Abstract
BACKGROUND
We examined the associations between muscular strength, markers of overall and central adiposity and cancer mortality in men.
METHODS
Prospective cohort study including 8,677 men aged 20-82 years followed from 1980 to 2003. Participants were enrolled in The Aerobics Centre Longitudinal Study, the Cooper Clinic in Dallas, Texas, U.S. Muscular strength was quantified by combining 1-repetition maximal measures for leg and bench presses. Adiposity was assessed by body mass index (BMI), percent body fat, and waist circumference.
RESULTS
Cancer death rates per 10,000 person-years adjusted for age and examination year were: 17.5, 11.0, and 10.3 across incremental thirds of muscular strength (P=0.001); 10.9, 13.4, and 20.1 across BMI groups of 18.5-24.9, 25.0-29.9, and ≥30kg/m2, respectively (P=0.008); 11.6 and 17.5 for normal (<25%) and high percent body fat (≥25%), respectively (P=0.006); and 12.2 and 16.7 for normal (≤102 cm) and high waist circumference (>102 cm), respectively (P=0.06). After adjusting for additional potential confounders, hazard ratios (95% confidence intervals) were 1.00 (referent), 0.65 (0.47-0.90), and 0.61 (0.44-0.85) across incremental thirds of muscular strength, respectively (P=0.003 for linear trend). Further adjustment for BMI, percent body fat, waist circumference, or cardiorespiratory fitness had little effect on the association. The associations of BMI, percent body fat, or waist circumference with cancer mortality did not persist after further adjusting for muscular strength (all P≥0.1).
CONCLUSIONS
Higher levels of muscular strength are associated with lower cancer mortality risk in men, independent of clinically established measures of overall and central adiposity, and other potential confounders.
Keywords: Muscular strength, obesity, cancer, cardiorespiratory fitness, resistance exercise
INTRODUCTION
Cancer is one of the leading causes of death for North-American as well as for European men, accounting for approximately 285,000 and 850,000 of deaths annually, respectively (1, 2). Lifestyle-related factors associated with cancer mortality include smoking and poor diet. More recently, it has been shown that overall and central obesity also increases the risk of cancer (3-5). Another important lifestyle factor is the level of physical activity (6, 7). The International Agency for Research on Cancer estimated in 2002 that up to one-third of several types of cancers could be attributed to excess of body fat and a sedentary lifestyle (8). A dose-response relationship for several cancers has been reported, such that engaging in longer exercise sessions, or exercising at higher intensities or for more years is associated with greater reductions in the risk of cancer development (8).
There is increasing evidence highlighting the beneficial effects of muscular strength in the prevention of chronic diseases, as well as in the performance of the activities of daily life (9). Resistance-type physical activities are major determinants of muscular strength, and are currently recommended by the most influential health organizations, such as the American Heart Association and the American Cancer Society, for improving both health and fitness (10-13).
The prospective association between muscular strength and cancer mortality has been examined in several studies (14-17), with inconsistent findings. These studies assessed muscular strength via a handgrip test, which provides information derived from only a small muscle group. Assessing additional muscle groups should provide a better overall index of muscular strength, especially when measured in large muscle groups. In addition, none of these studies accounted for cardiorespiratory fitness, which has been shown to be a strong predictor of cancer mortality (18-25). We have shown that muscular strength measured in large muscle groups from the upper and lower body is inversely and independently associated with all-cause (26, 27) and cancer (27) mortality in men, even after adjusting for cardiorespiratory fitness.
Data from The Aerobics Centre Longitudinal Study (ACLS) showed that higher levels of cardiorespiratory fitness are associated with lower cancer mortality risk in men, independent of overall and central adiposity measures, such as body mass index [BMI, weight (kg)/height (m)2], percent body fat, and waist circumference (20). From a public health perspective it is important to understand whether higher levels of muscular strength may counteract the negative consequences ascribed to adiposity. Studies examining the independent and joint associations among muscular strength, several established clinical measures of overall and central adiposity, and cancer mortality are scarce. Therefore we examined these associations in a cohort of middle-aged men enrolled in the ACLS.
MATERIAL AND METHODS
Study Population
Between 1980 and 1989, 10,265 men aged 20-82 years received a comprehensive medical examination and muscular strength tests at The Cooper Clinic in Dallas, Texas, United States, and were enrolled in the ACLS. Participants were predominantly European-Americans, well-educated, and belonged to middle to upper socioeconomic strata. Detailed information regarding to the study population has been published previously (19, 28). Participants came to the clinic for periodic preventive health examinations and for counseling regarding diet, exercise, and other lifestyle factors associated with increased risk of chronic disease. Participants thus were volunteers (i.e., were not paid for participation). Many were sent by their employers for the examination, some were referred by their doctors and others were self referred.
Participants performed a maximal graded treadmill test to assess their cardiorespiratory fitness, and had complete measures of height and weight (from which BMI was computed), percent body fat, and waist circumference. Participants were not included in the present study if, at baseline they were either younger than 20 years or older than 90 years (n=96), they did not achieve at least 85% of aged-predicted maximal heart rate (220-age) during the treadmill test (n=671), they had an abnormal resting or exercise electrocardiogram (29) (n=581), they reported history of myocardial infarction (n=51), stroke (n=6), or cancer (n=55), or they were underweight (BMI < 18.5 kg/m2) (n=128). These criteria resulted in 8,677 asymptomatic men aged 20-82 years, who were followed up from the date of their baseline examination until their date of death, or December 31st, 2003. Participants provided written consent to participate in the follow-up study, and The Cooper Institute Institutional Review Board approved the study annually.
Clinical Data
Participants completed a comprehensive health evaluation that included self-reported personal and family health history, anthropometry, a standardized medical examination by a physician, fasting blood chemistry assessment, muscular strength tests and a maximal graded treadmill exercise test. Body mass index was computed from measured weight and height (kg/m2). Percent body fat was assessed with hydrostatic weighing, the sum of 7 skinfolds, or both, following standardized protocols (30). Detailed description of our hydrodensitometry procedures has been published elsewhere (31). Waist circumference was measured level with the umbilicus. Adiposity exposure groups were based on standard clinical definitions for BMI (normal weight: 18.5-24.9 kg/m2, overweight: 25.0-29.9 kg/m2, obese: 30.0 kg/m2 or higher); percent body fat (normal: <25%; obese: ≥25% for men) (31); and waist circumference (normal: ≤ 102.0 cm; abdominal obesity: > 102.0 cm).
Blood pressure was measured with standard auscultatory methods after the participant had been seated for five minutes. Systolic and diastolic blood pressures were recorded as the first and fifth Korotkof sounds, respectively. Concentrations of total and high density lipoprotein cholesterol, triglycerides, and glucose were determined in the Cooper Clinic clinical chemistry laboratory, which participates in and meets the quality control standards of the Centers for Disease Control and Prevention Lipid Standardization Program. Baseline medical conditions, such as previous myocardial infarction, stroke, hypertension, diabetes, and hypercholesterolemia were defined as a history of physician diagnosis, measured phenotypes that met clinical thresholds for a specific condition, or when appropriate, the combination of both methods. Smoking habits (current smoker or not), and alcohol intake (number of drinks per week) were obtained from a standardized questionnaire.
Cardiorespiratory fitness was assessed by a maximal treadmill test using a modified Balke protocol (32), as previously described (19, 31). The mean (standard deviation) percentage of age-predicted maximal heart rate achieved during exercise was 101.6 (6.2), which indicates that most participants achieved a maximal effort. Exercise duration on this protocol is highly correlated with measured maximal oxygen uptake (r = 0.92) (33), and was used for the analyses (in minutes). To standardize interpretation of exercise test performance, maximal metabolic equivalents (METs, 1 MET = 3.5 ml oxygen uptake/kg/min) were also estimated based on the final treadmill speed and grade (34). Cardiorespiratory fitness was dichotomized as unfit (low) and fit (high) corresponding to the lower 20% and the upper 80%, respectively, of the age-specific distribution of treadmill exercise duration in the overall ACLS population (31, 35-39).
We assessed muscular strength in the upper and lower body following a standardized strength testing protocol using variable resistance weight machines (Universal Equipment, Cedar Rapids, IA) (40, 41). Upper body strength was assessed with a one-repetition (1-RM) maximum supine bench press, and lower body strength was assessed with a 1-RM maximum seated leg press. Initial loads (weights) were 70% of body weight for the bench press and 100% of body weight for the leg press. Increments of ~2-4 kg were added until maximal effort was achieved for each lift, usually after 5 trials or less. Participants were allowed to rest (~1-2 min) between trials. All participants were able to lift the initial load at least one time. Participants were instructed on proper breathing and lifting form for each movement. The intraclass correlation coefficient for 1-RM bench and leg press was 0.90 and 0.83, respectively, in a subgroup of 246 men who underwent two muscular strength assessments within a 1-year period (40).We computed a muscular strength score by combining the standardized values of bench and leg press (27). Each of these variables was standardized as follows: standardized value = (value - mean)/ standard deviation (SD). The score was calculated separately for each age group (20-29, 30-39, 40-49, 50-59, and ≥60 years). The score for muscular strength was calculated as the mean of the two standardized scores (bench and leg presses). For analysis we used thirds of the age-group specific composite strength score.
Mortality Surveillance
Vital status was ascertained using the National Death Index (NDI) and using death certificates from states in which participant death occurred. Over 95% of mortality follow-up is complete by these methods. The NDI has been shown to be an accurate method of ascertaining deaths in observational studies, with high sensitivity (96%) and specificity (100%) (42). Cancer deaths were identified using the International Classification of Diseases, Ninth Revision (codes 140-208) for deaths occurring before 1999, and Ten Revision (codes C00-C97) for deaths during 1999 to 2003. Cancers of the digestive and gastrointestinal (hereafter called digestive) system were identified using ICD-9/10 codes 150-159/C15-C26. For neoplasm of lymphoid, hematologic, and related tissues, ICD-9/10 codes 201.0 – 205.9 and 238.6/C81.0–C96 were used. For cancer in specific sites, the following ICD-9/10 codes were used: colon, 153/C18; rectum, 154/C19-C21; pancreas, 157/C25; lung, 162.2–163.0/C34; and prostate, 186/C61.
Statistical Analyses
The follow-up interval was computed from the date of a participant’s baseline examination until the date of death for decedents, or until December 31, 2003 for survivors. Descriptive statistics summarized baseline characteristics by muscular strength fitness levels. Groups were compared using χ2 analysis (for categorical variables, such as current smoker and hypertension) and general linear models with Bonferonni post hoc comparison tests (for continuous variables such as age and BMI). Cox proportional hazard models were used to estimate hazard ratios (HRs), 95% confidence intervals (CIs), and cancer mortality rates (deaths per 10,000 person-years of follow-up) according to exposure categories. Multivariate analyses included the following covariates: age (years), examination year, smoking status (current smoker or not), alcohol intake (≥5 drinks/week or not), cardiorespiratory fitness (entered as a continuous variable, in minutes) and baseline medical conditions (presence or not of hypertension, diabetes, or hypercholesterolemia). The proportional hazards assumption was examined by comparing the cumulative hazard plots grouped on exposure; no appreciable violations were noted. Tests of linear trends in mortality rates and risk estimates across exposure categories were computed using ordinal scoring for muscular strength thirds, BMI, percent body fat, and waist circumference groups. Models were also fitted with strength squared to assess non-linearity.
Finally, we examined the joint associations of muscular strength and adiposity exposures with cancer mortality, as well as the joint associations of muscular strength and cardiorespiratory fitness with cancer mortality. We assessed the interaction among exposure groups using likelihood ratio tests of nested models. We calculated two-sided P values and we considered those <0.05 as significant. Analyses were done using SAS statistical software, version 9.1 (SAS Inc., Cary, NC).
RESULTS
During an average follow-up of 18.8 years and 163,128 person-years of exposure, 211 cancer deaths occurred. Baseline characteristics of the overall cohort according to muscular strength categories are presented in Table 1. With the exception of alcohol intake, each of the other baseline characteristics were significantly (P < 0.05) associated with categories of muscular strength. There was a direct gradient of treadmill test duration across increasing thirds of muscular strength (P < 0.001).
Table 1.
Baseline characteristics according to thirds of muscular strength, Aerobics Center Longitudinal Study, 1980-2003.
| Muscular Strength Thirds |
|||||
|---|---|---|---|---|---|
| Characteristic | All (n = 8,677) |
Lowest (n = 2,892) |
Middle (n = 2,894) |
Upper (n = 2,891) |
P for linear trend |
| Age, mean (SD), y | 42.7 (9.5) | 43.3 (9.5) | 42.7 (9.4) | 42.2 (9.7) | < 0.0001 |
| Body mass index , mean (SD), (kg/m2) | 25.8 (3.5) | 26.9 (4.2) | 25.5 (3.0) | 25.1 (2.7) | < 0.0001 |
| Waist circumference, mean (SD), cm | 92.7 (10.1) | 97.5 (10.9) | 92.0 (8.8) | 88.6 (8.3) | < 0.0001 |
| Percent body fat, mean (SD) | 20.3 (6.2) | 23.6 (5.9) | 20.1 (5.3) | 17.1 (5.5) | < 0.0001 |
| Maximal METs, mean (SD) | 12.5 (2.5) | 11.5 (2.3) | 12.5 (2.3) | 13.4 (2.4) | < 0.0001 |
| Treadmill time, mean (SD), minutes | 19.7 (4.9) | 17.7 (4.8) | 19.7 (4.6) | 21.6 (4.5) | < 0.0001 |
| Bench press, mean (SD) | |||||
| Kg | 71.5 (17.4) | 61.6 (12.1) | 69.6 (12.6) | 83.3 (19.0) | < 0.0001 |
| Kg/kg of body weight | 0.9 (0.2) | 0.7 (0.1) | 0.9 (0.1) | 1.1 (0.2) | < 0.0001 |
| Leg press, mean (SD) | |||||
| Kg | 136.7 (27.0) | 124.8 (24.7) | 135.5 (23.4) | 149.9 (26.5) | < 0.0001 |
| Kg/kg of body weight | 1.7 (0.3) | 1.4 (0.2) | 1.7 (0.2) | 1.9 (0.3) | < 0.0001 |
| Lipids, mean (SD), mg/dL | |||||
| Total cholesterol | 211 (44) | 214 (41) | 212 (51) | 207 (40) | < 0.0001 |
| High density lipoprotein cholesterol | 46 (12) | 45 (12) | 46 (12) | 47 (12) | < 0.0001 |
| Triglycerides | 130 (106) | 142 (105) | 131 (123) | 117 (85) | < 0.0001 |
| Fasting blood glucose, mean (SD), mg/dL | 100 (14) | 101 (17) | 99 (13) | 98 (11) | < 0.0001 |
| Blood pressure, mean (SD), mmHg | |||||
| Systolic | 119 (13) | 120 (13) | 118 (12) | 119 (13) | < 0.0001 |
| Diastolic | 79 (9) | 80 (9) | 79 (9) | 79 (9) | < 0.0001 |
| Current smoker, No. (%) | 1313 (15.1) | 492 (17.0) | 455 (15.7) | 366 (12.7) | < 0.0001 |
| Alcohol intake (≥5 drinks/wk), No. (%) | 4300 (49.6) | 1431 (49.5) | 1446 (50.0) | 1423 (49.2) | 0.84 |
| Sedentary*, No. (%) | 1725 (19.9) | 746 (25.8) | 574 (19.8) | 405 (14.0) | < 0.0001 |
| Baseline medical conditions†, No. (%) | |||||
| Hypercholesterolemia | 1875 (21.6) | 687 (23.8) | 632 (21.8) | 556 (19.2) | < 0.0001 |
| Diabetes mellitus | 208 (2.4) | 100 (3.5) | 49 (1.7) | 59 (2.0) | 0.0005 |
| Hypertension | 2166 (25.0) | 848 (29.3) | 688 (23.8) | 630 (21.8) | < 0.0001 |
| Cardiovascular disease | 111 (1.3) | 49 (1.7) | 33 (1.1) | 29 (1.0) | 0.02 |
Abbreviations: METs indicates maximal metabolic equivalents achieved during the treadmill test; Kg, Kilograms; SD, standard deviation.
Participants were defined as sedentary if they reported no leisure-time physical activity in the 3 months before baseline examination.
Defined as the presence of hypercholesterolemia (history of physician-diagnosed high cholesterol level or measured fasting total cholesterol level ≥ 240 mg/dL [6.20 mmol/L]) or diabetes (history of physician diagnosis, use of insulin or measured fasting glucose level ≥ 126 mg/dL [7.0 mmol/L], or self-reported diabetes); or hypertension (history of physician diagnosis or resting systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg).
The death rates per 10,000 person-years, HRs and 95% CIs for muscular strength and cancer mortality, and for adiposity exposures and cancer mortality are shown in Table 2 and Table 3, respectively. The cancer mortality rates were 1.59 (17.5/11.0) and 1.70 (17.5/10.3) times greater for those in the lowest third of muscular strength than for those in the middle and upper third of muscular strength, respectively. After adjusting for age, examination year, smoking status, alcohol intake, and baseline medical conditions (Table 2), HRs of cancer mortality across incremental thirds of muscular strength were 1.00, 0.65, and 0.61 (P = 0.003 for linear trend). Further adjustment for BMI, percent body fat, waist circumference, or cardiorespiratory fitness had little effect on the association (Table 2). The test for non-linearity was not significant (P = 0.08 for quadratic trend). The HRs of cancer mortality were higher across incremental BMI categories (1.0, 1.17, and 1.71; P = 0.03 for linear trend) in models adjusted for age, examination year, smoking status, alcohol intake, and baseline medical conditions. Likewise, those with higher percent body fat (≥ 25.0% vs < 25.0%) had an increased risk of mortality (HR, 1.45; CI, 1.08-1.95; P = 0.01). There was a suggestion of a 30% increased risk of death among those with abdominal obesity (≤ 102 cm vs > 102 cm) (HR, 1.30; 95% CI, 0.93-1.82; P = 0.13). The associations of BMI, percent body fat, or waist circumference with cancer mortality did not persist after further adjustment for muscular strength or cardiorespiratory fitness (all P ≥ 0.1).
Table 2.
Risk of cancer mortality according to thirds of muscular strength, Aerobics Center Longitudinal Study, 1980-2003.
| Deaths | Rate* | HR (95% CI)† | HR (95% CI)‡ | HR (95% CI)§ | HR (95% CI)∥ | HR (95% CI)¶ | |
|---|---|---|---|---|---|---|---|
| Strength thirds | |||||||
| Lowest | 95 | 17.5 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Middle | 60 | 11.0 | 0.65 (0.47-0.90) | 0.67 (0.49-0.93) | 0.67 (0.48-0.94) | 0.69 (0.49-0.96) | 0.69 (0.49-0.95) |
| Upper | 56 | 10.3 | 0.61 (0.44-0.85) | 0.64 (0.46-0.90) | 0.64 (0.44-0.92) | 0.68 (0.48-0.97) | 0.70 (0.49-0.98) |
| P for linear trend | 0.001 | 0.003 | 0.008 | 0.01 | 0.03 | 0.03 |
Abbreviations: HR, hazard ratio; CI, confidence interval.
age and examination year adjusted death rate per 10,000 person-years.
adjusted for covariates: age, examination year, smoking status, alcohol intake, and medical conditions (presence or not of hypertension, diabetes, or hypercholesterolemia).
adjusted for covariates plus body mass index.
adjusted for covariates plus percent body fat.
adjusted for covariates plus waist circumference.
adjusted for covariates plus cardiorespiratory fitness.
Table 3.
Risk of cancer mortality according to clinical cut-points of adiposity measures, Aerobics Center Longitudinal Study, 1980-2003.
| Deaths | Rate* | HR (95% CI)† | HR (95% CI)‡ | HR (95% CI)§ | |
|---|---|---|---|---|---|
| Body mass index | |||||
| 18.5-24.9 kg/m2 | 83 | 10.9 | 1.00 | 1.00 | 1.00 |
| 25.0-29.9 kg/m2 | 100 | 13.4 | 1.17 (0.87-1.57) | 1.12 (0.83-1.51) | 1.05 (0.77-1.43) |
| ≥30.0 kg/m2 | 28 | 20.1 | 1.71 (1.10-2.66) | 1.51 (0.96-2.37) | 1.34 (0.82-2.20) |
| P for linear trend | 0.008 | 0.03 | 0.10 | 0.33 | |
| Percent body fat | |||||
| < 25.0 % | 139 | 11.6 | 1.00 | 1.00 | 1.00 |
| ≥ 25.0 % | 70 | 17.5 | 1.45 (1.08-1.95) | 1.27 (0.93-1.74) | 1.25 (0.90-1.75) |
| P for difference | 0.006 | 0.01 | 0.13 | 0.19 | |
| Waist circumference | |||||
| ≤ 102.0 cm | 167 | 12.2 | 1.00 | 1.00 | 1.00 |
| > 102.0 cm | 44 | 16.7 | 1.30 (0.93-1.82) | 1.13 (0.80-1.61) | 1.07 (0.74-1.55) |
| P for difference | 0.06 | 0.13 | 0.50 | 0.70 |
Abbreviations: HR, hazard ratio; CI, confidence interval.
age and examination year adjusted death rate per 10,000 person-years
adjusted for covariates: age, examination year, smoking status, alcohol intake, and medical conditions (presence or not of hypertension, diabetes, or hypercholesterolemia).
adjusted for covariates plus muscular strength.
adjusted for covariates plus cardiorespiratory fitness.
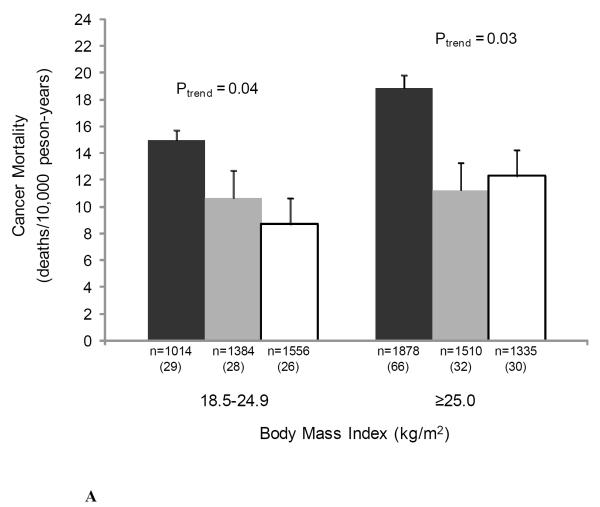
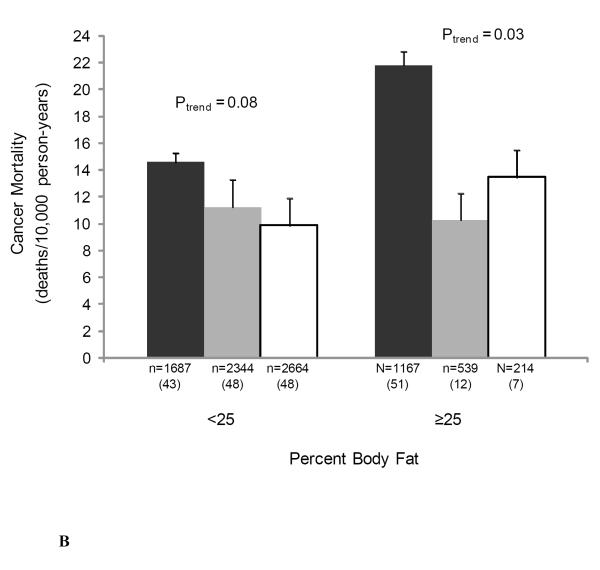
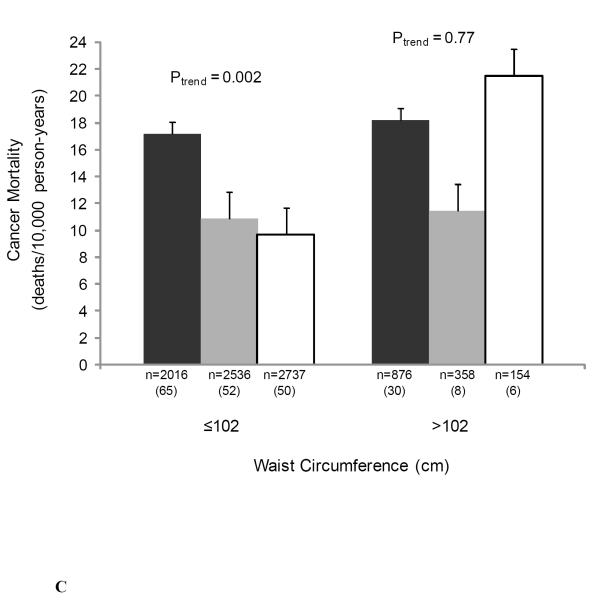
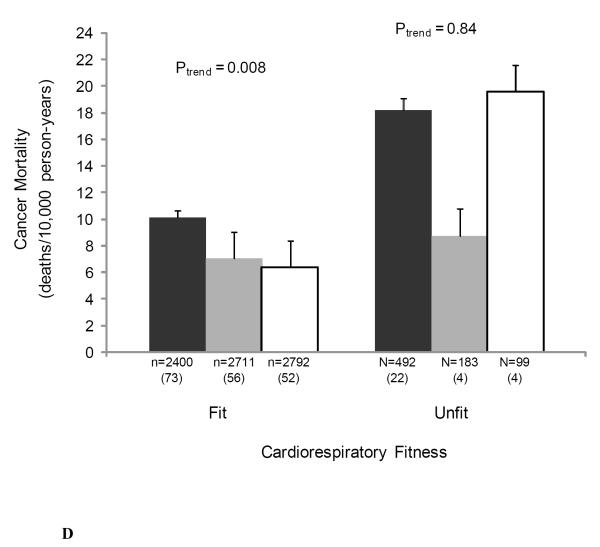
We also examined the joint associations of muscular strength and adiposity, and muscular strength and cardiorespiratory fitness with cancer mortality to provide greater clinical meaning for physicians and other health professionals (Figure 1). There were no significant interactions noted in analyses that included cross-product interaction terms. The likelihood ratio test for interaction was: χ2df=1 = 0.70, P = 0.40 for BMI-strength; χ2df=1 = 0.51, P = 0.48 for percent body fat-strength; χ2df=1 = 0.77, P = 0.38 for waist circumference-strength; and χ2df=1 = 1.74, P = 0.19 for cardiorespiratory fitness-strength. Muscular strength was inversely associated with cancer death rates in both normal weight and overweight men (both P < 0.05 for linear trend) (Figure 1A), in those of high percent body fat (P = 0.03 for linear trend) (Figure 1B), in those of normal waist circumference (P = 0.002 for linear trend) (Figure 1C), and in those with high levels of cardiorespiratory fitness (P = 0.008 for linear trend) (Figure 1D).
Figure 1.
Joint association of muscular strength and (1A) body mass index (BMI), (1B) percent body fat, (1C) waist circumference, and (1D) cardiorespiratory fitness with the age- and examination year-adjusted rates of cancer mortality, Aerobics Center Longitudinal Study, 1980-2003. Error bars represent standard error. Likelihood ratio test for interaction, χ2df=1 = 0.70, P = 0.40 for BMI-strength, χ2df=1 = 0.51, P = 0.48 for percent body fat-strength, χ2df=1 = 0.77, P = 0.38 for waist circumference-strength, and χ2df=1 = 1.74, P = 0.19 for cardiorespiratory fitness-strength. Black bars represent the lowest third; grey bars, the middle third; and white bars, the upper third of baseline muscular strength. Numbers under the bar are sample size (deaths from cancer).
Age and examination year-adjusted cancer mortality rates per 10,000 person-years in normal and overweight individuals were significantly higher among those in the low muscular strength category than among those in the middle and high strength categories (Figure 1A). Cancer mortality rates were significantly higher among individuals in the low muscular strength category versus those who were in the middle- and high-strength category within the abnormal percent body fat group (Figure 1B). The same pattern was observed in the normal percent body fat group; however, the association was only marginally statistically significant (P = 0.08). Finally, as shown in Figure 1C, cancer mortality rates were significantly higher among individuals with low muscular strength than those who were in the middle- and high-strength categories in the normal waist circumference group, whereas no association between muscular strength and cancer mortality rates was observed in the abdominal obese group (P = 0.77).
We focused primarily on all-cause cancer mortality because of the relatively small number of site-specific cancers deaths across strength levels on our cohort. However some exploratory analyses were performed for the associations between muscular strength and site-specific cancers (Table 4). In the current study, cancers in the digestive system accounted for 31% (n = 65) of total cancer deaths. After adjusting for age, and examination year, HRs (95% CI) were 1.00 (referent), 0.37 (0.19-0.70), and 0.49 (0.27-0.87) across incremental thirds of muscular strength, respectively (P = 0.007 for linear trend). The corresponding numbers of deaths were 35, 13, 17 for the low-, middle- and high-strength third, respectively. Excluding deaths that occurred during the first 2 years of follow-up did not materially change the results.
Table 4.
Risks of site-specific cancer mortality according to thirds of muscular strength, Aerobics Center Longitudinal Study, 1980-2003.
| Anatomic site- Strength thirds |
Deaths | HR (95% CI)* |
|---|---|---|
| All digestive system** | ||
| Low | 35 | 1.00 |
| Middle | 13 | 0.37 (0.19-0.70) |
| High | 17 | 0.49 (0.27-0.87) |
| P for linear trend | 0.007 | |
| Colorectal | ||
| Low | 11 | 1.00 |
| Middle | 2 | 0.18 (0.04-0.82) |
| High | 6 | 0.53 (0.20-1.45) |
| P for linear trend | 0.16 | |
| Pancreas | ||
| Low | 11 | 1.00 |
| Middle | 4 | 0.36 (0.11-1.13) |
| High | 6 | 0.54 (0.20-1.47) |
| P for linear trend | 0.18 | |
| Lung | ||
| Low | 17 | 1.00 |
| Middle | 17 | 0.998 (0.51-1.96) |
| High | 13 | 0.76 (0.37-1.57) |
| P for linear trend | 0.47 | |
| Prostate | ||
| Low | 3 | 1.00 |
| Middle | 7 | 2.56 (0.66-9.97) |
| High | 3 | 1.03 (0.21-5.16) |
| P for linear trend | 0.96 | |
| Hematopoietic/Lymph | ||
| Low | 11 | 1.00 |
| Middle | 8 | 0.71 (0.28-1.76) |
| High | 8 | 0.71 (0.28-1.76) |
| P for linear trend | 0.45 |
Abbreviations: HR, hazard ratio; CI, confidence interval.
Adjusted for age, and examination year.
Esophagus, n=7; Stomach, n=10; Colon, n=16; Rectum, n=3; Liver, n=6; Pancreas, n=21; Gall bladder, n=2.
DISCUSSION
There were three main findings from this study: First, muscular strength was significantly and inversely associated with cancer mortality risk in men independent of potential confounders such as age, smoking, alcohol intake, and health status. This association remained significant after further adjustment for measures of overall adiposity (i.e., BMI and percent body fat) and central adiposity (i.e., waist circumference). Additionally, adjusting for cardiorespiratory fitness had little effect on the associations. Second, BMI, percent body fat, and waist circumference were positively associated with rates of cancer mortality. However, the associations did not persist after adjusting for muscular strength or cardiorespiratory fitness. Third, analysis on the joint associations between muscular strength and adiposity revealed that cancer mortality rates in men with low levels of muscular strength (lowest third) and with high levels of adiposity were 40-50% higher (all P < 0.01) than the rates in the group of obese men with at least moderate (middle third) levels of muscular strength.
Higher levels of muscular strength were inversely associated with cancer mortality in both normal weight and overweight men, in those who have excessive percent body fat, in those with a normal waist circumference, and in those with high levels of cardiorespiratory fitness (Figure 1). Men with abdominal obesity (waist circumference > 102 cm) and low levels of muscular strength were not at higher risk for cancer mortality when compared to those with high levels of muscular strength and with abdominal obesity. The group of men with abdominal obesity as well as those with high levels of cardiorespiratory fitness had small sample sizes and fewer deaths (Figure 1C), resulting in a lack of statistical power. Therefore, these results should be confirmed in studies with a larger number of outcomes.
Taken together, these findings indicate that having at least moderate age-adjusted levels of muscular strength may counteract the deleterious consequences attributed to adiposity. To place our findings into a more public health perspective, the recommendation is to avoid falling into the low age-adjusted muscular strength category. Efforts should then focus not only on reducing levels of adiposity but also on increasing the muscular strength level.
Our findings are in accordance with those published by Gale et al. (15), but not with others (14, 16, 17). These studies measured muscular strength in only one small muscle group (handgrip strength), which may have masked the strength-cancer association. Additionally, they did not adjust for cardiorespiratory fitness. A thorough assessment of muscular strength should include testing of several major muscle groups. In addition, previous studies were either short-term follow-ups (5-6 years) (14, 16), or included only older adults (≥65 years) (15, 16). Our study group is unique in that we standardized the measures of muscular strength by testing the major muscle groups of the upper and lower body. Also, we included measures of adiposity and cardiorespiratory fitness in a large cohort of men aged 20-82, including an extensive follow-up, and with a comprehensive baseline clinical examination.
Higher levels of cardiorespiratory fitness are strongly associated with lower risk of cancer mortality in men and women, young or older people, and in diabetic or non-diabetic persons, independently of their weight status and tobacco use (18-25). It is worth noting that in the present study muscular strength and cardiorespiratory fitness were moderately correlated (age-adjusted partial r = 0.33). This suggests that the association between muscular strength and cancer mortality risk works, at least partially, through different mechanisms than those associated with the salutatory effects attributed to cardiorespiratory fitness. The apparent protective effect of muscular strength against cancer is likely to be due to a consequence of regular physical exercise, specifically resistance exercise. Muscular strength has a genetic component, yet there is convincing evidence that resistance-type physical activities are major determinants of muscular strength (11). We have observed a strong and direct association between self-reported participation in resistance exercises and muscular strength in men from the ACLS (41), that is, the higher the participation in resistance exercise the higher the muscular strength. This suggests that the muscular strength measurements obtained in the present study provide an adequate representation of the physical activities that involve resistance, such as daily work, lifting or carrying things, etc.
There are plausible biological mechanisms that may explain the lower risk of cancer mortality seen in men with higher levels of muscular strength, such as regulation in the metabolism of insulin, and insulin-like growth factors (IGFs), which have been linked to increased risk of several types of cancer (43, 44). There is compelling evidence that physical activity improves insulin sensitivity and increases glucose uptake by skeletal muscle, even in persons with type 2 diabetes mellitus (45). Intervention studies have shown that resistance training improves both insulin sensitivity and glycemic control (46-48). A decrease in the levels of IGF-1 has been observed after a resistance training period, concurrently with an increase in IGF binding protein-3 (49). IGF binding protein-3 binds to circulating IGF in the blood and decreases its ability to nurture potential cancer sites (50). Other potential mechanisms associated with higher levels of muscular strength include reduced exposure to systemic inflammation (51, 52), sex hormones (53, 54), improved antioxidant defense (55, 56) and immune function (57), and reduced overall and central adiposity (11).
Resistance exercise is an important complement for weight control, mainly due to the increases in metabolically active muscle mass (9). Resting energy expenditure is the largest component of total energy expenditure, especially when physically inactive. The energy expenditure related to muscle metabolism is the only component of resting energy expenditure that varies considerably (9). Therefore, the maintenance of a large muscle mass and consequent muscle protein turnover across a relatively long period of time may contribute to the prevention of obesity. Consequently, it is presumable that when sustained over time, resistance exercise training should help prevent increases in body fat (11). This fact has important public health implications given that the prevalence of overweight and obesity exceeds 70% in the U.S. men (58), and 65% of men from the U.K. (59). Moreover, the prevalence of these conditions are expected to increase, in the U.K., for example, to 75% of men by 2010 (59).
The observed positive association of BMI, percent body fat, and waist circumference with cancer mortality confirm the results of numerous studies reporting that overweight and obesity are associated with increased risk of common and less common cancers (3-5). That the associations of BMI, percent body fat, and waist circumference with cancer mortality did not persist after adjusting for muscular strength or cardiorespiratory fitness is noteworthy. In addition, the observation that cancer mortality rates of obese men with at least moderate levels of muscular strength are 40-50% lower than their obese peers in the lowest strength third have important public health implications and should inform the exploration of biological mechanisms that link obesity and muscular strength with cancer. These findings acquire special relevance because cigarette smoking (which is the largest cause of cancers in developed countries) is decreasing, and therefore adiposity and sedentariness may become the dominant lifestyle factors contributing to cancer occurrence in such countries (5). The key role of muscle mass in a number of metabolic processes and in the prevention of many common pathologic conditions and chronic diseases has been highlighted (9). Therefore, it is reasonable to hypothesize that increased muscle mass in those men with higher levels of muscular strength may partially explain their reduced cancer mortality rates compared to those men with lower strength levels.
Limitations of the current study include the fact that participants were predominantly male, white, well-educated, and middle to upper socioeconomic status. This may limit the ability to generalize the study results but does not affect the study’s internal validity. Although cancer death rates seem to vary by both level of education and by race (60), there is little reason to assume that the benefits of muscular strength would be different in other racial/ethnic or socioeconomic groups. Due to a limited number of women, who contributed relatively few cancer deaths to the current study, we were unable to perform a meaningful parallel analysis on women. Therefore, women were not included in this study.
None of the participants reported family history of cancer, which might be another limitation of the ACLS due to self-selection bias. In fact, only 1.16% of men in the entire cohort reported a family history of cancer. Therefore, our cohort might be considered to be at the positive end of the health spectrum, that is, a group with the greatest chance of cancer survival. That we saw a significant association between muscular strength and cancer mortality is then remarkable. These findings indicate that even in men with the best chance of cancer survival, having higher levels of muscular strength is associated with lower risk of cancer mortality compared with those men with low levels of strength. We identified an inverse association between muscular strength and cancers in the digestive system, yet, the findings should be interpreted cautiously because of the small number of deaths in our cohort. The small number of site-specific digestive system cancers, or other site-specific cancers also precluded us performing further analyses.
No detailed information about medication use, or dietary habits was available which may have biased the results through residual confounding. However, given that adjusting for BMI, percent body fat, or waist circumference did not diminish the strength-cancer mortality association, it is unlikely that accounting for dietary behaviors would have a major influence on the results.
In this cohort, we had only a single baseline assessment of muscular strength, adiposity measurements, and cardiorespiratory fitness; thus, whether changes in any of these variables occurred during follow-up and whether this may have influenced the study results is not known. It is important to bear in mind that, aside from its association with lifestyle-related variables, at present it is difficult to know how muscular fitness functions with respect to the molecular/genetic mechanisms involved in the carcinogenesis and tumor growth and development.
In conclusion, the present study showed that higher levels of muscular strength were associated with lower cancer mortality risk in men, independent of clinically established measures of overall and central adiposity, cardiorespiratory fitness, and other potential confounders. Mortality rates were lower for men with moderate/high muscular strength compared to individuals with low strength. While each adiposity measure was positively associated with cancer mortality, the association was eliminated after adjusting for either muscular strength or cardiorespiratory fitness. These findings suggest that attaining a moderate to high level of muscular strength may attenuate some of the cancer mortality risks associated with increased adiposity. Maintaining a healthy weight should continue to be a cornerstone in the prevention of chronic diseases and premature death. However, in the light of the results obtained in the present and other studies, it is equally important to maintain healthy muscular strength levels, and most importantly, to prevent falling into the lower strength categories.
It is biologically plausible to reduce cancer mortality death rates among men by promoting regular resistance training involving the major muscle groups of the upper and lower extremities at least 2 days per week (10-13). Resistance and aerobic exercise should complement each other. The recommendation for moderate to vigorous physical activity and resistance training are supported by the current research showing a reduction in all-cause and cancer mortality associated with increased cardiorespiratory fitness, muscular strength, or both.
Acknowledgements
We thank the doctors and technicians at The Cooper Clinic who collected the baseline data and staff at the Cooper Institute for data entry and data management.
Financial support: This work was supported by National Institutes of Health grants AG06945 and HL62508; The Spanish Ministry of Education (EX-2007-1124); the Swedish Council for Working Life and Social Research, the European Union, in the framework of the Public Health Programme (ALPHA project, Ref: 2006120); the American Heart Association Predoctoral Fellowship; and the American College of Sports Medicine Paffenbarger-Blair Fund for Epidemiological Research on Physical Activity.
Footnotes
Conflict of interest: none
REFERENCES
- 1.Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol. 2005;16:481–8. doi: 10.1093/annonc/mdi098. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society . Cancer Facts & Figures 2006. American Cancer Society; Atlanta, GA: 2006. [Google Scholar]
- 3.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 4.Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117:1658–67. doi: 10.1161/CIRCULATIONAHA.107.739714. [DOI] [PubMed] [Google Scholar]
- 5.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 6.McTiernan A. Mechanisms linking physical activity with cancer. Nat Rev Cancer. 2008;8:205–11. doi: 10.1038/nrc2325. [DOI] [PubMed] [Google Scholar]
- 7.World Cancer Research Fund / American Institute for Cancer Research . Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. AICR; Washington DC: 2007. [Google Scholar]
- 8.Vainio H, Kaaks R, Bianchini F. Weight control and physical activity in cancer prevention: international evaluation of the evidence. Eur J Cancer Prev. 2002;11(Suppl 2):S94–100. [PubMed] [Google Scholar]
- 9.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84:475–82. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- 10.Kraemer WJ, Adams K, Cafarelli E, et al. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2002;34:364–80. doi: 10.1097/00005768-200202000-00027. [DOI] [PubMed] [Google Scholar]
- 11.Williams MA, Haskell WL, Ades PA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;116:572–84. doi: 10.1161/CIRCULATIONAHA.107.185214. [DOI] [PubMed] [Google Scholar]
- 12.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–93. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 13.Kushi LH, Byers T, Doyle C, et al. American Cancer Society Guidelines on Nutrition and Physical Activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2006;56:254–81. doi: 10.3322/canjclin.56.5.254. quiz 313-4. [DOI] [PubMed] [Google Scholar]
- 14.Fujita Y, Nakamura Y, Hiraoka J, et al. Physical-strength tests and mortality among visitors to health-promotion centers in Japan. J Clin Epidemiol. 1995;48:1349–59. doi: 10.1016/0895-4356(95)00533-1. [DOI] [PubMed] [Google Scholar]
- 15.Gale CR, Martyn CN, Cooper C, Sayer AA. Grip strength, body composition, and mortality. Int J Epidemiol. 2007;36:228–35. doi: 10.1093/ije/dyl224. [DOI] [PubMed] [Google Scholar]
- 16.Rantanen T, Volpato S, Ferrucci L, Heikkinen E, Fried LP, Guralnik JM. Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J Am Geriatr Soc. 2003;51:636–41. doi: 10.1034/j.1600-0579.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki H, Kasagi F, Yamada M, Fujita S. Grip strength predicts cause-specific mortality in middle-aged and elderly persons. Am J Med. 2007;120:337–42. doi: 10.1016/j.amjmed.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 18.Lee CD, Blair SN. Cardiorespiratory fitness and smoking-related and total cancer mortality in men. Med Sci Sports Exerc. 2002;34:735–9. doi: 10.1097/00005768-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr., Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 20.Farrell SW, Cortese GM, LaMonte MJ, Blair SN. Cardiorespiratory fitness, different measures of adiposity, and cancer mortality in men. Obesity (Silver Spring) 2007;15:3140–9. doi: 10.1038/oby.2007.374. [DOI] [PubMed] [Google Scholar]
- 21.Thompson AM, Church TS, Janssen I, Katzmarzyk PT, Earnest CP, Blair SN. Cardiorespiratory fitness as a predictor of cancer mortality among men with pre-diabetes and diabetes. Diabetes Care. 2008;31:764–9. doi: 10.2337/dc07-1648. [DOI] [PubMed] [Google Scholar]
- 22.Sui X, Laditka JN, Hardin JW, Blair SN. Estimated functional capacity predicts mortality in older adults. J Am Geriatr Soc. 2007;55:1940–7. doi: 10.1111/j.1532-5415.2007.01455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farrell SW, Braun L, Barlow CE, Cheng YJ, Blair SN. The relation of body mass index, cardiorespiratory fitness, and all-cause mortality in women. Obes Res. 2002;10:417–23. doi: 10.1038/oby.2002.58. [DOI] [PubMed] [Google Scholar]
- 24.Kampert JB, Blair SN, Barlow CE, Kohl HW., 3rd Physical activity, physical fitness, and all-cause and cancer mortality: a prospective study of men and women. Ann Epidemiol. 1996;6:452–7. doi: 10.1016/s1047-2797(96)00059-2. [DOI] [PubMed] [Google Scholar]
- 25.Oliveria SA, Kohl HW, 3rd, Trichopoulos D, Blair SN. The association between cardiorespiratory fitness and prostate cancer. Med Sci Sports Exerc. 1996;28:97–104. doi: 10.1097/00005768-199601000-00020. [DOI] [PubMed] [Google Scholar]
- 26.FitzGerald SJ, Barlow CE, Kampert JB, Morrow JR, Jackson AW, Blair SN. Muscular fitness and all-cause mortality: Prospective observations. J Phys Act Health. 2004;1:7–18. [Google Scholar]
- 27.Ruiz JR, Sui X, Lobelo F, et al. Association between muscular strength and mortality in men: prospective cohort study. BMJ. 2008;337:a439. doi: 10.1136/bmj.a439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macera CA, Jackson KL, Hagenmaier GW, Kronenfeld JJ, Kohl HW, Blair SN. Age, physical activity, physical fitness, body composition, and incidence of orthopedic problems. Res Q Exerc Sport. 1989;60:225–33. doi: 10.1080/02701367.1989.10607444. [DOI] [PubMed] [Google Scholar]
- 29.Gibbons LW, Mitchell TL, Wei M, Blair SN, Cooper KH. Maximal exercise test as a predictor of risk for mortality from coronary heart disease in asymptomatic men. Am J Cardiol. 2000;86:53–8. doi: 10.1016/s0002-9149(00)00827-4. [DOI] [PubMed] [Google Scholar]
- 30.Jackson AS, Pollock ML. Practical assessment of body composition. Phys Sports Med. 1985;13:76–90. doi: 10.1080/00913847.1985.11708790. [DOI] [PubMed] [Google Scholar]
- 31.Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr. 1999;69:373–80. doi: 10.1093/ajcn/69.3.373. [DOI] [PubMed] [Google Scholar]
- 32.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. U S Armed Forces Med J. 1959;10:675–88. [PubMed] [Google Scholar]
- 33.Pollock ML, Bohannon RL, Cooper KH, et al. A comparative analysis of four protocols for maximal treadmill stress testing. Am Heart J. 1976;92:39–46. doi: 10.1016/s0002-8703(76)80401-2. [DOI] [PubMed] [Google Scholar]
- 34.American College of Sports Medicine . ACSM’s Guidelines For Exercise Testing And Prescription. 7th ed Lippincott Williams and Wilkins; Philadelphia: 2005. [Google Scholar]
- 35.Sui X, LaMonte MJ, Blair SN. Cardiorespiratory fitness as a predictor of nonfatal cardiovascular events in asymptomatic women and men. Am J Epidemiol. 2007;165:1413–23. doi: 10.1093/aje/kwm031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sui X, LaMonte MJ, Blair SN. Cardiorespiratory fitness and risk of nonfatal cardiovascular disease in women and men with hypertension. Am J Hypertens. 2007;20:608–15. doi: 10.1016/j.amjhyper.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blair SN, Kampert JB, Kohl HW, 3rd, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205–10. [PubMed] [Google Scholar]
- 38.Blair SN, Kohl HW, 3rd, Barlow CE, Paffenbarger RS, Jr., Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA. 1995;273:1093–8. [PubMed] [Google Scholar]
- 39.Wei M, Kampert JB, Barlow CE, et al. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA. 1999;282:1547–53. doi: 10.1001/jama.282.16.1547. [DOI] [PubMed] [Google Scholar]
- 40.Jurca R, Lamonte MJ, Barlow CE, Kampert JB, Church TS, Blair SN. Association of muscular strength with incidence of metabolic syndrome in men. Med Sci Sports Exerc. 2005;37:1849–55. doi: 10.1249/01.mss.0000175865.17614.74. [DOI] [PubMed] [Google Scholar]
- 41.Jurca R, Lamonte MJ, Church TS, et al. Associations of muscle strength and fitness with metabolic syndrome in men. Med Sci Sports Exerc. 2004;36:1301–7. doi: 10.1249/01.mss.0000135780.88930.a9. [DOI] [PubMed] [Google Scholar]
- 42.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol. 1984;119:837–9. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 43.Ma J, Pollak MN, Giovannucci E, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 1999;91:620–5. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- 44.Chan JM, Stampfer MJ, Giovannucci E, et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–6. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 45.Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286:1218–27. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- 46.Klimcakova E, Polak J, Moro C, et al. Dynamic strength training improves insulin sensitivity without altering plasma levels and gene expression of adipokines in subcutaneous adipose tissue in obese men. J Clin Endocrinol Metab. 2006;91:5107–12. doi: 10.1210/jc.2006-0382. [DOI] [PubMed] [Google Scholar]
- 47.Ibanez J, Izquierdo M, Arguelles I, et al. Twice-weekly progressive resistance training decreases abdominal fat and improves insulin sensitivity in older men with type 2 diabetes. Diabetes Care. 2005;28:662–7. doi: 10.2337/diacare.28.3.662. [DOI] [PubMed] [Google Scholar]
- 48.Dunstan DW, Vulikh E, Owen N, Jolley D, Shaw J, Zimmet P. Community center-based resistance training for the maintenance of glycemic control in adults with type 2 diabetes. Diabetes Care. 2006;29:2586–91. doi: 10.2337/dc06-1310. [DOI] [PubMed] [Google Scholar]
- 49.Izquierdo M, Ibanez J, Gonzalez-Badillo JJ, et al. Differential effects of strength training leading to failure versus not to failure on hormonal responses, strength, and muscle power gains. J Appl Physiol. 2006;100:1647–56. doi: 10.1152/japplphysiol.01400.2005. [DOI] [PubMed] [Google Scholar]
- 50.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472–89. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 51.Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2000;55:M709–15. doi: 10.1093/gerona/55.12.m709. [DOI] [PubMed] [Google Scholar]
- 52.Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–32. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 53.Raastad T, Bjoro T, Hallen J. Hormonal responses to high- and moderate-intensity strength exercise. Eur J Appl Physiol. 2000;82:121–8. doi: 10.1007/s004210050661. [DOI] [PubMed] [Google Scholar]
- 54.Willoughby DS, Taylor L. Effects of sequential bouts of resistance exercise on androgen receptor expression. Med Sci Sports Exerc. 2004;36:1499–506. doi: 10.1249/01.mss.0000139795.83030.d1. [DOI] [PubMed] [Google Scholar]
- 55.Garcia-Lopez D, Hakkinen K, Cuevas MJ, et al. Effects of strength and endurance training on antioxidant enzyme gene expression and activity in middle-aged men. Scand J Med Sci Sports. 2007;17:595–604. doi: 10.1111/j.1600-0838.2006.00620.x. [DOI] [PubMed] [Google Scholar]
- 56.Parise G, Phillips SM, Kaczor JJ, Tarnopolsky MA. Antioxidant enzyme activity is up-regulated after unilateral resistance exercise training in older adults. Free Radic Biol Med. 2005;39:289–95. doi: 10.1016/j.freeradbiomed.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 57.Simonson SR, Jackson CG. Leukocytosis occurs in response to resistance exercise in men. J Strength Cond Res. 2004;18:266–71. doi: 10.1519/R-12572.1. [DOI] [PubMed] [Google Scholar]
- 58.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 59.Zaninotto P, Wardle H, Stamatakis E, Mindell J, Head J. Forecasting Obesity to 2010. National Centre for Social Research, Department of Health; London, UK: [Accessed July 3, 2008]. 2006. http://www.dh.gov.uk/en/Publicationsandstatistics/Statistics/DH_4138630. [Google Scholar]
- 60.Albano JD, Ward E, Jemal A, et al. Cancer mortality in the United States by education level and race. J Natl Cancer Inst. 2007;99:1384–94. doi: 10.1093/jnci/djm127. [DOI] [PubMed] [Google Scholar]