Abstract
The regulatory effects of the immune system on the skeleton during homeostasis and activation have been appreciated for years. In the past decade it has become evident that bone tissue can also regulate immune cell development. In the bone marrow, the differentiation of hematopoietic progenitors requires specific microenvironments, called niches, provided by various subsets of stromal cells, many of which are of mesenchymal origin. Among these stromal cell populations, cells of the osteoblast lineage serve a supportive function in the maintenance of normal hematopoiesis, and B lymphopoiesis in particular. Within the osteoblast lineage, distinct differentiation stages exert differential regulatory effects on hematopoietic development. In this review we will highlight the critical role of osteoblast progenitors in the perivascular B lymphocyte niche.
B lymphocyte development is dependent upon growth factors and cytokines provided by the stromal microenvironment
In mammals B lymphocyte development occurs in the fetal liver during embryonic development, then migrates to the bone marrow in the perinatal period, where B lymphocytes are continually generated throughout the entire lifespan [1]. The successful development of lymphoid precursors into committed B cells expressing antigen-specific receptors requires signals from the specialized microenvironment constituted by non-lymphoid stromal cells that interact intimately with the developing lymphocytes, providing paracrine support by producing humoral factors such as cytokines and growth factors, and physical support by cell-surface molecular interactions. These stromal cell subsets participate in the establishment of specific bone marrow microenvironments, also called bone marrow niches, required for the generation and maintenance of B lymphopoiesis and hematopoiesis in general.
Each step during B cell differentiation is regulated by growth factors and cytokines as well as by cell-cell contact [2]. Within the bone marrow, B lymphocyte development begins with the differentiation of hematopoietic stem cells (HSCs) into common lymphoid progenitors (CLPs), which in turn give rise to the earliest identifiable B cell precursors, prepro-B cells. Prepro-B cells progressively differentiate into intermediate pro-B and pre-B cell stages, followed by immature/naïve IgM+ B cells and finally by terminally differentiated antibody-secreting plasma cells [3–8].
The progression of B lymphocyte development within the bone marrow is critically dependent upon the actions of various cytokines, particularly chemokines and interleukins, which act at specific steps of differentiation. Gene targeting studies have revealed that among chemokines, CXCL12 and its receptor CXCR4 are essential for B cell development. Experimental deficiency of CXCL12 or its receptor CXCR4 in the developing murine embryo results in early lethality due to defective organ vascularization, cardiac and nervous system malformations, as well as lack of BM seeding by immature hematopoietic progenitor cells with resulting impaired B lymphopoiesis and bone marrow myelopoiesis [9–13]. Interleukin (IL)-7 has also been shown to be a critical cytokine for lymphoid development, and in particular is essential for differentiation of pro-B cells. However, IL-7 is ineffective as a regulator of the differentiation of B lineage cells beyond pre-B cell development since more mature B lymphocytes lack the IL-7 receptor [14–16]. Both IL-7 and IL-7 receptor KO mice exhibit an arrest in B cell development at the pro B cell stage in bone marrow [16, 17].
These supporting cytokines are supplied to hematopoietic progenitors by a network of non-hematopoietic stromal cells, and distinct populations of stromal cells appear to play key roles at unique stages of differentiation. Tokoyoda et al. localized prepro-B cells in close contact to CXCL12-expressing stromal cells in vivo [2]. Stromal CXCL12-expressing cells have been described as having a reticular morphology, and are dispersed throughout bone marrow in particular surrounding sinusoidal endothelial cells [2, 18]. In contrast, mitotically active pro-B cells are found near IL-7-expressing cells, which are distinct from both CXCL12-expressing cells and bone-lining osteoblasts [2]. IL7-expressing cells are also closely apposed to the vasculature [19]. As B cell precursors mature further into pre-B cells, however, they are found at some distance from both CXCL12 and IL-7 expressing stromal cells [2]. A specific niche constituted by galectin-1 expressing stromal cells, distinct from IL-7 expressing cells, has been reported for pre-B cells [19–21]. Finally, naïve IgM+ B cells enter the blood circulation and complete their differentiation in peripheral lymphoid organs. Of note, terminally differentiated B lymphocytes, or antibody-producing plasma cells, return to the bone marrow where they are found in contact with the cell bodies and processes of CXCL12-expressing cells and perivascular dendritic cells [22]. These findings demonstrate that B cell precursors migrate between specific niches during the course of differentiation, and that these niches are comprised of distinct populations of stromal cells.
The importance of bone to hematopoiesis and B lymphopoiesis
Beyond B lymphocytes, specific niches have been identified in the bone marrow for hematopoietic stem cells (HSCs) (reviewed elsewhere in this issue by Calvi and Link) as well as maturing lineage committed precursors, including those that will give rise to neutrophils and platelets [23, 24]. The cellular identity of the stromal cells supporting these various stages of hematopoiesis is of significant interest. In the bone marrow, the stroma is composed of a heterogeneous group of cells; many are of mesenchymal origin, including fibroblasts, osteoblasts, adipocytes, reticular cells, endothelial cells and perivascular cells, but sympathetic neurons, glial cells and macrophages have also been shown to be part of the supportive microenvironment [25–34]. In this review we will highlight the role of cells of the osteoblast lineage, increasingly recognized as essential for supporting B lymphopoiesis at various stages in the bone marrow [35, 36].
Hematopoietic bone marrow is contained in bones, and therefore osteoblastogenesis is necessary for bone formation and subsequent bone marrow development. In mice lacking either Runx2 or Osterix, transcription factors required for osteoblast development, the absence of bone tissue and resulting failure of bone marrow formation results in the outsourcing of hematopoiesis to extramedullary organs [37–41]. With respect to B lymphocyte differentiation, a crucial role for the osteoblast lineage was revealed by ablation of osteoblasts in vivo. In transgenic mice with thymidine kinase targeted to maturing osteoblasts by the 2.3 kb type I collagen promoter, ablation of osteoblasts by administration of gancyclovir resulted in decreased bone marrow cellularity with an acute loss of B lymphocytes followed by progressive loss of hematopoietic stem cell (HSC) subsets [42]. Zhu et al. further demonstrated that calvarial osteoblasts in culture could support the full differentiation program of hematopoietic stem/progenitor cells into the B lymphocyte lineage, confirming a direct interaction between osteoblastic cells and B cell precursors [43].
Cells of the osteoblast lineage at differing stages of differentiation serve distinct functions in the bone marrow hematopoietic niche
The osteoblast lineage itself is quite heterogeneous, representing a spectrum of differentiation stages from newly committed osteoblast progenitors (hereafter referred to as osteoprogenitors) to terminally differentiated osteoblasts. Commitment to the osteoblast lineage from mesenchymal stem cells begins with the expression of Runx2, a transcription factor required for osteoblastogenesis [37, 38, 44]. Downstream of Runx2, osteoblast commitment is reinforced by expression of Osterix (Osx), which is expressed in proliferative osteoprogenitors [39]. Differentiation of the osteoblast lineage is marked by the progressive expression of markers of osteoblast maturation, alkaline phosphatase, type I collagen, bone sialoprotein, osteopontin and osteocalcin [45].
With such a diversity of populations within the osteoblast lineage, it is likely that cells at various stages of differentiation will serve unique functions in the hematopoietic niche. Indeed, increasing evidence suggests that earlier stages of the osteoblast lineage are especially crucial to hematopoietic support. Mice lacking either Runx2 or Osx fail to develop a mineralized skeleton and die soon after birth with a complete absence of bone marrow inside long bones [37, 38, 40]. In contrast, mice deficient in osteocalcin, a marker of terminally differentiated osteoblasts, are phenotypically normal with stronger bones and greater mineral density than their control littermates and no hematopoietic alterations were reported [46].
In vitro studies have also shown that maturational status of osteoblasts influences their hematopoietic supporting ability. In co-cultures with hematopoietic stem/progenitor cells, freshly isolated calvarial osteoblasts, which expressed higher levels of immature osteoblast markers, were better able to support hematopoietic expansion than calvarial osteoblasts that had been previously cultured and expressed markers of mature osteoblasts [47]. In follow-up studies this group demonstrated that expression of CD166 on cultured osteoblasts correlated with high levels of Runx2 expression, low levels of osteocalcin expression, and high hematopoiesis enhancing activity. Furthermore, loss of CD166 expression with osteoblast differentiation and maturation was associated with loss of support for hematopoietic function [48]. Consistent with the idea that earlier stages of the osteoblast lineage are more relevant to the hematopoietic niche, the CD105+Thy1− fraction of fetal mesenchymal progenitors can direct formation of a hematopoiesis-supporting microenvironment when transplanted under the renal capsule. In contrast, the CD105+Thy1+ fraction, enriched in more committed osteoblast lineage cells, can form only ectopic bone lacking a hematopoietic marrow [49].
Mesenchymal progenitors are perivascular and support B lymphopoiesis
Mature osteoblasts are easily identified in situ on bone surfaces by their cuboidal morphology. Upon terminal differentiation osteoblasts may remain on the bone surface as flattened, quiescent lining cells, be incorporated into mineralized matrix as osteocytes, or die by apoptosis [50]. In contrast to mature osteoblasts and osteocytes, however, earlier mesenchymal progenitors and osteoblast precursors have been more difficult to identify within the non-hematopoietic stroma in vivo. The absence of specific surface antigen markers capable of unequivocally distinguishing the different stromal subpopulations and their stages of maturation has been a challenge in the field. In bone marrow and other tissues, mesenchymal stem cells share features with perivascular cells termed pericytes [51, 52]. Recently, perivascular cells variously described as expressing PDGFRα and Sca-1, Nestin, the leptin receptor, or Prx1 and exhibiting multipotent mesenchymal capacity have been identified as crucial components of the HSC niche [28, 53–56].
While the perivascular niche does not contain mature osteoblasts, growing evidence suggests that osteoprogenitors are also perivascular. During embryonic development Osx-expressing osteoprogenitors, but not type I collagen-expressing osteoblasts, are found in close proximity to invading blood vessels [57]. In humans and rodents, expression of CD146 marks a population of reticular cells lining the sinusoids of the bone marrow microvasculature that express alkaline phosphatase and can be induced to differentiate into osteoblasts. Upon transplantation in vivo, CD146+ positive cells generate bone (CD146-negative osteoblasts) and self-renew into CD146+ reticular cells lining on the sinusoids, demonstrating that they are functional osteoprogenitors [58]. Since CXCL12- and IL7-producing stromal cells and osteoprogenitors have all been localized near the vasculature, and cells of the osteoblast lineage can support B lymphopoiesis, these studies suggest that osteoprogenitors may be an important source of CXCL12 and/or IL-7 in vivo. Indeed, both CXCL12 and IL-7 can be produced by cells of the osteoblast lineage [43, 59, 60].
CXCL12+ abundant reticular cells (CAR cells) are located close to bone marrow sinusoids, express osteogenic genes and have the potential to differentiate into osteoblasts in culture; they also appear to have the capacity to differentiate into the adipocyte lineage [18, 26]. Furthermore, short-term ablation of CAR cells in vivo impairs both osteogenic and adipogenic differentiation potential of bone marrow cells [26]. Interestingly, Osx-expressing osteoprogenitors have recently been demonstrated to have adipogenic potential as well [61]. Taken together, perivascular mesenchymal progenitors with at least osteogenic and adipogenic potential play an important role in supporting B lymphocyte differentiation. Two recent studies have confirmed the importance of CXCL12 production by mesenchymal and osteoprogenitors but not mature osteoblasts in supporting bone marrow B lymphoid development [55, 56]. Deletion of CXCL12 in Prx1-expressing mesenchymal progenitors or in Osx- or Col2.3-expressing osteoprogenitors but not in osteocalcin-expressing mature osteoblasts leads to depletion of early lymphoid progenitors with a particular reduction in committed B-lineage progenitors [55, 56]. CD146+ reticular cells produce large amounts of CXCL12 in humans and rodents, can establish a hematopoietic niche upon transplantation, and have been shown to sustain primitive hematopoietic stem and progenitor cells (HSPCs) in vitro through cell-to-cell contact [62]. Taken together, these results demonstrate that perivascular mesenchymal progenitors with osteogenic (and possibly adipogenic) potential are an important source of CXCL12 in the hematopoietic, and specifically B lymphocyte, bone marrow niche.
IL-7-expressing stromal cells, while distinct from CXCL12+ cells, have also been localized to the perivascular space [2, 19]. While IL-7 has not been specifically deleted from osteoblast lineage cells, the targeted overexpression of human IL-7 to maturing osteoblasts can rescue the osteopenia and B cell development of IL-7 KO mice, without interfering with T lymphopoiesis [63].
Gsα signaling in osteoblast progenitors is required for production of IL-7 and support of B lymphopoiesis
Signaling mediated by the parathyroid hormone (PTH)/PTH-related peptide receptor (PPR) in osteoblasts is an important regulator of the hematopoietic stem cell niche [59]. Expression of both CXCL12 and IL-7 is increased in response to parathyroid hormone (PTH) stimulation by its actions on the PTH receptor PPR [43, 59, 60]. When stimulated with PTH in vitro, calvarial osteoblasts showed marked increases in CXCL12 and IL-7 levels and are able to support B lymphoid commitment and sustain B cell differentiation from the earliest B cell progenitors to the generation of mature IgM+ B cells. Of note, this inductive effect is not shared by adipocytes or endothelial cells [43].
The PPR is a G protein-coupled receptor, and the Gsα subunit of the heterotrimeric Gs protein is a major downstream mediator of PPR signaling [64]. We have demonstrated that the ablation of Gsα early in the osteoblast lineage, in osterix-expressing osteoprogenitors (GsαOsxKO mice), leads to severe osteoporosis with a dramatic reduction in trabecular bone [65, 66]. Notably, GsαOsxKO mice exhibit impaired B lymphopoieis with a dramatic reduction in B cell precursors. There is a specific block in the transition from pro-B to pre-B cells, whereas prepro-B cells are unaffected. This transition to pre-B cells is dependent upon IL-7, and IL-7 mRNA levels were significantly reduced in osteoprogenitors from GsαOsxKO mice. In contrast, and consistent with the unchanged frequency of prepro-B cells, CXCL12 mRNA levels were not altered. Moreover, the stage-specific differentiation block could be rescued in GsαOsxKO mice with exogenous treatment of IL-7, and transplantation of KO bone marrow cells into a wild-type host restored the hematopoietic phenotype, confirming the microenvironmental defect in GsαOsxKO mice [65].
While ablation of Gsα in osteoprogenitors leads to impaired B cell development, deletion of Gsα in terminally differentiated osteocytes does not affect B lymphopoiesis despite an osteopenic phenotype with reduced osteoblast numbers [65, 67]. Instead there is a dramatic increase in myeloid cells, mediated by production of granulocyte colony stimulating factor (GCSF) by osteocytes [67]. These results again highlight the importance osteoprogenitors specifically in regulating bone marrow B lymphopoiesis.
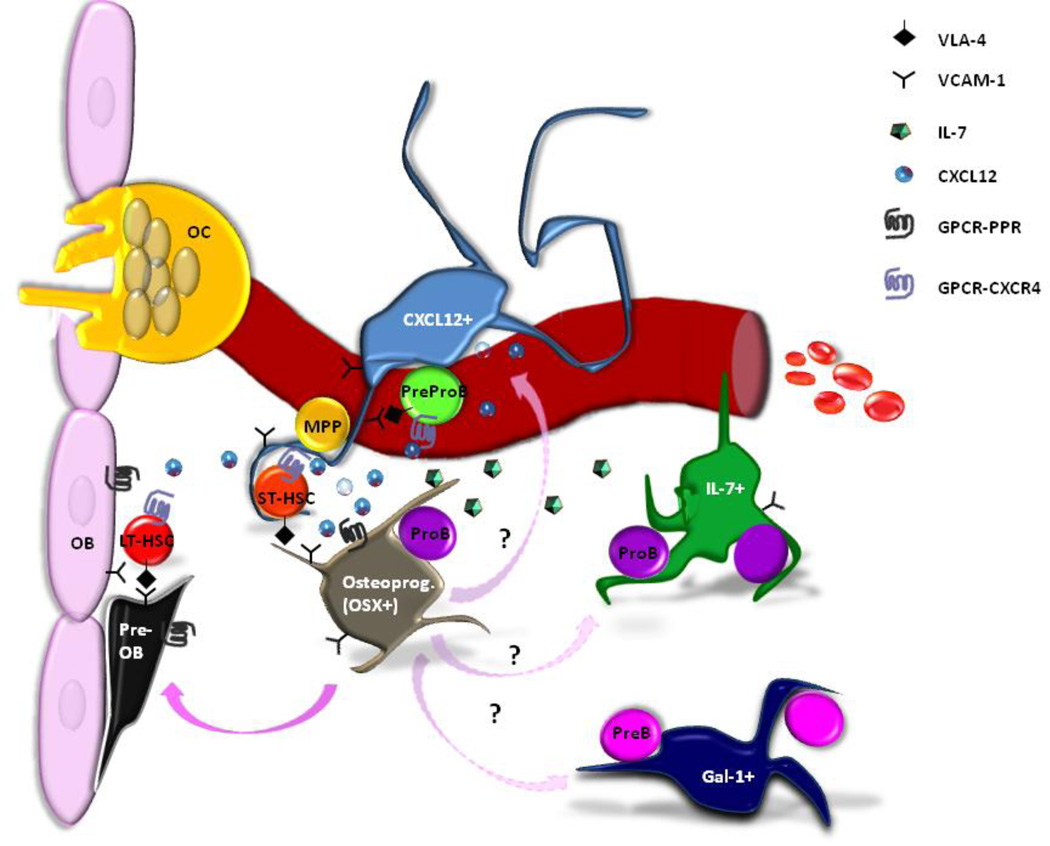
Stromal cells with osteogenic capacity and perivascular location expressing CXCL12, IL-7, CD146, osterix and galectin-1 have therefore all been implicated in regulating various stages of B cell precursor differentiation within the bone marrow. How these cellular subsets overlap and differ remains to be determined by careful studies comparing these populations (Figure 1). For example, CXCL12+ cells, IL-7+ cells and osteoblasts have all been described to be VCAM1+, CD54+, PDGFRα+, CD45−, and CD31− [2, 54, 68, 69]. In contrast, the majority of Gal-1 expressing cells are VCAM1−, PDGFRα− and CD31+, indicating that these cells constitute a different stromal cell subset [19]. Of note, VCAM-1, together with its ligand VLA-4, has been described to be involved in the maintenance of HSCs in their quiescent state inside their niche. Tokoyoda et al. found that CXCL12 induces a significant increase in adhesion of prepro-B cells but not pro-B or pre-B to VCAM-1 [2]. This suggests that CXCL12 acts selectively on prepro-B cells to increase adhesiveness of VLA-4 ligand through the control of the integrin α4, which has been shown to be essential for early B lymphopoiesis in bone marrow [70].
Figure 1.
A variety of stromal cell populations expressing CXCL12, IL-7 and galectin-1 have been identified as integral components of the bone marrow B lymphocyte niches. CXCL12+ and IL-7+ stromal cells are perivascular and support B cell precursor prepro-B and pro-B cell populations, respectively. Osterix+ osteoprogenitors can produce both CXCL12 and IL-7; how these subsets differ or overlap and whether they give rise to mature osteoblasts lining the bone surface remains to be determined.
Additional animal models of impaired osteoblastic regulation of B lymphopoieis
Recently additional studies have noted impaired B lymphopoiesis in the setting of an abnormal bone microenvironment, confirming the crucial role of the osteoblast lineage in the B lymphocyte niche. For example, an impaired B lymphocyte niche can result from altering the balance between bone formation and bone resorption. Defective B lymphocyte differentiation has been identified in various mouse models of impaired osteoclastic resorption, or osteopetrosis [71–75]. Osteopetrotic oc/oc mice exhibit a block at the pro-B to pre-B cell transition associated with reduced levels of IL-7; this phenotype can be partly reversed by exogenous IL-7 or restoration of osteoclast function [76, 77]. Treatment with zoledronic acid (ZA), a potent bisphosphonate, to inhibit osteoclast activity also results in an osteopetrotic phenotype with increased bone mass. In response to ZA treatment, the number of B cells is decreased by 50% in the bone marrow. The reduction in B lymphocytes affected all B cell subsets from the prepro-B stage but was restricted to bone marrow, and was not due to a direct effect of ZA on B lymphocytes or osteoblast/stromal cells [78]. Osteoclastic resorption plays an important role in recruitment of mesenchymal and osteoblast progenitors [79], and indeed in the absence of functioning osteoclasts there is a significant reduction in osteoprogenitors as determined by the colony forming-unit-alkaline phosphatase (CFU-ALP) assay. In keeping with osteoprogenitors as a major source of CXCL12 and IL-7 in the hematopoietic niche, the mRNA levels of CXCL12 and IL-7 were dramatically reduced in BM of ZA treated mice [78].
In a different model of high bone mass, sclerostin (SOST) KO mice exhibit a marked reduction of B lymphocytes [80]. Sclerostin is a secreted inhibitor of canonical Wnt signaling primarily expressed by mature osteocytes and acts on osteoblasts as a negative regulator of bone growth [81, 82]. Mice with deletion of the SOST coding region display highly mineralized bone due to increased osteoblast activity without altered osteoclast activity [83]. Despite the high bone mass and increased activation of osteoblasts, SOST KO mice display a specific reduction in all B cell stages but not in HSCs or other hematopoietic progenitor populations in the BM. In this case the significant decline of all committed B cell developmental stages was associated with increased apoptosis at the precursor, immature and recirculating B cell stages, rather than with impaired differentiation [80]. Whether osteoprogenitors are specifically implicated has not been investigated. The effects of sclerostin ablation on osteoprogenitors remain to be characterized. Perhaps in the presence of a hypermineralized bone mainly mature osteoblasts are expanded while early mesenchymal stromal cells or osteoprogenitor populations might be deficient. The reduction in CXCL12 mRNA levels in stromal cells of SOST KO mice gives support to this idea. Alternatively, Wnt signaling restrains CXCL12 expression in bone marrow stromal cells in vitro, so perhaps overactive Wnt signaling in stromal cells in the absence of SOST results in a reduction of CXCL12 to levels that are not conducive for B cell survival [84].
A role for macrophages in the osteoblastic B lymphocyte niche
In addition to direct interactions between B lymphocyte precursors and osteoblast lineage cells, several recent studies have highlighted a role for macrophages in regulating the hematopoietic niche via osteoblasts. Tissue-resident macrophages, termed osteomacs, can be identified in close association with areas of bone remodeling and are required for optimal bone forming activity in vitro and in vivo [85]. Mobilization of hematopoietic stem/progenitor cells by G-CSF is associated with suppression of endosteal bone formation and decreased expression of CXCL12 [29, 33]. However, osteoblast lineage cells do not express G-CSF receptors; instead the actions of G-CSF on HSPCs and osteoblasts are mediated by macrophages. Several in vivo studies have demonstrated that loss of macrophages negatively affected the growth and/or survival of osteoblasts and allowed egress of HSPCs to the blood stream [32–34]. In addition, mobilizing doses of G-CSF also impaired medullary B lymphopoiesis with reduction of all B cell precursors, immature and mature B lymphocytes and increased levels of apoptotic B cells in BM [86]. Since B lymphocytes and their precursors do not express the G-CSF receptor, this phenomenon may also reflect a role for macrophages in the regulation of B lymphopoiesis by cells of the osteoblast lineage.
Conclusions
In summary, the complexity of the crosstalk between cells involved in bone homeostasis and hematopoiesis has become increasingly apparent, especially in the regulation of bone marrow B lymphocyte development. Further studies are needed to characterize different stromal populations involved in order to better understand their contribution to the generation of unique bone marrow niches, their roles in the crosstalk with hematopoietic cells and the temporal and spatial mechanisms by which they can regulate the cell cycle and differentiation program of hematopoietic cells. In particular, a clearer understanding of the interactions between hematopoietic and mesenchymal cell populations at specific stages of differentiation is needed.
Acknowledgements
This work was supported by the Harvard Stem Cell Institute (Cambridge, MA) and NIH grant DP2OD008466 to J.Y.W.
Footnotes
The authors have stated that they have no conflict of interest.
References
- 1.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112(5):1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tokoyoda K, et al. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20(6):707–718. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Nagasawa T. Microenvironmental niches in the bone marrow required for B-cell development. Nat Rev Immunol. 2006;6(2):107–116. doi: 10.1038/nri1780. [DOI] [PubMed] [Google Scholar]
- 4.Otero DC, Rickert RC. CD19 function in early and late B cell development. II. CD19 facilitates the pro-B/pre-B transition. J Immunol. 2003;171(11):5921–5930. doi: 10.4049/jimmunol.171.11.5921. [DOI] [PubMed] [Google Scholar]
- 5.Hardy RR, et al. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173(5):1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osmond DG, Rolink A, Melchers F. Murine B lymphopoiesis: towards a unified model. Immunol Today. 1998;19(2):65–68. doi: 10.1016/s0167-5699(97)01203-6. [DOI] [PubMed] [Google Scholar]
- 7.Li YS, et al. Identification of the earliest B lineage stage in mouse bone marrow. Immunity. 1996;5(6):527–535. doi: 10.1016/s1074-7613(00)80268-x. [DOI] [PubMed] [Google Scholar]
- 8.Ogawa M, ten Boekel E, Melchers F. Identification of CD19(–)B220(+)c-Kit(+)Flt3/Flk- 2(+)cells as early B lymphoid precursors before pre-B-I cells in juvenile mouse bone marrow. Int Immunol. 2000;12(3):313–324. doi: 10.1093/intimm/12.3.313. [DOI] [PubMed] [Google Scholar]
- 9.Zou YR, et al. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393(6685):595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 10.Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci U S A. 1994;91(6):2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peled A, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283(5403):845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 12.Tachibana K, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393(6685):591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 13.Nagasawa T, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382(6592):635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 14.Lee G, et al. Recombinant interleukin-7 supports the growth of normal B lymphocyte precursors. Curr Top Microbiol Immunol. 1988;141:16–18. doi: 10.1007/978-3-642-74006-0_3. [DOI] [PubMed] [Google Scholar]
- 15.Miller JP, et al. The earliest step in B lineage differentiation from common lymphoid progenitors is critically dependent upon interleukin 7. J Exp Med. 2002;196(5):705–711. doi: 10.1084/jem.20020784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Freeden-Jeffry U, et al. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181(4):1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peschon JJ, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180(5):1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugiyama T, et al. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Mourcin F, et al. Galectin-1-expressing stromal cells constitute a specific niche for pre-BII cell development in mouse bone marrow. Blood. 2011;117(24):6552–6561. doi: 10.1182/blood-2010-12-323113. [DOI] [PubMed] [Google Scholar]
- 20.Gauthier L, et al. Galectin-1 is a stromal cell ligand of the pre-B cell receptor (BCR) implicated in synapse formation between pre-B and stromal cells and in pre-BCR triggering. Proc Natl Acad Sci U S A. 2002;99(20):13014–13019. doi: 10.1073/pnas.202323999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Espeli M, et al. Impaired B-cell development at the pre-BII-cell stage in galectin-1-deficient mice due to inefficient pre-BII/stromal cell interactions. Blood. 2009;113(23):5878–5886. doi: 10.1182/blood-2009-01-198465. [DOI] [PubMed] [Google Scholar]
- 22.Sapoznikov A, et al. Perivascular clusters of dendritic cells provide critical survival signals to B cells in bone marrow niches. Nat Immunol. 2008;9(4):388–395. doi: 10.1038/ni1571. [DOI] [PubMed] [Google Scholar]
- 23.Taichman RS, Emerson SG. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J Exp Med. 1994;179(5):1677–1682. doi: 10.1084/jem.179.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avecilla ST, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10(1):64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 25.Naveiras O, et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460(7252):259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Omatsu Y, et al. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33(3):387–399. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 27.Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 28.Ding L, et al. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katayama Y, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124(2):407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 30.Mendez-Ferrer S, et al. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452(7186):442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 31.Yamazaki S, et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147(5):1146–1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 32.Winkler IG, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116(23):4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- 33.Christopher MJ, et al. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J Exp Med. 2011;208(2):251–260. doi: 10.1084/jem.20101700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chow A, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208(2):261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porter RL, Calvi LM. Communications between bone cells and hematopoietic stem cells. Arch Biochem Biophys. 2008;473(2):193–200. doi: 10.1016/j.abb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu JY, Scadden DT, Kronenberg HM. Role of the osteoblast lineage in the bone marrow hematopoietic niches. J Bone Miner Res. 2009;24(5):759–764. doi: 10.1359/jbmr.090225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Komori T, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89(5):755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 38.Otto F, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89(5):765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 39.Nakashima K, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108(1):17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 40.Zhou X, et al. Multiple functions of Osterix are required for bone growth and homeostasis in postnatal mice. Proc Natl Acad Sci U S A. 2010;107(29):12919–12924. doi: 10.1073/pnas.0912855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deguchi K, et al. Excessive extramedullary hematopoiesis in Cbfa1-deficient mice with a congenital lack of bone marrow. Biochem Biophys Res Commun. 1999;255(2):352–359. doi: 10.1006/bbrc.1999.0163. [DOI] [PubMed] [Google Scholar]
- 42.Visnjic D, et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103(9):3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- 43.Zhu J, et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109(9):3706–3712. doi: 10.1182/blood-2006-08-041384. [DOI] [PubMed] [Google Scholar]
- 44.Ducy P, et al. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89(5):747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 45.Aubin JE. Regulation of osteoblast formation and function. Rev Endocr Metab Disord. 2001;2(1):81–94. doi: 10.1023/a:1010011209064. [DOI] [PubMed] [Google Scholar]
- 46.Ducy P, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382(6590):448–452. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 47.Cheng YH, et al. Impact of maturational status on the ability of osteoblasts to enhance the hematopoietic function of stem and progenitor cells. J Bone Miner Res. 2011;26(5):1111–1121. doi: 10.1002/jbmr.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chitteti BR, et al. Hierarchical organization of osteoblasts reveals the significant role of CD166 in hematopoietic stem cell maintenance and function. Bone. 2013;54(1):58–67. doi: 10.1016/j.bone.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan CK, et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457(7228):490–494. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21(2):115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 51.Crisan M, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 52.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21(2):193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 53.Mendez-Ferrer S, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morikawa S, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206(11):2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Greenbaum A, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495(7440):227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maes C, et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19(2):329–344. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sacchetti B, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 59.Calvi LM, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 60.Jung Y, et al. Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone. 2006;38(4):497–508. doi: 10.1016/j.bone.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 61.Song L, et al. Loss of wnt/beta-catenin signaling causes cell fate shift of preosteoblasts from osteoblasts to adipocytes. J Bone Miner Res. 2012;27(11):2344–2358. doi: 10.1002/jbmr.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Corselli M, et al. Perivascular support of human hematopoietic stem/progenitor cells. Blood. 2013;121(15):2891–2901. doi: 10.1182/blood-2012-08-451864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aguila HL, et al. Osteoblast-specific overexpression of human interleukin-7 rescues the bone mass phenotype of interleukin-7-deficient female mice. J Bone Miner Res. 2012;27(5):1030–1042. doi: 10.1002/jbmr.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Juppner H, et al. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science. 1991;254(5034):1024–1026. doi: 10.1126/science.1658941. [DOI] [PubMed] [Google Scholar]
- 65.Wu JY, et al. Osteoblastic regulation of B lymphopoiesis is mediated by Gs{alpha}-dependent signaling pathways. Proc Natl Acad Sci U S A. 2008;105(44):16976–16981. doi: 10.1073/pnas.0802898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu JY, et al. Gsalpha enhances commitment of mesenchymal progenitors to the osteoblast lineage but restrains osteoblast differentiation in mice. J Clin Invest. 2011;121(9):3492–3504. doi: 10.1172/JCI46406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fulzele K, et al. Myelopoiesis is regulated by osteocytes through Gsalpha-dependent signaling. Blood. 2012 doi: 10.1182/blood-2012-06-437160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Link A, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8(11):1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 69.Nagasawa T, Omatsu Y, Sugiyama T. Control of hematopoietic stem cells by the bone marrow stromal niche: the role of reticular cells. Trends Immunol. 2011;32(7):315–320. doi: 10.1016/j.it.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 70.Arroyo AG, et al. Differential requirements for alpha4 integrins during fetal and adult hematopoiesis. Cell. 1996;85(7):997–1008. doi: 10.1016/s0092-8674(00)81301-x. [DOI] [PubMed] [Google Scholar]
- 71.Franzoso G, et al. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev. 1997;11(24):3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kong YY, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397(6717):315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 73.Tagaya H, et al. Intramedullary and extramedullary B lymphopoiesis in osteopetrotic mice. Blood. 2000;95(11):3363–3370. [PubMed] [Google Scholar]
- 74.Blin-Wakkach C, et al. Hematological defects in the oc/oc mouse, a model of infantile malignant osteopetrosis. Leukemia. 2004;18(9):1505–1511. doi: 10.1038/sj.leu.2403449. [DOI] [PubMed] [Google Scholar]
- 75.Scimeca JC, et al. The gene encoding the mouse homologue of the human osteoclast-specific 116-kDa V-ATPase subunit bears a deletion in osteosclerotic (oc/oc) mutants. Bone. 2000;26(3):207–213. doi: 10.1016/s8756-3282(99)00278-1. [DOI] [PubMed] [Google Scholar]
- 76.Blin-Wakkach C, et al. Characterization of a novel bipotent hematopoietic progenitor population in normal and osteopetrotic mice. J Bone Miner Res. 2004;19(7):1137–1143. doi: 10.1359/JBMR.040318. [DOI] [PubMed] [Google Scholar]
- 77.Blin-Wakkach C, et al. Interleukin-7 partially rescues B-lymphopoiesis in osteopetrotic oc/oc mice through the engagement of B220+ CD11b+ progenitors. Exp Hematol. 2006;34(7):851–859. doi: 10.1016/j.exphem.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 78.Mansour A, et al. Osteoclast activity modulates B-cell development in the bone marrow. Cell Res. 2011;21(7):1102–1115. doi: 10.1038/cr.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tang Y, et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009;15(7):757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cain CJ, et al. Absence of sclerostin adversely affects B-cell survival. J Bone Miner Res. 2012;27(7):1451–1461. doi: 10.1002/jbmr.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Semenov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280(29):26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- 82.Choi HY, et al. Lrp4, a novel receptor for Dickkopf 1 and sclerostin, is expressed by osteoblasts and regulates bone growth and turnover in vivo. PLoS ONE. 2009;4(11):e7930. doi: 10.1371/journal.pone.0007930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li X, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23(6):860–869. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 84.Tamura M, Sato MM, Nashimoto M. Regulation of CXCL12 expression by canonical Wnt signaling in bone marrow stromal cells. Int J Biochem Cell Biol. 2011;43(5):760–767. doi: 10.1016/j.biocel.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 85.Chang MK, et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 2008;181(2):1232–1244. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- 86.Winkler IG, et al. B-lymphopoiesis is stopped by mobilizing doses of G-CSF and is rescued by overexpression of the anti-apoptotic protein Bcl2. Haematologica. 2013;98(3):325–333. doi: 10.3324/haematol.2012.069260. [DOI] [PMC free article] [PubMed] [Google Scholar]