Abstract
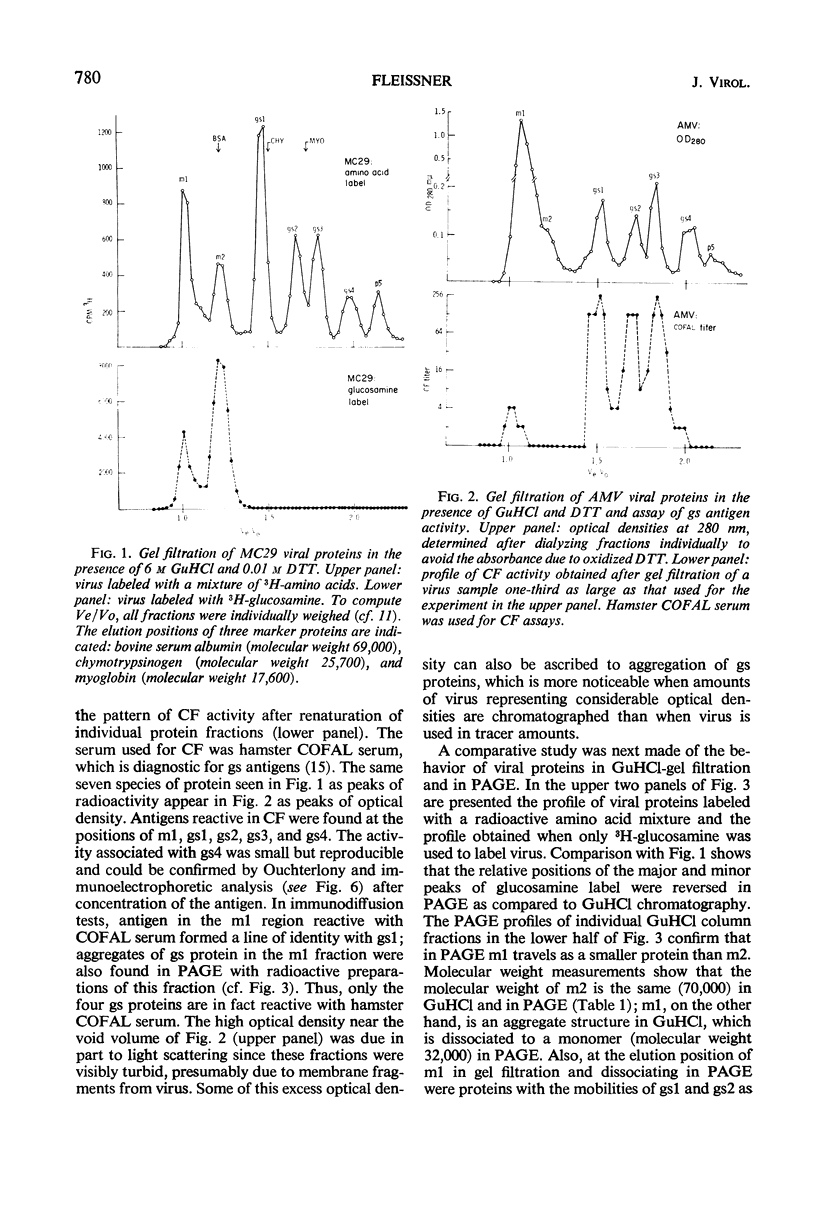
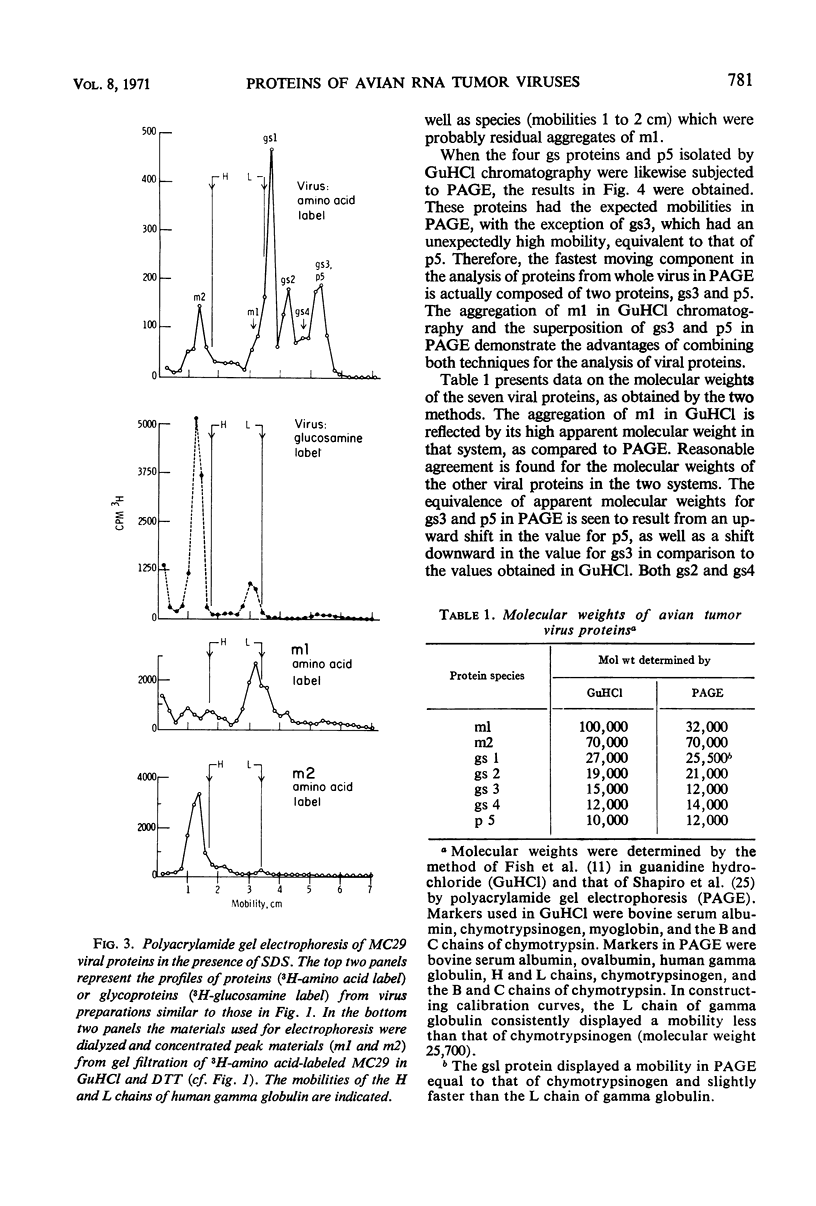
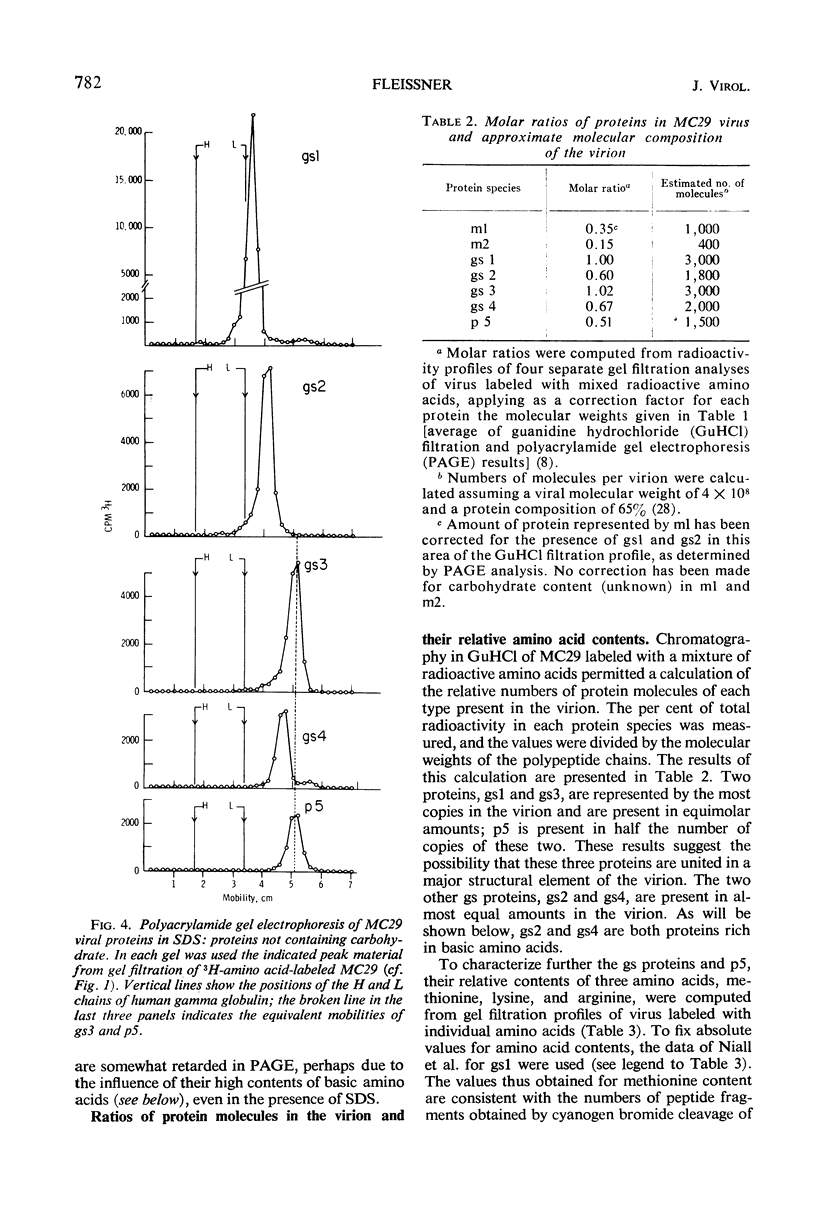
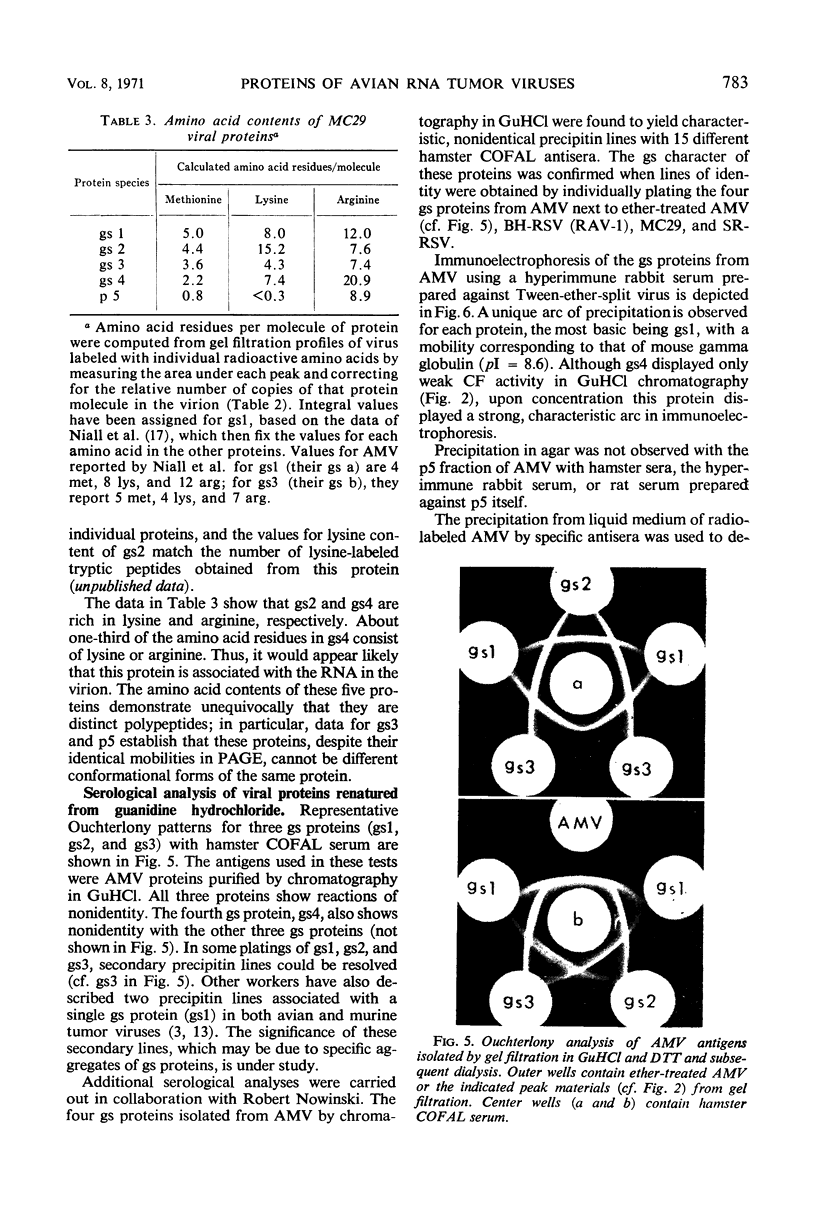
Gel filtration of avian tumor virus proteins in 6 m guanidine hydrochloride clearly resolved seven major protein species. The antigenic activity of these proteins was recovered in good yield after removal of the denaturing solvent, permitting a correlation of specific polypeptides with the principal antigens of the virion. Two of the proteins, of molecular weights 70,000 and 32,000, contain carbohydrate and are situated on the viral membrane, as shown by their being accessible in the intact virus to specific antibodies. Four proteins, with molecular weights (in guanidine) of 27,000, 19,000, 15,000, and 12,000, have different group-specific (gs) antigens and are enclosed within the viral membrane. The smallest protein, with a molecular weight of 10,000, has not previously been described; it is not detectable with antisera and possesses a mobility identical to that of one gs protein when subjected to electrophoresis in polyacrylamide gels in the presence of sodium dodecyl sulfate. Of the proteins lacking carbohydrate, three are present in the virion in a molecular ratio of 2:2:1, and the two others, present in almost equal amount, are rich in lysine and arginine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. W., Sarma P. S., Niall H. D., Sauer R. Isolation of a second avian leukosis group-specific antigen (gs-b) from avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1970 Oct;67(2):837–842. doi: 10.1073/pnas.67.2.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Bauer H., Bolognesi D. P. Polypeptides of avian RNA tumor viruses. II. Serological characterization. Virology. 1970 Dec;42(4):1113–1126. doi: 10.1016/0042-6822(70)90358-2. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Bauer H. Polypeptides of avian RNA tumor viruses. 1. Isolation and physical and chemical analysis. Virology. 1970 Dec;42(4):1097–1112. doi: 10.1016/0042-6822(70)90357-0. [DOI] [PubMed] [Google Scholar]
- Chen C., Compans R. W., Choppin P. W. Parainfluenza virus surface projections: glycoproteins with haemagglutinin and neuraminidase activities. J Gen Virol. 1971 Apr;11(1):53–58. doi: 10.1099/0022-1317-11-1-53. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Dimmock N. J. An electron microscopic study of single-cycle infection of chick embryo fibroblasts by influenza virus. Virology. 1969 Nov;39(3):499–515. doi: 10.1016/0042-6822(69)90098-1. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Klenk H. D., Caliguiri L. A., Choppin P. W. Influenza virus proteins. I. Analysis of polypeptides of the virion and identification of spike glycoproteins. Virology. 1970 Dec;42(4):880–889. doi: 10.1016/0042-6822(70)90337-5. [DOI] [PubMed] [Google Scholar]
- Compans R. W. Location of the glycoprotein in the membrane of Sindbis virus. Nat New Biol. 1971 Jan 27;229(4):114–116. doi: 10.1038/newbio229114a0. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Martin G. S., Vogt P. K. Glycoprotein components of avian and murine RNA tumor viruses. Virology. 1970 Aug;41(4):631–646. doi: 10.1016/0042-6822(70)90428-9. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Robinson H. L., Robinson W. S., Huebner R. J., Turner H. C. Proteins of Rous sarcoma virus. Virology. 1968 Sep;36(1):73–86. doi: 10.1016/0042-6822(68)90118-9. [DOI] [PubMed] [Google Scholar]
- Fish W. W., Mann K. G., Tanford C. The estimation of polypeptide chain molecular weights by gel filtration in 6 M guanidine hydrochloride. J Biol Chem. 1969 Sep 25;244(18):4989–4994. [PubMed] [Google Scholar]
- Fleissner E. Virus-specific antigens in hamster cells transformed by Rous sarcoma virus. J Virol. 1970 Jan;5(1):14–21. doi: 10.1128/jvi.5.1.14-21.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden R. V., Oroszlan S., Huebner R. J. Coexistence of intraspecies and interspecies specific antigenic determinants on the major structural polypeptide of mammalian C-type viruses. Nat New Biol. 1971 May 26;231(21):107–108. doi: 10.1038/newbio231107a0. [DOI] [PubMed] [Google Scholar]
- Hung P. P., Robinson H. L., Robinson W. S. Isolation and characterization of proteins from Rous sarcoma virus. Virology. 1971 Jan;43(1):251–266. doi: 10.1016/0042-6822(71)90243-1. [DOI] [PubMed] [Google Scholar]
- Kelloff G., Vogt P. K. Localization of avian tumor virus group-specific antigen in cell and virus. Virology. 1966 Jul;29(3):377–384. doi: 10.1016/0042-6822(66)90213-3. [DOI] [PubMed] [Google Scholar]
- Langlois A. J., Bolognesi D. P., Fritz R. B., Beard J. W. Strain MC29 avian leukosis virus release by chick embryo cells infected with the agent. Proc Soc Exp Biol Med. 1969 May;131(1):138–143. doi: 10.3181/00379727-131-33823. [DOI] [PubMed] [Google Scholar]
- Niall H. D., Sauer R., Allen D. W. The N-terminal amino acid sequence of two avian leukosis group specific antigens. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1804–1809. doi: 10.1073/pnas.67.4.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowinski R. C., Old L. J., Sarkar N. H., Moore D. H. Common properties of the oncogenic RNA viruses (oncornaviruses). Virology. 1970 Dec;42(4):1152–1157. doi: 10.1016/0042-6822(70)90367-3. [DOI] [PubMed] [Google Scholar]
- RUBIN H. An analysis of the assay of Rous sarcoma cells in vitro by the infective center technique. Virology. 1960 Jan;10:29–49. doi: 10.1016/0042-6822(60)90004-0. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. The gross conformation of protein-sodium dodecyl sulfate complexes. J Biol Chem. 1970 Oct 10;245(19):5161–5165. [PubMed] [Google Scholar]
- Robinson W. S., Hung P., Robinson H. L., Ralph D. D. Proteins of avian tumor viruses with different coat antigens. J Virol. 1970 Nov;6(5):695–698. doi: 10.1128/jvi.6.5.695-698.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. S., Robinson H. L. DNA polymerase in defective Rous sarcoma virus. Virology. 1971 May;44(2):457–462. doi: 10.1016/0042-6822(71)90278-9. [DOI] [PubMed] [Google Scholar]
- SARMA P. S., TURNER H. C., HUEBNER R. J. AN AVIAN LEUCOSIS GROUP-SPECIFIC COMPLEMENT FIXATION REACTION. APPLICATION FOR THE DETECTION AND ASSAY OF NON-CYTOPATHOGENIC LEUCOSIS VIRUSES. Virology. 1964 Jul;23:313–321. doi: 10.1016/0042-6822(64)90253-3. [DOI] [PubMed] [Google Scholar]
- Schulze I. T. The structure of influenza virus. I. The polypeptides of the virion. Virology. 1970 Dec;42(4):890–904. doi: 10.1016/0042-6822(70)90338-7. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr, Darnell J. E., Jr Evidence for virus-specific noncapsid proteins in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1965 Aug;54(2):505–513. doi: 10.1073/pnas.54.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- Vogt P. K. Avian tumor viruses. Adv Virus Res. 1965;11:293–385. doi: 10.1016/s0065-3527(08)60549-7. [DOI] [PubMed] [Google Scholar]