Abstract
Objectives
RhoA and its main downstream effector, Rho-kinase (ROCK) are important in maintaining the penis in the flaccid state. The pathophysiology of Sickle cell disease-associated priapism is not well defined. We hypothesize that RhoA/ROCK vasoconstrictive pathways may be involved in the development of priapism. Therefore, the objective of this study was to evaluate molecular changes in RhoA and ROCK in an established transgenic sickle cell mouse model of priapism.
Methods
Two groups of mice were utilized: 1) wild type (WT; C57BL/6), and 2) transgenic Sickle cell mice (Sickle). We evaluated RhoA GTPase and total ROCK activities as well as ROCK1 and ROCK2 protein expression in WT and Sickle mice penes. We also evaluated in vivo erectile responses to cavernous nerve stimulation (CNS) and the frequency and duration of spontaneous erections both pre- and post-CNS.
Results
Sickle mice demonstrated significantly (p<0.05) enhanced erectile responses to CNS and frequency of spontaneous erections both pre- and post-CNS when compared to WT. Sickle mice penes had a significant decline in RhoA GTPase (p<0.01) and total ROCK activities (p<0.05) when compared to WT mice. There was a significant (p<0.05) reduction in ROCK2 protein expression in Sickle mice penes when compared to WT mice protein expression. No change in ROCK1 protein expression was observed in both cohort’s of mice penes.
Conclusion
These data suggest that Sickle cell disease associated-priapism may be contributed by a lack of RhoA/ROCK mediated vasoconstriction and highlight a novel molecular mechanism in the pathophysiology of priapism.
Keywords: RhoA, priapism, sickle cell disease, vasoconstriction
Introduction
Priapism is an erectile disorder in which erection persists uncontrollably and without sexual purpose.1 In its low-flow or ischemic form the corporal bodies are exposed to intermittent anoxic events, eventually contributing to progressive erectile dysfunction (ED) and fibrosis of the penile vascular bed. Ischemic priapism is associated with a variety of disease states including malignancy, neurological conditions and hematological dyscrasias.1,2 Its prevalence is particularly high among men with Sickle cell disease (30–45%) in whom the rate of resultant ED exceeds 30%.2,3
The molecular mechanisms involved in the development of ischemic priapism, both in general and in men with Sickle cell disease are only beginning to be characterized. Principally, ischemic priapism represents the imbalance of vasoconstrictive and vasodilatory mechanisms that govern penile vascular tone. To date, most studies have focused on vasodilatory mechanisms, mediated primarily by nitric oxide (NO).4 More recently however, vasoconstrictive pathways, primarily those activated by RhoA and its downstream effector Rho-kinase (ROCK, existing in two isoforms of ROCK1 and ROCK2), have been shown to be important in maintaining the penis in a flaccid state.5,6 Furthermore, the RhoA/Rho-kinase pathway has been shown to be aberrantly activated in states of ED.7–11 In contrast, in endothelial nitric oxide synthase null (eNOS KO) mice which have an exaggerated erectile response to cavernous nerve stimulation and display phenotypic changes in erectile function consistent with priapism, ROCK activity is significantly reduced.12,13 To date, the role of RhoA/ROCK signaling in Sickle cell disease related ischemic priapism or other priapism-related disease states has not been explored.
Recently, a transgenic Sickle cell disease mouse model has been developed to study the pathophysiology of Sickle cell disease-associated priapism.14,15 Here we use this experimental animal model of Sickle cell disease priapism to examine the status of the RhoA/ROCK signaling, with the hypothesis that the RhoA/ROCK vasoconstrictive pathways would be down-regulated and thus contribute to priapism.
Material and Methods
Mouse model of human Sickle cell disease
Transgenic Sickle cell (Sickle) mice (4 to 6 months old; n=8) with knockout of all mouse hemoglobin genes and expressing exclusively human Sickle hemoglobin were developed at Lawrence Berkeley National Laboratory.14,16 A breeding colony at the National Institutes of Health (NIH) generated animals for this study by mating Sickle male mice to hemizygous females (approximately 15 generations). Because C57BL/6 is one of the background strains for the transgenic Sickle mice, C57BL/6 was chosen as wild-type (WT) control (n=7). Mice were pathogen free and received routine NIH rodent chow and water. Studies were approved by the animal care and use committees of Johns Hopkins University.
Collection of Tissue Specimens
Penile specimens were obtained following a lethal dose of sodium pentobarbital (80 mg/kg intraperitoneally). The mouse penis was removed by cutting the crura of the corpus cavernosum at the point of adhesion to the lower pubic bone, snap frozen in liquid nitrogen, and stored at −70° C until processing for molecular analyses. All molecular analyses were determined under basal conditions.
Physiologic erection studies
In vivo erectile function in response to cavernous nerve stimulation (CNS) was studied in WT and Sickle anesthetized mice as previously described.14 Induction of anesthesia was achieved by placing the animal in a jar containing gauze soaked with isoflurane. The mice were then intubated and placed on a thermoregulated surgical table. The animals were ventilated with 95% O2/5% CO2 and 2% isoflurane using a custom-designed, constant-flow mouse ventilator with tidal volume set to 6.7 μl/g at 140 breaths/min. The shaft of the penis was freed of skin and fascia, and by removing part of the overlying ischiocavernous muscle exposure of the right crus was performed. A 27-gauge needle filled with 250 U/ml of heparin and connected to PE-50 tubing was inserted into the right crura and connected to a pressure transducer to permit continuous measurement of intracavernosal pressure (ICP). The bladder and prostate were exposed through a midline abdominal incision. The right major pelvic ganglion and cavernous nerve were identified posterolateral to the prostate on one side, and an electrical stimulator with a stainless steel bipolar hook was placed around the cavernous nerve. ICP was measured with a pressure transducer connected to a data acquisition system (Biopac) for continuous measurement of ICP. The cavernous nerve was stimulated with a square pulse stimulator (Grass Instruments, Quincy, MA). Each mouse underwent CNS at a frequency of 15 Hz and pulse width of 30 milliseconds. The application of 1 and 2 volts was used in the current protocol to achieve a significant and consistent erectile response. The duration of stimulation was approximately 30 seconds with rest periods of 2 to 15 mins between subsequent stimulations depending on the prolonged erections that occurred in the Sickle mice. The frequency of spontaneous erections per hour was calculated pre and post CNS (2 V) as previously described.12,14 In the present study, we also calculated the duration (minutes) of the prolonged erections in Sickle mice. This was defined as the time of onset of spontaneous erections (increase in ICP) from baseline corporal pressure until corporal pressure returned to baseline levels to the nearest 15 second time point pre and post CNS (2 V). The erection in this analysis was defined as two times the baseline corporal pressure.
Western Blot Analysis
Penes were excised and homogenized in a buffer containing 50 mM Tris-HCl (pH 7.5), 2 mM EDTA, 2 mM EGTA, 150 mM NaCl, 50 mM NaF, 10% glycerol, 10 μg/ml leupeptin, 2 μg/ml aprotinin, 10 μg/ml trypsin inhibitor, 1 mM phenylmethylsulfornyl fluoride (PMSF) and 1 mM Na3VO4. Cytosolic fractions were isolated for Rho-kinase (ROCK) 1 and 2 isoforms Western blot analysis.8,13 Protein concentration was determined by the BCA kit (Pierce), and equal amounts of protein were loaded to 4–20% Tris-HCl gel (Bio-Rad). After their separation by SDS-polyacrylamide gel electrophoresis, the proteins were transferred to polyvinylidene fluoride membranes and incubated with primary antibodies (ROCK1, ROCK2, and GAPDH from BD Bioscience and Amersham) overnight at 4°C. The membranes were incubated with a horseradish peroxidase-linked secondary antibody and visualized using an enhanced chemiluminescence kit (Amersham). The densitometry results were normalized by GAPDH expression. The intensities of the resulting bands were quantified by using software Image J 1.43s (NIH).
RhoA GTPase and ROCK Activity Assay
Penes were homogenized in a buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, and 1 μg/ml leupeptin. ROCK activity was analyzed in the presence of 0.1 mM ATP by a Rho-kinase activity assay kit according to manufacturer’s instructions, using a peroxidase coupled anti-phospho-MYPT1 Thr696 monoclonal antibody (Cyclex). RhoA GTPase activity (Cytoskeleton, Denver, CO) was assayed in whole penile lysates by 96-well activity assays according to manufacturer specifications and read on a Molecular Devices M-5 microplate reader. RhoA-GTPase and ROCK activities were expressed as a percentage of sham ROCK activity as previously described.13
Statistical Analysis
Data are presented as mean ± SEM. Comparisons between baseline variables in WT and Sickle mice were performed using paired or unpaired t tests, as appropriate. Comparisons between groups were made using ANOVA analysis with repeated measures and Neumann-Kuels post hoc test for multiple group comparisons. Statistical calculations were performed using GraphPad Prism version 5.00 for Windows, (GraphPad Software, San Diego California USA, www.graphpad.com).
Results
In Vivo Erectile Responses
Erectile responses to CNS were conducted to evaluate in vivo erectile physiology and determine if the Sickle mice displayed a priapic phenotype (Fig. 1). Sickle mice (n=8) had elevated resting ICP (p<0.01) when compared to WT mice (n=7) suggesting increased corporeal perfusion at baseline (Fig. 1A). Compared to WT, Sickle mice displayed priapic activity (Fig. 1B) and increased erectile responses to CNS at all voltage settings studied (Fig. 1C). The frequency of spontaneous erections pre-CNS in Sickle mice was 4 fold greater than their WT counterparts (p<0.01) (Fig. 1B). In addition, erectile frequency post-CNS (2 V) in Sickle mice was even further increased almost 5 fold compared to WT (p<0.01; Fig. 1B). Sickle mice were highly responsive to CNS even at low voltages, achieving significantly increased ICP levels when compared to WT erectile responses at all voltage settings studied (1 and 2 V; p<0.01; Fig. 1C). We also calculated the duration (minutes) of prolonged spontaneous erections in Sickle mice both pre- and post-CNS (Fig. 1D). Sickle mice had prolonged spontaneous erections that ranged from 45 sec to 4 mins pre-CNS and 1.5 mins to 15 mins post-CNS (mean duration 2.4 ± 0.2 mins and 5.2 ± 0.4 mins respectively). WT mice had no spontaneous prolonged erections that met our criteria for analysis. These data establish in vivo evidence of priapism in Sickle mice and are consistent with our previous observation using transgenic Sickle cell mice to study the pathophysiology of priapism.14
Figure 1.
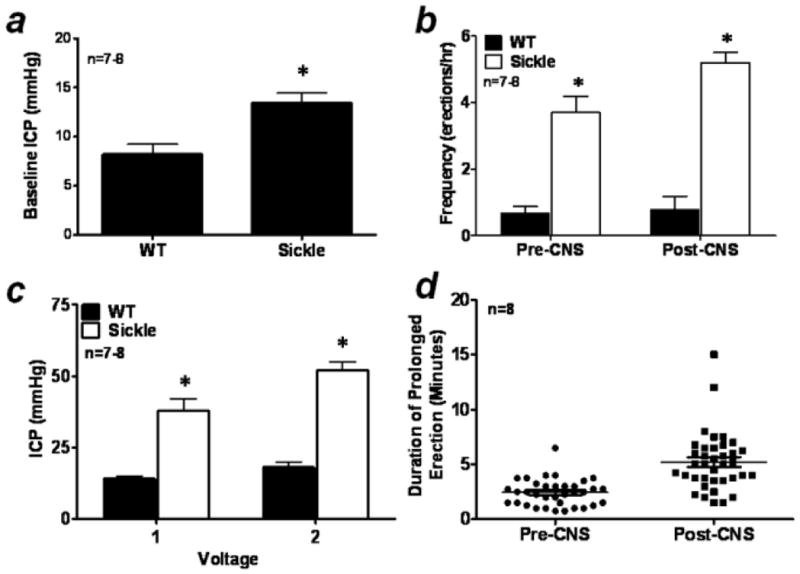
(A) Baseline resting intracavernous pressure (ICP); (B) bar graph demonstrating the frequency of spontaneous erections (erections/hr) pre- and post- cavernous nerve stimulation (CNS; 2 V) in WT and transgenic Sickle cell (Sickle) mice; (C) voltage dependent erectile responses to CNS showing peak ICP; (D) duration (mins) of spontaneous prolonged erections in Sickle mice. n = number of experiments; * p<0.05 vs WT. For all panels, data expressed as mean values ± SEM.
Western Blot Analysis of ROCK
ROCK1 (cytosol) and ROCK2 (cytosol) protein expression were measured in penile tissue of WT and Sickle mice and these data are summarized in Figure 2. ROCK2 protein levels were significantly lower in the Sickle mice penes when compared to WT (Fig. 2A) while there was no change in ROCK1 protein expression (Fig. 2A). When ROCK levels were analyzed by densitometry, ROCK2 protein levels were significantly reduced (p=0.04) in the Sickle mice penes when compared to WT mice penile expression (Fig. 2C). There was no change in ROCK1 protein expression as measured by densitometry in the Sickle mice penes (Fig. 2B).
Figure 2.
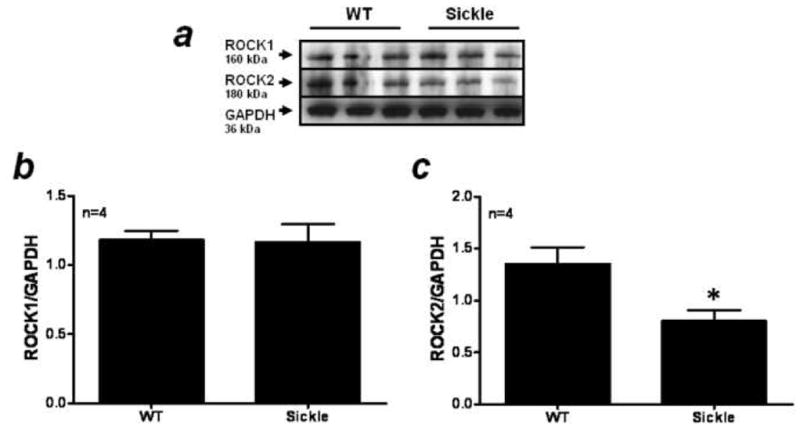
(A) Western blot analysis demonstrating expression of ROCK1, ROCK2 and GAPDH protein in penes of WT (lanes 1–3) and Sickle (lanes 4–6) mice. Densitometry analysis of the ratio of ROCK1 (B) and ROCK2 (C) to GAPDH protein expression in WT and Sickle mice penes. n indicates number of tissue samples; * p<0.05 when compared to WT.
Activated RhoA GTPase and ROCK Activity
Activated RhoA GTPase and ROCK activity were measured in WT and Sickle mice penes, and these data are summarized in Figure 3. Penile RhoA GTPase (Fig. 3A) and total ROCK (Fig. 3B) activity levels were significantly lower (p<0.01 and p=0.03, respectively) in the Sickle mice penes when compared to WT activity levels (Fig. 3).
Figure 3.
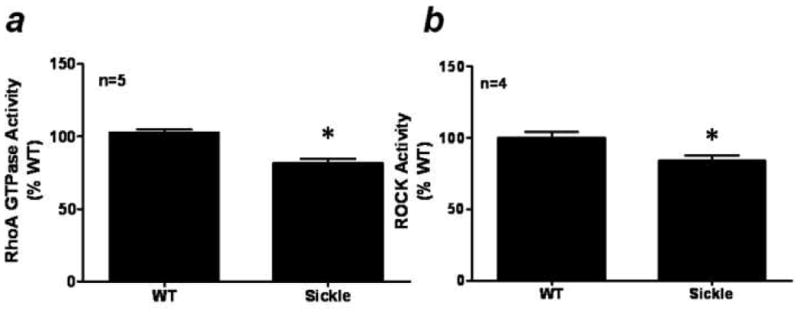
(A) Activated RhoA GTPase and (B) ROCK activity in WT and Sickle mice penes. n indicates number of tissue samples; * p<0.05 when compared to WT.
Discussion
The results of the present study confirm that transgenic Sickle mice display a priapic phenotype, characterized by an increased frequency of erections both pre- and post- CNS as well as enhanced erectile response to neurogenic stimuli. We further demonstrate that both RhoA GTPase activity and the activity of its downstream effector ROCK are decreased in Sickle mice penes. Decreased ROCK activity was associated with a decrease in ROCK2 protein expression while ROCK1 expression remained unchanged. These data suggest that priapism in transgenic Sickle mice is associated with a lack of RhoA/ROCK mediated vasoconstriction of the penile vasculature.
While the association between priapism and Sickle cell disease is well established, little is known about the molecular mechanisms contributing to the development of Sickle cell disease-associated priapism. Previous investigations in experimental animal models have demonstrated that priapism may be caused by phosphodiesterase type 5 (PDE5) dysregulation, NO imbalance, and adenosine overproduction.12–15,17,18 In eNOS KO mice, an animal model with phenotypic in vivo evidence of priapism similar to transgenic Sickle mice, chronic NO deficiency is associated with PDE5 downregulation and unchecked cGMP accumulation allowing for uncontrolled penile vasodilation.12,13 Further studies in these mice revealed that ROCK activity was reduced, offering the first in vivo suggestion that decreased vasoconstriction contributes to priapism.13
The RhoA/ROCK signal transduction pathway has been shown to influence erectile function in vivo through an array of mechanisms, including vasoconstriction of the penile vasculature via smooth muscle contraction and regulation of eNOS.5,6,8 RhoA, a member of the Ras low molecular weight of GTP-binding proteins, mediates agonist-induced activation of ROCK.19 The exchange of GDP for GTP on RhoA and translocation of RhoA from the cytosol to the membrane are markers of its activation, and enable the downstream stimulation of various effectors such as ROCK1 and ROCK2, protein kinase N, phosphatidylinositol (PI) 3-kinase and tyrosine phosphorylation. There are three major classes of regulators of the Rho family of GTPases: guanine nucleotide factors (GEFs), GTPase-activating proteins (GAPs), and guanine nucleotide dissociation inhibitors.19 ROCK exerts a contractile effect on smooth muscle by calcium independent promotion of myosin light chain (MLC) phosphorylation (via phosphorylation of the myosin light chain phosphatase targeting subunit (MYPT1) and subsequent inhibition of myosin light chain phosphatase. In the present study, we have demonstrated that both RhoA GTPase and total ROCK activities are significantly reduced in the Sickle mice penes suggesting that alterations in penile vascular tone and reduced corporal smooth muscle contraction predispose enhanced corporal smooth muscle dilation to any degree of erectogenic stimuli which results in priapism in vivo. The putative molecular event which causes a decrease in RhoA GTPase in the Sickle mouse penis remains unknown at this time but may be a result of GEF and GAP expression in the Sickle penile vasculature.
In Sickle mice penes we found a selective decrease in ROCK2 protein expression. ROCK1 and ROCK2 share 65% homology and have similar tissue distribution.20 Both isoforms are expressed in the penis and both have been implicated in penile vasoconstriction however isoform specific roles of ROCK are not well established.6,8,9 Recent in vitro work by Wang et al. demonstrates that ROCK2 is the predominant isoform regulating vascular smooth muscle contraction. Interestingly the authors found that inhibition of ROCK2 by RNA interference increased ROCK1 expression and vice versa suggesting close interplay between the two isoforms.21 The present study results demonstrate that ROCK2 protein expression as well as total ROCK activity declines in Sickle mice penes highlighting for the first time a molecular mechanism of Sickle cell disease-related priapism which is associated with decreased vasoconstrictor activity in the penis. Further studies are warranted to determine phosphorylation states of MLC in Sickle mice penes in order to support the hypothesis that less smooth muscle contraction may be a mechanism of priapism in Sickle cell disease.
Recent pre-clinical evidence in established mouse models of priapism have identified a role for NO/cGMP as well as RhoA/ROCK signaling in the pathophysiology of priapism.12–14,17 In the penis, vascular smooth muscle cells are continuously subjected to vasodilatory action of basally released NO from the vascular endothelium and vasoconstrictor factors such as RhoA/Rho-kinase.4 Endothelium-derived NO can regulate the vascular tone in the penis by controlling downstream targets of NO (cGMP, protein kinase G, or PDE5) as well as regulating other signaling pathways (RhoA/ROCK). Previously, we demonstrated that recurrent priapism is a manifestation of defective PDE5 regulatory function in the penis, resulting from altered endothelial nitric oxide/cGMP signaling in the organ and the unchecked accumulation of cGMP.12 We also demonstrated that chronic endothelial NO deficiency in eNOS KO mice influences other signaling molecules in the penis, in particular the RhoA/ROCK pathway.13 In the current investigation we show that reduced RhoA and ROCK activities may contribute to the susceptibility of corporal tissue to excessive relaxation via less contractile effects of ROCK in the corpora cavernosa during episodes of Sickle cell disease-associated priapism.
Conventional treatment of priapism is usually administered after an acute episode has occurred. As such, therapies have been primarily reactive rather than preventive. Novel pharmacotherapies which prevent Sickle cell disease-associated priapism are currently being evaluated in men with stuttering ischemic priapism.22–24 However, a large proportion of men still need emergent treatment of their ischemic priapism. Our data indicate that a lack of vasoconstriction may contribute to priapism in men with Sickle cell disease. As such, direct intracavernous injection of RhoA/ROCK activators may represent a novel therapeutic approach to treat Sickle cell patients who present with ischemic priapism.
It is worth noting a couple of limitations of this study. Sickle mice demonstrate a wide spectrum of hematologic findings that are similar to those found in the human phenotype but there are differences.16,25 Therefore, the changes in RhoA/ROCK signaling in the Sickle mouse penis may not represent human Sickle cell pathology that governs penile hemodynamics and ultimately priapism. Also, we have only investigated molecular changes in RhoA/ROCK signaling at one time point (4–6 months) in the Sickle mouse penis with relatively small sample numbers. It is plausible that changes may be occurring either earlier or later in the lifespan of the mouse that may contribute to development of priapism and influence end organ damages that occur in the Sickle cell mouse penes (fibrosis) and eventual erectile failure.14 Also, we have previously shown that in eNOS KO mice penes there is less total ROCK activity with no change in RhoA activity.13 Unlike eNOS KO mice, RhoA GTPase activity are significantly decreased in the Sickle mice penes. The difference may reflect species differences and method used to measure molecular alterations of penile RhoA in the eNOS KO and Sickle mouse. We believe that the results of the present study are a better representation of human Sickle cell disease since they are found in a mouse model of human Sickle cell disease pathology.
Conclusion
The priapic phenotype of Sickle cell disease can be faithfully recapitulated in vivo using a transgenic Sickle cell mouse model. In these mice, RhoA activity, and the activity of its downstream effector ROCK are decreased. The decline in total ROCK activity can be attributed to a reduction in ROCK2 protein expression. These data indicate that Sickle cell disease associated-priapism may be contributed by a lack of RhoA/ROCK mediated vasoconstriction and suggests a role for activators of this pathway in the treatment of this disease process.
Acknowledgments
The present study was funded in part from an American Urological Association Foundation Astellas USA Foundation MD/PhD grant to TJB, National Institute of Health grant RO1DK67223 to TJB and ALB, and Sickle Cell Program Project grant U54HL90515 to ALB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Montague DK, Jarow J, Broderick GA, et al. American Urological Association guideline on the management of priapism. J Urol. 2003;170:1318–1324. doi: 10.1097/01.ju.0000087608.07371.ca. [DOI] [PubMed] [Google Scholar]
- 2.Burnett AL, Bivalacqua TJ. Priapism: current principles and practice. Urol Clin North Am. 2007;34:631–642. doi: 10.1016/j.ucl.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Adeyoju AB, Olujohungbe AB, Morris J, et al. Priapism in sickle-cell disease; incidence, risk factors and complications – an international multicentre study. BJU Int. 2002;90:898–902. doi: 10.1046/j.1464-410x.2002.03022.x. [DOI] [PubMed] [Google Scholar]
- 4.Burnett AL. Nitric oxide in the penis--science and therapeutic implications from erectile dysfunction to priapism. J Sex Med. 2006;3:578–582. doi: 10.1111/j.1743-6109.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 5.Chitaley K, Wingard CJ, Clinton Webb R, et al. Antagonism of Rho-kinase stimulates rat penile erection via a nitric oxide-independent pathway. Nat Med. 2001;7:119–122. doi: 10.1038/83258. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Eto M, Steers WD, et al. RhoA-mediated Ca2+ sensitization in erectile function. J Biol Chem. 2002;277:30614–3021. doi: 10.1074/jbc.M204262200. [DOI] [PubMed] [Google Scholar]
- 7.Gao BH, Zhao ST, Meng FW, et al. Y-27632 improves the erectile dysfunction with ageing in SD rats through adjusting the imbalance between nNo and the Rho-kinase pathways. Andrologia. 2007;39:146–150. doi: 10.1111/j.1439-0272.2007.00782.x. [DOI] [PubMed] [Google Scholar]
- 8.Bivalacqua TJ, Champion HC, Usta MF, et al. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes-associated erectile dysfunction. Proc Natl Acad Sci U S A. 2004;101:9121–9126. doi: 10.1073/pnas.0400520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang S, Hypolite JA, Changolkar A, et al. Increased contractility of diabetic rabbit corpora smooth muscle in response to endothelin is mediated via Rho-kinase beta. Int J Impot Res. 2003;15:53–62. doi: 10.1038/sj.ijir.3900947. [DOI] [PubMed] [Google Scholar]
- 10.Wingard CJ, Moukdar F, Prasad RY, et al. Reversal of voltage-dependent erectile responses in the Zucker obese-diabetic rat by rosuvastatin-altered RhoA/Rho-kinase signaling. J Sex Med. 2009;6 (Suppl 3):269–278. doi: 10.1111/j.1743-6109.2008.01184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin L, Liu T, Lagoda GA, Champion HC, Bivalacqua TJ, Burnett AL. Elevated RhoA/Rho-kinase activity in the aged rat penis: mechanism for age-associated erectile dysfunction. FASEB J. 2006;20:536–538. doi: 10.1096/fj.05-4232fje. [DOI] [PubMed] [Google Scholar]
- 12.Champion HC, Bivalacqua TJ, Takimoto E, et al. Phosphodiesterase-5A dysregulation in penile erectile tissue is a mechanism of priapism. Proc Natl Acad Sci U S A. 2005;102:1661–1666. doi: 10.1073/pnas.0407183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bivalacqua TJ, Liu T, Musicki B, et al. Endothelial nitric oxide synthase keeps erection regulatory function balance in the penis. Eur Urol. 2007;51:1732–1740. doi: 10.1016/j.eururo.2006.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bivalacqua TJ, Musicki B, Hsu LL, et al. Establishment of a Transgenic Sickle Cell Mouse Model to Study the Pathophysiology of Priapism. J Sex Med. 2009;6:2494–2504. doi: 10.1111/j.1743-6109.2009.01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mi T, Abbasi S, Zhang H, et al. Excess adenosine in murine penile erectile tissues contributes to priapism via A2B adenosine receptor signaling. J Clin Invest. 2008;118:1491–1501. doi: 10.1172/JCI33467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu LL, Champion HC, Campbell-Lee SA, et al. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood. 2007;109:3088–3098. doi: 10.1182/blood-2006-08-039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claudino MA, Franco-Penteado CF, Corat MA, et al. Increased cavernosal relaxations in sickle cell mice priapism are associated with alterations in the NO-cGMP signaling pathway. J Sex Med. 2009;6:2187–2196. doi: 10.1111/j.1743-6109.2009.01337.x. [DOI] [PubMed] [Google Scholar]
- 18.Lin G, Xin ZC, Lue TF, et al. Up and down-regulation of phosphodiesterase-5 as related to tachyphylaxis and priapism. J Urol. 2003;170:S15–S19. doi: 10.1097/01.ju.0000075500.11519.e8. [DOI] [PubMed] [Google Scholar]
- 19.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa O, Fujisawa K, Ishizaki T, et al. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996;392:189–93. doi: 10.1016/0014-5793(96)00811-3. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Zheng XR, Riddick N, et al. ROCK isoform regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circ Res. 2009;104:531–540. doi: 10.1161/CIRCRESAHA.108.188524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burnett AL, Bivalacqua TJ, Champion HC, et al. Long-term oral phosphodiesterase 5 inhibitor therapy alleviates recurrent priapism. Urology. 2006;67:1043–1048. doi: 10.1016/j.urology.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 23.Burnett AL, Bivalacqua TJ, Champion HC, et al. Feasibility of the use of phosphodiesterase type 5 inhibitors in a pharmacologic prevention program for recurrent priapism. J Sex Med. 2006;3:1077–1084. doi: 10.1111/j.1743-6109.2006.00333.x. [DOI] [PubMed] [Google Scholar]
- 24.Abern MR, Levine LA. Ketoconazole and prednisone to prevent recurrent ischemic priapism. J Urol. 2009;182:1401–1406. doi: 10.1016/j.juro.2009.06.040. [DOI] [PubMed] [Google Scholar]
- 25.Manci EA, Hillery CA, Bodian CA, et al. Pathology of Berkeley sickle cell mice: similarities and differences with human sickle cell disease. Blood. 2006;107:1651–1658. doi: 10.1182/blood-2005-07-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]


