Abstract
Parkinson’s disease (PD) is characterized by disorders of visuospatial function that can impact everyday functioning. Visuospatial difficulties are more prominent in those whose motor symptoms begin on the left body side (LPD) than the right body side (RPD) and have mainly been attributed to parietal dysfunction. The source of visuospatial dysfunction is unclear, as in addition to subcortical–cortical changes, there are irregularities of visual scanning and potentially of retinal-level vision in PD. To assess these potential contributors, performance on a visuospatial task—line bisection—was examined together with retinal structure (nerve fiber layer thickness, measured by optical coherence tomography [OCT]), retinal function (contrast sensitivity, measured by frequency-doubling technology [FDT]), and visual scanning patterns. Participants included 20 nondemented patients (10 LPD, 10 RPD) and 11 normal control (NC) adults. Relative to the other groups, LPD were expected to show rightward bias on horizontal line bisection, especially within the left visual hemispace, and downward bias on vertical bisection. LPD relative rightward bias was confirmed, though not mainly within the left hemispace and not correlated with retinal structure or function. Retinal thinning was seen in LPD relative to RPD. Qualitative visualization of eye movements suggested greater LPD exploration of the right than left side of the line during horizontal bisection, and some overall compression of scanning range in RPD (both orientations) and LPD (primarily vertical). Results indicated that rightward visuospatial bias in our LPD sample arose not from abnormalities at the retinal level but potentially from attentional biases, reflected in eye movement patterns.
Keywords: visuospatial, retina, Parkinson’s disease, optical coherence tomography, frequency doubling technology
Individuals with Parkinson’s disease (PD) without dementia exhibit deficits in multiple domains of visuospatial functioning (reviewed in Cronin-Golomb, 2010), including line bisection (Barber, Tomer, Sroka, & Myslobodsky, 1985; Davidsdottir, Wagenaar, Young, & Cronin-Golomb, 2008; Lee, Harris, Atkinson, & Fowler, 2001a; Lee, Harris, Atkinson, Nithi, & Fowler 2002; Starkstein, Leiguarda, Gershanik, & Berthier, 1987). It has been found that PD patients with initial motor symptoms on the right side of the body (RPD; predominant left basal ganglia dysfunction) perform similarly to healthy control adults, whereas those whose initial symptoms originate on the left side of the body (LPD; predominant right basal ganglia dysfunction) tend to deviate to the right of center on horizontal line bisection tasks (Lee et al., 2001a; Davidsdottir et al., 2008) and downward on vertical line bisection tasks (Lee et al., 2002). This pattern may be conceived of as a tendency of LPD patients to perceive visual space as compressed in the leftward and upper visual fields. This type of visuospatial distortion has also been considered a form of visuospatial neglect, and may relate to observations that LPD patients have a greater bias toward visual exploration on the right than the left side of space (Ebersbach et al., 1996) and toward perceiving objects in the left visual field as smaller than objects in the right visual field (Harris, Atkinson, Lee, Nithi, & Fowler, 2003). Such visuospatial difficulties can interfere with daily life. For example, PD patients experience problems when attempting to move through narrow spaces such as doorways (Davidsdottir, Cronin-Golomb, & Lee, 2005; Lee & Harris, 1999). Those with LPD, but not those with RPD or healthy control adults, were found to misjudge the size of virtual doorways through compression, overestimating by 10% the space they would need to pass through the opening (Lee, Harris, Atkinson, & Fowler, 2001b).
The cause of LPD patients’ difficulty with line bisection is unknown. Although dysfunction in cortical (including parietal) and subcortical regions relevant to visuospatial function has been reported (e.g., Clower, Dum, & Strick, 2005; Cronin-Golomb, 2010; Witt, Kopper, Deuschl, & Krack, 2006), there are other potential factors that could be contributing to the behavioral deficits. As one example, in cases of patients with right-hemisphere lesions, the visual field placement of the horizontal line for bisection can significantly affect performance (i.e., more rightward bisection is observed on lines placed farther leftward in the visual field; Mennemeier, Vezey, Chatterjee, Rapcsak, & Heilman, 1997). The effect of visual field placement has been studied to only a limited extent in PD. In regard to vertical line bisection, one study found no performance differences across left, center, and right visual fields when comparing LPD, RPD, and NC groups, but performance was not examined within the upper and lower visual fields (Lee et al., 2002). It may be that effects of visual field placement would be found if stimuli were positioned in line with the orientation of the visuospatial task. That is, just as horizontal line bisection biases in those with putative right hemisphere dysfunction are increased or decreased when stimuli are positioned more leftward or rightward on the horizontal axis, vertical line bisection biases may be increased or decreased if stimuli are positioned higher or lower on the vertical axis.
Another potential contributor to deficits in line bisection is irregularities in scanning strategies, with oculomotor abnormalities being well established in PD (e.g., Hunt, Sadun & Bassi, 1995; Nakamura et al., 1991; Shibasaki, Tsuji, & Kuroiwa, 1979). In one study, patterns of eye movements in PD patients differed from those of a control group on an executive function task (computerized Tower of London) and appeared to be associated with attentional and working memory deficits (Hodgson, Tiesman, Owen, & Kennard, 2002). More recently, Clark and colleagues documented differences between PD patients and control adults in how visual scanning variables were related to frontal-mediated executive dysfunction in regard to facial emotion recognition (Clark, Neargarder, & Cronin-Golomb, 2010). In that study, a significant correlation between scanning patterns and measures of basic visual function (acuity, contrast sensitivity) were found in PD but not in the control group.
Such findings of a relation between basic vision and higher-order cognition in PD raise another factor that may contribute to visuospatial dysfunction, namely, retinal abnormalities. Retinal dopamine may be reduced in PD (reviewed in Archibald, Clarke, Mosimann, & Burn, 2009), and such a deficiency may influence functional vision. As well, the thinning of the retinal nerve fiber layer (RNFL) is likely to have an impact on vision. In PD, abnormal loss of retinal nerve cells has been found to result in areas of RNFL thinning (Altintaş, Işeri, Ozkan, & Cağlar, 2008; Hajee et al., 2009; Inzelberg, Ramirez, Nisipeanu, & Ophir, 2004; though not in Archibald, Clarke, Mosimann, & Burn, 2011). The degree of thinning of the inner retinal layer in PD has been shown to be independent of intraocular pressure, arguing against an explanation based on a simple glaucomatous process (Hajee et al., 2009). It is not known if retinal dopamine and the thinning of the RNFL may affect visuospatial performance such as on line bisection tasks. It is also currently unknown if other aspects of retinal functioning, such as contrast sensitivity across the retina, may correlate with RNFL thinning or contribute to visuospatial dysfunction in PD.
The present study examined the possible contributions of visual field placement, scanning patterns, and retinal structure and function to visuospatial function as assessed through vertical and horizontal line bisection. We expected that, compared with NC and RPD, LPD patients would show a bias to bisect a line more toward the right of true center for horizontal stimuli, and more below true center for vertical stimuli, as can be expected from the literature. We anticipated that the bisection bias would be especially pronounced in the presumably more-affected hemispace—that is, the left visual field for horizontally oriented stimuli and the upper visual field for vertical stimuli. We hypothesized that thinning of specific RNFL areas (revealed by optical coherence tomography [OCT]) and impairment in its functional correlate, decreased contrast sensitivity in portions of the visual field (revealed by frequency doubling technology [FDT]), would correlate with performance on the bisection task in the associated field of view, and that LPD would show a different correlation profile than RPD or NC. Further, it was predicted that patterns of scanning during the bisection task would differ across groups, with LPD spending relatively less time looking at areas of the line that were on the side of the hypothesized more-affected hemispace. These comparisons of scanning patterns, as well as analysis of line bisection across the visual field with functional and structural measures of retinal quadrants, are unique to this study.
Method
Participants
Participants were nondemented individuals with idiopathic PD whose prominent initial motor symptom or symptoms were present on the left (LPD; n = 10, 3 women) or right (RPD; n = 10, 6 women) sides of their body, and healthy NC participants (n = 11, 7 women). LPD, RPD, and NC participants were matched for age and education, F(2, 28) = 1.88, p = .17 and F(2, 28) = 0.84, p = .44, respectively. The three groups performed similarly on the Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975) or Modified Mini-Mental State Examination (Stern, Sano, Paulson, & Mayeux, 1987) with scores converted to standard MMSE scores, F(2, 26) = .54, p = .59. Two LPD and 1 RPD patients were left-handed, an additional 2 RPD patients self-identified as ambidextrous, and all other participants were right-handed (see Table 1).
Table 1.
Participant Characteristics
| Age, mean years (SD) |
Male: Female |
Education, mean years (SD) |
Disease duration, mean years (SD; range) |
MMSE, mean score (SD) |
|
|---|---|---|---|---|---|
| NC (n= 11) | 70 (5.5) | 4:7 | 17 (2.0) | N/A | 28.4 (1.0) |
| LPD (n= 10) | 64 (8.6) | 7:3 | 16 (2.1) | 9.5 (4.4; 4–16) | 27.9 (1.5) |
| RPD (n= 10) | 67 (8.0) | 4:6 | 16 (1.4) | 8.8 (5.6; 1–21) | 28.4 (0.9) |
Note. LPD = left-body-onset Parkinson’s disease; MMSE = Mini-Mental State Examination or Modified Mini-Mental State Examination converted equivalent; NC = normal control participants; RPD = right-body-onset Parkinson’s disease; SD = standard deviation.
Exclusion criteria for all groups included coexisting serious chronic medical illnesses (including psychiatric or neurological illness); use of psychoactive medication besides antidepressants and anxiolytics in the PD groups; use of any psychoactive medications in the NC group; and history of intracranial surgery, traumatic brain injury, and alcohol or other substance abuse or dependence. Participants received a detailed neuro-ophthalmological examination to rule out visual disorders arising from dysfunction of the anterior pathways, including cataracts, glaucoma, and macular degeneration. No participant met or exceeded predetermined cutoff scores on measures of depression for either the Beck Depression Inventory II (Beck, Steer, & Brown, 1996; administered to those aged 64 years or younger; cutoff = 14) or the Geriatric Depression Scale (Yesavage, 1988; administered to those 65 or older; cutoff = 17; mood data unavailable for 2 NC, 2 LPD, 1 RPD). Initial side of motor symptom onset information was gathered in a review of neurology records or by patient self-report. Motor disability as indexed by Hoehn and Yahr (H&Y) stage (Hoehn & Yahr, 1967) was similar for the LPD and RPD groups (Kolmogorov-Smith [K-S], Z = 1.07, p = .21). Seven patients (3 LPD, 4 RPD) were H&Y Stage 1.5 (unilateral), 5 patients (3 LPD, 2 RPD) were Stage 2 or 2.5 (mild bilateral), and 3 patients (1 LPD, 2 RPD) were Stage 3 (moderate bilateral with postural instability). H&Y data were unavailable for 3 LPD and 1 RPD. Mean PD duration was 9.1 years (SD = 4.9, range 1 to 21) with no difference between LPD and RPD, t(17) = 0.301, p = .77. Disease duration information was unavailable for 1 LPD. PD patients were tested while taking antiparkinsonian medications as prescribed.
Procedures
Study procedures were reviewed and approved by the Charles River Campus Institutional Review Board of Boston University. All participants gave written informed consent.
Line bisection
The line bisection (Landmark-like) test was presented to participants binocularly, with each trial presented within one of nine grid positions on an LCD computer display (approximately 19 in. diagonal, 12 in. vertical, and 15 in. horizontal). Visual field placement of the line was systematically varied. Stimuli consisted of an approximately 6-in. line (subtending 15.5 degrees of visual angle at a 22-in. viewing distance) intersected at a right angle by a 1.5-in. target line. The target line was initially presented as offset from the center of the long line by 8% to 12% of the total long line length (Davidsdottir et al., 2008). To minimize bias associated with the side from which the target line initially appeared (Davidsdottir et al., 2008), the starting position of the target line was alternated to either side of true center across trials. The long line was oriented either horizontally or vertically, in blocks of 6 trials each, at each of 9 grid positions on the computer display (see Figure 1). Four orders of presentation were used, with each participant receiving one order. Across orders, the following elements were varied: the sequence of where on the grid the blocks of stimuli were presented (randomly determined); the order of presentation of orientation (horizontal or vertical; random within the constraint of equal numbers of each), and the starting direction of the target line (whether the block began with the target line above or below center for vertical trials; or left or right of center for horizontal trials; random within the constraint of equal numbers of each). For each trial, the experimenter moved the target line in the initial direction toward the center of the long line in small steps (each being 0.5% of the long line length) and participants were instructed to verbally indicate when the target line reached the exact center. Trials were untimed and participants could request that the position of the target line be adjusted in either direction until they were satisfied with the position. Participants used a chinrest, essentially fixing the position of the head and body. Eye movements were unrestrained and recorded using remote eye tracking equipment.
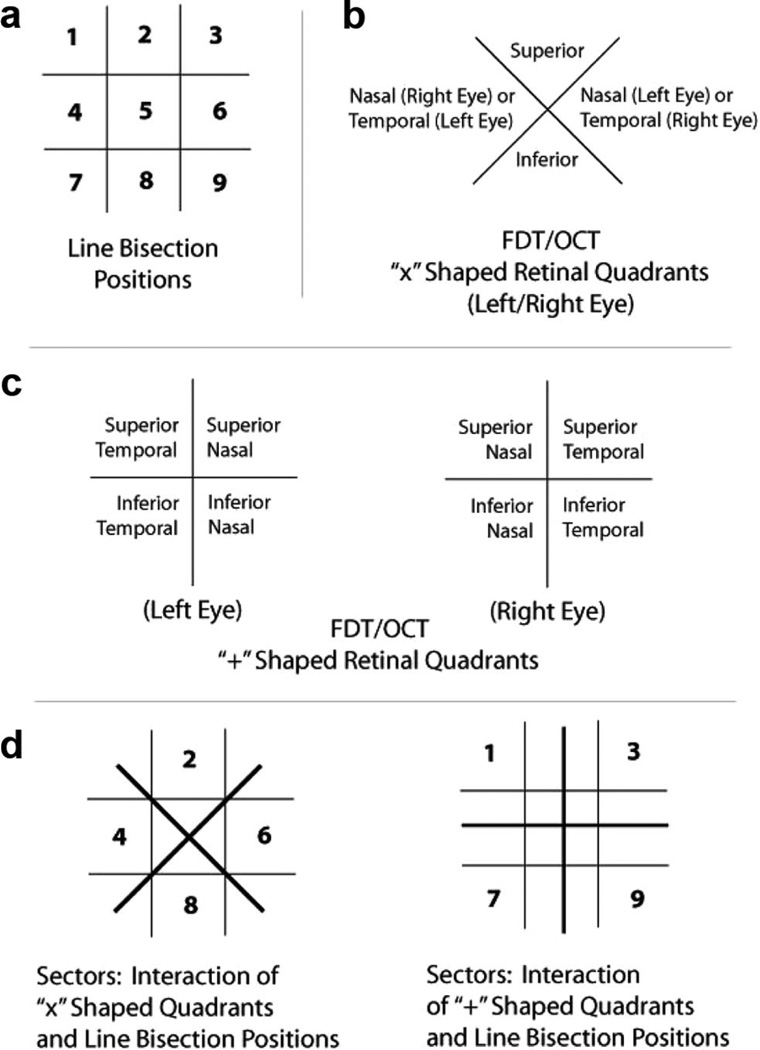
Figure 1.
Visualization of line bisection positions, frequency-doubling technology (FDT) and optical coherence tomography (OCT) retinal quadrants, and schematics of sectors. Grid lines are for illustrative purposes in this figure and were not visible to the participants. Note: the optics of the lens of the eye invert (top to bottom) and reverse (left to right) an external image that is projected onto the retina. Therefore, in analyses that relate retinal quadrants to line bisection positions (i.e., Figure 1d), retinal quadrants were matched to their corresponding image source location.
For analysis, the bisected line was conceptualized as a number line. The true center was represented by zero, and increasingly greater distances from center were represented by more positive numbers in one direction and more negative numbers in the other direction. The values of all responses were averaged for a given condition. This “bias parameter” (Çiçek, Deouell, & Knight 2009) was used for the major analyses and correlations of line bisection performance with other variables. Differences were also examined in the magnitude of error, taking into account the side of bias (referred to here as “side-specific error magnitude”). This measure allows a comparison of magnitude of error between the two sides of center.
Frequency-doubling technology (FDT)
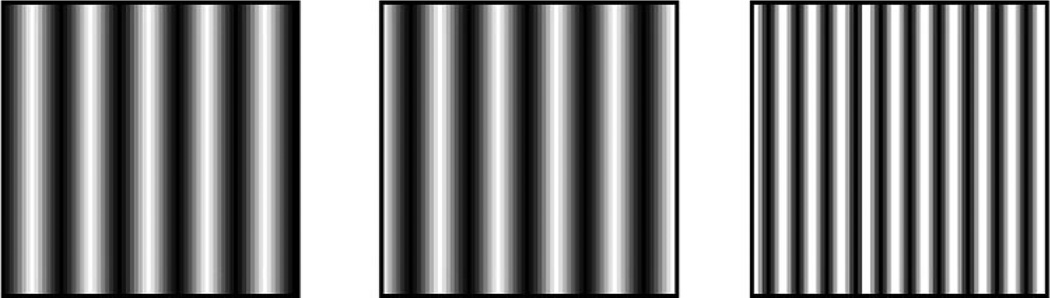
FDT is a method of measuring visual function across the retina. “Frequency doubling” refers to what is perceived when a low-spatial-frequency sinusoidal grading (i.e., a striped stimulus) rapidly alternates in contrast: It appears to approximately double in its spatial frequency (i.e., more stripes are perceived than are actually present; Anderson & Johnson, 2003; Rosli, Bedford, & Maddess, 2009; see Figure 2). Magnocellular retinal ganglion cells are specifically tuned to this frequency-doubling stimulation. The FDT equipment uses frequency doubling stimuli as a measure of contrast sensitivity across the retina (i.e., perimetry). A small patch of frequency-doubling grating is projected at specific points across the retina. Participants fixate on a central point and are asked to respond by button press when they see a grating appear. The contrast of the stimuli is varied to determine contrast thresholds (in dB) at each retinal position. A Humphrey Matrix with Welch Allyn FDT machine (Zeiss Series 715) was used. Because testing was done at the end of a full eye examination, participants’ pupils were dilated during the FDT testing.
Figure 2.
An example of the concept of frequency-doubling technology (FDT) stimuli. Rapid alternation of light and dark gradient stripes (represented by the left and center patches) creates the perceived doubling illusion (represented by the patch on the right). The FDT test measures contrast sensitivity across the retina using the “doubled” stimuli. Figure modeled after Levin, 2005
Automated validity measures for the FDT machine are not always accurate. False fixation errors can be triggered if a participant’s optic nerve is located outside of a statistically normative location on the eye, even if the person is well fixated. In PD patients, occasional involuntary motor movements in the hands may trigger excess response “errors,” yet the overall performance may still be valid. Using a strict cutoff based on automated error information, especially in a neurodegenerative sample such as PD, can systematically exclude participants who have valid tests and potentially bias and limit the generalization of the findings. Hence, the administering examiner performed a determination of clinical validity of tests for our participants.
Participants were tested monocularly, right eye followed by left eye, as per standard optometric practice. They were shown a 30–2 stimulus presentation pattern, which refers to the grid upon which the FDT samples the functioning of areas of the central 30° of the retina. The samples represent responses to 5° targets, 17 in each of four retinal quadrants and one central macular target. One participant who received a comprehensive eye examination from his personal ophthalmologist had been given a 24–2 pattern, which samples the central 24° of the retina and uses three or four (depending on the quadrant) fewer targets in the outer edge of each quadrant compared with the 30–2 pattern, and one central macular target. Threshold readings were averaged for a given quadrant. The FDT output divides the measured retinal surface into quadrants in a “+” formation (i.e., two upper, two lower quadrants; see Figure 1b). To better match OCT quadrant distribution and the nine positions of line bisection, for some comparisons output was also divided into quadrants in an “X” formation (i.e., upper/lower/ nasal/temporal; see Figure 1c), with threshold readings averaged within the given areas, and readings that fell on the border of two quadrants weighted at one-half weight.
Optical coherence tomography (OCT)
The structure of the retina was assessed with OCT, which is an imaging technique in which the equipment emits light toward the retina and interprets the backscatter of the reflected light to create a high-resolution image (Hee et al., 1995; Huang et al., 1991). The OCT uses a superluminescent diode to project a broad bandwidth near-infrared light beam (820 nm) onto the retina while the observer looks at a fixation point. A Zeiss Stratus OCT (Model 3000; third-generation model) machine was used. A Fast RNFL scan was performed on each eye of each participant (right eye first). The protocol consisted of 360° circular scans with a diameter of 3.4 mm centered on the optic disk. Software in the OCT machine calculated parapapillary RNFL thickness for temporal quadrant thickness (316°–45°), superior quadrant thickness (46°–135°), nasal quadrant thickness (136°–225°), and inferior quadrant thickness (226°–315°; Altintaş et al., 2008). Fast retinal thickness maps and fast RNLF thickness readouts were produced. Participants’ pupils were dilated during the procedure. Usable scans were obtained from all participants.
Relation between measures of line bisection, FDT, and OCT
As noted, line-bisection stimuli were presented in one of nine regions, referred to as “positions” (Positions 1 to 9) within a participant’s field of view (Figure 1a), and retinal results (OCT, FDT) were divided into quadrants corresponding to sectors of the visual field (Figure 1b, 1c). Quadrants of RNFL cells represent an inexact but roughly analogous topographic measure of corresponding macular and foveal quadrants. Here, a “position” refers to a grid-like section of the computer monitor (visual field) in which line bisection stimuli were presented, a “quadrant” refers to a single section of the retina that is measured (directly or indirectly) by the OCT or FDT, and a “sector” or “field of view” refers to the interaction—that is, the portion of the visual field in which a position is viewed by a corresponding retinal quadrant or quadrants (see Figure 1d). Figure 1 includes diagrams of all screen positions and retinal quadrants.
Eye tracking
Tracking and recording of eye movements was accomplished with an Applied Science Laboratories (ASL) eye tracking system. A model D6 camera array was located underneath the stimulus monitor and used infrared light to discern the participant’s pupil and corneal reflection. These two reflection data points were constantly monitored through Eye-Trac software (user interface software version 1.58.4.0) to discern the position of the eye. Viewing was binocular; the left eye was tracked (as per Clark et al., 2010; Wong, Cronin-Golomb, & Neargarder, 2005). The system used an ASL EYE-TRAC 6 Control unit (control unit software version 6.40.03), and the camera recorded at 60 Hz. The manufacturer reports the system accuracy as 0.5-degree visual angle, and the resolution as 0.25-degree visual angle. A 9-point calibration sequence was used at the beginning of each session and as needed during testing to allow the equipment to accurately and continually calculate the participant’s point of gaze relative to the display. Line bisection stimuli were presented using Microsoft Power-Point (version 2000 SP-3), which was synched to the Eye-Trac software through custom software programming by ASL. Eye tracking data were processed using ASL Results software (version 1.17.09).
Eye tracking was attempted on all participants. Data were not usable when the eye tracking equipment was unable to automatically or manually make or maintain a consistent “lock” on the eye reference points. If this happened for more than approximately half of the six trials in any block, the participant was excluded, as occurred for 2 NC and 1 RPD participants, leaving an eye tracking subsample of 9 NC, 10 LPD, and 9 RPD. Occasional brief loss of eye lock was common for the remaining participants.
Areas of interest
The ASL Results software allows the creation of areas of interest (AOIs) that are designated regions of the stimulus display. Calculations can be made of the percentage of time the participant spent looking within an AOI region. Three equal-sized rectangular AOIs were created for each line bisection stimulus. They encompassed the center portion of the line, and the rightward or leftward side of the line (for horizontal bisection) or the upward or downward side of the line (for vertical bisection). The three AOIs were located directly next to each other, were nonoverlapping, and together encompassed the entire bisection line stimulus being displayed plus a small amount of space surrounding the stimulus. The software treated the rest of the display that did not contain one of the described AOIs as a fourth region. Percentage of time the participant spent looking within each AOI or the fourth region was calculated by the software. Demarcations for AOIs were never visible to the participant.
Results
Line Bisection
The hypotheses were that relative to the other groups, LPD would be biased to the right on horizontal line bisection and downward on vertical line bisection. Significant main effects are summarized in Table 2.
Table 2.
Significant Main Effects
| Measure | Main effect | F value | p value |
|---|---|---|---|
| Line bisection | Group | F(2, 28) = 4.2 | <.05 |
| Position | F(5.9, 21) = 2.2 | <.05 | |
| Frequency | |||
| Doubling technology | Eye | F(1, 28) = 13.9 | <.001 |
| Optical coherence | |||
| Tomography | Eye | F(1, 27) = 5.33 | <.05 |
| Quadrant | F(3, 25) = 43.9 | <.001 |
Note. Eye = left eye, right eye. Group = left-body-onset Parkinson’s disease (LPD), normal control participants (NC); right-body-onset Parkinson’s disease (RPD). Position = location of presentation of stimuli at nine positions on a grid. Quadrant = inferior, superior, nasal, temporal quadrants of retinal nerve layer fiber thickness.
Main effects: Bias parameter analysis
A 2 × 3 × 9 (Orientation [horizontal, vertical] × Group [LPD, RPD, NC] × Screen Position) repeated measures ANOVA revealed a main effect of group, F(2, 28) = 4.2, p < .05, and of position, F (5.9, 21) = 2.2, p < .05, Huynh-Feldt corrected (ε = .738). Tukey’s HSD tests showed that LPD differed from RPD (p < .05). No other main effects or interactions were significant.
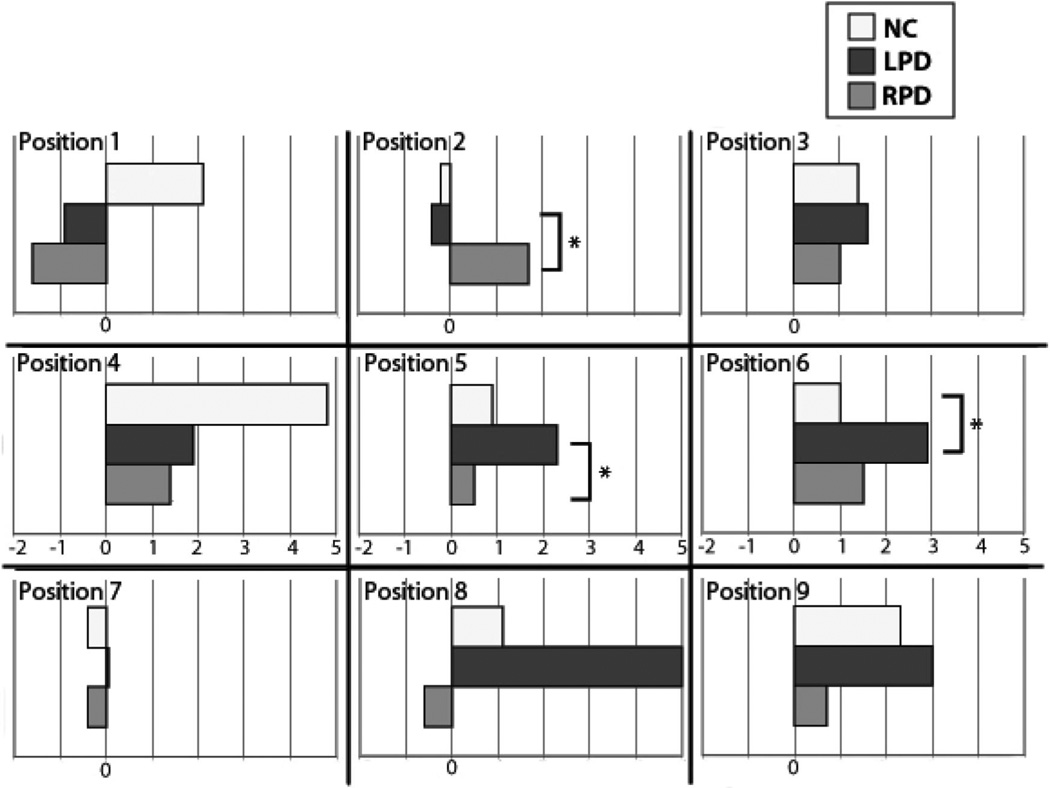
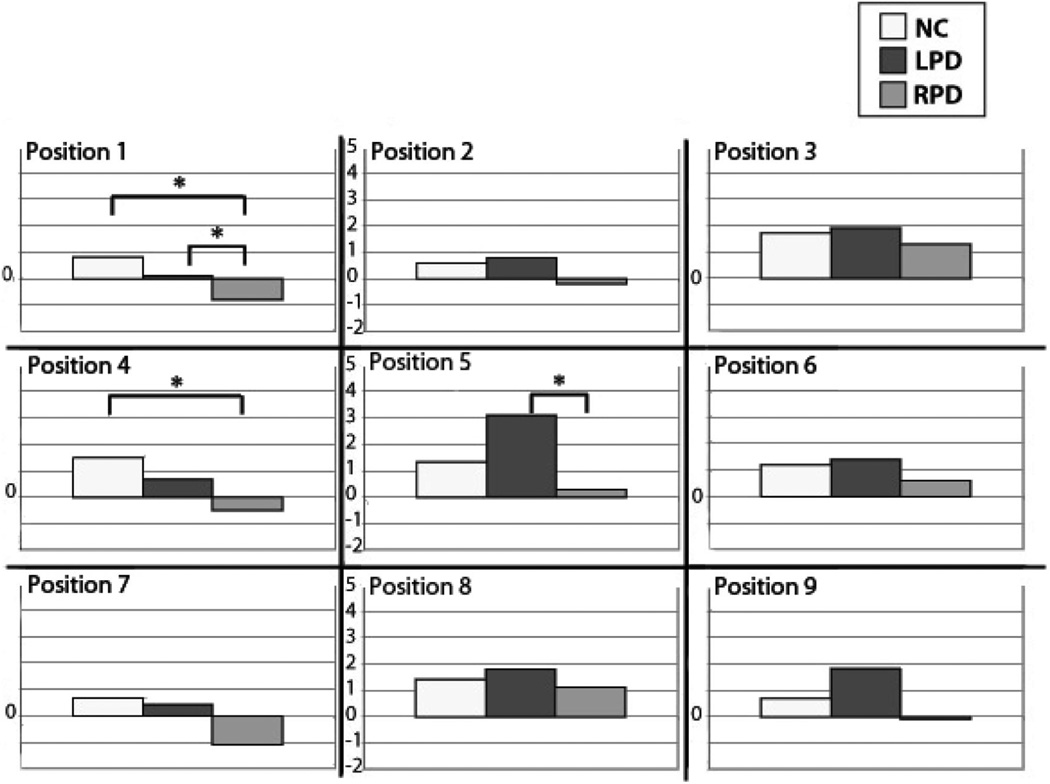
Additional a priori analyses were performed with independent samples t tests for line bisection performance for each orientation. Though we might have used one-tailed comparisons in accordance with our hypotheses (rightward and downward bisection by LPD relative to other groups), we retained the more conservative .05 alpha in light of the number of positions under investigation. For the horizontal orientation, LPD bisected the lines more rightward than NC at Position 6 (center-right), t(19) = 2.21, p < .05; more rightward than RPD at Position 5 (center), t(18) = 2.12, p < .05; and more leftward than RPD at Position 2 (upper-center), t(18) = 2.36, p < .05 (see Figure 3). For the vertical orientation, RPD bisected lines more downward than LPD at Position 1 (upper-left), t(18) = 2.31, p < .05, and also at Position 5 (center), t(18) = 2.47, p < .05. RPD bisected lines more downward than NC at Position 1 (upper-left), t(19) = 2.09, p < .05, and also at Position 4 (center-left), t(19) = 2.12, p < .05 (see Figure 4). There were no other significant group effects for horizontal or vertical line bisection. In sum, the hypothesis that LPD would bisect horizontal lines more rightward than the other groups was supported at two positions (center and center-right), but not specifically for the left visual hemispace. The hypothesis that LPD would bisect vertical lines more downward than the other groups was not supported; in fact, it was the RPD group that exhibited relative downward bisection. As seen in Figures 3 and 4, line bisection bias that we examined was in one group relative to other groups, and was not necessarily relative to the true center.
Figure 3.
Horizontal line bisection results by screen position and by group. Screen position is indicated (also see Figure 1a). Positive values on the x-axis represent greater rightward bisection bias; negative numbers represent greater leftward bisection bias; zero represents true center. LPD showed a more rightward bisection bias at Positions 5 and 6 than did RPD and NC, respectively. At Position 2, RPD bisected more rightward than LPD. LPD = left-body-onset Parkinson’s disease; NC = normal control participants; RPD = right-body-onset Parkinson’s disease. *p < .05.
Figure 4.
Vertical line bisection results by screen position and by group. Screen position is indicated (also see Figure 1a). Positive values on the y-axis represent greater upward bisection bias; negative numbers represent greater downward bisection bias; zero represents true center. LPD bisected more upward than RPD at Position 5. RPD showed a downward bias at Position 1 (compared with NC and LPD) and at Position 4 (compared with NC). LPD = left-body-onset Parkinson’s disease; NC = normal control participants; RPD = right-body-onset Parkinson’s disease. *p < .05.
FDT
A 2 × 3 × 4 (Eye [left, right] × Group × Retinal Quadrant [superior-temporal, superior-nasal, inferior-temporal, inferior-nasal]) repeated measures ANOVA showed a main effect of eye, F(1, 28) = 13.9, p < .001, Huynh-Feldt corrected (ε = 1.0). Across all groups, the left eye (M [SE] = 19.71 [0.75] dB) had worse sensitivity than the right eye (M [SE] = 21.51 [.64] dB). There was no effect of group, and inspection of the individual group means indicated that this relative trend of mean sensitivity was seen in each of the groups. No other main effects or interactions were statistically significant.
OCT
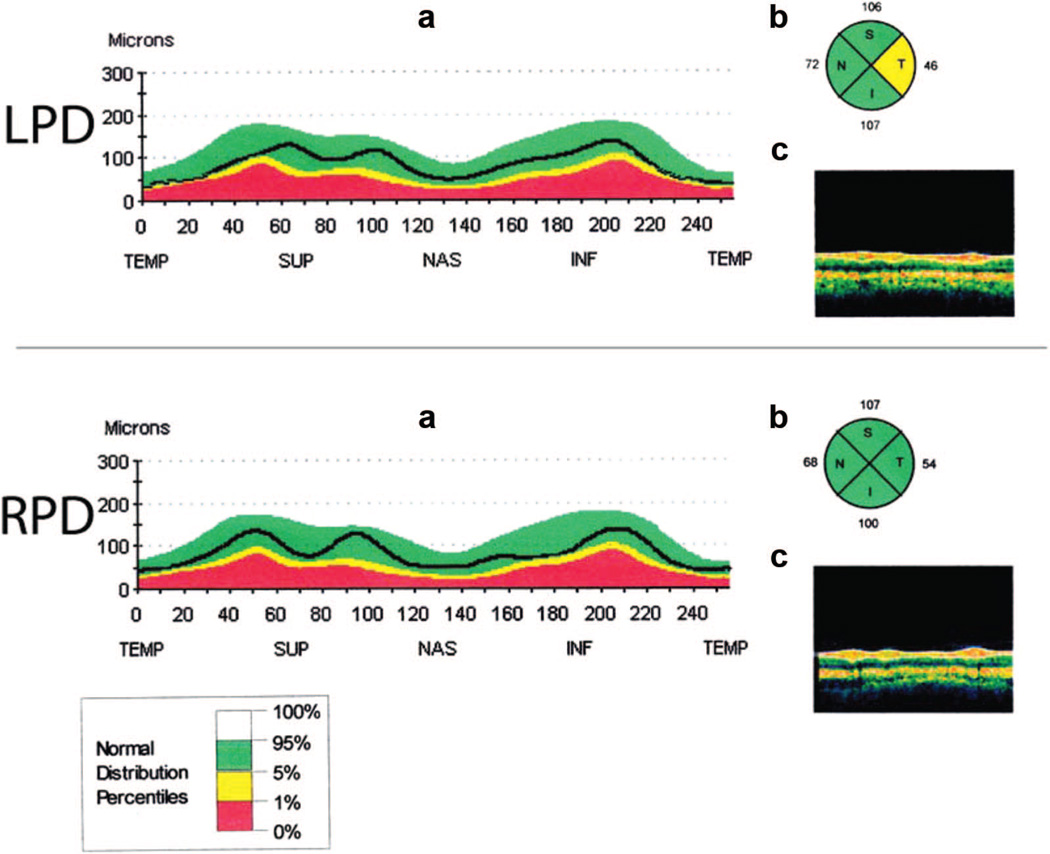
A 2 × 3 × 4 (Eye [left, right] × Group × RNFL Quadrant) repeated measures ANOVA showed a main effect of eye, F(1, 27) = 5.33,p < .05 (right RNFL [M (SE) = 90.9 (2.7) µm] was thicker than left [M (SE) = 87.7 (2.5) µm]). There was no effect of group; inspection of the group means indicated that this pattern was seen for NC and RPD, with LPD means being relatively similar across eyes. A main effect of quadrant was also found, F(3, 25) = 43.9, p < .001. Post hoc paired samples t tests showed that the superior quadrant (M [SE] = 104.4 [3.4] µm) was thicker than temporal (M [SE] =73.1 [2.6] µm; p < .001) and nasal (M[SE] = 70.1 [4.7] µm, p < .001); the inferior quadrant (M [SE] = 109.8 [3.6] µm) was thicker than temporal (p < .001) and nasal (p < .001). This relative pattern of means was seen in each of the groups. The Quadrant × Group interaction was significant, F(6, 27) = 3.5, p < .01. Post hoc independent sample t tests showed that the RNFL in the temporal quadrant was significantly thinner in LPD than in RPD, t(17) = 2.85, p < .05 (see Figure 5). No other main effects or interactions were significant.
Figure 5.
Retinal nerve fiber layer (RNFL) depictions. Selected elements of an OCT scan output for an individual LPD participant (upper set of figures) and RPD participant (lower set of figures). (a) A linear representation of the RNFL. The black line represents the participant’s RNFL thickness. Colored areas are comparison ranges of a normal distribution (see legend at bottom of figure). Abbreviations of RNFL quadrant names are noted on the lower line of the x-axis. Note the reduced thickness in the temporal quadrant (the black line, far left and far right of the representation) in the LPD but not the RPD example. (b) A circular representation of the RNFL, divided into quadrants. The numbers next to each quadrant represent average thickness in microns. Colors represent normal distribution percentiles (see legend at bottom of figure). The temporal quadrant is indicated by “T” and is reduced in the LPD, but not RPD, example. (c) Representation of the measured retinal layers of the eye. These individual scans were chosen because they are illustrative of group findings (i.e., greater retinal thinning in the temporal quadrant in LPD than in RPD) and are not representative of all members of the group. Pictured scans are of the left eye and are from men. INF or I = inferior RNFL quadrant; LPD = left-body-onset Parkinson’s disease; NAS or N = nasal RNFL quadrant; OCT = optical coherence tomography; RPD = right-body-onset Parkinson’s disease; SUP or S = superior RNFL quadrant; TEMP or T = temporal RNFL quadrant.
Correlations of FDT, OCT, and line bisection bias
It was predicted that thinning of the RNFL (revealed by OCT) and impairment in its functional correlate in contrast sensitivity (revealed by FDT) would correlate with bias on the bisection task in the associated field of view, and that LPD would show a different correlation profile than RPD or NC. We performed the correlations with horizontal line bisection, which elicited a relative rightward bias at some positions for LPD. Vertical line bisection did not elicit hypothesized group differences in bisection bias and therefore we did not perform correlations with other structure–function variables.
Correlations of line bisection bias and contrast sensitivity as measured by FDT within a given sector
There were no significant correlations for any group between direction/magnitude of bisection bias on horizontal line bisection and FDT contrast sensitivity for any sector.
Correlations of line bisection bias and RNFL thickness as measured by OCT within a given sector
For NC, there was a significant correlation in the left eye between RNFL thickness in the superior nasal quadrant and the direction/magnitude of bisection bias of horizontal bisection at Position 7 (lower left; r[9] = .65; p < .05). RNFL thickness in the nasal quadrant of the right eye was correlated with line bisection bias at Position 6 (center right; r[9] = .74, p = .01). For both sectors, the thinner the RNFL, the more leftward bias on line bisection. RPD also showed correlations between RNFL and horizontal line bisection, with nasal quadrant thickness of the left eye correlated with performance at Position 4 (center left; r[9] = .83, p < .01); and nasal quadrant thickness of the right eye correlated with performance at Position 6 (center right; r[9] = .84, p < .01). As was seen for NC, for both sectors, the thinner the RNFL, the more leftward bias on line bisection. There were no correlations between RNFL thickness and horizontal line bisection for LPD.
Correlations of retinal function (FDT) and RNFL thickness (OCT) for the same retinal quadrant
For NC, RNFL greater thickness of the superior quadrant in the right eye was significantly correlated with better FDT sensitivity in the same superior quadrant of the same eye (r = .68, p < .05). For LPD, there was a significant correlation between RNFL thickness of the superior temporal quadrant of the left eye and FDT sensitivity in that same quadrant of the same eye (r = .68, p < .05). For RPD, RNFL thickness was correlated with FDT sensitivity in the nasal quadrant (r = .68, p < .05) and also in the inferior quadrant (r = .73, p < .05) of the left eye. That is, for all three groups, there was some evidence that thinner RNFL was associated with poorer contrast sensitivity.
In sum, the analyses of retinal structure and function indicated the following: (a) for all groups, there were significant correlations between retinal structure and function; (b) in regard to LPD relative rightward bisection bias, retinal function (FDT) was not a predictor; (c) retinal structure (OCT) was also not a predictor; and (d) for NC and RPD, but not for LPD, more leftward line bisection at some positions was predicted by RNFL thinness. The structural pattern does not provide a clear explanation of the line-bisection bias results, because it was the LPD group that differed from the others by bisecting more rightward at some positions.
Eye tracking
We encountered technical difficulties with eye tracking that led to noninclusion of data for three of the 31 individuals tested (1 RPD, 2 NC), as well as occasional eye gaze paths that appeared to be shifted so that they fell outside of the AOIs due to loss of calibration or other minor technical difficulties. This percentage of noninclusion accords with that reported in other eye tracking studies with healthy older adults (e.g., 10% inaccurate files in Circelli, Clark, & Cronin-Golomb, 2012; 8.9% of files with partially removed data in Clark et al., 2010), and patients with PD (17.7% of files with partially removed data in Clark et al., 2010). No obvious systematic patterns were noted regarding data loss within or across participant groups, nor regarding the exclusion of those for whom there were calibration or other technical difficulties.
Data are the percentage of the participants’ time spent looking at areas of the bisection line (i.e., within the left AOI, center AOI, right AOI, or region outside of those AOIs) while performing the horizontal line bisection task. As noted, the results with vertical line bisection are not analyzed further here.
Our hypothesis was that patterns of scanning during the bisection task would differ across groups, with LPD spending relatively less time looking at areas on the left side of the stimulus, particularly when the stimulus was located within the left visual field.
Between-groups comparisons
Student’s t tests for unequal sample sizes and unequal variance were calculated for each AOI for pairs of participant groups for line bisection stimuli at screen Positions 4, 5, and 6. No significant differences were found between groups.
Within-group comparisons
Dependent Student’s t tests were calculated for a specific AOI, comparing each of the two positions on opposite sides of the screen (Positions 4 and 6, which were, respectively, the central left and central right positions of the visual field). Comparisons were also made of the difference in time spent looking within each of the three AOIs at these screen positions. Patterns of relative time spent in AOIs can be compared across positions within a group in order to ascertain whether looking strategy for a group is different in various areas across the visual field. Because of the exploratory nature of these analyses, a conservative alpha value of 0.01 was used.
Comparisons of a Single AOI in the Left and Right Visual Field
When stimuli were in the left side of the visual field compared with when they were in the right side of the visual field, NC and LPD spent less time looking within the center AOI and more time looking within the left AOI. When stimuli were in the left side of the visual field, RPD spent less time looking within the right AOI (see Table 3 for significant comparisons).
Table 3.
Eye Tracking Area of Interest (AOI) Comparisons for Horizontal Line Bisection
| Group | AOIs compared | Horizontal, Position 4 | Horizontal, Position 5 | Horizontal, Position 6 |
|---|---|---|---|---|
| NC | Center & Right | C > R | n.s. | C > R |
| Center & Left | C > L | L > C | C > L | |
| Right & Left | L > R | L > R | L > R | |
| LPD | Center & Right | C >R | C > R | C > R |
| Center & Left | C > L | L > C | C > L | |
| Right & Left | L > R | L > R | P > L | |
| RPD | Center & Right | C > R | n.s. | C > R |
| Center & Left | C > L | L > C | C > L | |
| Right & Left | n.s. | L > R | R > L |
Note. For each participant group, pairs of AOIs (each representing percentage of time participants looked in a region) were compared at the specified screen position. Within-group AOI pairs that were statistically different (p < .01) are included in the table. The AOI that was viewed proportionally more is indicated as being “greater than” (>) the other AOI. C = center AOI; Horizontal = horizontal line bisection orientation; L = left AOI; LPD = left-body-onset Parkinson’s disease; NC = normal control; n.s. = not significant; position = screen position (see Figure 1a); R = right AOI; RPD = right-body-onset Parkinson’s disease.
Comparisons of the Three AOIs of a Bisection Line, Calculated in the Left, Center, and Right Visual Field
When viewing stimuli in the left side of the visual field, all groups showed a similar pattern of spending more time looking within the center AOI of the target line compared with time spent looking within either the left AOI or right AOI. When comparing time spent within the left AOI with that spent within the right AOI, the NC and LPD both looked more within the left AOI and the RPD showed no difference. For stimuli in the central visual field, all groups showed a similar pattern of looking more within the left AOI than either the center AOI or right AOI. LPD spent more time in the center AOI than in the right AOI, but NC and RPD showed no difference. Finally, when line bisection was presented in the right visual field, all groups spent more time in the center AOI than either of the left AOI or right AOI, but LPD and RPD differed from the NC. LPD and RPD spent more time looking within the right AOI, and NC spent more time within the left AOI.
Differences between LPD and the other groups were seen, but there was no unique LPD pattern of looking less at the left side of a stimulus, including when the stimulus was in the left side of the visual field.
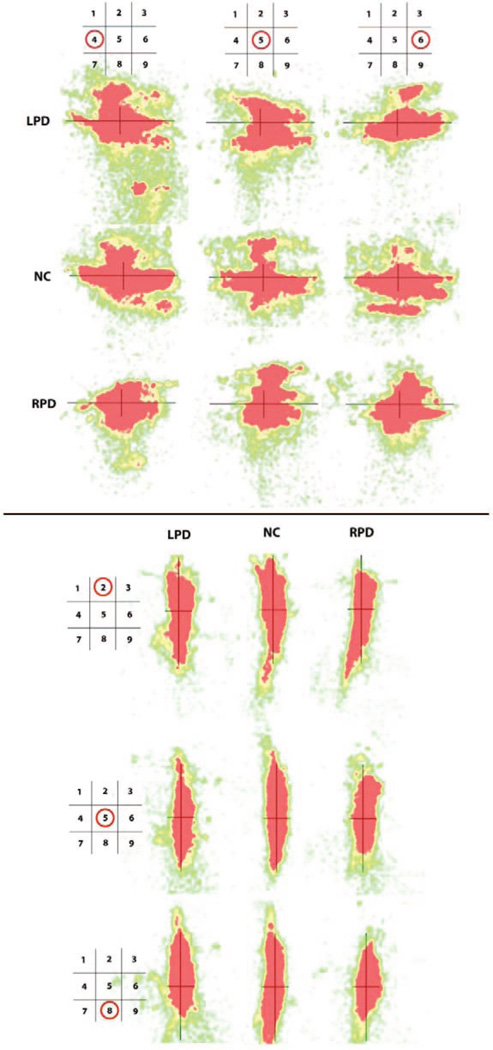
“Heat map” visualizations of the relative time at locations where participants looked while performing the line bisection task were created from the eye tracking data. These maps provide a qualitative representation of overall viewing patterns during each condition for each participant group. Selected visual field positions were examined for each line bisection orientation. For horizontal line bisection, LPD and NC patterns appeared similar at the left visual field position, but at the center and right position, LPD appeared to explore more on the absolute right side of the line than the left. The pattern supports the main hypothesis that LPD would look less at the left than right hemispace, but not the secondary hypothesis that this pattern would be accentuated by placement of the stimulus within the left visual field. Relative to NC, the RPD group’s overall range of scanning manifested as somewhat compressed along the horizontal axis at each of the visual field positions examined, and LPD showed mild relative compression at the right and possibly also at the central visual field position (Figure 6, top). To examine whether looking preference or compression was a general feature of PD scanning, we provide heat maps for the vertical line bisection task as well (Figure 6, bottom). RPD appeared to have a central compression of scanning along the vertical axis in the central and lower positions. As is especially evident at the lowest position, RPD and LPD appeared to spend more time looking above than below the midline, in contrast to the more balanced NC pattern. For LPD, this pattern did not support the hypothesis of a tendency to spend less time exploring upper regions of the line bisection stimulus, particularly in the superior visual field.
Figure 6.
Eye tracking “heat map” representations for horizontal (top) and vertical (bottom) line bisection at three visual field positions each. Each of the nine diagrams for each orientation represents where participants looked while performing the line bisection task. Each diagram contains a line bisection stimulus (black lines) for reference and associated eye tracking data. Diagrams are shown for selected screen positions (see schematics at top of columns [horizontal line condition] or left side of rows [vertical line condition] for visual field position), and for LPD, NC, and RPD (by row [horizontal line condition] or column [vertical line condition]). Colors closer to the red end of the spectrum indicate the most time spent looking at those areas, and “cooler” colors indicate progressively less time looking at an area. Each map represents performance across all group members. At some positions, LPD scanning appears to be shifted off-center compared with NC, and compression of scanning area along the line is seen in RPD and LPD compared with NC at some positions. See text for details. LPD = left-body-onset Parkinson’s disease; NC = normal control participants; RPD = right-body-onset Parkinson’s disease.
Discussion
In this study, we confirmed a rightward bias of LPD patients in horizontal line bisection that others have reported (e.g., Davidsdottir et al., 2008; Lee et al., 2001a), and investigated factors that may contribute to this visuospatial bias: placement of the line stimuli in the visual field, retinal structure and function, and visual scanning patterns. We found that the horizontal LPD bisection pattern did not appear as hypothesized in the left visual hemispace, and it did not correlate with retinal structure or function. Examination of time spent looking at different areas of the line during the bisection task did not show a clear pattern of differential exploration that would support the hypothesis of an LPD bias toward looking away from an affected hemispace. Qualitative visualization of eye movements, however, suggested that LPD explored the right side of the stimulus more than the left, and also suggested a compression of scanning range in RPD.
Line Bisection and Effects of Visual Field Placement
LPD bisected the lines more rightward than RPD at the center position of the field (Position 5) and more rightward than NC at the center right position (Position 6) as well. We did not find that LPD showed more rightward horizontal line bias in the left visual field, nor did we find the downward bias exhibited by LPD on vertical bisection that was reported by Lee et al. (2002), possibly owing to methodological differences (e.g., larger lines used in the study by Lee et al.).
Retinal Structure and Function
Examination of retinal structure (RNFL thickness measured by OCT) revealed thinning of the temporal quadrant in LPD relative to RPD. A prior study of RNFL thickness in PD (not distinguished by side of onset) reported a reduction in the inferior retinal quadrant compared with a control group (Inzelberg et al., 2004). This inferior retinal thinning was not found in either of our PD subgroups. The sample studied by Inzelberg and colleagues was on average about 8 years younger and the duration of disease was about 2 years shorter than in our sample. Other researchers have found thinning in PD retinal areas that were not a focus of our examination, including sections of the inner and outer macula (Altintaş et al., 2008) and the paramacular inner retinal layer but not outer retinal layer (Hajee et al., 2009). Across all our groups, the RNFL was thicker in the right eye than in the left. This finding has also been reported for samples of normal adults and is not dependent on the order in which the eyes were scanned (Budenz, 2008; Mwanza, Durbin, & Budenz, 2011), but it is of unclear origin. Correlations were found between RNFL thickness and horizontal line bisection only for NC and RPD, where a thinner retina was associated with bisecting the line further leftward.
Retinal function (contrast sensitivity), as measured by FDT, did not differ across groups in our study, although overall the right eye was significantly more sensitive than the left. This finding is in line with an FDT screening of over 14,000 people in the general population in Japan that found the same result (Tatemichi et al., 2003). Others have found greater contrast sensitivity variability among quadrants in PD than in healthy control adults, with disease progression correlated with worse function across the retina, particularly for nasal visual quadrants (Silva et al., 2005).
We did not find any correlation of contrast sensitivity (FDT) with line bisection bias for any of our groups. We did, however, find a relation between contrast sensitivity and RNFL thickness. Because OCT and FDT are able to measure a roughly topographically analogous area of retina, structure–function relations can accordingly be examined. In LPD, we found a significant correlation between greater RNFL thickness of the superior temporal quadrant of the left eye and better contrast sensitivity (FDT) in that same quadrant of the same eye. For NC, thickness of the superior quadrant in the right eye was significantly correlated with FDT sensitivity in the same superior quadrant of that eye. For RPD, in the left eye, RNFL thickness was correlated with FDT sensitivity in the nasal quadrant and in the inferior quadrant. Though different sectors showed significant results by group, in general, the results are in accord with findings from other disorders in which retinal thinning as measured with OCT is typically thought to be associated with functional loss as measured with FDT (e.g., in HIV, Faria E Arantes, Garcia, Mello, & Muccioli, 2010; in glaucoma, El Beltagi et al., 2003).
Scanning Patterns
Nothing apparent in the relative scanning patterns (as measured by AOIs) explains the LPD rightward bias on line bisection. It is noteworthy, however, that LPD and RPD showed some differences in relative scanning across the visual field, which has implications for PD studies using visual scanning in general. Comparing PD to a control group without accounting for the composition of the PD group in regard to side of onset may produce quite different results from study to study because of different proportions of LPD and RPD in each. The “heat map” representations of eye movements suggested that those with RPD, and LPD for some positions, tended to focus on narrower, more central areas of the stimuli than did the control group. Those with LPD appeared to be biased more than control adults to look toward the right side (i.e., relative neglect of left side) on horizontal bisection for lines that were presented in the central and right visual fields. Both PD groups, but not NC, tended to focus on the upper rather than lower portion of the line during vertical bisection that was at the central bottom area of the visual field. Overall, LPD and RPD scanning patterns appeared to be more somewhat more similar to each other than to those of control adults in regard to compression, suggesting that those with PD use different scanning strategies to solve visuospatial line bisection tasks.
Taken together, the results of the present study indicate factors of potential importance in considering visuospatial difficulties in PD—in particular, rightward bias in line bisection in LPD. We found this bias when the line to be bisected was in the center or right of center of the display. In LPD relative to RPD, there was thinning of the temporal quadrant of the RNFL. Retinal structure and function as measured by OCT and FDT did not predict LPD rightward bias in line bisection. In regard to visual scanning, LPD did not show a distinct pattern on AOI analysis that we could readily relate to their line bisection bias, but there were differences between LPD and RPD scanning that may be important to consider in PD studies in general. Qualitative visualization of eye movements (“heat maps”) on the horizontal axis suggested that LPD explored the right side of the stimulus more than the left, and suggested a compression of the area of visual field scanned in RPD. The difference between the AOI and qualitative data may reflect a relative insensitivity of using large AOIs. It is possible that smaller areas, with their positioning informed by the qualitative data collected here, may lead to a more sensitive quantitative measure in future studies. Further work is required in this area in order to draw firmer conclusions about the role of visual scanning in visuospatial task performance. Together, our results indicate that rightward visuospatial bias in LPD is not explained primarily by changes at the retinal level. The possibility is left open that LPD visuospatial task performance is influenced by attentional biases that are reflected in eye movement patterns.
The present study was subject to limitations. Although our PD sample was large enough to reveal group differences, participation was restricted to nondemented individuals, and the present results may not reflect the status of retinal vision, scanning patterns, or visuospatial function in patients in the more severe stages of the disease including in patients with dementia. Further, the eye tracking technology produced unusable results for about 10% of our sample, which may have introduced additional error or bias. The loss of data did not, however, appear to be systematic within or across our groups and the percent of data loss was similar to that reported in other studies. We also recognize that by not requiring participants to centrally fixate while performing our visuospatial task, it was possible that spontaneous eye movements shifted the gaze so that no matter where the stimulus was physically located in external space (what we refer to as “visual field”), it would be viewed centrally (foveally) rather than viewed across a much larger area of retina. This design choice was based on previous research showing an effect of PD on line bisection (Davidsdottir et al., 2008; Lee et al., 2001a, 2002) and on patient reports of visual dysfunction (Davidsdottir et al., 2005), both occurring despite unrestrained eye movements. Although we do not consider this to be a limitation of the study, but rather an attempt at ecological validity, we believe that the use of a design requiring fixation would possibly have provided different results.
This research provides a first examination of retinal function, retinal structure, and eye scanning across the visual field in the service of explaining higher-order visuospatial processes in PD and healthy age-matched adults. The use of these complementary methods of examining visuospatial functioning has the potential to further the understanding of the impact of PD on the visual system, such as in regard to perceptual compression of space. In turn, this knowledge may lay the groundwork for patient-centric education and intervention. The individuals who participated in the present study received a debriefing in which the hypothesis of visual field compression was explained. After the debriefing, an LPD patient’s eyes widened and he exclaimed, “Is that why I hit mailboxes on the side of the road when I drive? The side of my car is all scratched up!” It is hoped that increased insight may lead to real-world compensation for those who experience these visuospatial deficits.
Acknowledgments
This research was supported by National Institute of Neurological Disorders and Stroke grants R01 NS052914 and R01 NS050446 to Alice Cronin-Golomb, and by the Parkinson’s Disease Foundation and Sigma Xi to Thomas M. Laudate. We are grateful to Palavi Joshi for aiding in the early project design; Dan Norton for technical and conceptual assistance with the eye-tracking equipment and help with test administration; Alison Lee for fruitful discussions in conceptualizing the project; Chelsea Toner and Bruce Reese for expert technical support; Jessica Ryder and the other laboratory members who helped with data entry and processing; and Mark O’Donoghue and colleagues at the New England Eye Institute, Boston, Massachusetts, for conducting the eye examinations. Denise Valenti first introduced OCT and FDT equipment to our laboratory.
Contributor Information
Thomas M. Laudate, Department of Psychology, Boston University
Sandy Neargarder, Department of Psychology, Boston University, and Department of Psychology, Bridgewater State University.
Alice Cronin-Golomb, Department of Psychology, Boston University.
References
- Altintaş O, Işeri P, Ozkan B, Cağlar Y. Correlation between retinal morphological and functional findings and clinical severity in Parkinson’s disease. Documenta Opthalmologica. Advances in Ophthalmology. 2008;116:137–146. doi: 10.1007/s10633-007-9091-8. [DOI] [PubMed] [Google Scholar]
- Anderson AJ, Johnson CA. Frequency-doubling technology perimetry. Ophthalmology Clinics of North America. 2003;16:213–225. doi: 10.1016/s0896-1549(03)00011-7. [DOI] [PubMed] [Google Scholar]
- Archibald NK, Clarke MP, Mosimann UP, Burn DJ. The retina in Parkinson’s disease. Brain: A Journal of Neurology. 2009;132:1128–1145. doi: 10.1093/brain/awp068. [DOI] [PubMed] [Google Scholar]
- Archibald NK, Clarke MP, Mosimann UP, Burn DJ. Retinal thickness in Parkinson’s disease. Parkinsonism & Related Disorders. 2011;17:431–436. doi: 10.1016/j.parkreldis.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Barber J, Tomer R, Sroka H, Myslobodsky MS. Does unilateral dopamine deficit contribute to depression? Psychiatry Research. 1985;15:17–24. doi: 10.1016/0165-1781(85)90035-6. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory. 2nd ed. San Antonio, TX: The Psychological Corporation; 1996. . [Google Scholar]
- Budenz DL. Symmetry between the right and left eyes of the normal retinal nerve fiber layer measured with optical coherence tomography (an AOS thesis) Transactions of the American Ophthalmological Society. 2008;106:252–275. [PMC free article] [PubMed] [Google Scholar]
- Çiçek M, Deouell LY, Knight RT. Brain activity during landmark and line bisection tasks. Frontiers in Human Neuroscience. 2009;3:7. doi: 10.3389/neuro.09.007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Circelli KS, Clark US, Cronin-Golomb A. Visual scanning patterns and executive function in relation to facial emotion recognition in aging. Aging, Neuropsychology, and Cognition. 2012 doi: 10.1080/13825585.2012.675427. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark US, Neargarder S, Cronin-Golomb A. Visual exploration of emotional facial expressions in Parkinson’s disease. Neuropsy-chologia. 2010;48:1901–1913. doi: 10.1016/j.neuropsychologia.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clower DM, Dum RP, Strick PL. Basal ganglia and cerebellar inputs to “AIP.”. Cerebral Cortex. 2005;15:913–920. doi: 10.1093/cercor/bhh190. [DOI] [PubMed] [Google Scholar]
- Cronin-Golomb A. Parkinson’s disease as a disconnection syndrome. Neuropsychology Review. 2010;20:191–208. doi: 10.1007/s11065-010-9128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidsdottir S, Cronin-Golomb A, Lee A. Visual and spatial symptoms in Parkinson’s disease. Vision Research. 2005;45:1285–1296. doi: 10.1016/j.visres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Davidsdottir S, Wagenaar R, Young D, Cronin-Golomb A. Impact of optic flow perception and egocentric coordinates on veering in Parkinson’s disease. Brain: A Journal of Neurology. 2008;131:2882–2893. doi: 10.1093/brain/awn237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersbach G, Trottenberg T, Hättig H, Schelosky L, Schrag A, Poewe W. Directional bias of initial visual exploration. A symptom of neglect in Parkinson’s disease. Brain: A Journal of Neurology. 1996;119:79–87. doi: 10.1093/brain/119.1.79. [DOI] [PubMed] [Google Scholar]
- El Beltagi TA, Bowd C, Boden C, Amini P, Sample PA, Zangwill LM, Weinreb RM. Retinal nerve fiber layer thickness measured with optical coherence tomography is related to visual function in glaucomatous eyes. Ophthalmology. 2003;110:2185–2191. doi: 10.1016/S0161-6420(03)00860-1. [DOI] [PubMed] [Google Scholar]
- Faria E, Arantes TE, Garcia CR, Mello PA, Muccioli C. Structural and functional assessment in HIV-infected patients using optical coherence tomography and frequency-doubling technology perimetry. American Journal of Ophthalmology. 2010;149:571–576. doi: 10.1016/j.ajo.2009.11.026. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Hajee ME, March WF, Lazzaro DR, Wolintz AH, Shrier EM, Glazman S, Bodis-Wollner IG. Inner retinal layer thinning in Parkinson disease. Archives of Ophthalmology. 2009;127:737–741. doi: 10.1001/archophthalmol.2009.106. [DOI] [PubMed] [Google Scholar]
- Harris JP, Atkinson EA, Lee AC, Nithi K, Fowler MS. Hemispace differences in the visual perception of size in left hemiPar-kinson’s disease. Neuropsychologia. 2003;41:795–807. doi: 10.1016/s0028-3932(02)00285-3. [DOI] [PubMed] [Google Scholar]
- Hee MR, Izatt JA, Swanson EA, Huang D, Schuman JS, Lin CP, Fujimoto JG. Optical coherence tomography of the human retina. Archives of Ophthalmology. 1995;113:325–332. doi: 10.1001/archopht.1995.01100030081025. [DOI] [PubMed] [Google Scholar]
- Hodgson TL, Tiesman B, Owen AM, Kennard C. Abnormal gaze strategies during problem solving in Parkinson’s disease. Neuropsychologia. 2002;40:411–422. doi: 10.1016/s0028-3932(01)00099-9. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: Onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Fujimoto JG. Optical coherence tomography. Science. 1991 Nov 22;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt LA, Sadun AA, Bassi CJ. Review of the visual system in Parkinson’s disease. Optometry and Vision Science. 1995;72:92–99. doi: 10.1097/00006324-199502000-00008. [DOI] [PubMed] [Google Scholar]
- Inzelberg R, Ramirez JA, Nisipeanu P, Ophir A. Retinal nerve fiber layer thinning in Parkinson disease. Vision Research. 2004;44:2793–2797. doi: 10.1016/j.visres.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Lee AC, Harris JP. Problems with perception of space in Parkinson’s disease: A questionnaire study. Neuro-ophthalmology. 1999;22:1–15. [Google Scholar]
- Lee AC, Harris JP, Atkinson EA, Nithi K, Fowler MS. Dopamine and the representation of the upper visual field: Evidence from vertical bisection errors in unilateral Parkinson’s disease. Neuropsychologia. 2002;40:2023–2029. doi: 10.1016/s0028-3932(02)00055-6. [DOI] [PubMed] [Google Scholar]
- Lee AC, Harris JP, Atkinson L, Fowler MS. Evidence from a line bisection task for visuospatial neglect in left Hemiparkin-son’s disease. Vision Research. 2001a;41:2677–2686. doi: 10.1016/s0042-6989(01)00129-8. [DOI] [PubMed] [Google Scholar]
- Lee AC, Harris JP, Atkinson L, Fowler MS. Disruption of estimation of aperture width in Hemiparkinson’s disease. Neuropsychologia. 2001b;39:1097–1104. doi: 10.1016/s0028-3932(01)00032-x. [DOI] [PubMed] [Google Scholar]
- Levin GM. Glaucoma: Part I–Diagnosis. 2005 Retrieved from http://www.cyberounds.com/cmecontent/art269.html?pf=yes. [Google Scholar]
- Mennemeier M, Vezey E, Chatterjee A, Rapcsak SZ, Heilman KM. Contributions of the left and right cerebral hemispheres to line bisection. Neuropsychologia. 1997;35:703–715. doi: 10.1016/s0028-3932(96)00114-5. [DOI] [PubMed] [Google Scholar]
- Mwanza J-C, Durbin MK, Budenz DL. Interocular symmetry in peripapillary retinal nerve fiber layer thickness measured with the Cirrus HD-OCT in healthy eyes. American Journal of Ophthalmology. 2011;151:514–521. doi: 10.1016/j.ajo.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Kanayama R, Sano R, Ohki M, Kimura Y, Aoyagi M, Koike Y. Quantitative analysis of ocular movements in Parkinson’s disease. Acta oto-laryngologica Supplementum. 1991;481:559–562. doi: 10.3109/00016489109131470. [DOI] [PubMed] [Google Scholar]
- Rosli Y, Bedford SM, Maddess T. Low-spatial-frequency channels and the spatial frequency-doubling illusion. Investigative Ophthalmology and Visual Science. 2009;50:1956–1963. doi: 10.1167/iovs.08-1810. [DOI] [PubMed] [Google Scholar]
- Shibasaki H, Tsuji S, Kuroiwa Y. Oculomotor abnormalities in Parkinson’s disease. Archives of Neurology. 1979;36:360–364. doi: 10.1001/archneur.1979.00500420070009. [DOI] [PubMed] [Google Scholar]
- Silva MF, Faria P, Regateiro FS, Forjaz V, Januário C, Freire A, Castelo-Branco M. Independent patterns of damage within magno-, parvo-, and koniocellular pathways in Parkinson’s disease. Brain: A Journal of Neurology. 2005;128:2260–2271. doi: 10.1093/brain/awh581. [DOI] [PubMed] [Google Scholar]
- Starkstein S, Leiguarda R, Gershanik O, Berthier M. Neuropsychological disturbances in hemiparkinson’s disease. Neurology. 1987;37:1762–1764. doi: 10.1212/wnl.37.11.1762. [DOI] [PubMed] [Google Scholar]
- Stern Y, Sano M, Paulson J, Mayeux R. Modified Mini-Mental State Examination: Validity and reliability [abstract] Neurology. 1987;37:179. [Google Scholar]
- Tatemichi M, Nakano T, Tanaka K, Hayashi T, Nawa T, Iwasaki A, Sugita M. Laterality of the performance of glaucoma mass screening using frequency-doubling technology. Journal of Glaucoma. 2003;12:221–225. doi: 10.1097/00061198-200306000-00007. [DOI] [PubMed] [Google Scholar]
- Witt K, Kopper F, Deuschl G, Krack P. Subthalamic nucleus influences spatial orientation in extra-personal space. Movement Disorders. 2006;21:354–361. doi: 10.1002/mds.20728. [DOI] [PubMed] [Google Scholar]
- Wong B, Cronin-Golomb A, Neargarder S. Patterns of visual scanning as predictors of emotion identification in normal aging. Neuropsychology. 2005;19:739–749. doi: 10.1037/0894-4105.19.6.739. [DOI] [PubMed] [Google Scholar]
- Yesavage JA. Geriatric Depression Scale. Psychopharmacology Bulletin. 1988;24:709–711. [PubMed] [Google Scholar]