Abstract
Meningioangiomatosis consists of benign hamartomatous lesions of the brain and the leptomeninges, which typically present with seizure. Management is predicated on resection and control of seizures with medication. Lesions are typically solitary. Multifocal meningioangiomatosis is extremely rare, with only 2 cases reported in adults and none in children. The authors report the first case, to their knowledge, of multifocal meningioangiomatosis in a child. This unique case highlights therapeutic challenges associated with these lesions and demonstrates that multifocality is possible in the pediatric population. This finding has implications for diagnosis and follow-up for children afflicted with these tumors.
Keywords: multifocal, meningioangiomatosis, multiple, solitary, meningioma
INTRODUCTION
Meningioangiomatosis consists of benign hamartomatous lesions of the brain and the leptomeninges that typically present with seizure. Found both sporadically and in association with NF2, they have nearly exclusively been described as single lesions, with only 2 reported cases of multiple tumors. Both of these multifocal cases were present in adult patients. In this report we describe a case of pathologically confirmed multifocal meningioangiomatosis in a child, with the lesions separated in both location and time of presentation.
HISTORY, PHYSICAL EXAM AND IMAGING
This previously healthy 3-year-old girl presented with right-sided focal motor seizures. She had no other known medical conditions, previous operations, allergies, or relevant family history. Specifically, there was no known history of NF2, other tumors, or seizures. On examination at the time of presentation, the child was neurologically intact, with age appropriate fluent speech, full strength bilaterally, and normal sensation in all extremities. The findings of a general physical examination were unremarkable. As part of her seizure evaluation, MRI studies of the brain and spine were obtained. These studies revealed a lesion of the left sylvian fissure, with avid Gd enhancement (Fig. 1). No other abnormalities were noted in the brain or spine, including no evident lesion in the region of the gyrus rectus. First Operation. Following multidisciplinary review of the case, the child was taken to the operating room for resection of the lesion via a left frontotemporal craniotomy. At surgery, a subtotal resection was performed, with complete removal thwarted by extensive encasement of branches of the middle cerebral artery by the tumor. The child did well, with no morbidity related to the operation and with subsequent control of her seizures (including the use of antiepileptic medication). Pathological Findings. Pathological analysis of the lesion confirmed the diagnosis of meningioangiomatosis. Dispersed throughout the specimen was a network of mildly to moderately hyalinized blood vessels associated with perivascular meningothelial cells and rare microcalcifications (Fig. 2A). Adjacent to the foci of meningioangiomatosis were distinct regions of meningioma (WHO Grade I) characterized by moderate cellularity and composed of cells with ovoid- to spindle-shaped nuclei and moderate to abundant amounts of eosinophilic cytoplasm (Fig. 2C). The tumor showed no evidence of mitotic activity, sheetlike growth, prominent nucleoli, or necrosis, which was consistent with the assigned low histological grade. Postoperative Course. The child was followed with annual clinic visits and MRI sessions. Four years after the resection, routine follow-up imaging revealed a new lesion in the region of the gyrus rectus, distant from the site of the original tumor (Fig. 1). The characteristics were identical to the original sylvian fissure lesion. On retrospective review, it may have been evident on the scan obtained 3 years after surgery, but was not detectable on any other pre- or postoperative images. Second Operation and Outcome. Given this finding (albeit without clinical symptoms), the child was taken for resection of the lesion via a left frontal craniotomy. A gross-total resection was achieved, with pathological findings identical to those in the original lesion (Fig. 2).
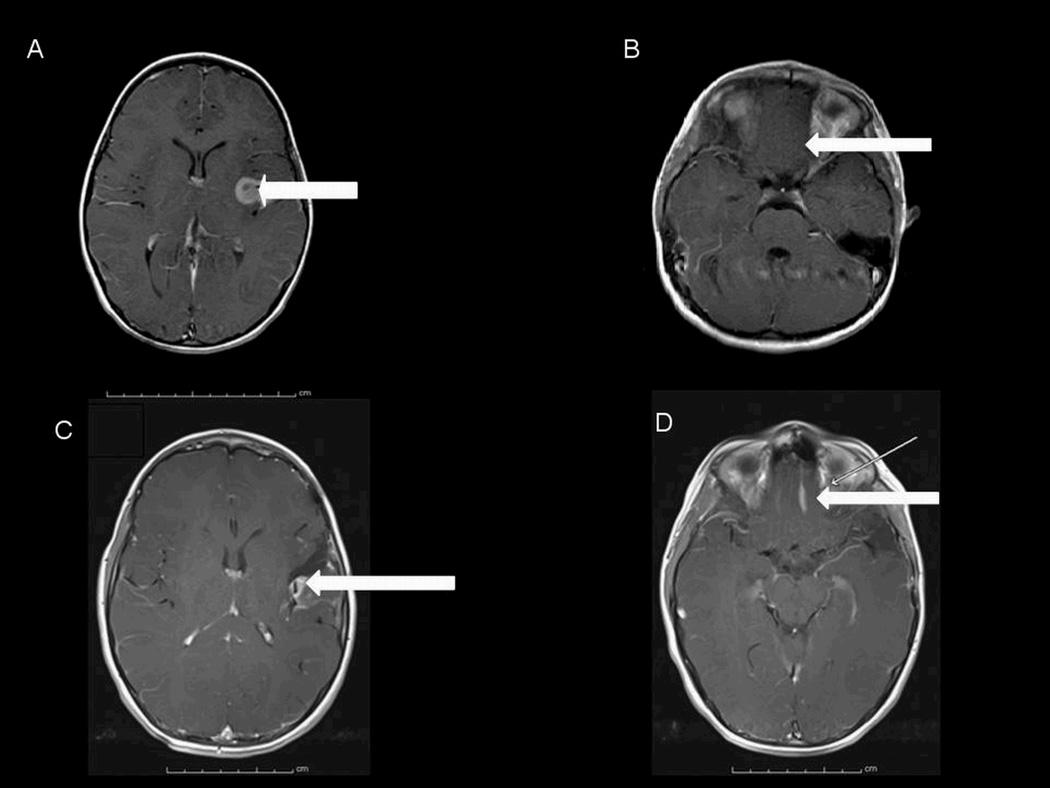
Figure 1.
Axial MRI studies obtained with contrast, in 2005, showing an enhancing lesion in the left sylvian fissure (A) and no obvious lesion in the left gyrus rectus (B). Axial postcontrast MRI studies obtained in 2009 showing a partially resected left sylvian fissure lesion (C) and a newly apparent and distinct lesion in the left gyrus rectus (D). The arrows designate the lesions (or in the case of panel B, the lack thereof).
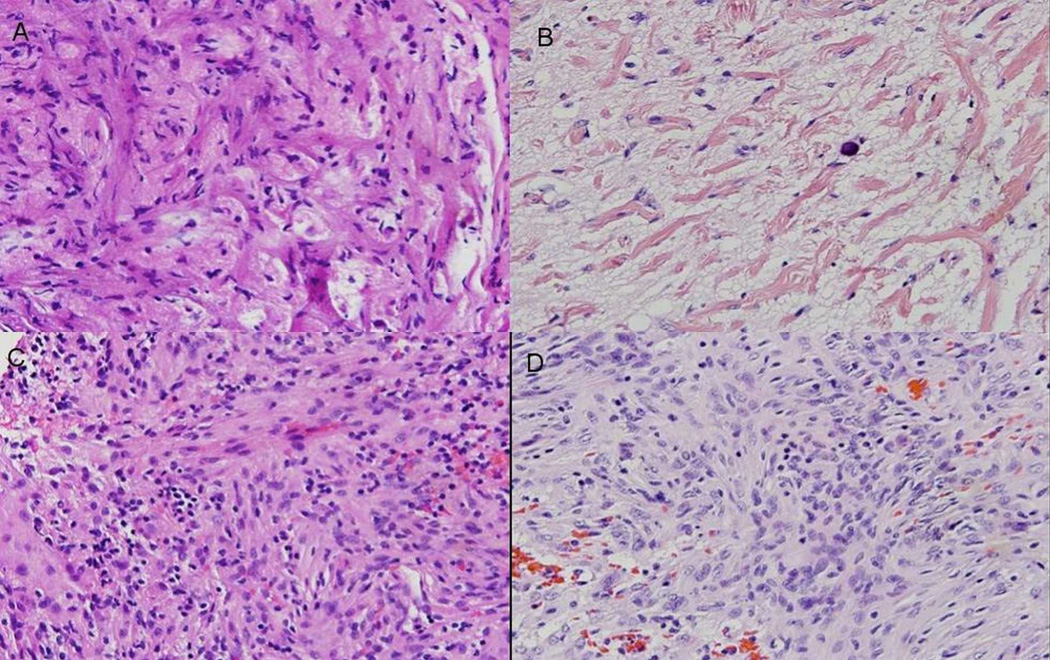
Figure 2.
Photomicrographs of histopathological specimens obtained during the 2005 and 2009 resections. A: Meningioangiomatosis characterized by mildly to moderately hyalinized blood vessels associated with scattered perivascular meningothelial cells involving reactive cortex (specimen from the 2005 resection). B: Meningioangiomatosis with prominent vascular hyalinization and infrequent meningothelial cells in association with rarefied cortex showing neuronal depletion (specimen from the 2009 resection). Moderately cellular meningioma occurring adjacent to meningioangiomatosis in 2005 (C) and in 2009 (D). H & E, original magnification ×40.
The child had no morbidity from the surgery. After 2 years of follow-up from the second operation, the child remains symptom free on antiepileptic medication. Radiologically, the residual lesion in the sylvian fissure has minimally increased in size, whereas the area of the gyrus rectus remains disease free. No other lesions have been identified in the CNS. Genetic testing yielded negative results for NF2.
DISCUSSION
Meningioangiomatosis was initially described in 1915 and remains a rare entity, with fewer than 100 cases described in the literature. 2,16 The pathognomonic finding of this tumor is its invasive nature, characterized pathologically by invasive meningiovascular proliferation, perivascular cuffs of spindle cell proliferation, perivascular connective tissue proliferation, and neurofibrillary tangles, often interspersed with more discrete meningiomas (3,4,9,10,12,16–21,24,26).
Radiographically, the lesions of meningioangiomatosis are characterized by calcifications on CT scans, with MRI studies revealing low intensity on T1-weighted imaging, high intensity on T2-weighted imaging, and avid enhancement with administration of Gd. The differential diagnosis on imaging includes meningioma, oligodendroglioma, granulomatous meningitis, parasitic diseases, and calcified vascular malformation (1,5,10,17,22,23). Although the majority of cases of meningioangiomatosis are sporadic, particular note should be made of the association between meningioangiomatosis and NF2. Sporadic and NF2-associated lesions are histologically identical, but differ in their clinical presentation. Sporadic cases are usually symptomatic, almost exclusively with seizures. In contrast, NF2-associated lesions are most commonly detected as incidental findings (6,7,10,13,16). Given the identical pathological characteristics, it is reasonable to presume that the asymptomatic nature of the NF2 lesions is probably secondary to incidental discovery on screening imaging studies. Sporadic, non-NF2 cases of meningioangiomatosis have also been found in association with other types of brain tumors, including astrocytomas, ependymomas, oligodendrogliomas, primitive neuroectodermal tumors, schwannomas, and hamartomas (10,11,14). Individual case reports have also cited the presence of meningioangiomatosis with anterior cerebral aneurysms, venous angiomas, and encephaloceles (11,25). Meningioangiomatosis occurs in patients of all ages, although more commonly in adults than in children. Sporadic meningioangiomatosis rarely occurs in children younger than 3 years of age. Anatomically, lesions are most frequently identified in the temporal lobe, followed by the frontal, parietal, and occipital lobes in descending order (4,16,21). Clinically, meningioangiomatosis usually presents with seizures and headaches. 1,3,8,10,11,17 Seizures are usually simple or complex partial, and are notably and remarkably refractory to treatment (1,3,8,10,11). This condition is benign and treatable surgically, although resection can be complicated by the invasive nature of the tumor (1,3,8,10,11,17). When possible, complete resection can result in both cure of the tumor and resolution of seizures. However, careful follow-up of treated patients is necessary because the biology of these lesions increases the risk of local recurrence. Literature on this tumor is limited, with the largest series to date composed of only 6 patients, reported by Jallo et al. (7) in 2005. All 6 patients presented with seizure: 4 with simple partial seizures, 1 with complex partial seizures, and 1 with a generalized tonic-clonic seizure. Contrary to other studies, which usually cite temporal lesions, in this series there were 4 frontal and 2 parietal tumors. With resection, patients were seizure free, citing an average of more than 6 years of follow-up. The next largest series, by Kim et al. (10) reviewed 5 patients who also presented with seizure. In a PubMed review of meningioangiomatosis only 2 cases of multifocal disease were identified, both of which were in adult patients. 17 Both presented with headaches and seizures. In one patient the lesions were left frontoparietal and right parietal, and the other patient was found to have left frontal and parietal lobe lesions. In both cases the tumors were initially identified as contemporaneous lesions.
We report on a case of meningioangiomatosis that is unique in being a pediatric report of multifocal disease and also in being a case of 2 lesions growing at different times. The 2 lesions arose in completely different locations and were documented radiographically and confirmed pathologically. This is important because it suggests that these tumors can be multifocal, even inchildren—a finding that has immediate implications for follow-up in affected patients. In addition, this report offers proof of the principle that subtypes of this tumor with different biological behaviors may exist. Although acknowledging that this is a rare entity in any case, we hope that our reporting of this heretofore unwitnessed phenomenon may serve to inform treating physicians about the potential of this tumor.
CONCLUSION
Meningioangiomatosis is a rare, benign, surgically correctible cause of seizures. This report demonstrates the presence of 2 anatomically and temporally distinct lesions in a child with meningioangiomatosis. This finding is important because it reveals the capacity of these tumors to be multifocal, and it underscores the need for continued long-term follow-up in affected patients.
Acknowledgments
Financial support: None
Footnotes
Parts of the current manuscript were presented as an electronic poster presentation at the 79th Annual Scientific Meeting of the AANS held on April 9–13, 2011 in Denver, Colorado.
DISCLOSURES
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper. Author contributions to the study and manuscript preparation include the following. Conception and design: Smith, Jamil. Acquisition of data: all authors. Drafting the article: Jamil. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Smith.
REFERENCES
- 1.Bassoe P, Nuzum F. Report of a case of central and peripheral neurofibromatosis. J Nerv Ment Dis. 1915;42:785–796. [Google Scholar]
- 2.Aizpuru RN, Quencer RM, Norenberg M, Altman N, Smirniotopoulos J. Meningioangiomatosis: clinical, radiologic, and histopathologic correlation. Radiology. 1991 Jun;179(3):819–821. doi: 10.1148/radiology.179.3.2027998. [DOI] [PubMed] [Google Scholar]
- 3.Blumenthal D, Berho M, Bloomfield S, Schochet SS, Jr, Kaufman HH. Childhood meningioma associated with meningio-angiomatosis. Case report. J Neurosurg. 1993 Feb;78(2):287–289. doi: 10.3171/jns.1993.78.2.0287. [DOI] [PubMed] [Google Scholar]
- 4.Deb P, Gupta A, Sharma MC, Gaikwad S, Singh VP, Sarkar C. Meningioangiomatosis with meningioma: an uncommon association of a rare entity--report of a case and review of the literature. Childs Nerv Syst. 2006 Jan;22(1):78–83. doi: 10.1007/s00381-004-1074-4. [DOI] [PubMed] [Google Scholar]
- 5.Fedi M, Kalnins RM, Shuey N, Fitt GJ, Newton M, Mitchell LA. Cystic meningioangiomatosis in neurofibromatosis type 2: an MRI-pathological study. Br J Radiol. 2009 Jul;82(979):e129–e132. doi: 10.1259/bjr/56536580. [DOI] [PubMed] [Google Scholar]
- 6.Fujimoto K, Nikaidoh Y, Yuasa T, Nagata K, Ida Y, Fujioka M, Ohnishi H, Kurokawa S. Meningioangiomatosis not associated with von Recklinghausen's disease--case report. Neurol Med Chir (Tokyo) 1993 Sep;33(9):651–655. doi: 10.2176/nmc.33.651. [DOI] [PubMed] [Google Scholar]
- 7.Jallo GI, Kothbauer K, Mehta V, Abbott R, Epstein F. Meningioangiomatosis without neurofibromatosis: a clinical analysis. J Neurosurg. 2005 Oct;103(4 Suppl):319–324. doi: 10.3171/ped.2005.103.4.0319. [DOI] [PubMed] [Google Scholar]
- 8.Jorge CL, Nagahashi-Marie SK, Pedreira CC, Rosemberg S, Valério RM, Valente KD, Yacubian EM. Clinical characteristics and surgical outcome of patients with temporal lobe tumors and epilepsy. Arq Neuropsiquiatr. 2000 Dec;58(4):1002–1008. doi: 10.1590/s0004-282x2000000600004. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi H, Ishii N, Murata J, Saito H, Kubota KC, Nagashima K, Iwasaki Y. Cystic meningioangiomatosis. Pediatr Neurosurg. 2006;42(5):320–324. doi: 10.1159/000094071. [DOI] [PubMed] [Google Scholar]
- 10.Kim NR, Cho SJ, Suh YL. Allelic loss on chromosomes 1p32, 9p21, 13q14, 16q22, 17p, and 22q12 in meningiomas associated with meningioangiomatosis and pure meningioangiomatosis. J Neurooncol. 2009 Sep;94(3):425–430. doi: 10.1007/s11060-009-9879-3. [DOI] [PubMed] [Google Scholar]
- 11.Kim NR, Choe G, Shin SH, Wang KC, Cho BK, Choi KS, Chi JG. Childhood meningiomas associated with meningioangiomatosis: report of five cases and literature review. Neuropathol Appl Neurobiol. 2002 Feb;28(1):48–56. doi: 10.1046/j.1365-2990.2002.00365.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim SH, Yoon SH, Kim JH. A case of infantile meningioangiomatosis with a separate cyst. J Korean Neurosurg Soc. 2009 Sep;46(3):252–256. doi: 10.3340/jkns.2009.46.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.López JI, Ereño C, Oleaga L, Areitio E. Meningioangiomatosis and oligodendroglioma in a 15-year-old boy. Arch Pathol Lab Med. 1996 Jun;120(6):587–590. [PubMed] [Google Scholar]
- 14.Mut M, Söylemezoğlu F, Firat MM, Palaoğlu S. Intraparenchymal meningioma originating from underlying meningioangiomatosis. Case report and review of the literature. J Neurosurg. 2000 Apr;92(4):706–710. doi: 10.3171/jns.2000.92.4.0706. [DOI] [PubMed] [Google Scholar]
- 15.Omeis I, Hillard VH, Braun A, Benzil DL, Murali R, Harter DH. Meningioangiomatosis associated with neurofibromatosis: report of 2 cases in a single family and review of the literature. Surg Neurol. 2006 Jun;65(6):595–603. doi: 10.1016/j.surneu.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 16.Park MS, Suh DC, Choi WS, Lee SY, Kang GH. Multifocal meningioangiomatosis: a report of two cases. AJNR Am J Neuroradiol. 1999 Apr;20(4):677–680. [PMC free article] [PubMed] [Google Scholar]
- 17.Saad A, Folkerth R, Poussaint T, Smith E, Ligon K. Meningioangiomatosis associated with meningioma: a case report. Acta Cytol. 2009 Jan-Feb;53(1):93–97. doi: 10.1159/000325091. [DOI] [PubMed] [Google Scholar]
- 18.Liu SS, Johnson PC, Sonntag VK. Meningioangiomatosis: a case report. Surg Neurol. 1989 May;31(5):376–380. doi: 10.1016/0090-3019(89)90070-0. [DOI] [PubMed] [Google Scholar]
- 19.Stemmer-Rachamimov AO, Horgan MA, Taratuto AL, Munoz DG, Smith TW, Frosch MP, Louis DN. Meningioangiomatosis is associated with neurofibromatosis 2 but not with somatic alterations of the NF2 gene. J Neuropathol Exp Neurol. 1997 May;56(5):485–489. doi: 10.1097/00005072-199705000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Tacconi L, Thom M, Symon L. Cerebral meningioangiomatosis: case report. Surg Neurol. 1997 Sep;48(3):255–260. doi: 10.1016/s0090-3019(96)00464-8. [DOI] [PubMed] [Google Scholar]
- 21.Takeshima Y, Amatya VJ, Nakayori F, Nakano T, Sugiyama K, Inai K. Meningioangiomatosis occurring in a young male without neurofibromatosis: with special reference to its histogenesis and loss of heterozygosity in the NF2 gene region. Am J Surg Pathol. 2002 Jan;26(1):125–129. doi: 10.1097/00000478-200201000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Tien RD, Osumi A, Oakes JW, Madden JF, Burger PC. Meningioangiomatosis: CT and MR findings. J Comput Assist Tomogr. 1992;16:361–365. [PubMed] [Google Scholar]
- 23.Wang Y, Gao X, Yao ZW, Chen H, Zhu JJ, Wang SX, Gao MS, Zhou LF, Zhang FL. Histopathological study of five cases with sporadic meningioangiomatosis. Neuropathology. 2006 Jun;26(3):249–256. doi: 10.1111/j.1440-1789.2006.00668.x. [DOI] [PubMed] [Google Scholar]
- 24.Whiting DM, Awad IA, Miles J, Chou SS, Lüders H. Intractable complex partial seizures associated with occult temporal lobe encephalocele and meningoangiomatosis: a case report. Surg Neurol. 1990 Nov;34(5):318–322. doi: 10.1016/0090-3019(90)90007-c. [DOI] [PubMed] [Google Scholar]
- 25.Wiebe S, Munoz DG, Smith S, Lee DH. Meningioangiomatosis. A comprehensive analysis of clinical and laboratory features. Brain. 1999 Apr;122(Pt 4):709–726. doi: 10.1093/brain/122.4.709. [DOI] [PubMed] [Google Scholar]
- 26.Wilson D, Dempsey RJ, Clark DB. Meningioma developing from underlying from underlying meningioangiomatosis. J Neuropathol Exp Neurol. 1991;50:371. (Abstract). [Google Scholar]