Abstract
Background
To investigate the clinical characteristics, exercise training response, beta-blocker selectivity and outcomes in heart failure (HF) patients with chronic obstructive pulmonary disease (COPD).
Methods
We performed an analysis of HF-ACTION, which randomized 2,331 HF patients with ejection fraction ≤35% to usual care with or without aerobic exercise training. We examined clinical characteristics and outcomes [mortality/hospitalization, mortality, cardiovascular (CV) mortality/CV hospitalization, and CV mortality/HF hospitalization] by physician-reported COPD status using adjusted Cox models and explored an interaction with exercise training. The interaction between beta-blocker cardioselectivity and outcomes was investigated.
Results
Of patients with COPD status documented (N=2311), 11% (N=249) had COPD. COPD patients were older, had more comorbidities, and lower use of beta-blockers compared to those without COPD. At baseline, COPD patients had lower peak VO2 and higher VE/VCO2 slope. During a median follow-up of 2.5 years, COPD was associated with increased mortality/hospitalization, mortality, and CV mortality/HF hospitalization. After multivariable adjustment, the risk of CV mortality/HF hospitalization remained increased (Hazard Ratio [HR] 1.46, 95% Confidence Interval [CI]: 1.14–1.87), while mortality/hospitalization (HR 1.15, 95% CI: 0.96–1.37) and mortality (HR 1.33, 95% CI: 0.99–1.76) were not significantly increased. There was no interaction between COPD and exercise training on outcomes or between COPD and beta-blocker selectivity on mortality/hospitalization (all P>0.1).
Conclusions
COPD in HF patients was associated with older age, more comorbidities, reduced exercise capacity, and increased CV mortality/HF hospitalization, but not a differential response to exercise training. Beta-blocker selectivity was not associated with differences in outcome for patients with versus without COPD.
Keywords: heart failure, COPD, exercise training
Chronic obstructive pulmonary disease (COPD) is a common comorbidity seen in ~30% of patients with heart failure (HF)1, yet its impact on clinical outcomes is unclear. Until recently, few studies have focused on the prognosis of HF patients with COPD. HF predictive models have been inconsistent regarding the impact of COPD on mortality. Several studies support worse outcomes in HF patients with COPD4–6, while others have been neutral. The primary effect of COPD in HF patients may be increased non-cardiovascular (CV) mortality in the acute HF setting9 with similar outcomes following hospital discharge10.
In COPD patients with concomitant HF, clinicians underuse evidence-based HF therapies. Concerns exist about beta-blockers, particularly non-cardioselective beta-blockers, precipitating bronchospasm or attenuating the benefit of inhaled beta-2 agonists in such patients11. However, the impact of beta-blocker selectivity in patients with concomitant HF and COPD is not well-characterized. Similarly, while data suggest a positive effect of pulmonary rehabilitation on patients’ symptoms and outcomes in the general COPD population14, limited data exist on the impact of exercise training in COPD patients with HF.
We aimed to describe the baseline clinical characteristics and long-term outcomes of HF patients with COPD in a cohort of ambulatory HF patients enrolled in the HF-ACTION study (Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing). We were interested in the baseline quality of life and exercise capacity of HF patients with and without COPD. Our secondary aims were to explore interactions with exercise training and beta-blocker selectivity on outcomes and health status. We hypothesized that COPD would be associated with increased hospitalization, but not increased mortality or a differential exercise training response. We also hypothesized that non-cardioselective beta-blockers would not be associated with worse outcomes compared with cardioselective beta-blockers in HF patients with COPD.
Methods
The design, rationale, and primary results of the HF-ACTION study have been published. Briefly, HF-ACTION was a randomized trial evaluating the effect of exercise training and usual care versus usual care alone on outcomes in patients with chronic HF (New York Heart Association [NYHA] class II–IV, left ventricular ejection fraction [LVEF]≤35%). The primary endpoint was mortality/hospitalization over a median follow-up of 2.5 years. An independent clinical events committee adjudicated CV hospitalizations; if a HF hospitalization for a patient was confirmed by the committee, no future hospitalizations were adjudicated for that patient. COPD status was prospectively recorded on study enrollment by the clinician-investigator based on clinical evidence and knowledge by past medical history of COPD. HF-ACTION was approved by local institutional review boards, and all enrolled patients provided written informed consent.
Statistical Methods
Baseline characteristics were described by COPD status. Continuous variables were summarized with the median and 25th and 75th percentiles and compared using the Wilcoxon rank-sum statistic. Categorical variables are presented as percentages and compared with a Pearson chisquared statistic or exact test when appropriate. The change in exercise capacity (indexed by 6-minute walk distance, and the cardiopulmonary exercise testing [CPX] parameters of peak oxygen consumption [VO2] and exercise duration) and health status (as reported on the Kansas City Cardiomyopathy Questionnaire [KCCQ]), from baseline to 3 months was examined in the usual care and exercise training study arms. Emphasis was given to the 3-month time point due to the greater amount of missing data at later time points.
The primary outcome was hospitalization/mortality. We also evaluated the secondary outcomes of time to cardiovascular (CV) death/CV hospitalization, CV death/HF hospitalization, and mortality. We analyzed the relationship between COPD and outcomes using Cox proportional hazards models, including adjustment for a comprehensive set of predictors17. We explored the interaction between COPD and treatment assignment for the primary and secondary outcomes using adjusted Cox models.
Linear regression modeling adjusted for baseline covariates was used to explore an interaction between COPD and treatment as a predictor of the change in exercise parameters and health status variables from baseline to 3 months. Baseline adjustment covariates were determined via stepwise selection from a list of candidate covariates. To account for increased missing data at 3 months, possibly correlated with treatment or COPD status, subjects in the linear regression model were inversely weighted by their probability of having a missing response.
We examined the interaction between COPD and beta-blocker cardioselectivity as a predictor of mortality/hospitalization using adjusted Cox models. A two-tailed P value <0.05 was considered statistically significant for all analyses, bearing in mind the exploratory nature of this study. All statistical analyses were performed by the Duke Clinical Research Institute using SAS (Cary, NC) system version 9.2. No extramural funding was used. The authors are solely responsible for the study analyses, the drafting and editing of the paper and its final contents.
Results
Of patients with baseline determination of COPD status (N=2311), 11% (N=249) had COPD. Table 1 presents the baseline characteristics by COPD status. COPD patients were significantly older, with more ischemic etiology, compared to those without COPD. COPD patients more often had hypertension, atrial fibrillation/flutter and prior myocardial infarction. The COPD group had a similar LVEF to those without COPD. Blood pressure and heart rate were similar between those with and without COPD as were basic laboratory values. Over 90% of patients with and without COPD were receiving ACE-inhibitors (ACE-Is)/angiotensin II receptor blockers (ARBs) at baseline, with approximately half as many receiving aldosterone antagonists. There were no significant between-group differences in the use of ACE-Is/ARBs or aldosterone antagonists, but COPD patients were less likely to receive beta-blockers. Patients with COPD were more likely to receive cardioselective than non-cardioselective beta-blockers (54% vs. 38% of patients on a beta-blocker). COPD patients received lower doses of both cardioselective and non-cardioselective beta-blockers compared to non-COPD patients.
Table 1.
Baseline Characteristics by COPD Status.
| Variable | No COPD (n=2062, 89%) | COPD (n=249, 11%) |
|---|---|---|
| Age, years* | 59 (51, 68) | 64 (56, 71) |
| Female Sex | 29 | 25 |
| Race | ||
| Black or African American | 33 | 24 |
| White | 60 | 70 |
| Other | 5 | 6 |
| New York Heart Association Class III–IV* | 35 | 50 |
| Ischemic Etiology* | 50 | 62 |
| Left Ventricular Ejection Fraction, % | 25 (20, 30) | 25 (20, 30) |
| Diabetes Mellitus | 32 | 34 |
| Previous Myocardial Infarction* | 41 | 53 |
| Hypertension* | 59 | 66 |
| Atrial Fibrillation/Flutter* | 20 | 27 |
| Systolic Blood Pressure, mmHg | 110 (100, 126) | 112 (104, 124) |
| Heart Rate, bpm | 70 (63, 77) | 72 (64, 79) |
| Sodium, mmol/L | 139 (137, 141) | 139 (137, 141) |
| Blood Urea Nitrogen, mg/dL | 20 (15, 28) | 21 (16, 31) |
| Serum Creatinine, mg/dL | 1.2 (1.0, 1.5) | 1.2 (1.0, 1.5) |
| ACE-Inhibitor/Angiotensin II Receptor Blocker | 95 | 92 |
| Beta-blocker* | 95 | 88 |
| Cardioselective* | 38 | 54 |
| Cardioselective dose†, mg/day* | 50 (25, 75) | 25 (13, 50) |
| Non-Cardioselective* | 62 | 46 |
| Non-Cardioselective dose†, mg/day | 38 (19, 50) | 25 (13, 50) |
| Aldosterone Antagonist | 45 | 44 |
| Loop Diuretic | 77 | 82 |
| Digoxin | 44 | 49 |
| Implantable Cardioverter Defibrillator | 40 | 41 |
| Biventricular Pacemaker | 18 | 20 |
Expressed as median (IQR) or percentage
P-value<0.05
Carvedilol dose equivalent
Table 2 presents the baseline health status and exercise parameters in patients with and without COPD. Compared with non-COPD patients, those with COPD had worse quality of life. COPD patients also had worse baseline exercise capacity as evidenced by shorter CPX duration and 6-minute walk distance compared to those without COPD. Those patients with COPD had lower peak VO2 and higher VE/VCO2 slope and were significantly less likely to achieve a respiratory exchange ratio (RER) >1.10, a value suggestive of near-maximal effort18.
Table 2.
Baseline Health Status and Exercise Parameters by COPD Status.
| Variable | No COPD (n=2062, 89%) | COPD (n=249, 11%) | P-value |
|---|---|---|---|
| Beck Depression Inventory II Score | 8 (4, 15) | 9 (5, 16) | 0.023 |
| Kansas City Cardiomyopathy Questionnaire Score | 69 (52, 84) | 61 (49, 78) | <0.001 |
| 6-Minute Walk Distance, meters | 376 (303, 439) | 326 (259, 405) | <0.001 |
| Cardiopulmonary Exercise Duration, min | 10.0 (7.1, 12.2) | 7.4 (5.6, 10.2) | <0.001 |
| Peak VO2, mL/kg/min | 14.6 (11.6, 17.8) | 13.1 (10.1, 15.9) | <0.001 |
| VE/VCO2 Slope | 32 (28, 38) | 34 (29, 41) | 0.003 |
| % with Respiratory Exchange Ratio >1.10 | 44 | 36 | 0.024 |
| Heart Rate at Peak Exercise, bpm | 120 (105, 135) | 114 (97, 126) | <0.001 |
Expressed as median (IQR) unless noted
Exercise training adherence as measured by total exercise minutes per week for months 1–3 (excluding the initial three weeks) was similar in those with and without COPD [median of 77 minutes (40, 118) versus 77 (29, 109), respectively]. The absolute change in exercise capacity and health status outcomes from baseline to 3 months in those with and without COPD is presented in Table 3. After adjusting for baseline characteristics, there was insufficient evidence to suggest an interaction between COPD status and exercise training for each of the exercise and health status variables (all P>0.1) (Table 3).
Table 3.
Change in Exercise and Health Status Outcomes from Baseline to 3 Months.
| Test | No COPD (n=2062, 89%) | COPD (n=249, 11%) | P-value for Interaction between COPD, Treatment Assignment and Outcome* | |
|---|---|---|---|---|
| Change in KCCQ Score | Usual Care | 2.1 (−5.2, 9.4) | 3.9 (−5.2, 13.5) | 0.52 |
| Exercise | 5.2 (−2.1, 13.5) | 2.1 (−4.9, 13.3) | ||
|
|
||||
| Change in 6-Minute Walk, m | Usual Care | 5 (−28, 37) | 1 (−41, 40) | 0.16 |
| Exercise | 20 (−16, 55) | 19 (−9, 69) | ||
|
|
||||
| Change in CPX Duration, min | Usual Care | 0.3 (−0.6, 1.5) | 0.1 (−1.1, 1.0) | 0.51 |
| Exercise | 1.5 (0.3, 3.0) | 1.4 (0.2, 2.4) | ||
|
|
||||
| Change in Peak VO2, mL/kg/min | Usual Care | 0.2 (−1.2, 1.5) | 0.1 (−1.0, 1.2) | 0.82 |
| Exercise | 0.7 (−0.8, 2.4) | 0.2 (−0.6, 1.5) | ||
|
|
||||
Expressed as median (interquartile range)
KCCQ, Kansas City Cardiomyopathy Questionnaire; CPX, cardiopulmonary exercise test
The interaction analyses have been adjusted as follows:
Change in KCCQ score: baseline KCCQ overall summary score, age, peripheral vascular disease, biventricular pacemaker, atrial fibrillation/flutter, Beck Depression score, BUN, BMI, CCS angina class, and peak VO2.
Change in 6-minute walk: baseline 6-minute walk distance, number of HF hospitalizations in the previous 6 months, resting heart rate, LVEF, KCCQ clinical summary score, BUN, peak respiratory exchange ratio, smoking status, CPX duration, and peak VO2.
Change in CPX duration: baseline CPX duration, age, sex, number of HF hospitalizations in the previous 6 months, ischemic etiology, atrial fibrillation/flutter, diastolic blood pressure, KCCQ clinical summary score, BUN, BMI, peak respiratory exchange ratio, heart rate, income, and peak VO2.
Change in peak VO2: baseline peak VO2, age, sex, number of HF hospitalizations in the previous 6 months, ischemic etiology, insulin use, pacemaker, LVEF, BUN, BMI, peak respiratory exchange ratio, race, and CPX duration.
COPD was associated with increased mortality/hospitalization, mortality, and CV mortality/HF hospitalization on unadjusted analysis, but not with increased CV mortality/CV hospitalization (Table 4). After multivariable adjustment, the increased risk of CV mortality/HF hospitalization remained statistically significant (Hazard Ratio [HR] 1.46, 95% Confidence Interval [CI]: 1.14–1.87), while mortality/hospitalization (HR 1.15, 95% CI: 0.96–1.37) and mortality (HR 1.33, 95% CI: 0.99–1.76) were not significantly increased. There was no evidence to suggest an interaction between exercise training and COPD status for any of the clinical endpoints (all P>0.15).
Table 4.
Outcome Hazard Ratios for Patients with Concomitant COPD.
| Outcome | Chi-Square | p-value | Hazard Ratio (95% CI) | P-value for Interaction between COPD, Treatment Assignment and Outcome |
|---|---|---|---|---|
| All-Cause Mortality or Hospitalization (# events=1542) | ||||
| Unadjusted | 14.0 | <0.001 | 1.34 (1.15 – 1.56) | 0.62 |
| Adjusted* [N = 1756] | 2.4 | 0.13 | 1.15 (0.96 – 1.37) | |
| All-Cause Mortality (# events=384) | ||||
| Unadjusted | 21.7 | <0.001 | 1.89 (1.45 – 2.48) | 0.17 |
| Adjusted†[N = 2220] | 3.8 | 0.051 | 1.33 (0.99 – 1.76) | |
| CV Mortality or CV Hospitalization (# events=1298) | ||||
| Unadjusted | 1.7 | 0.20 | 1.12 (0.94 – 1.33) | 0.88 |
| Adjusted‡ [N = 1728] | <0.1 | 0.93 | 0.99 (0.81 – 1.21) | |
| CV Mortality or HF Hospitalization (# events=731) | ||||
| Unadjusted | 14.3 | <0.001 | 1.51 (1.22 – 1.86) | 0.30 |
| Adjusted§ [N = 1692] | 8.9 | 0.003 | 1.46 (1.14 – 1.87) | |
Abbreviations: COPD, chronic obstructive pulmonary disease; CV, cardiovascular; HF, heart failure
Adjusted for Weber class, KCCQ symptom stability score, BUN, country, LVEF, sex, beta-blocker dosage, mitral regurgitation grade, and ventricular conduction prior to baseline CPX test.
Adjusted for exercise duration on baseline CPX, serum creatinine level, BMI, sex, loop diuretic dose, LVEF, CCS angina classification, and ventricular conduction prior to baseline CPX.
Adjusted for LVEF, mitral regurgitation grade, ventricular conduction on baseline CPX, KCCQ symptom stability score, BUN, race, heart rate at peak exercise on baseline CPX, sex, nitrates at baseline, Weber class, and KCCQ total symptom score.
Adjusted for loop diuretic dose, LVEF, mitral regurgitation grade, ventricular conduction prior to baseline CPX, KCCQ symptom stability score, BUN, race, sex, age, Weber class, and VE/VCO2 on baseline CPX.
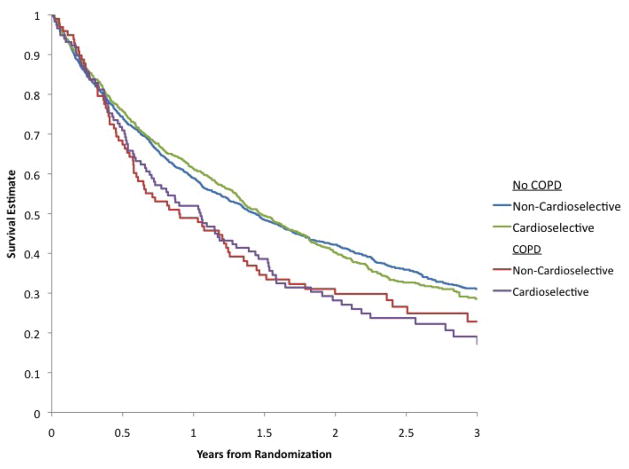
For the overall cohort, there was no significant difference in mortality/hospitalization by beta-blocker type (65% vs. 68%, non-cardioselective vs. cardioselective beta-blockers, P=0.09; adjusted HR 0.97; 95% CI, 0.86–1.10). Figure 1 presents the Kaplan-Meier survival estimates for mortality/hospitalization by beta-blocker selectivity and COPD status. The adjusted HR for mortality/hospitalization in the COPD cohort with cardioselective beta-blockers vs. noncardioselective beta-blockers was 0.91; 95% CI, 0.64–1.29; P=0.58). There was insufficient evidence to suggest an interaction between COPD status and beta-blocker selectivity associated with mortality/hospitalization following risk adjustment (P=0.72).
Figure 1.
Kaplan-Meier Survival Estimates for All-Cause Mortality/Hospitalization by Beta-blocker Selectivity and COPD Status.
Discussion
The prognosis for patients with COPD and HF have been conflicting and limited data exist with respect to the impact of exercise training and beta-blocker selectivity on outcomes in this population. In HF-ACTION, the largest randomized trial of exercise training in chronic, stable HF patients with reduced EF16, we demonstrated that COPD was associated with older age, an increased burden of comorbidities, worse baseline clinical status, and reduced exercise capacity. In the adjusted analysis, COPD was associated with a 46% increased risk for CV mortality/HF hospitalization, but not with the other clinical endpoints, nor did it confer a differential response to exercise training. In addition, beta-blocker selectivity was not associated with differences in outcome for HF patients with COPD versus those without.
HF patients with COPD had an increased burden of comorbidities, including hypertension, atrial fibrillation/flutter and coronary disease compared to those without COPD. The underlying pathophysiology may be due, in part, to the shared risk factor of smoking with low-grade systemic inflammation accelerating the progression of atherosclerosis, ischemic heart disease and COPD19. The diagnosis and management of comorbidities should be targeted to this high-risk population, since the risk for adverse outcomes for HF patients substantially increases with the number of chronic conditions5.
In contrast to previous studies, HF-ACTION patients with and without COPD had similar baseline vital signs, laboratory values, and ACE-inhibitor/aldosterone antagonist use. In EVEREST and OPTIMIZE-HF, COPD patients tended to have lower blood pressure, higher serum creatinine and underuse of ACE-inhibitors/aldosterone antagonists. Our findings may be due, in part, to the relatively stable chronic HF population in HF-ACTION. Therefore, while HF patients with COPD may demonstrate different clinical profiles compared to those without COPD in the acute setting, the between-group differences may be less marked following HF stabilization. These findings suggest greater attention to the initiation and uptitration of HF therapies in the COPD population following HF stabilization. Nevertheless, we found that COPD patients with HF were significantly less likely to receive beta-blockers than those without COPD. Guidelines recommend beta-blocker therapy in patients with coexistent COPD and HF with reduced EF. Notably, the usage of beta-blockers in COPD patients in HF-ACTION (88%) was greater than in previous studies (e.g. ~65%). This finding was likely related to the HF-ACTION protocol which encouraged the use of an optimal guideline-based HF regimen for 6 weeks before enrollment.
It has been suggested that improved utilization of beta-blockers in HF patients with COPD should involve cardioselective beta-blockers pending additional data. We found that COPD patients more often received cardioselective beta-blockers compared to non-COPD patients, but those with COPD still tended to receive lower doses of cardioselective beta-blockers. Careful uptitration of beta-blocker dosage is warranted in HF patients22 including in those with concomitant COPD. Despite concerns about beta-blocker therapy in HF patients with COPD, there was no evidence that beta-blocker selectivity was associated with differences in clinical outcomes for the overall cohort or for those with COPD. Similar findings have been demonstrated in OPTIMIZE-HF23.
Functional limitation as quantified by 6-minute walk distance and CPX testing has been associated with poor quality of life and worse outcomes in both COPD patients and HF patients24–31. However, baseline functional capacity and the impact of exercise training in patients with concomitant COPD and HF have not been well characterized. We demonstrated that, compared with non-COPD patients, HF patients with COPD had a significant reduction in functional capacity at baseline, both during CPX testing and the 6-minute walk test. COPD patients were also significantly less likely to achieve a peak RER >1.10, a value generally considered to indicate near-maximal effort18. However, a RER <1.10 may also be seen in those with a pulmonary limitation to exercise18. There was insufficient evidence to suggest an interaction between COPD status and the exercise training effect on health status and CPX variables after adjusting for baseline characteristics. Nonetheless, the differences in exercise variables observed between those with and without COPD should be taken into account when interpreting functional evaluations for clinical risk stratification and in the design and analysis of trials in HF and COPD populations.
Our finding of no association between COPD and the primary outcome of mortality/hospitalization has several possible explanations. First, it may be that COPD is not associated with increased mortality/hospitalization. Other factors such as neurohormonal activation, renal dysfunction and metabolic status may play a more prominent role than COPD in determining outcomes. Second, the difficulty of diagnosing COPD in patients with HF may have limited the ability to detect between-group differences in outcome. Alternatively, our neutral findings may be related to selecting a lower risk COPD cohort in the setting of an exercise training clinical trial.
There was a significant 46% increased risk for CV mortality/HF hospitalization associated with COPD. COPD may worsen HF due to mechanisms including low-grade inflammation, underuse of beta-blockers and adverse CV effects of bronchodilators 9. The increased risk for hospitalization in HF patients with COPD may also be related to the reduction in HF-related mortality with contemporary therapies. With the improved survival of HF patients with COPD, there is a larger population at risk for hospitalization.
Our study should be interpreted in the context of several limitations. This was a retrospective analysis. Outside the context of a prospective, controlled trial, definitive cause-and-effect relationships are difficult to determine. The study population had strict inclusion and exclusion criteria for a trial of exercise training in HF, such that these findings may not apply to those with different baseline characteristics; the percentage of patients with COPD in this study (11%) was substantially lower than in the general HF community (~30%). COPD was defined by the clinician-investigator at patient enrollment based on clinical evidence or knowledge by past medical history. While objective criteria such as pulmonary function tests (PFTs) were not explicitly used, our stratification is strengthened by the fact that clinicians prospectively collected the data in the controlled setting of a clinical trial. Since the majority of HF patients have not had PFTs,33 which may be confounded by abnormal respiratory physiology due to HF, the classification of COPD in the present analysis represents the method frequently used in clinical practice. Given the exclusion of individuals on supplemental oxygen and lack of information on forced expiratory volume in 1 second and bronchodilator therapy, future prospective studies are warranted9. Since patients with COPD are commonly excluded from HF clinical trials (and vice-versa), this analysis provides background for future trials that incorporate PFT criteria and document bronchodilator and steroid use (inhaled and oral). Despite covariate adjustment, other measured and unmeasured factors may have influenced these findings. Given the potential for type II error, future prospective studies with longer follow-up duration will need to externally validate these associations.
Conclusion
COPD in patients with HF due to systolic dysfunction was associated with older age, more comorbidities, worse clinical status, lower beta-blocker use, reduced exercise capacity, and increased CV mortality/HF hospitalization, but not with the primary endpoint of mortality/hospitalization. We did not observe a differential response to aerobic exercise training in those with and without COPD. Beta-blocker selectivity was not associated with differences in long-term outcome. However, given baseline differences in these patient groups, COPD status should be taken into account in the design and analysis of trials of exercise training in HF patients.
Acknowledgments
Funding Support: HF-ACTION was funded by the NHLBI. Dr. Schulte was supported by NIH grant P01 CA142538.
Footnotes
Conflict of Interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hawkins NM, Petrie MC, Jhund PS, Chalmers GW, Dunn FG, McMurray JJ. Heart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiology. Eur J Heart Fail. 2009;11(2):130–9. doi: 10.1093/eurjhf/hfn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Staszewsky L, Wong M, Masson S, Barlera S, Carretta E, Maggioni AP, et al. Clinical, neurohormonal, and inflammatory markers and overall prognostic role of chronic obstructive pulmonary disease in patients with heart failure: data from the Val-HeFT heart failure trial. J Card Fail. 2007;13(10):797–804. doi: 10.1016/j.cardfail.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Macchia A, Monte S, Romero M, D’Ettorre A, Tognoni G. The prognostic influence of chronic obstructive pulmonary disease in patients hospitalised for chronic heart failure. Eur J Heart Fail. 2007;9(9):942–8. doi: 10.1016/j.ejheart.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290(19):2581–7. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 5.Braunstein JB, Anderson GF, Gerstenblith G, Weller W, Niefeld M, Herbert R, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42(7):1226–33. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 6.Jong P, Vowinckel E, Liu PP, Gong Y, Tu JV. Prognosis and determinants of survival in patients newly hospitalized for heart failure: a population-based study. Arch Intern Med. 2002;162(15):1689–94. doi: 10.1001/archinte.162.15.1689. [DOI] [PubMed] [Google Scholar]
- 7.O’Connor CM, Abraham WT, Albert NM, Clare R, Gattis Stough W, Gheorghiade M, et al. Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) American heart journal. 2008;156(4):662–73. doi: 10.1016/j.ahj.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 8.Siirila-Waris K, Lassus J, Melin J, Peuhkurinen K, Nieminen MS, Harjola VP. Characteristics, outcomes, and predictors of 1-year mortality in patients hospitalized for acute heart failure. Eur Heart J. 2006;27(24):3011–7. doi: 10.1093/eurheartj/ehl407. [DOI] [PubMed] [Google Scholar]
- 9.Mentz RJ, Fiuzat M, Wojdyla DM, Chiswell K, Gheorghiade M, Fonarow GC, et al. Clinical characteristics and outcomes of hospitalized heart failure patients with systolic dysfunction and chronic obstructive pulmonary disease: findings from OPTIMIZE-HF. Eur J Heart Fail. 2012;14(4):395–403. doi: 10.1093/eurjhf/hfs009. [DOI] [PubMed] [Google Scholar]
- 10.Mentz RJ, Schmidt PH, Kwasny MJ, Ambrosy AP, O’Connor CM, Konstam MA, et al. The Impact of Chronic Obstructive Pulmonary Disease in Patients Hospitalized for Worsening Heart Failure with Reduced Ejection Fraction: An Analysis of the EVEREST Trial. J Card Fail. 2012;18(7):515–523. doi: 10.1016/j.cardfail.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Hawkins NM, Petrie MC, Macdonald MR, Jhund PS, Fabbri LM, Wikstrand J, et al. Heart failure and chronic obstructive pulmonary disease the quandary of Beta-blockers and Beta-agonists. Journal of the American College of Cardiology. 2011;57(21):2127–38. doi: 10.1016/j.jacc.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Jabbour A, Macdonald PS, Keogh AM, Kotlyar E, Mellemkjaer S, Coleman CF, et al. Differences between beta-blockers in patients with chronic heart failure and chronic obstructive pulmonary disease: a randomized crossover trial. J Am Coll Cardiol. 2010;55(17):1780–7. doi: 10.1016/j.jacc.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 13.Lainscak M, Podbregar M, Kovacic D, Rozman J, von Haehling S. Differences between bisoprolol and carvedilol in patients with chronic heart failure and chronic obstructive pulmonary disease: a randomized trial. Respir Med. 2011;105 (Suppl 1):S44–9. doi: 10.1016/S0954-6111(11)70010-5. [DOI] [PubMed] [Google Scholar]
- 14.Ries AL, Bauldoff GS, Carlin BW, Casaburi R, Emery CF, Mahler DA, et al. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest. 2007;131(5 Suppl):4S–42S. doi: 10.1378/chest.06-2418. [DOI] [PubMed] [Google Scholar]
- 15.Whellan DJ, O’Connor CM, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007;153(2):201–11. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 16.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1439–50. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Connor CM, Whellan DJ, Wojdyla D, Leifer E, Clare RM, Ellis SJ, et al. Factors related to morbidity and mortality in patients with chronic heart failure with systolic dysfunction: the HF-ACTION predictive risk score model. Circ Heart Fail. 2012;5(1):63–71. doi: 10.1161/CIRCHEARTFAILURE.111.963462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, et al. Clinician’s Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122(2):191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 19.Dahlstrom U. Frequent non-cardiac comorbidities in patients with chronic heart failure. Eur J Heart Fail. 2005;7(3):309–16. doi: 10.1016/j.ejheart.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 21.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012 doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 22.Fiuzat M, Wojdyla D, Kitzman D, Fleg J, Keteyian SJ, Kraus WE, et al. Relationship of Beta-Blocker Dose With Outcomes in Ambulatory Heart Failure Patients With Systolic Dysfunction: Results From the HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) Trial. J Am Coll Cardiol. 2012 doi: 10.1016/j.jacc.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mentz RJ, Wojdyla D, Fiuzat M, Chiswell K, Ahmad T, Fonarow G, et al. The Association of Beta-blocker Use and Selectivity with Outcomes in HF patients with COPD: Findings from OPTIMIZE-HF. J Am Coll Cardiol. 2012;59(13_Suppl_S):E862. [Google Scholar]
- 24.Enfield K, Gammon S, Floyd J, Falt C, Patrie J, Platts-Mills TA, et al. Six-minute walk distance in patients with severe end-stage COPD: association with survival after inpatient pulmonary rehabilitation. J Cardiopulm Rehabil Prev. 2010;30(3):195–202. doi: 10.1097/HCR.0b013e3181c565e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Torres JP, Casanova C, Cote CG, Lopez MV, Diaz O, Maria Marin J, et al. Six-minute walking distance in women with COPD. COPD. 2011;8(4):300–5. doi: 10.3109/15412555.2011.589870. [DOI] [PubMed] [Google Scholar]
- 26.Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–12. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 27.Brown CD, Benditt JO, Sciurba FC, Lee SM, Criner GJ, Mosenifar Z, et al. Exercise testing in severe emphysema: association with quality of life and lung function. COPD. 2008;5(2):117–24. doi: 10.1080/15412550801941265. [DOI] [PubMed] [Google Scholar]
- 28.Myers J, Arena R, Dewey F, Bensimhon D, Abella J, Hsu L, et al. A cardiopulmonary exercise testing score for predicting outcomes in patients with heart failure. Am Heart J. 2008;156(6):1177–83. doi: 10.1016/j.ahj.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Francis DP, Shamim W, Davies LC, Piepoli MF, Ponikowski P, Anker SD, et al. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO(2)slope and peak VO(2) Eur Heart J. 2000;21(2):154–61. doi: 10.1053/euhj.1999.1863. [DOI] [PubMed] [Google Scholar]
- 30.Curtis JP, Rathore SS, Wang Y, Krumholz HM. The association of 6-minute walk performance and outcomes in stable outpatients with heart failure. J Card Fail. 2004;10(1):9–14. [PubMed] [Google Scholar]
- 31.Arslan S, Erol MK, Gundogdu F, Sevimli S, Aksakal E, Senocak H, et al. Prognostic value of 6-minute walk test in stable outpatients with heart failure. Tex Heart Inst J. 2007;34(2):166–9. [PMC free article] [PubMed] [Google Scholar]
- 32.O’Connor CM, Mentz RJ, Cotter G, Metra M, Cleland JG, Davison BA, et al. The PROTECT in-hospital risk model: 7-day outcome in patients hospitalized with acute heart failure and renal dysfunction. Eur J Heart Fail. 2012;14(6):605–12. doi: 10.1093/eurjhf/hfs029. [DOI] [PubMed] [Google Scholar]
- 33.Cleland JG, Swedberg K, Follath F, Komajda M, Cohen-Solal A, Aguilar JC, et al. The EuroHeart Failure survey programme-- a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. European heart journal. 2003;24(5):442–63. doi: 10.1016/s0195-668x(02)00823-0. [DOI] [PubMed] [Google Scholar]
- 34.Light RW, George RB. Serial pulmonary function in patients with acute heart failure. Archives of internal medicine. 1983;143(3):429–33. [PubMed] [Google Scholar]
- 35.Naum CC, Sciurba FC, Rogers RM. Pulmonary function abnormalities in chronic severe cardiomyopathy preceding cardiac transplantation. The American review of respiratory disease. 1992;145(6):1334–8. doi: 10.1164/ajrccm/145.6.1334. [DOI] [PubMed] [Google Scholar]