Abstract
Background
Although genetic influences on bipolar I disorder are well established, localization of genes that predispose to the illness has been difficult. Some genes predisposing to bipolar I disorder may be transmitted without expression of the categorical clinical phenotype. One strategy to overcome this obstacle is the use of quantitative endophenotypes as has been done for other psychotic disorders.
Methods
We analyzed 30 bipolar I extended families (300 subjects, average family size 10.34 members, range: 2–31) and 20 unrelated healthy controls from a Costa Rican sample. Heritability and genetic correlation of the state and trait scale from the Anxiety State and Trait Inventory was computed by using the general linear model (Solar package software). We also assessed variation of both scores among groups (patients, relatives and controls) and tested independence of affection status.
Results
Heritability for state is 0.45 (SE=0.11, p=0.0000001) and for trait is 0.89 (SE=0.06, p=6.22e–29). Genetic correlation for state and trait is 0.29, (SE=0.12, p=0.038–3.19e-8). Bipolar I patients showed the highest trait score (F=12.17,[5,24], p=0.002), (bipolar I patients > relatives with other pathologies, > healthy relatives > unrelated healthy controls) with normal distribution in healthy individuals and no difference regarding depression and mania current status, (F=0.230, df=1, p=0.632) and (F=1.401, df=1, p=0.238) respectively, contrary to the state score.
Limitations
Confounding factors such as comorbid disorders could affect the interaction of subclinical anxiety with mania. Due to our limited budget we were not able to re-evaluate the subjects and conduct a test retest to assess the STAI reliability and mood state independence of anxiety traits over different times. Further research need to evaluate if anxiety traits are specially related to bipolar I disorder in comparison with other traits such as anger, attention or response inhibition deficit, pathological impulsivity or low self-directedness.
Conclusions
Anxiety state and trait are heritable and share some genetic factors but only trait showed normal distribution in healthy subjects, mood current status independence and significant liability for bipolar I disorder. A stair-step distribution of trait anxiety scores in the family members and controls based on their genetic proximity to affected individuals and diagnostic status suggests that trait anxiety could be an endophenotype in these bipolar I families.
Keywords: Bipolar disorder, Endophenotype, Genetics, Family studies, Subclinical anxiety
Introduction
Estimates of the prevalence of bipolar I disorder have ranged from 0.8% to 1.6% of the general population (Berns et al., 2003). The disorder is primarily defined by a history of a manic episode, although many patients experience both manic and depressive episodes. Many bipolar I patients show anxiety symptoms that can be very disabling and as in the case of bipolar disorder alone, this phenotype is caused by an interaction of biopsychosocial factors, including genetic vulnerability, stress, or trauma to produce clinically significant syndromes by the action of norepinephrine and serotonin (Schinka et al., 2004; Sen et al., 2004; Unschuld et al., 2007). High commorbidity rates for anxiety have been documented in bipolar I disorder (MacKinnon et al., 2002; Chen et al., 1995; McElroy et al., 2001; Feske et al., 2000; Keck et al., 1998); however, subclinical levels of anxiety have also been associated with bipolar I patients who did not meet criteria for a categorical Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) anxiety disorder (Mantere et al., 2008). These individuals have poor outcome with longer, more frequent, and more difficult to treat mood episodes, are less responsive to lithium therapy, have earlier onset of symptoms and have greater functional impairment (McElroy et al., 2001).
Candidate gene association studies on the comorbidity of these two disorders have been controversial and replication of findings has always been difficult (Rotondo et al., 2002). To date, most genetic research has focused on the categorical classification and few have assessed anxiety as a quantitative phenotype (Wozniak et al., 2002).
Imprecision of psychiatric phenotyping might explain the failure of genetic research to identify genes that contribute to susceptibility of these two disorders where subjective assessments form the basis of both clinical and research diagnoses (Bearden et al., 2004). This lack of objective markers has, in turn, promoted substantial disagreements about the specific criteria to define diagnostic categories, and the interpretation of results from genetic studies. The need for a new approach to psychiatric genetics has lead to the introduction of the concept of endophenotypes (internal phenotype that lies intermediate between the gene and the disease itself) (Gottesman et al., 1973). It is assumed that genes involved in endophenotypic variation are likely to represent more elementary phenomena than those involved in complex psychiatric diagnostic entities. It is also used interchangeably with the term ‘intermediate trait,’ describing a heritable quantitative phenotype believed to be closer in the chain of causality to the genes underlying the disease (Bearden et al., 2006).
Many candidate endophenotypes for bipolar I disorder (e.g. neurocognitive functions, behavioral traits, sleep abnormalities) have been proposed (Gottesman et al., 2003; Hasler et al., 2006). To our knowledge, no study has assessed anxiety traits in bipolar I extended pedigrees in an isolated population, such as the Costa Rican Central Valley.
To determine whether quantitative anxiety symptomatology is a candidate endophenotype for bipolar I disorder, we tested heritability of the state scale (measurement of the level of anxiety the individual is currently experiencing that may be expected to change over time) and trait scale (measurement of the general level of anxiety experienced over the lifetime) from the Anxiety State and Trait Inventory (STAI) in a sample of extended pedigrees from the Central Valley of Costa Rica. We will demonstrate that patients and their relatives show higher scores on the measures, and establish genetic correlation between the measure and affection status.
Methods
Participants
Subjects were originally recruited for a multi-site bipolar sibling pair study (Genetics of Bipolar disorder in Latino Populations NIMH 1 R01 MH069856-01A2). The study was explained to each subject and written informed consent was obtained. This study was reviewed and approved by the Institutional Review Boards of the University of Costa Rica and the University of Texas (UTHSCSA).
The sample was composed of 30 extended families (300 subjects, average family size 10.34 members, range: 2–31) and 20 unrelated healthy controls. Each family had at least one member diagnosed with bipolar disorder type I.
Diagnostic assessment
The subjects were diagnosed based on the diagnostic criteria of DSM-IV through a best estimation process (Leckman et al., 1982), utilizing clinical information obtained from the Diagnostic Interview for Genetic Studies (Nurnberger et al., 1994), a Family Interview for Genetic Studies (Maxwell et al., 1992) and psychiatric records. Final diagnoses were determined through a consensus process where two independent psychiatrists reviewed all available information, arrived at independent diagnoses and, if there were differences, reached a consensus diagnoses. The Lifetime Dimensions of Psychosis Scale (LDPS) was used to capture affective and psychotic symptoms over the lifetime and a consensus process of two best estimators per case was utilized as well.
Anxiety Assessment
Subclinical anxiety was assessed by means of the STAI to measure anxiety scores in each individual. The STAI is a self-rated instrument that contains two 20-item scales (4 response choices per item, higher scores indicate higher anxiety) (Spielberger et al., 1983). One scale measures state anxiety (i.e. the extent to which respondents experience anxiety symptoms as they complete the measure) (Vigneau et al., 2008). The second scale measures trait anxiety (i.e. the extent to which respondents generally experience anxiety symptoms). This instrument has been validated in Spanish (Rodrigo et al., 1988).
The instrument was applied within the same month period after the psychiatric interview in 285 subjects, while 35 individuals were assessed with the STAI at a different time (later than a month after psychiatric interview) due to availability of the participants.
Statistical Analysis
General linear model (GLM) techniques were used to test endophenotype criteria (e.g. heritability, sensitivity to the illness, genetic correlation). Heritability was assessed with variance component methods by using the SOLAR package software. Genetic correlation was also computed for the scales of the STAI (state and trait) and quantifiable mania symptoms score from the M-1 item (duration × severity) of the LDPS. Models were analyzed with cofactors age, sex, the square of age, and interactions between age and sex, to allow for different age effects in males and females and non-linear change with age. Bivariate analyses provide genetic and environmental correlations as a means of examining how these traits vary together. Such analyses allowed us to ask whether scores on anxiety correlated with each other and with mania in the bipolar I subjects because they are influenced by the same genes.
To test association of anxiety traits with the illness, we examined multiple dependent, independent and covariates variables (e.g. age, gender). We tested the anxiety scores of bipolar I individuals and their relatives to determine whether they differed from scores in controls, suggesting an underlying genetic correlation between anxiety score and bipolar I disorder. This hypothesis was tested through GLM methods modeling anxiety scores as function of genetic proximity to an affected individual (bipolar I disorder > relatives with other psychiatric illness (different than bipolar I disorder) > healthy relatives > healthy unrelated controls). All calculations were adjusted for age and gender by using the Statistical Package for the Social Sciences (SPSS) Software v.15.
Results
Sample characteristics
Of the 300 subjects from extended pedigrees 63 had bipolar disorder type I, 79 major depressive disorder, 23 specific phobia, 15 panic disorder, 7 schizoaffective disorder, 7 psychosis not otherwise specified, 4 social phobia, 4 bipolar disorder type II, 3 obsessive compulsive disorder, 1 generalized anxiety disorder and 101 had no axis I. One-hundred and seventy-four (54%) were females and the average age of the whole sample was 39.3 (SD=16.2).
Only 46 individuals (14.37% of the total sample) met criteria for a DSMIV categorical anxiety diagnosis. In contrast, out of the 274 subjects with no categorical anxiety diagnosis after best estimation diagnostic process, 72 (26%) were over percentile 75 (37 points) of the trait scale.
The dimensional index of lifetime mania (M-1 severity × duration of the LDPS) was 2.55. The average anxiety state score was 21.46 (SD=7.5) out of a maximum of 60 and the trait score is 31.07 (SD=8.1) out of a maximum of 60.
Heritability Analysis
The heritability for state is 0.45 (SE=0.11, p=0.0000001) and for trait is 0.89 (SE=0.06, p=6.22e-29). The interaction between age and sex was significant only for state (although the two individual covariates were not statistically significant). The computed genetic correlation for state and trait is 0.29, (SE=0.12, p: correlation is different from zero=0.038 and p: correlation is different from one=3.19e-8).
Sensitivity to Liability for Bipolar I Disorder
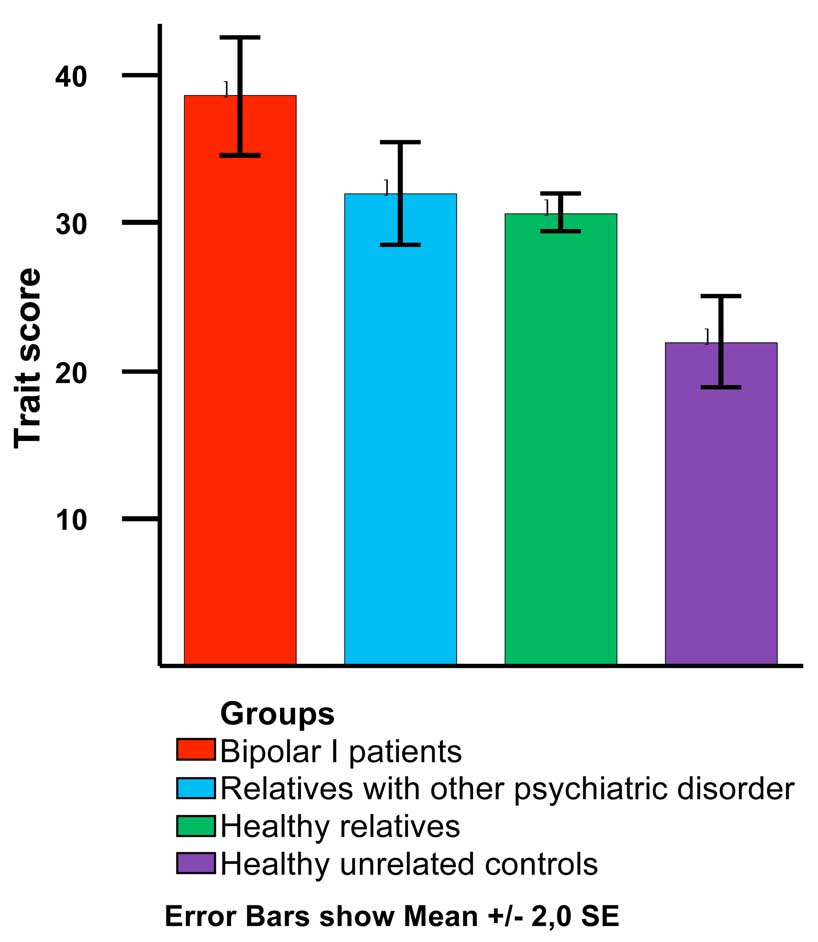
As seen in table 1 and figure 1, bipolar I patients showed significantly higher anxiety trait scores than their relatives and healthy controls (F=12.17 [5,24], p=.002), (bipolar I patients > relatives with other pathologies, > healthy relatives > unrelated healthy controls). State score did not show any significant difference between groups (F=1.18, p=0.32).
Table 1.
STAI score by group.
| Variable | BPI (n=30) | RELs+ (n=40) | RELs− (n=230) | CON (n=20) |
|---|---|---|---|---|
| Trait mean (SD) | 37.13 (12.07) | 33.10 (10.80) | 30.72 (9.46) | 21.95 (7.04) |
| State mean (SD) | 23.23 (12.02) | 23.49 (10.09) | 20.87 (9.65) | 21.45 (7.49) |
Abbreviations: BPI = Bipolar disorder type I. RELs+ = Relatives with other psychiatric disorders. RELs−= Healthy relatives. CON= Unrelated healthy controls.
Figure 1.
Trait score by group
To confirm the overall difference between groups on trait anxiety scores we assessed the difference between each group by conducting the post-hoc pairwise comparisons test. Since variances of trait mean were unequal (Levene’s statistic=2.45, df=3, p=0.06), Dunnet C test was used. As seen in table 2, means differences between each group remained statistically significant.
Table 2.
Pairwise differences for trait anxiety between groups (post hoc comparison pairwise test).
| (I) Group | (J) Group | Mean Difference (95%CI) | SE (p) |
|---|---|---|---|
| BPI | RELs+ | 6,53 (0.34, 12.72) | 2,331 (0.03) |
| RELs− | 7,90 (2.90, 12.90) | 1,884 (0.0001) | |
| CON | 16,65 (9.21, 24.09) | 2,801 (0.0001) | |
| RELs+ | BPI | −6,53 (−12.72, −0.34) | 2,331 (0.03) |
| RELs− | 1,37 (−3.00, 5.74) | 1,645 (1.0) | |
| CON | 10,12 (3.10, 17.15) | 2,646 (0.001) | |
| RELs- | BPI | −7,90 (−12.90,−2.90) | 1,884 (0.0001) |
| RELs+ | −1,37 (−5.74, 3.00) | 1,645 (1.0) | |
| CON | 8,75 (2.75, 14.76) | 2,262 (0.001) | |
| CON | BPI | −16,65 (−24.09, −9.21) | 2,801 (0.0001) |
| RELs+ | −10,12 (−17.15,−3.10) | 2,646 (0.001) | |
| RELs− | −8,75 (−14.76, −2.75) | 2,262 (0.001) |
Abbreviations: BPI = Bipolar disorder type I. RELs+ = Relatives with other psychiatric disorders. RELs− = Healthy relatives. CON = Unrelated healthy controls. SE = standard error.
Dunnet C for unequal n’s and variances was utilized as a post-hoc test to compare specific groups.
Significant mean differences are in bold.
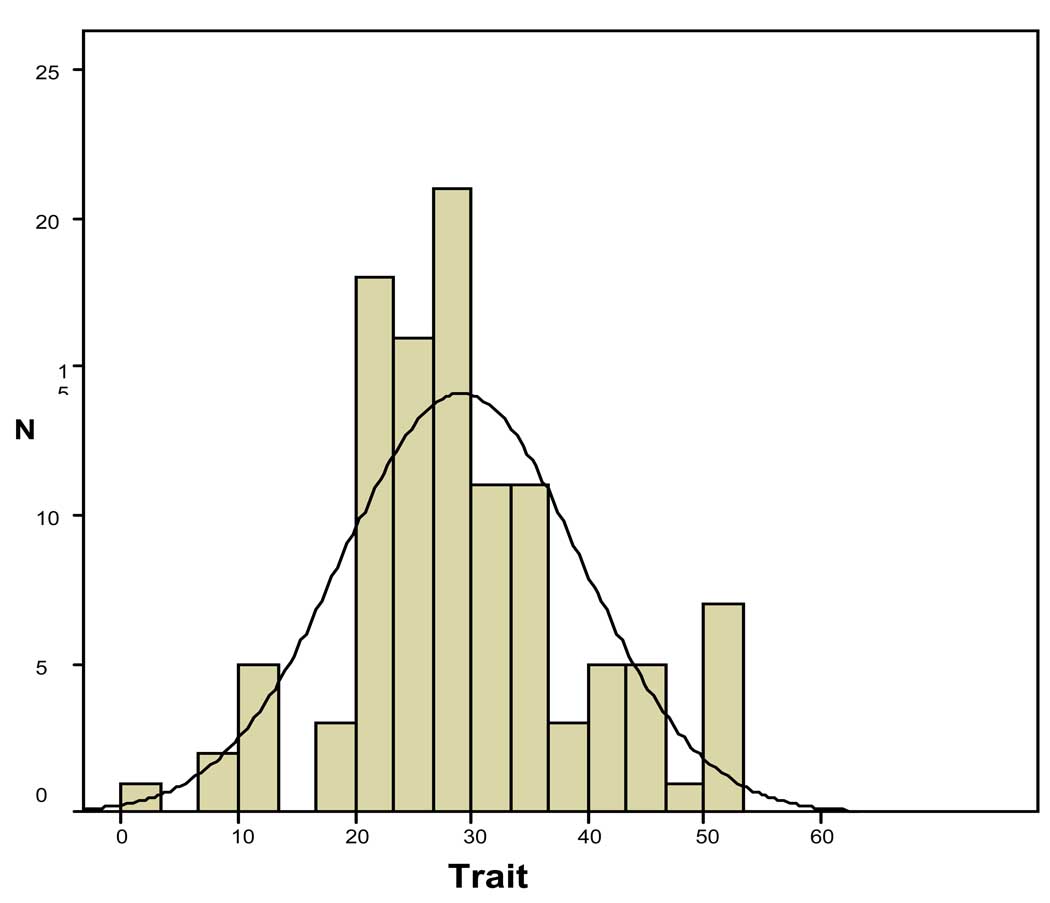
As shown in figure 2, we found a normal distribution for trait scores in the healthy subjects (healthy relatives and healthy unrelated controls) but not for the state scores.
Figure 2.
Trait score distribution in healthy subjects
The trait score was correlated with lifetime mania (LDPS M-1 duration × severity) r=0.21(p<.0001) after controlling for age contrary to lifetime depression (LDPS DE-1 duration × severity) r=0.06 (p=0.26). The state score does not show correlation with mania.
Of the 285 subjects (90% of the whole sample) evaluated at the same time (during a month period after psychiatric evaluation), 27 met criteria for a current depressive syndrome (five or more depressive symptoms within the last four weeks of psychiatric assessment) and 11 for a current manic syndrome (elated mood + three or more manic symptoms or irritability + four or more manic symptoms within the last four weeks of psychiatric evaluation). Only 27 subjects (9%) were depressed during the time of assessment and their trait score mean was 31.26 (SD=10.95) compared to 30.32 (SD=9.62) of the non-depressed group. Eleven individuals (3%) had mania during the time of assessment. Their trait score mean was 27.0 (SD=7.34) in contrast to 30.54 (SD=9.80) of the non-manic group. We found no significant differences in the trait scores regarding current mood status (currently depressed or currently manic), (F=0.230, df=1, p=0.632) and (F=1.401, df=1, p=0.238) respectively.
Discussion
Quantitative measures of anxiety represent a window into the genetically influenced biological processes underlying bipolar I disorder and can facilitate the identification of some of its susceptibility genes.
With the current categorical diagnostic system, anxiety symptoms are often under-diagnosed. In our sample, more than one quarter of patients with high trait anxiety scores (above percentile 75) did not receive any anxiety diagnosis after best estimation diagnostic process. The main reason is that many individuals who endorse anxiety do not meet all criteria for a full blown anxiety disorder. This finding strongly encourages the use of anxiety quantitative measures in psychiatric genetics research.
Regarding trait anxiety, individuals with bipolar I disorder showed the highest scores followed by relatives with other psychiatric disorders, healthy relatives and healthy unrelated controls. These results are similar to previous publications. For instance, Vázquez et al. (2008) found that healthy relatives of bipolar patients exhibited higher scores on the anxious temperament subscale of the TEMPS-A than controls. In a study by Evans et al. (2005) unaffected relatives of bipolar patients and controls showed differences on the hyperthymic scale and on the first extracted factor, anxious/reactive of the TEMPS-A and TCI-125. All of the above evidence is consistent with temperament and personality anxiety related traits having a genetic basis rather than resulting from a bipolar disorder.
A positive significant correlation was also found between trait scores and quantitative lifetime mania whereas no correlation was observed between trait scores and lifetime depression. As expected, this finding is frequently seen in clinical practice when bipolar I patients complaint of anxiety symptoms not always identified as a specific anxiety disorder according to the current diagnostic classification. Likewise, many healthy relatives of bipolar I patients endorse anxiety symptoms sometimes perceived as personality anxiety related traits.
We found significant heritability for quantitative anxiety measures in a set of multiplex, multigenerational families affected by bipolar I disorder, suggesting that some of the variations in the traits are affected by genetic factors. Even though both scales (state and trait) showed significant heritability, state was less heritable and it was affected by the interaction between age and sex whereas trait score was more stable within the sample. Consequently, lifetime measurement seems to be more useful to capture quantitative anxiety symptoms in this population while current quantitative anxiety is more affected by other variants. We also found genetic correlation significantly different from zero between these scores suggesting that they are influenced by some of the same genes. Based on these results, we conclude that both measures (state and trait score) are heritable and share some genetic factors (overlapping genetic influences) in the Costa Rican sample.
We found a stair-step distribution of trait anxiety scores in the family members and controls based on their genetic proximity to affected individuals and diagnostic status. This encourages our research focus of studying trait anxiety as an endophenotype in this isolated population of families with bipolar I disorder.
From the two STAI scales, only anxiety trait showed normal distribution in healthy subjects (healthy relatives and healthy unrelated matched controls), current mood status independence and significant liability for bipolar I disorder not only per groups but also as a continuum quantitative variable (lifetime mania). All of these evidence allow us to postulate anxiety trait as an intermediate phenotype for bipolar I disorder in this Costa Rican sample.
It is worth noting that in liability threshold model analyses, significant results are difficult to obtain when working with dichotomous traits. In our study we used continuous traits (state scale, trait scale and quantitative lifetime mania) that allowed us to reach significantly strong results. Another strength of this work is provided by the study design; besides bipolar I patients we assessed individuals without overt symptom expression (healthy relatives) but with high genetic risk for bipolar I disorder and healthy unrelated controls. The diagnosis of each subject was obtained thru the best-estimate procedure which is considered the current standard in many psychiatric genetic research studies. All participants were from an environmentally and genetically homogeneous sample reducing etiological heterogeneity and confusing interactions of genes and environment.
Some methodological differences between our work and two recent publications that we cited above can be found. In the Argentinean study, Vázquez et al. (2008) used the Mood Disorder Questionnaire to rule out bipolarity and a best-estimate procedure was not mentioned as done with each of our subjects. In a study with a larger sample of 85 BP families and 63 controls conducted by Evans et al. (2005), bipolar I and bipolar II subjects from San Diego, Vancouver, and Cincinnati were placed in the same group. Their controls were recruited through advertisements (for participation in sleep studies and other studies at the UCSD Mental Health Clinical), which may have introduced an ascertainment bias. They did not assess mood state at the time of completing the questionnaire which could affect subjects’ rating. Some limitations of our work need to be taken into consideration for further replication of our findings. Confounding factors such as substance abuse, medical illness and medication history could affect the interaction of measured anxiety with mania. Due to our limited budget we were not able to re-evaluate the subjects and conduct a test retest to assess the instrument reliability and mood state independence of anxiety traits over different times. Such a test is necessary to ensure that this scale can be used as a measurable trait and to better understand, if it does vary with time, the relationship of the anxiety trait to other core symptoms of bipolar I disorder (i.e. depression, mania, psychosis). Additional assessment needs to be done to identify how many patients with high anxiety score have been treated and to evaluate current functioning and effect of medication on clinical course. Our findings raise several questions that with our available data we were not able to answer. It is crucial to know if anxiety traits are specially related to bipolar I disorder in comparison with other traits such as anger frequently associated with bipolar II and bipolar family history, attention or response inhibition deficit, pathological impulsivity or others character dimensions such as low self-directedness (ability to adapt one’s behaviors to achieve chosen goals). All of these issues will be addressed in our future research project in addition to identification of regions containing genes specific for these quantitative measures by mean of Quantitative Trait Loci linkage and association analyses. This will improve our understanding of the pathophysiology of affective and anxiety disorders in the Costa Rican population.
The relevance of our work can be summarized as follows: (1) Quantitative anxiety measures as an endophenotype may facilitate the identification genes which predispose individuals to develop bipolar I disorder. (2) Confirmation of this result will aid researchers to understand the essential pathophysiology underlying bipolar spectrum disorders. (3) If this trait is probed to be an endophenotype, it will be of help in diagnosing and treating bipolar I patients in a more reliable and biologically valid manner than our current classification allows. This will also have direct epidemiological implication on public health policies. (4) As other bipolar endophenotypes, anxiety traits raise the possibility to be modeled in animal research. Several genetic, pharmacological, and behavioral animal models have long been used to establish animal anxiety-like phenotypes, as well as to assess their memory, learning, and other cognitive functions (Ennaceur et al., 2006; Kalueff et al., 2007; Waikar et al., 1997; Wang et al., 2007; Yokoyama et al., 2009). Specifically, chronic icv oxytocin has been used to attenuate the high level of trait anxiety in rats (Slattery et al., 2009). Some innate fear responses may also underlie natural tendencies manifested as high anxiety levels as we found in Costa Rican bipolar I patients.
Acknowledgments
Financial support for this study was provided by Fogarty (TW006152) training grant and NIMH 1 R01 MH069856-01A2 grant. SOLAR is supported by MH59490 (J. Blangero). The authors wish to thank Evelyn Duran, MD, Marcela Barguil, MA and Lara Mora, MA for assistance in data collection and Mercedes Ramirez, MD, Salvador Contreras, MD, Juan Zavala MD, and Lorena Jimenez, MD for assistance in best estimation. None of the authors have financial disclosers and portions of these data were presented at World Congress of Biological Psychiatry (Paris, 2009) where Dr. Javier Contreras received the Young Investigator Best Poster Award. We are also grateful to the families who have made this research possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors:
Drs. Contreras, Hare, Escamilla and Raventos take responsibility for the integrity of the data and the accuracy of the statistical analyses. The project was conducted by Dr. Contreras, Adriana Pacheco and Dr. Raventos. Dr. Hare and Dr. Escamilla contributed to statistical analysis. All authors had full access to all of the data in the study.
Conflict of interest:
None of the authors have financial disclosers.
References
- Bearden CE, Freimer NB. Endophenotypes for psychiatric disorders: ready for primetime? Trends. Genet. 2006;22(6):306–313. doi: 10.1016/j.tig.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Reus VI, Freimer NB. Why genetic investigation of psychiatric disorders is so difficult? Curr. Opin. Genet. Dev. 2004;14(3):280–286. doi: 10.1016/j.gde.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Berns GS, Nemeroff CB. The Neurology of Bipolar Disorder. Am. J. Med. Genet. C. (Semin. Med. Genet.) 2003;123C:76–84. doi: 10.1002/ajmg.c.20016. [DOI] [PubMed] [Google Scholar]
- Chen YW, Dilsaver SC. Comorbidity of panic disorder in bipolar illness: evidence from the Epidemiologic Catchment Area Survey. Am. J. Psychiatry. 1995;152(2):280–282. doi: 10.1176/ajp.152.2.280. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Michalikova S, Chazot PL. Models of anxiety: responses of rats to novelty in an open space and an enclosed space. Behav. Brain. Res. 2006;171(1):26–49. doi: 10.1016/j.bbr.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Evans L, Akiskal HS, Keck PE, Jr, McElroy SL, Sadovnick AD, Remick RA, Kelsoe JR. Familiality of temperament in bipolar disorder: support for a genetic spectrum. J. Affect. Disord. 2005;85(1–2):153–168. doi: 10.1016/j.jad.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Feske U, Frank E, Mallinger AG, Houck PR, Fagiolini A, Grochocinski VJ, Kupfer DJ. Anxiety as a correlate of response to the acute treatment of bipolar I disorder. Am. J. Psychiatry. 2000;157:956–962. doi: 10.1176/appi.ajp.157.6.956. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The Endophenotype Concept in Psychiatry: Etymology and Strategic Intentions. Am. J. Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Shields J. Genetic theorizing and schizophrenia. Br. J. Psychiatry. 1973;122:15–30. doi: 10.1192/bjp.122.1.15. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets W, Gould T, Gottesman I, Manji H. Toward constructing an endophenotype strategy for bipolar disorders. Biol. Psychiatry. 2006;60(2):93–105. doi: 10.1016/j.biopsych.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Murphy DL. The importance of cognitive phenotypes in experimental modeling of animal anxiety and depression. Neural. Plast. 2007 doi: 10.1155/2007/52087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck PE, Jr, McElroy SL, Strakowski SM, West SA, Sax KW, Hawkins JM, Bourne ML, Haggard P. 12-month outcome of patients with bipolar disorder following hospitalization for a manic or mixed episode. Am. J. Psychiatry. 1998;155:646–652. doi: 10.1176/ajp.155.5.646. [DOI] [PubMed] [Google Scholar]
- Leckman J, Sholomskas D, Thompson W, Belanger A, Weissman M. Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch. Gen. Psychiatry. 1982;39:879–883. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- MacKinnon DF, Zandi PP, Cooper J, Potash JB, Simpson SG, Gershon E, Nurnberger J, Reich T, DePaulo JR. Comorbid bipolar disorder and panic disorder in families with a high prevalence of bipolar disorder. Am. J. Psychiatry. 2002;159:30–35. doi: 10.1176/appi.ajp.159.1.30. [DOI] [PubMed] [Google Scholar]
- Mantere O, Suominen K, Valtonen HM, Arvilommi P, Isometsä E. Only half of bipolar I and II patients report prodromal symptoms. J. Affect. Disord. 2008;111(2–3):366–371. doi: 10.1016/j.jad.2008.03.011. 2008. [DOI] [PubMed] [Google Scholar]
- Maxwell M. Manual for the FIGS. Wasington, DC: Intramural Research Program, National Institute of Mental Health; 1992. [Google Scholar]
- McElroy SL, Altshuler LL, Suppes T, Keck PE, Jr, Frye MA, Denicoff KD, Nolen WA, Kupka RW, Leverich GS, Rochussen JR, Rush AJ, Post RM. Axis I psychiatric comorbidity and its relationship to historical illness variables in 288 patients with bipolar disorder. Am. J. Psychiatry. 2001;158:420–426. doi: 10.1176/appi.ajp.158.3.420. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch. Gen. Psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Rodrigo G, Lusiardo M. Note on the reliability and concurrent validity of the Spanish version of the State-Trait Anxiety Inventory. Percept. Mot. Skills. 1988;67(3):926. doi: 10.2466/pms.1988.67.3.926. [DOI] [PubMed] [Google Scholar]
- Rotondo A, Mazzanti C, Dell'Osso L, Rucci P, Sullivan P, Bouanani S, Gonnelli C, Goldman D, Cassano GB. Catechol o-methyltransferase, serotonin transporter, and tryptophan hydroxylase gene polymorphisms in bipolar disorder patients with and without comorbid panic disorder. Am. J. Psychiatry. 2002;159(1):23–29. doi: 10.1176/appi.ajp.159.1.23. [DOI] [PubMed] [Google Scholar]
- Schinka JA, Busch RM, Robichaux-Keene N. A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Mol. Psychiatry. 2004;9:197–202. doi: 10.1038/sj.mp.4001405. [DOI] [PubMed] [Google Scholar]
- Sen S, Burmeister M, Ghosh D. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. Am. J. Med. Genet. 2004;127B:85–89. doi: 10.1002/ajmg.b.20158. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Neumann ID. Chronic icv oxytocin attenuates the pathological high anxiety state in female high anxiety-related behaviour rats. Neuropharmacology. 2009 doi: 10.1016/j.neuropharm.2009.06.038. in press. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Palo Alto, CA: Consulting Psychologists Press; 1983. Manual for the State-Trait Anxiety Inventory (Form Y) [Google Scholar]
- Unschuld PG, Ising M, Erhardt A, Lucae S, Kloiber S, Kohli M, Salyakina D, Welt T, Kern N, Lieb R, Uhr M, Binder EB, Müller-Myhsok B, Holsboer F, Keck ME. Polymorphisms in the serotonin receptor gene HTR2A are associated with quantitative traits in panic disorder. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2007;144(4):424–429. doi: 10.1002/ajmg.b.30412. [DOI] [PubMed] [Google Scholar]
- Vázquez GH, Kahn C, Schiavo CE, Goldchluk A, Herbst L, Piccione M, Saidman N, Ruggeri H, Silva A, Leal J, Bonetto GG, Zaratiegui R, Padilla E, Vilapriño JJ, Calvó M, Guerrero G, Strejilevich SA, Cetkovich-Bakmas MG, Akiskal KK, Akiskal HS. Bipolar disorders and affective temperaments: a national family study testing the "endophenotype" and "subaffective" theses using the TEMPS-A Buenos Aires. J. Affect. Disord. 2008;108(1–2):25–32. doi: 10.1016/j.jad.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Vigneau F, Cormier S. The factor structure of the State-Trait Anxiety Inventory: an alternative view. J. Pers. Assess. 2008;90(3):280–285. doi: 10.1080/00223890701885027. [DOI] [PubMed] [Google Scholar]
- Waikar SV, Craske MG. Cognitive correlates of anxious and depressive symptomatology: an examination of the Helplessness/Hopelessness model. J. Anxiety. Disord. 1997;11(1):1–16. doi: 10.1016/s0887-6185(96)00031-x. [DOI] [PubMed] [Google Scholar]
- Wang D, Noda Y, Tsunekawa H, Zhou Y, Miyazaki M, Senzaki K, Nabeshima T. Behavioural and neurochemical features of olfactory bulbectomized rats resembling depression with comorbid anxiety. Behav. Brain. Res. 2007;178(2):262–273. doi: 10.1016/j.bbr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Wozniak J, Biederman J, Monuteaux MC, Richards J, Faraone SV. Parsing the comorbidity between bipolar disorder and anxiety disorders: a familial risk analysis. J. Child. Adolesc. Psychopharmacol. 2002;12(2):101–111. doi: 10.1089/104454602760219144. [DOI] [PubMed] [Google Scholar]
- Yokoyama F, Yamauchi M, Oyama M, Okuma K, Onozawa K, Nagayama T, Shinei R, Ishikawa M, Sato Y, Kakui N. Anxiolytic-like profiles of histamine H3 receptor agonists in animal models of anxiety: a comparative study with antidepressants and benzodiazepine anxiolytic. Psychopharmacology. (Berl) 2009;205(2):177–187. doi: 10.1007/s00213-009-1528-1. [DOI] [PubMed] [Google Scholar]