie HH, Hou H, Veis A, Butler WT. J Biol Chem. 1994;269:3698–3702. [PubMed] [Google Scholar]
- 21.Yamakoshi Y, Hu JC, Liu S, Zhang C, Oida S, Fukae M, Simmer JP. Eur J Oral Sci. 2003;111:60–67. doi: 10.1034/j.1600-0722.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- 22.Dong J, Gu T, Jeffords L, MacDougall M. Am J Med Genet. 2005;132:305–309. doi: 10.1002/ajmg.a.30460. [DOI] [PubMed] [Google Scholar]
- 23.Qin C, Baba O, Butler WT. Crit Rev Oral Biol Med. 2004;15:126–136. doi: 10.1177/154411130401500302. [DOI] [PubMed] [Google Scholar]
- 24.Yamakoshi Y, Hu JC, Iwata T, Kobayashi K, Fukae M, Simmer JP. J Biol Chem. 2006;281:38235–38243. doi: 10.1074/jbc.M607767200. [DOI] [PubMed] [Google Scholar]
- 25.Ibaraki K, Shimokawa H, Sasaki S. Matrix. 1991;11:115–124. doi: 10.1016/s0934-8832(11)80215-5. [DOI] [PubMed] [Google Scholar]
- 26.Jonsson M, Fredriksson S. J Chromatogr. 1978;157:234–242. doi: 10.1016/s0021-9673(00)92338-0. [DOI] [PubMed] [Google Scholar]
- 27.Termine JD, Belcourt AB, Miyamoto MS, Conn KM. J Biol Chem. 1980;255:9769–9772. [PubMed] [Google Scholar]
- 28.Chang SR, Chiego D, Jr, Clarkson BH. Calcif Tissue Int. 1996;59:149–153. doi: 10.1007/s002239900101. [DOI] [PubMed] [Google Scholar]
- 29.George A, Bannon L, Sabsay B, Dillon JW, Malone J, Veis A, Jenkins NA, Gilbert DJ, Copeland NG. J Biol Chem. 1996;271:32869–32873. doi: 10.1074/jbc.271.51.32869. [DOI] [PubMed] [Google Scholar]
- 30.Gu K, Chang S, Ritchie HH, Clarkson BH, Rutherford RB. Eur J Oral Sci. 2000;108:35–42. doi: 10.1034/j.1600-0722.2000.00765.x. [DOI] [PubMed] [Google Scholar]
- 31.Hansen JC, Lu X, Ross ED, Woody RW. J Biol Chem. 2006;281:1853–1856. doi: 10.1074/jbc.R500022200. [DOI] [PubMed] [Google Scholar]
- 32.Yamakoshi Y. J Oral Biosciences. 2008 doi: 10.2330/joralbiosci.50.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cross KJ, Huq NL, Reynolds EC. J Pept Res. 2005;66:59–67. doi: 10.1111/j.1399-3011.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 34.Thomas LJ, Panneerselvam K, Beattie DT, Picard MD, Xu B, Rittershaus CW, Marsh HC, Jr, Hammond RA, Qian J, Stevenson T, Zopf D, Bayer RJ. Glycobiology. 2004;14:883–893. doi: 10.1093/glycob/cwh112. [DOI] [PubMed] [Google Scholar]