Abstract
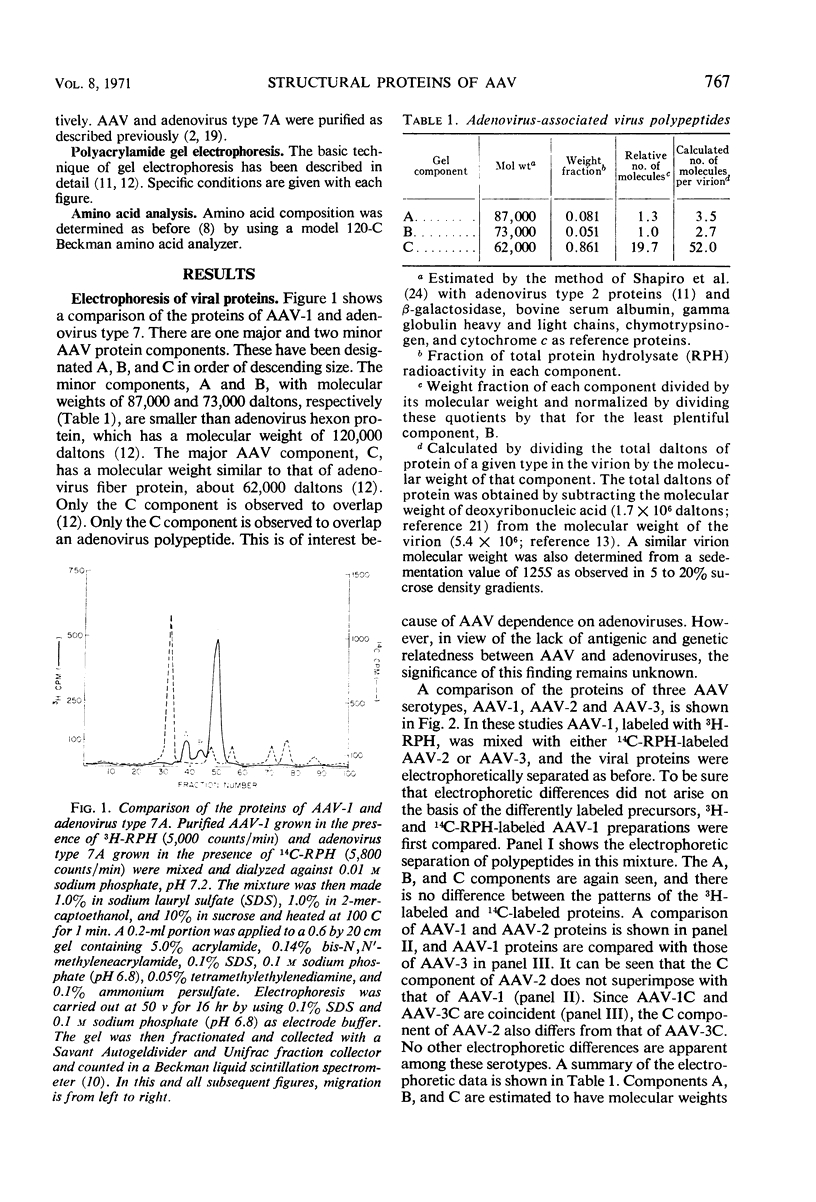
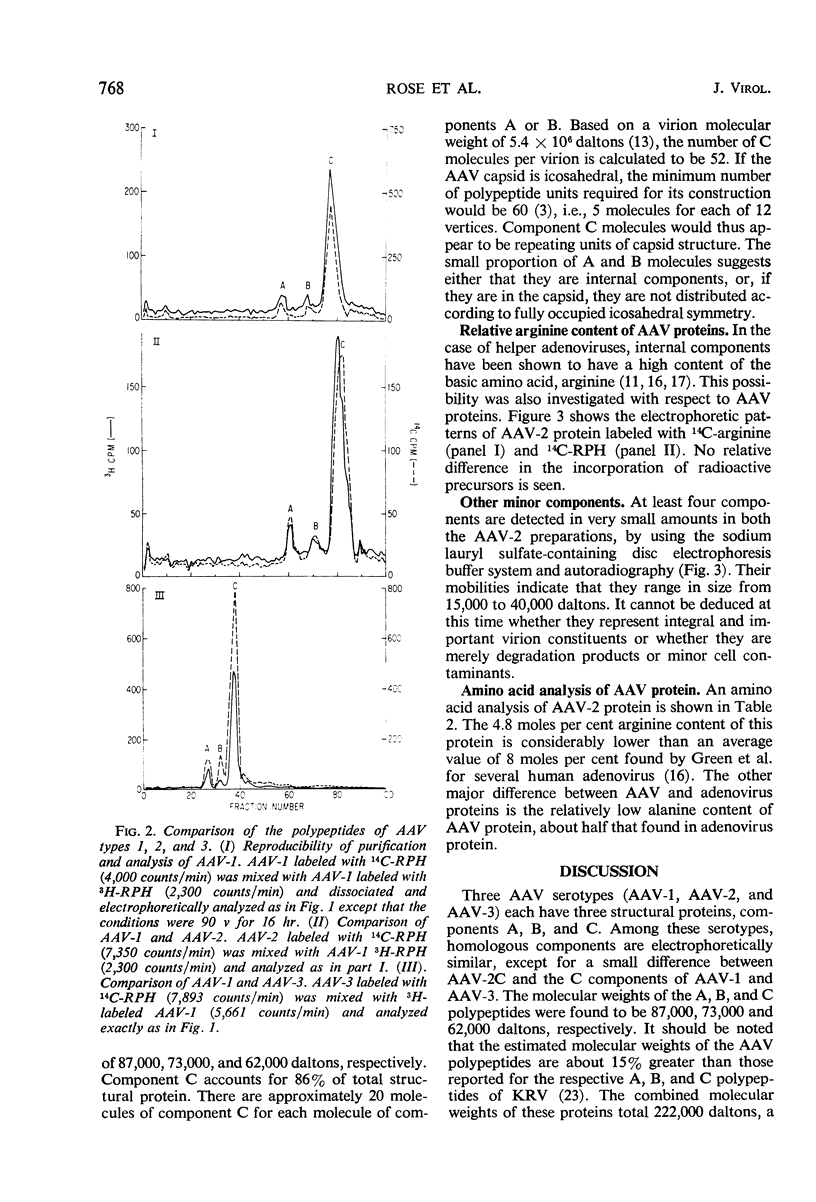
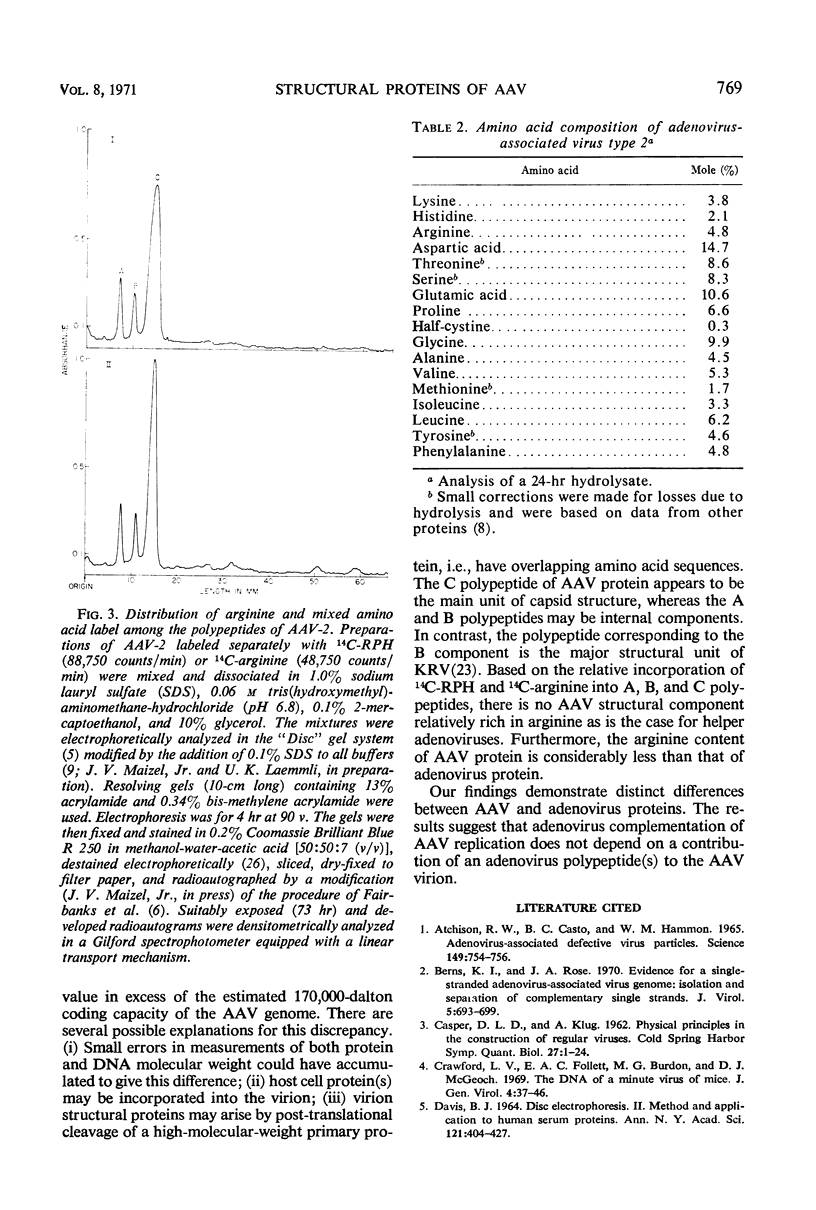
The structural proteins of adenovirus-associated virus (AAV) types 1, 2, and 3 were analyzed by acrylamide gel electrophoresis. In each case, one major protein (C) and two minor proteins (A and B) were identified. Component C had an estimated molecular weight of 62,000 daltons, and the molecular weights of components A and B were found to be 87,000 and 73,000 daltons, respectively. Coelectrophoresis of adenovirus and AAV proteins revealed an overlap only between the adenovirus fiber-penton component and the AAV C polypeptide. Among AAV serotypes, homologous components were electrophoretically identical, except that the C component of AAV-2 was of slightly lower molecular weight than the C components of AAV-1 and AAV-3. The relative incorporation of 14C-arginine and 14C-mixed amino acids into the three polypeptides of AAV-2 was similar, indicating an absence of an arginine-rich component. In addition, AAV-2 was found to have a substantially lower arginine content than helper adenoviruses.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATCHISON R. W., CASTO B. C., HAMMON W. M. ADENOVIRUS-ASSOCIATED DEFECTIVE VIRUS PARTICLES. Science. 1965 Aug 13;149(3685):754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Rose J. A. Evidence for a single-stranded adenovirus-associated virus genome: isolation and separation of complementary single strands. J Virol. 1970 Jun;5(6):693–699. doi: 10.1128/jvi.5.6.693-699.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Follett E. A., Burdon M. G., McGeoch D. J. The DNA of a minute virus of mice. J Gen Virol. 1969 Jan;4(1):37–46. doi: 10.1099/0022-1317-4-1-37. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Jr, Levinthal C., Reeder R. H. Analysis of C14-labeled proteins by disc electrophoresis. Biochem Biophys Res Commun. 1965 Aug 16;20(4):393–399. doi: 10.1016/0006-291x(65)90589-9. [DOI] [PubMed] [Google Scholar]
- Hoggan M. D., Blacklow N. R., Rowe W. P. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1467–1474. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968 Sep;36(1):115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. II. Soluble proteins, cores, top components and the structure of the virion. Virology. 1968 Sep;36(1):126–136. doi: 10.1016/0042-6822(68)90122-0. [DOI] [PubMed] [Google Scholar]
- Mayor H. D., Torikai K., Melnick J. L., Mandel M. Plus and minus single-stranded DNA separately encapsidated in adeno-associated satellite virions. Science. 1969 Dec 5;166(3910):1280–1282. doi: 10.1126/science.166.3910.1280. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Green M., Piña M., Melnick J. L. Physicochemical characterization of adeno-associated satellite virus type 4 and its nucleic acid. J Virol. 1967 Oct;1(5):980–987. doi: 10.1128/jvi.1.5.980-987.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Melnick J. L., Rongey R., Mayor H. D. Physical assay and growth cycle studies of a defective adeno-satellite virus. J Virol. 1967 Feb;1(1):171–180. doi: 10.1128/jvi.1.1.171-180.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polasa H., Green M. Adenovirus proteins. I. Amino acid composition of oncogenic and nononcogenic human adenoviruses. Virology. 1967 Mar;31(3):565–567. doi: 10.1016/0042-6822(67)90242-5. [DOI] [PubMed] [Google Scholar]
- Prage L., Pettersson U., Philipson L. Internal basic proteins in adenovirus. Virology. 1968 Nov;36(3):508–511. doi: 10.1016/0042-6822(68)90178-5. [DOI] [PubMed] [Google Scholar]
- Rose J. A., Berns K. I., Hoggan M. D., Koczot F. J. Evidence for a single-stranded adenovirus-associated virus genome: formation of a DNA density hybrid on release of viral DNA. Proc Natl Acad Sci U S A. 1969 Nov;64(3):863–869. doi: 10.1073/pnas.64.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Hoggan M. D., Koczot F., Shatkin A. J. Genetic relatedness studies with adenovirus-associated viruses. J Virol. 1968 Oct;2(10):999–1005. doi: 10.1128/jvi.2.10.999-1005.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Hoggan M. D., Shatkin A. J. Nucleic acid from an adeno-associated virus: chemical and physical studies. Proc Natl Acad Sci U S A. 1966 Jul;56(1):86–92. doi: 10.1073/pnas.56.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Reich P. R., Weissman S. M. RNA production in adenovirus-infected KB cells. Virology. 1965 Dec;27(4):571–579. doi: 10.1016/0042-6822(65)90183-2. [DOI] [PubMed] [Google Scholar]
- Salzman L. A., Jori L. A. Characterization of the Kilham rat virus. J Virol. 1970 Feb;5(2):114–122. doi: 10.1128/jvi.5.2.114-122.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman L. A., White W. L. Structural proteins of Kilham rat virus. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1551–1556. doi: 10.1016/0006-291x(70)90564-4. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Smith K. O., Gehle W. D., Thiel J. F. Properties of a small virus associated with adenovirus type 4. J Immunol. 1966 Dec;97(6):754–766. [PubMed] [Google Scholar]
- Ward S. An improved transverse destaining apparatus for acrylamide gels. Anal Biochem. 1970 Feb;33(2):259–262. doi: 10.1016/0003-2697(70)90295-2. [DOI] [PubMed] [Google Scholar]



