Abstract
Normally, non-native polypeptides are not transported through the secretory pathway. Rather, they are translocated from the endoplasmic reticulum (ER) lumen into the cytosol where they are degraded by proteasomes. Here we characterize the function in ER quality control of two proteins derived from alternative splicing of the OS-9 gene. OS-9.1 and OS-9.2 are ubiquitously expressed in human tissues and are amplified in tumors. They are transcriptionally induced upon activation of the Ire1/Xbp1 ER-stress pathway. OS-9 variants do not associate with folding-competent proteins. Rather, they selectively bind folding-defective ones thereby inhibiting transport of non-native conformers through the secretory pathway. The intralumenal level of OS-9.1 and OS-9.2 inversely correlates with the fraction of a folding-defective glycoprotein, the Nullhong kong (NHK) variant of α1-antitrypsin that escapes retention-based ER quality control. OS-9 up-regulation does not affect NHK disposal, but reduction of the intralumenal level of OS-9.1 and OS-9.2 substantially delays disposal of this model substrate. OS-9.1 and OS-9.2 also associate transiently with non-glycosylated folding-defective proteins, but association is unproductive. Finally, OS-9 activity does not require an intact mannose 6-P homology domain. Thus, OS-9.1 and OS-9.2 play a dual role in mammalian ER quality control: first as crucial retention factors for mis-folded conformers, and second as promoters of protein disposal from the ER lumen.
About 30% of the eukaryotic gene products are synthesized by ribosomes attached at the cytosolic face of the endoplasmic reticulum (ER)2 (1). The nascent polypeptide chains are translocated into the ER lumen where molecular chaperones and folding enzymes assist their maturation. Native proteins are transported at their intra- or extracellular destination through the secretory pathway. The ER lumen also contains chaperones and enzymes that retain and appropriately tag terminally mis-folded proteins for destruction. Due to the facility of manipulating the yeast genome, many aspects and components of ERAD have been discovered in Saccharomyces cerevisiae (2–4). Yos9p is no exception. It was initially reported that deletion of Yos9p from the yeast ER selectively inhibits degradation of glycosylated ERAD substrates (5). Subsequent work revealed that Yos9p is required for disposal of substrates with luminal folding defects, whereas it is dispensable for disposal of proteins with defects in the transmembrane and cytosolic domains (6–10). Studies on the involvement of the mammalian ortholog OS-9 in ERAD have been hampered by data showing that OS-9 is a cytosolic protein (11). This study was followed by a series of publications in which experimental design and interpretation of the data were based on the assumption that OS-9 is a cytosolic protein (12–14).
Our analysis shows that OS-9 is a N-glycosylated protein expressed in two splice variants in the ER lumen. Transcription of both OS-9 variants is enhanced upon activation of the Ire1/Xbp1 pathway in cells exposed to acute ER stress. OS-9 variants do not associate with folding-competent proteins, but form non-covalent complexes with misfolded ones. OS-9 association prevents secretion from the ER of misfolded NHK conformers and facilitates NHK disposal. OS-9 variants play a crucial role in maintaining the tightness of retention-based ER quality control and in promoting disposal of misfolded proteins from the mammalian ER.
EXPERIMENTAL PROCEDURES
Antibodies, Expression Plasmids, and OS-9 Mutagenesis—The pcDNA3 plasmids encoding mouse OS-9.1 and OS-9.2 were a kind gift of L. Litovchick. The nucleotide sequences of all plasmids used in this study were verified on both strands. Mutagenesis of the OS-9 MRH domain was carried out using a PCR-based site-directed mutagenesis Quick Gene® kit (Stratagene). The following primers were used: 5′-CTCAACGGGAAGCCCG-CAGAAGCTGAAGTTCG-3′ (sense) and 5′-CGAACTTC-AGCTTCTGCGGGCTTCCCGTTGAG-3′ (antisense).
The anti-OS-9 used for immunoprecipitation, immunoblots, and immunofluorescence was from Novus Biologicals (BC100-519). The anti-protein-disulfide isomerase (PDI) for immunofluorescence was a commercial monoclonal antibody from ABR (MA3-018). The anti-EDEM1 for immunoprecipitation was purchased from Santa Cruz Biotechnology Inc. (C-19, sc-27391). The monoclonal anti-amyloid precursor protein (15) was a kind gift of P. Paganetti. For immunoblots, all primary antibodies were used at 1:1000–1:2000 dilutions. Secondary antibodies for immunoblots were horseradish peroxidase-conjugated anti-rabbit antibodies (1:5000). The ECL Plus detection system was from Amersham Biosciences. DNA preparations were obtained using commercially available purification kits (Sigma). Secondary antibodies for immunofluorescence (Alexa 488-labeled goat anti-rabbit, Alexa 594-labeled goat anti-mouse) were used at 1:100 dilution.
Cell Lines, Transient Transfections, Metabolic Labeling, Analysis of Data—All cell lines used in the study were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. Xbp1−/− cells were a kind gift of L. Glimcher. Cells at 80–90% confluence in a 6-cm tissue culture plate were transfected with the expression plasmid of interest (4 μg for single transfections, 6 μg of total DNA for transfections with two plasmids) using Lipofectamine 2000 (Invitrogen) according to the instructions of the manufacturer. Experiments were performed 17 h after transfection. Metabolic labeling is described in Ref. 16. Gels were exposed to Bio-Max (Kodak) films and scanned with an Agfa scanner. Relevant bands were quantified by ImageQuant software (Molecular Dynamics).
Subcellular Fractionation and Separation of Membrane Versus Luminal Content—35S-Labeled cells were extensively washed in a 10-cm dish with isotonic buffer. They were detached with a rubber policeman and resuspended in 800 μl of homogenization buffer (10 mm triethanolamine, 10 mm acetic acid, 250 mm sucrose, 20 mm N-ethylmaleimide, 1 mm EDTA and a mixture of protease inhibitors, pH 7.4). Cells were broken with 10 passages through a 25-gauge 1 needle. Postnuclear supernatants were subjected to ultracentrifugation (45 min, 200,000 × g in TLA 120.2) to separate endomembranes from the cytosol. The endomembrane-containing pellet was extensively washed, resuspended in 500 μl of 100 mm Na2CO3, and incubated for 25 min on ice for carbonate extraction. After an additional ultracentrifugation step (45 min, 200,000 × g), the supernatant was harvested (ER lumen in Fig. 1G). The endomembrane fraction (ER memb in Fig. 1G) was washed once with 100 mm Na2CO3 (35 min, 200,000 × g) and then resuspended in 800 μl of lysis buffer. Insoluble material was removed after 25 min on ice by 10 min centrifugation at 200,000 × g. The ER luminal content and the endomembrane fraction were subjected to immunoprecipitation against calreticulin, calnexin, OS-9, and EDEM1.
FIGURE 1.
OS-9 is a glycosylated, stress-inducible ER resident protein. A, schematic view of OS-9, numbering is for the human protein. The signal peptide (SP), the mannose 6-phosphate receptor homology domain (MRH), and the putative splice regions (gray part of exon 11 and the entire exon 13) are shown. B, whole cDNA pool from HEK293 cells was amplified with specific primers (depicted in panel A) to determine the expression of OS-9 splice variants. Theoretical lengths are shown on the right, positions of 100-, 200-, 300-, 400-, and 500-bp markers are shown on the left. C, expression of endogenous (lane 1) and ectopic OS-9.1 (lane 2) and OS-9.2 (lane 3) is revealed by immunoblot (IB). D, EndoH treatment to confirm modification with N-linked oligosaccharides of the endogenous OS-9 variants revealed by IB and ectopically expressed, labeled OS-9 variants immunoisolated (IP) from cell-lysates. E, the presence of intramolecular disulfide bond was confirmed by changes in OS-9 mobility in non-reducing (NR) versus reducing (R) gels. F, immunofluorescence showing co-localization between PDI (left panel) and ectopically expressed OS-9 (right panel). G, endomembranes were purified from metabolically labeled HEK293 cells. The select proteins (calreticulin (crt), calnexin (cnx), OS-9, and EDEM1) were immunoisolated from luminal (lanes 1– 4) or membrane fractions (lanes 5–8). The OS9 and EDEM1 mobility is shown in lanes 9 and 10, respectively. H, HEK293, wt, and Xbp1−/− MEF were exposed (+) or not (−) to 2.5 μg/ml tunicamycin for 12 h to induce ER stress. The levels of OS-9, EDEM1, Synoviolin (Xbp1-dependent), BiP and Sel1L (ATF6-dependent), and Actin (loading control) have been assessed by semi-quantitative RT-PCR. Depletion of Xpb1 prevents stress induction of both OS-9 transcripts. I, quantitative RT-PCR analysis of induction of BiP, EDEM1, OS-9, Sel1L, and Synoviolin upon ER stress in wt and Xbp1−/− MEF. Actin was used as endogenous control (n = 2).
Immunofluorescence Microscopy—2 × 105 HEK293 cells were seeded in a 6-cm Petri dishes containing 1% Alcian blue-treated glass coverslips. Coverslips were rinsed with phosphate-buffered saline, and cells were fixed in 3.7% formaldehyde. After 2 short washings with 10 mm Hepes serum-free medium, and two additional washings with phosphate-buffered saline, the antigen accessibility was improved by a 20-min incubation with 0.05% saponin, 10% goat serum, 10 mm Hepes, and 15 mm glycine. Images were viewed on a Nikon eclipse E-800 fluorescent microscope, captured by a Hamamatsu EM-CCD Digital camera C9100, and analyzed with the Open lab 3 software (Improvision, Inc., Lexington, MA).
Semi-quantitative and Quantitative Reverse Transcriptase-Polymerase Chain Reactions (RT-PCR)—Primers used for identification of OS-9 splice variants expressed in HEK293 cells were as follows: primers amplifying all 4 putative splice variants (Fig. 1B, left panel): CTTCCGTCAGACCGAGACC (forward), GGGCGGACAATTTTGATCT (reverse); primers amplifying only OS-9.1 and OS-9.4 (Fig. 1B, right): CTTCCGTCAGACCGA-GACC (forward), CTGATTAGGGCCTCCGAGA (reverse). For analysis of variations in gene transcription upon ER stress, HEK293, wt, and Xbp1−/− mouse embryonic fibroblasts (MEF) were plated in 6-cm dishes without (mock) or with tunicamycin (Tun, 2.5 μg/ml). After 12 h cells were lysed in TRIzol reagent (Invitrogen) and RNA was isolated according to the instructions of the manufacturer. Two μg of RNA were used for cDNA synthesis using SuperScriptII reverse transcriptase (Invitrogen) and oligo(dT) (Invitrogen). Semi-quantitative PCR was performed using Taq DNA polymerase (Invitrogen) with transcript-specific primers using three different cycle numbers for each gene, all within the linear phase of template amplification. Quantitative RT-PCR was performed using the 7900HT Fast Real-time PCR System. The PCR were performed in a 10-μl Power SYBR Green PCR master mixture (Applied Biosystem), 5 μl of cDNA, 4 μl of ddH2O, and 1 μl of primer mixture (0.5 μm final). The housekeeping gene β-actin was used as reference. Data were analyzed using the SDS 2.2.2 software.
The primers were as follows (m, mouse; h, human): m-β-Actin, CTTTCTGGGTATGGAATCCT (forward), GGAGCAATGAT-CTTGATCTT (reverse); h-β-Actin, CTTCCTGGGCATGGAG-TCCT (forward), GGAGCAATGATCTTGATCTT (reverse); m/h-BiP, GAGTTCTTCAATGGCAAGGA (forward), CCAGT-CAGATCAAATGTACCC (reverse); m-EDEM1, TGGAATTT-GGGATTCTGAGC (forward), CTGCAGTCCAGGGAAG-AAAG (reverse); h-EDEM1, AAGATTCCACCGTCCAAGTC (forward), GTATCATTGCTCCGGAGGTT (reverse); m/hOS-9, GGAGGAGCTGAGTGAGATGC (forward), GTCTGGC-TGTGGTAGCGTTT (reverse) m-Sel1L; CCTCGACCAGAGA-GAAGCACC (forward), GCGGTCTCATAATCCACATCA (rev); m-Synoviolin, CGTGTGGACTTTATGGAACGC (forward), CGGGTCAGGATGCTGTGATAA (reverse).
Lentiviral Transduction and Stable Cell Line Establishment—Vesicular stomatitis virus g pseudotyped lentiviral vectors were produced by transient transfection of 293FT cells (Invitrogen) with an HIV-1-based packaging vector, vesicular stomatitis virus g envelope vector, and transfer vector containing either the short hairpin for human OS-9 or Luciferase. Forty-eight hours post-transfection, viral supernatants were filtered through a 0.45-μm filter, and added to HEK293 cells. Transduced cells were selected with puromycin gradually, from 1 to 30 μg/ml over 2 weeks. OS-9 short hairpin RNA targeting sequence, targeting the 3′ untranslated region, is as follows: GCTGCCTACCTGGAGATTCAG.
RESULTS
Two Splice Variants of OS-9 Are Expressed in Mammalian Cells—The OS-9 gene is composed of 15 exons (17). Alternative splicing events that conform to the GT-AG rule could generate 4 variants of the protein. The full-length version of OS-9.1 comprises 667 amino acids in humans; OS-9.2, which lacks the entire exon 13 and is 55 residues shorter with a glutamate to glycine conversion in the splice region; OS-9.3, which lacks exon 13 and the final part of exon 11 and is 70 residues shorter; OS-9.4, which lacks the final part of exon 11 resulting in a 15-residues deletion (schematics in Fig. 1A and Ref. 18). The expression of OS-9.1 and OS-9.2, two splice variants derived from a single transcript, has been shown in osteosarcoma cell lines (18).
To determine which splice variants are expressed in HEK293 cells, we amplified the total cellular cDNA pool with specific primers. Our analysis revealed expression of transcripts for OS-9.1 (Fig. 1B, amplification products of 461 in the left panel and 333 bp in the right panel) and OS-9.2 (Fig. 1B, amplification products of 296 bp in the left panel). Transcripts for variants 3 and 4 were not expressed (Fig. 1B, lack of amplification products of 416 and 251 bp in the left and 288 bp in the right panel, respectively). To confirm expression of OS-9.1 and OS-9.2 at the protein level, HEK293 cells were detergent-solubilized and the proteins contained in the post-nuclear supernatant were separated by SDS-PAGE, transferred onto polyvinylidene difluoride membrane, and probed with polyclonal antibodies raised against OS-9. The antibodies recognized two polypeptide bands (Fig. 1C, lane 1). The upper band co-migrated with ectopically expressed OS-9.1 (Fig. 1C, lane 2) and the lower with OS-9.2 (Fig. 1C, lane 3). Similar results were obtained with MEF, thus showing that mammalian-cultured cells contain two splice variants of OS-9: OS-9.1 and OS-9.2.
OS-9 Is a N-Glycosylated Protein of the ER Lumen—Yos9p, the yeast ortholog of mammalian OS-9 has an amino-terminal signal sequence for protein targeting into the ER lumen and a COOH-terminal HDEL signal for ER retrieval. Consistent with intralumenal localization, Yos9p displays 4 N-linked glycans, a hallmark of proteins synthesized in the ER. The human ortholog OS-9 has been reported to be associated with the cytosolic face of the ER membrane (11). Instead, our results clearly showed that OS-9 variants are soluble proteins in the ER. First, as for the yeast protein, the hidden Markov model used by SignalP (19) shows that both the human and mouse OS-9 have a cleavable NH2-terminal signal sequence, a characteristic of proteins translocated into the ER (20).
Second, endogenous and ectopically expressed OS-9.1 and OS-9.2 are N-glycosylated, a covalent modification of proteins translocated into the ER (21) that would not occur if OS-9 would face the cytosol as claimed in Ref. 11. For the endogenous proteins, this was established by subjecting detergent lysates of HEK293 cells to mock or EndoH treatments. EndoH specifically removes N-linked oligosaccharides from ER proteins (22). Cellular proteins from mock- and EndoH-treated detergent lysates were separated in SDS-PAGE, transferred onto polyvinylidene difluoride membranes, and probed with antibodies raised against endogenous OS-9 (eOS9) (Fig. 1D, lane 1 (Mock) and 2 (EndoH)). EndoH treatment reduced the apparent molecular mass of both OS-9 variants of about 4 kDa compared with untreated samples. The faster electrophoretic mobility is consistent with removal of 1 N-linked glycan from asparagine 177, which is the only acceptor site for oligosaccharides in the OS-9.1 and OS-9.2 sequences. N-Glycosylation was also confirmed for ectopically expressed OS-9 (rOS9). In this case, HEK293 cells were transfected with plasmids for expression of OS-9.1 (Fig. 1D, lanes 3 and 4) or OS-9.2 (lanes 5 and 6). Seventeen h post-transfection, cells were metabolically labeled with [35S]methionine and -cysteine. Labeled OS-9.1 and OS-9.2 were immunoisolated with specific antibodies from detergent lysates and the immunocomplexes were mock-treated (lanes 3 and 5) or were incubated for 1 h with EndoH (lanes 4 and 6). EndoH treatment enhanced the electrophoretic mobility of the ectopically expressed proteins, thus confirming their glycosylated status (compare lane 3 with 4 in Fig. 1D for OS-9.1 and lane 5 with 6 for OS-9.2).
Third, the electrophoretic mobility of both OS-9 variants is slower when the proteins are separated under reducing (R, Fig. 1E) rather than under non-reducing conditions. This shows that OS-9 cysteines are engaged in intramolecular disulfide bonds (23), another hallmark of proteins translocated in the ER lumen. Fourth, immunofluorescence analysis in HEK293 cells revealed the co-localization of ectopically expressed OS-9 with PDI, a canonical ER marker (Fig. 1F).
To determine whether OS-9 variants are membrane-bound or luminal proteins, HEK293 cells were metabolically labeled and extensively washed. Cells were broken with 10 passages through a 25-gauge 1 needle. Postnuclear supernatants were subjected to ultracentrifugation to separate endomembranes from the cytosol. The endomembrane pellet was washed twice to remove cytosolic contaminants and was subsequently extracted with carbonate to separate the luminal content (Fig. 1G, lanes 1–4) from the membrane-bound proteins (lanes 5–8). Calreticulin and EDEM1 served as luminal markers (24, 25). Consistently, they mostly partitioned in the luminal fraction (Fig. 1G, lanes 1 and 4, respectively) and were only weakly detected in the membrane fraction (lanes 5 and 8). On the other hand, the ER membrane marker calnexin partitioned in the membrane fraction (lane 6) and was not found in the luminal fraction (lane 2). The distribution of OS-9 variants reflected the distribution of the luminal markers calreticulin and EDEM1 as shown by their enrichment in the luminal fraction (Fig. 1G, lane 3).
OS-9 Is an Ire1/Xbp1-inducible ER Stress-regulated Gene—We next determined whether OS-9 transcription is induced in cells experiencing acute ER stress. RT-PCR analysis in HEK293 and MEF cells revealed that the basal level of OS-9.1 transcripts is substantially lower than that of OS-9.2 transcripts (Fig. 1H, lane 1 for HEK and lane 3 for MEF). This confirms data showing that transcripts for isoform 2 are the most abundantly expressed in human sarcomas (18). Cell exposure to tunicamycin, a potent ER-stress inducer, enhanced the expression of OS-9 transcripts without affecting the splicing reaction generating OS-9.2. OS-9.2 remained the most abundant splice variant amplified in both HEK293 (Fig. 1H, lane 2) and MEF (Fig. 1H, lane 4) subjected to acute ER stress. As positive controls, tunicamycin also enhanced expression of EDEM1, BiP, Synoviolin, and Sel1L transcripts (Fig. 1H). Deletion of the Ire1-activated transcription factor Xbp1 did not prevent induction of BiP and Sel1L, two genes regulated by the ATF6 ER-stress pathway (26), but substantially inhibited induction of EDEM1, OS-9, and Synoviolin transcription (Fig. 1H, lanes 5–6 and also refer to Ref. 27 for Xbp1-regulation of Synoviolin). These data were confirmed by quantitative real-time RT-PCR analysis (Fig. 1I). These results are intriguing because in S. cerevisiae Yos9p forms a functional complex with Hrd1p and Hrd3p, the orthologs of Synoviolin and Sel1L, respectively. It seems therefore that components of the same functional complex are regulated by distinct stress-induced pathways. Altogether, Fig. 1 shows that OS-9 variants are soluble, highly oxidized, N-glycosylated proteins of the ER lumen, which are transcriptionally induced upon activation of the Ire1/Xbp1 ER-stress pathway.
OS-9 Variants Associate with NHK and Inhibit Secretion of Extensively Misfolded Conformers from the ER Lumen—Human α1-antitrypsin is a serine protease inhibitor secreted from hepatocytes (28). The NHK variant (29) is a truncated, folding defective form of α1-antitrypsin (30). We report that in transiently transfected mammalian cultured cells, only about 75% of ectopically expressed NHK becomes an ERAD substrate. About 25% of the newly synthesized NHK escapes ER retention and is secreted from cells as aberrant disulfide-bonded dimers (see below and supplemental Fig. S1).
To determine the function of OS-9 variants in the ER lumen, HEK293 cells expressing NHK were mock transfected (Fig. 2, lanes 1–3), and transfected with OS-9.1 (lanes 4–6) or OS-9.2 (lanes 7–9). The proteins were metabolically labeled for 10 min with [35S]methionine and cysteine. After various chase times with unlabeled amino acids, the labeled intracellular NHK (Fig. 2, A and B) and the labeled secreted NHK escaping retention-based ER quality control (Fig. 2, C and D) were immunoisolated with specific antibodies from cell lysates and from the culture media, respectively. Quantifications were performed by band densitometry in reducing SDS-PAGE. These experiments revealed first that OS-9.1 and OS-9.2 associate with NHK. In fact, they did co-precipitate with the ERAD substrate (arrows in Fig. 2A, lanes 4–9). Second, that co-expression of both OS-9 variants similarly delayed disappearance of labeled NHK from the ER lumen (Fig. 2, A and B). In fact, after a 120-min chase, 30% of the labeled NHK was still retained in cells with physiologic OS-9 content (Fig. 2, A, lane 3, and B) versus 55% in cells expressing high levels of OS-9.1 (lane 6) or OS-9.2 (lane 9).
FIGURE 2.
Consequences of OS-9 up-regulation on secretion and degradation of NHK and on secretion of α 1-antitrypsin. A, radiolabeled NHK has been immunoisolated after the indicated chase times from detergent extract of cells with normal (lanes 1–3) or elevated levels of OS-9.1 (lanes 4 – 6) or OS-9.2 (lanes 7–9). Note the co-precipitation of ectopic OS-9.1 and ectopic OS-9.2. B, quantification of intracellular NHK (n = 4). C, secretion of labeled NHK from cells with the normal (lanes 1–3) or elevated content of OS-9.1 (lanes 4 – 6) or OS-9.2 (lanes 7–9). D, quantification of C (n = 3). E, same as A for α1-antitrypsin. F, quantification of E. G, same as C for α1-antitrypsin. H, quantification of G. I, NHK co-precipitates with OS-9.1 (lanes 4 – 6) and OS-9.2 (lanes 7–9). L, α1-antitrypsin does not co-precipitate with OS-9 variants.
The intracellular persistence of labeled NHK did not result from substantial inhibition of NHK disposal in response to ectopic OS-9 expression. Rather, it resulted from the efficient inhibition of secretion of non-native NHK. In fact, whereas roughly 25% of the labeled NHK normally escapes retention-based ER quality control and is secreted in the extracellular media (reducing gel shown in Fig. 2, C, lanes 1–3, and D, Mock), this percentage dropped to less than 5% when OS-9.1 (lanes 4–6) or OS-9.2 (lanes 7–9) were individually up-regulated (Fig. 2D). Note that non-native NHK is secreted as monomers or non-covalent oligomers (NHKMONOMER) and as disulfide-bonded dimers or oligomers (NHKDIMER) as shown when the proteins are separated in non-reducing gels (supplemental Fig. S1).
OS-9 Variants Do Not Associate with α1-Antitrypsin and Do Not Affect Secretion of Endogenous and Ectopic Native Proteins—OS-9-mediated ER retention was specific for mis-folded conformers because OS-9 overexpression did not interfere with secretion of the amyloid precursor protein, an endogenous secretory protein of HEK293 cells (arrowhead in Fig. 2C) nor with secretion of ectopically expressed α1-antitrypsin (Fig. 2, E–H). Consistent with a selective association of OS-9 variants with misfolded proteins (Fig. 2A), the immunoisolation of α1-antitrypsin did not result in the co-precipitation of ectopic OS-9.1 and OS-9.2 (Fig. 2E). Moreover, immunoisolation of OS-9 variants resulted in the co-precipitation of NHK (Fig. 2I, lanes 4–9), but not α1-antitrypsin (Fig. 2L, lanes 4–9), thus confirming the specificity of the associations. Note that the fraction of labeled NHK co-precipitating with OS-9.1 (Fig. 2I, lanes 4–6) and OS-9.2 (lanes 7–9) increased with progression of chase as if association would start only after a lag phase during which NHK is subjected to unproductive folding attempts.
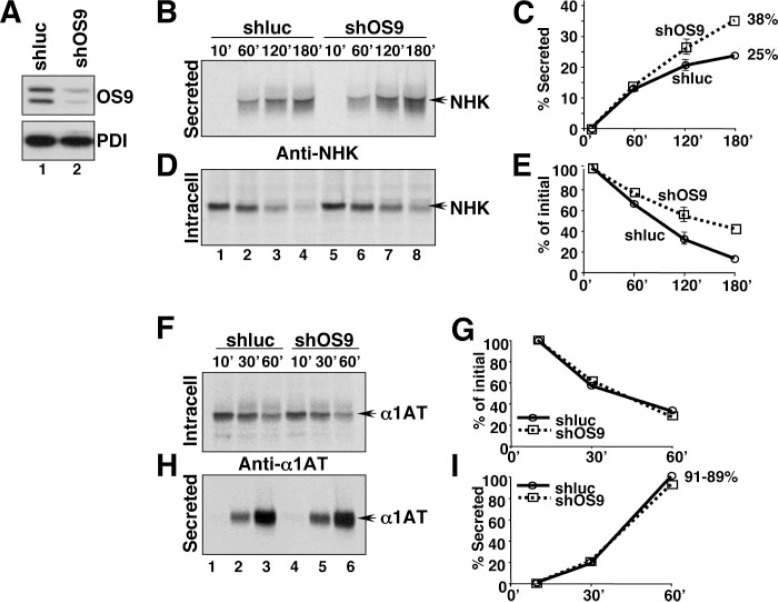
Reduction of the Intralumenal Level of OS-9 Reduces Tightness of Retention-based ER Quality Control—Consistent with a role of OS-9 in ER retention of non-native polypeptides, OS-9 down-regulation upon specific RNA interference (Fig. 3A and “Experimental Procedures”) increased, by more than 50%, the secretion of misfolded NHK (from 25% of the labeled NHK in control cells (Fig. 2, C, lanes 1–3, and D) and in cells expressing an inactive RNA duplex (Fig. 3, B, lanes 1–4, and C, shluc), to almost 40% in cells expressing an OS-9-targeted interfering RNA (Fig. 3, B, lanes 5–8, and C, shOS9). Interestingly, the reduction of the intralumenal OS-9 content also delayed disappearance of intracellular NHK thus hinting at a role of endogenous OS-9 in facilitating NHK disposal (Fig. 3. D–E, shOS9, and comments to Fig. 4). OS-9 down-regulation did not change the intracellular fate of the folding-competent α1-antitrypsin (Fig. 3, F–I) confirming the selectivity of OS-9 for aberrant polypeptides. The specificity of the results was confirmed by back-transfection of OS-9.1 and OS-9.2 in the cells subjected to RNA interference (supplemental Fig. S2).
FIGURE 3.
Consequences of OS-9 down-regulation on secretion and degradation of NHK and on secretion of α 1-antitrypsin. A, comparison by immunoblot of OS-9.1 and OS-9.2 content in cells exposed to an inactive small interfering RNA (lane 1) and to a small interfering RNA targeting the 3′ untranslated region of the OS-9 gene (lane 2). PDI serves as a loading and specificity control. B, secretion of labeled NHK from cells with normal (shluc, lanes 1– 4) or reduced (shOS9, lanes 5– 8) OS-9 levels. C, quantification of B (n = 3). D, intracellular content of labeled NHK for cells with normal (lanes 1– 4) or reduced (lanes 5– 8) OS-9 levels. E, quantification of D (n = 2). F, same as D for α1-antitrypsin. G, quantification of F. H, same as B for α1-antitrypsin. I, quantification of H.
FIGURE 4.
Intracellular (Retention), secreted and degraded NHK after a 120-min chase has been determined in cells with normal (Mock, shluc), high (OS9, shOS9+OS9), and reduced (shOS9) OS-9 content. This figure summarizes the data shown in Figs. 2 and 3.
The pleiotropic functions of OS-9 in the mammalian ER lumen are better appreciated upon analysis of the data shown in Figs. 2 and 3 as summarized in Fig. 4. In cells with physiologic OS-9 content, about 30% of the labeled NHK was still retained in the ER lumen 120 min after synthesis, 22% had been secreted from cells and 48% had been degraded (Fig. 4, Mock, average of 3–5 independent experiments). Prolongation of the chase (e.g. 240 min in Fig. 2, B and D) did not increase the amount of aberrant NHK escaping ER quality control much (25%, Fig. 2D), but allowed slow disposal of the NHK fraction retained in the ER (Fig. 2B). Elevation of the intralumenal OS-9 level (Fig. 4, OS-9) significantly increased the fraction of protein retained in the ER (55%), mostly at the expense of the fraction escaping ER quality control (reduced from 22–25 to 4%).
The reduction of the intralumenal level of OS-9 (Fig. 4, shOS9) also increased the amount of NHK retained in the ER lumen (from 30 to 50%). In this case, however, at the expense of the part that should have been degraded, which was reduced from 48 to roughly 20%.
Thus, OS-9 fulfills dual function in the ER as ERAD regulator and as a crucial operator of retention-based ER quality control. Ectopic expression of OS-9 in cells subjected to RNA interference re-established the phenotype of cells overexpressing OS-9 because the ectopic gene lacks the 3′ untranslated region targeted by the small interfering RNA (Fig. 4, shOS9+OS9 and supplemental Fig. S2).
OS-9 Variants Unproductively Associate with Non-glycosylated ERAD Substrates—In S. cerevisiae, Yos9p associates with non-glycosylated polypeptides, but it does not affect their fate (5, 8). To assess whether OS-9 associates and regulates disposal of non-glycosylated polypeptides from the mammalian ER, we performed the same experiments shown above for the tri-glycosylated protein NHK (Fig. 2) but we used the non-glycosylated NHKQQQ as a model ERAD substrate. NHKQQQ is a variant of NHK in which the asparagine residues of the three consensus sequences for N-glycosylation have been replaced by glutamine residues. Fig. 5 shows that both OS-9.1 and OS-9.2 did co-precipitate with NHKQQQ (panel A). OS-9 association with this non-glycosylated ERAD substrate was unproductive because it did not affect kinetics of disappearance of the labeled protein from cells (Fig. 5B, note that NHKQQQ is not secreted from cells). Down-regulation of OS-9 variants only weakly delayed ERAD of NHKQQQ (Fig. 5, C and D). The co-precipitation of labeled NHKQQQ with OS-9 variants confirmed the specificity of the interactions (Fig. 5E). It seems relevant that kinetics of this unproductive association between OS-9 variants and NHKQQQ differ from the kinetics of the productive association between OS-9 variants and NHK. In fact, only for NHK, which is glycosylated and escapes to some extent retention-based ER quality control (Fig. 2, C and D), was the fraction of labeled protein associated with OS-9 increased during the chase (Fig. 2I).
FIGURE 5.
Consequences of OS-9 up-regulation and down-regulation on degradation of NHKQQQ. A, radiolabeled NHKQQQ has been immunoisolated after the indicated chase times from detergent extract of cells with normal (lanes 1–3) or elevated levels of OS-9.1 (lanes 4 – 6) or OS-9.2 (lanes 7–9). Note the co-precipitation of ectopic OS-9.1 and ectopic OS-9.2. B, quantification of intracellular NHKQQQ. C, intra-cellular content of labeled NHKQQQ for cells with normal (lanes 1–3) or reduced (lanes 4 – 6) OS-9 levels. D, quantification of C. E, NHKQQQ co-precipitates with OS-9.1 (lanes 4 – 6) and with OS-9.2 (lanes 7–9).
A Functional MRH Domain Is Dispensable for Substrate Association and Activity of OS-9—Next, we determined whether inactivation of the MRH domain upon mutation of the conserved arginine 188 residue into an alanine (8, 10) affected OS-9 association with substrates and OS-9 activity. To this end, cell lines with low intracellular levels of OS-9 (Fig. 3) were back-transfected with OS-9.2R188A. As shown above for the wild type protein, the mutated protein as well did co-precipitate with NHK (Fig. 6A, lanes 3–6). This confirmed data published for the yeast ortholog Yos9p (8), and showed that substrate association does not require an intact MRH domain. In mammalian cells, however, the mutation also did not affect the function of the protein as shown by the virtually complete inhibition of secretion of the folding-defective NHK in the extracellular media (Fig. 6, C and D). The co-precipitation of labeled NHK with OS-9.2R188A confirmed the specificity of the interactions and the kinetics previously observed for productive association characterized by an increased complex formation at later chase times (Fig. 6E).
FIGURE 6.
Consequences of overexpression of OS-9 with mutated MRH domain. A, consequences on the fate of NHK in OS-9.2R188A back-transfected shOS9 cells. B, quantification of A. C, secretion of labeled NHK. D, quantification of C. E, NHK co-precipitates with OS-9.2R188A (lanes 4 – 6).
DISCUSSION
Here we show that OS-9.1 and OS-9.2 are ER-resident glycoproteins. Their intralumenal level, which is increased upon activation of the Ire1/Xbp1 ER stress pathway, directly correlates with tightness of retention-based ER quality control. This was established by using NHK, a folding defective, truncated form of the secretory protein α1-antitrypsin as model substrate. In transiently transfected HEK293 cells (Figs. 2, 3, and 6 and supplemental Fig. S1) and in MEF,3 only about 75% of ectopically expressed NHK becomes an ERAD substrate. About 25% of the newly synthesized, misfolded NHK escapes ER retention and is secreted from transiently transfected cells. Elevation of the intralumenal OS-9 content abolishes secretion of misfolded NHK, whereas reduction of the intralumenal OS-9 content results in enhanced secretion of non-native NHK. On the other hand, variations in the intralumenal OS-9 content do not affect secretion of folding-competent proteins.
The yeast ortholog Yos9p has been shown to regulate ERAD (6–10, 31, 32), and while this manuscript was in preparation, a role for OS-9 in mammalian ERAD has been shown (33). We report that the physiologic OS-9 content insures maximal ERAD efficiency because OS-9 induction does not further enhance ERAD capacity. Enhancement of ERAD capacity would probably require coordinate overexpression of several components of the human synoviolin complex (see below). OS-9 down-regulation moderately inhibits disposal of folding-defective polypeptides.
Christianson et al. (33) recently reported that OS-9.1 does not bind transthyretin variants, which are non-glycosylated destabilized proteins. They concluded that OS-9 is not able to bind folding-defective lumenal proteins lacking N-glycans. This must be substrate-specific because our data showed that OS-9.1 and OS-9.2 transiently bind the non-glycosylated ERAD substrate NHKQQQ. As reported for the yeast ortholog Yos9p (5, 8), for OS-9 variants as well the association with non-glycosylated folding defective proteins was non-productive. NHKQQQ is tightly retained in the ER lumen. It was therefore not possible to determine with this substrate whether OS-9 plays a role in preventing secretion of extensively misfolded non-glycosylated polypeptides, as it does with N-glycosylated ones.
The role of the MRH domain of OS-9 also remains unclear. Christianson et al. (33, 34) suggested an interesting model in which this domain is involved directly or indirectly in a functional association with the transmembrane protein Sel1L in the synoviolin complex that regulates disposal from the ER lumen of polypeptides with lumenal defects. A direct involvement of the MRH domain of OS-9 in a complex with Sel1L would be a clear difference with the yeast system in which formation of the complex does not require an intact MRH domain and is independent on Sel1L glycosylation (7, 32). Our data showed that substrate binding and activity of OS-9 do not require a functional MRH domain. Moreover, mammalian OS-9 is N-glycosylated in the middle of the MRH domain. Hence, a direct role of the MRH domain in stabilizing a multiprotein complex regulating ERAD seems unlikely.
Yos9p and OS-9 belong to a luminal surveillance complex comprising Kar2p/BiP, Hrd3p/Sel1L, and Hrd1p/Synoviolin (6–10, 31, 33). Analysis of transcriptional regulation of the mammalian orthologs of the yeast Hrd1p complex reveals that all components are inducible upon ER stress, but only OS-9 variants and synoviolin require activation of the Ire1/Xbp1 pathway (Fig. 1, H and I, and Ref. 27). We postulate that the “retention of misfolded” versus “facilitation of disposal” functions of mammalian OS-9 proteins might depend on formation of distinct multiprotein complexes and that activation of individual stress-response pathways (Ire1-regulated versus ATF6-regulated) in specific tissues or under specific developmental or stress conditions could enhance one or the other function of OS-9. Altogether, our data show that OS-9.1 and OS-9.2 play a dual role in the mammalian ER as ERAD promoters and as crucial operators of the retention-based ER quality control machinery that inhibits release of non-native conformers from the ER lumen into the secretory pathway.
Acknowledgments
L. Ruddock is acknowledged for critical comments on the manuscript, L. Glimcher, L. Litovchick, N. Hosokawa, K. Nagata, and P. Paganetti for plasmids and cell lines used in this study. A special thanks to G. Noseda and S. Monti.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The abbreviations used are: ER, endoplasmic reticulum; PDI, protein-disulfide isomerase; RT, reverse transcriptase; MEF, mouse embryonic fibroblasts; HEK, human embryonic kidney; EndoH, endoglycosidase H; NHK, Nullhong kong; ERAD, ER-associated degradation.
R. Bernasconi, T. Pertel, J. Luban, and M. Molinari, unpublished data.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
REFERENCES
- 1.Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, O’Shea EK, Weissman JS. Nature. 2003;425:737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- 2.Brodsky JL. Biochem J. 2007;404:353–363. doi: 10.1042/BJ20061890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meusser B, Hirsch C, Jarosch E, Sommer T. Nat Cell Biol. 2005;7:766–772. doi: 10.1038/ncb0805-766. [DOI] [PubMed] [Google Scholar]
- 4.Molinari M. Nat Chem Biol. 2007;3:313–320. doi: 10.1038/nchembio880. [DOI] [PubMed] [Google Scholar]
- 5.Buschhorn BA, Kostova Z, Medicherla B, Wolf DH. FEBS Lett. 2004;577:422–426. doi: 10.1016/j.febslet.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho P, Goder V, Rapoport TA. Cell. 2006;126:361–373. doi: 10.1016/j.cell.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 7.Denic V, Quan EM, Weissman JS. Cell. 2006;126:349–359. doi: 10.1016/j.cell.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 8.Bhamidipati A, Denic V, Quan EM, Weissman JS. Mol. Cell. 2005;19:741–751. doi: 10.1016/j.molcel.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 9.Kim W, Spear ED, Ng DT. Mol. Cell. 2005;19:753–764. doi: 10.1016/j.molcel.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Szathmary R, Bielmann R, Nita-Lazar M, Burda P, Jakob CA. Mol. Cell. 2005;19:765–775. doi: 10.1016/j.molcel.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Litovchick L, Friedmann E, Shaltiel S. J Biol Chem. 2002;277:34413–34423. doi: 10.1074/jbc.M203986200. [DOI] [PubMed] [Google Scholar]
- 12.Baek JH, Mahon PC, Oh J, Kelly B, Krishnamachary B, Pearson M, Chan DA, Giaccia AJ, Semenza GL. Mol. Cell. 2005;17:503–512. doi: 10.1016/j.molcel.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Flashman E, McDonough MA, Schofield CJ. Mol. Cell. 2005;17:472–473. doi: 10.1016/j.molcel.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Fu X, Gaiser S, Kottgen M, Kramer-Zucker A, Walz G, Wegierski T. J Biol Chem. 2007;282:36561–36570. doi: 10.1074/jbc.M703903200. [DOI] [PubMed] [Google Scholar]
- 15.Paganetti P, Calanca V, Galli C, Stefani M, Molinari M. J Cell Biol. 2005;168:863–868. doi: 10.1083/jcb.200410047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molinari M, Helenius A. Methods Enzymol. 2002;348:35–42. doi: 10.1016/s0076-6879(02)48623-5. [DOI] [PubMed] [Google Scholar]
- 17.Kimura Y, Nakazawa M, Tsuchiya N, Asakawa S, Shimizu N, Yamada M. J. Biochem (Tokyo) 1997;122:1190–1195. doi: 10.1093/oxfordjournals.jbchem.a021880. [DOI] [PubMed] [Google Scholar]
- 18.Kimura Y, Nakazawa M, Yamada M. J. Biochem (Tokyo) 1998;123:876–882. doi: 10.1093/oxfordjournals.jbchem.a022019. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen H, Krogh A. In: Sixth International Conference on Intelligent Systems for Molecular Biology. Glasgow J, Littlejohn T, Major F, editors. AAAI Press; Menlo Park, CA: 1998. pp. 122–130. [Google Scholar]
- 20.Blobel G, Dobberstein B. J Cell Biol. 1975;67:835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hebert DN, Molinari M. Physiol Rev. 2007;87:1377–1408. doi: 10.1152/physrev.00050.2006. [DOI] [PubMed] [Google Scholar]
- 22.Rothman JE, Urbani LJ, Brands R. J Cell Biol. 1984;99:248–259. doi: 10.1083/jcb.99.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braakman I, Hoover-Litty H, Wagner KR, Helenius A. J Cell Biol. 1991;114:401–411. doi: 10.1083/jcb.114.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olivari S, Galli C, Alanen H, Ruddock L, Molinari M. J Biol Chem. 2005;280:2424–2428. doi: 10.1074/jbc.C400534200. [DOI] [PubMed] [Google Scholar]
- 25.Molinari M, Calanca V, Galli C, Lucca P, Paganetti P. Science. 2003;299:1397–1400. doi: 10.1126/science.1079474. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida H, Matsui T, Hosokawa N, Kaufman RJ, Nagata K, Mori K. Dev. Cell. 2003;4:265–271. doi: 10.1016/s1534-5807(03)00022-4. [DOI] [PubMed] [Google Scholar]
- 27.Kaneko M, Yasui S, Niinuma Y, Arai K, Omura T, Okuma Y, Nomura Y. FEBS Lett. 2007;581:5355–5360. doi: 10.1016/j.febslet.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 28.Carlson JA, Rogers BB, Sifers RN, Hawkins HK, Finegold MJ, Woo SL. J Clin Investig. 1988;82:26–36. doi: 10.1172/JCI113580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sifers RN, Brashears-Macatee S, Kidd VJ, Muensch H, Woo SL. J Biol Chem. 1988;263:7330–7335. [PubMed] [Google Scholar]
- 30.Liu Y, Choudhury P, Cabral CM, Sifers RN. J Biol Chem. 1999;274:5861–5867. doi: 10.1074/jbc.274.9.5861. [DOI] [PubMed] [Google Scholar]
- 31.Kanehara K, Kawaguchi S, Ng DT. Semin Cell Dev Biol. 2007;18:743–750. doi: 10.1016/j.semcdb.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Gauss R, Jarosch E, Sommer T, Hirsch C. Nat Cell Biol. 2006;8:849–854. doi: 10.1038/ncb1445. [DOI] [PubMed] [Google Scholar]
- 33.Christianson JC, Shaler TA, Tyler RE, Kopito RR. Nat Cell Biol. 2008;10:272–282. doi: 10.1038/ncb1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S, Ng DT. Nat Cell Biol. 2008;10:251–253. doi: 10.1038/ncb0308-251. [DOI] [PubMed] [Google Scholar]