Abstract
β-lapachone is a naturally occurring 1,2-naphthoquinone-based compound that has been advanced into clinical trials based on its tumor-selective cytotoxic properties. Previously, we focused on the related 1,4-naphthoquinone pharmacophore as a basic core structure for developing a series of potent indoleamine 2,3-dioxygenase 1 (IDO1) enzyme inhibitors. In this study, we identified IDO1 inhibitory activity as a previously unrecognized attribute of the clinical candidate β-lapachone. Enzyme kinetics-based analysis of β-lapachone indicated an uncompetitive mode of inhibition, while computational modeling predicted binding within the IDO1 active site consistent with other naphthoquinone derivatives. Inhibition of IDO1 has previously been shown to breach the pathogenic tolerization that constrains the immune system from being able to mount an effective anti-tumor response. Thus, the finding that β-lapachone has IDO1 inhibitory activity adds a new dimension to its potential utility as an anti-cancer agent distinct from its cytotoxic properties, and suggests that a synergistic benefit can be achieved from its combined cytotoxic and immunologic effects.
Keywords: indoleamine 2, 3-dioxygenase, IDO, beta-lapachone, naphthoquinone, inhibitor, cancer, immunotherapy, tryptophan
Introduction
Small molecules that interfere with the indoleamine 2,3-dioxygenase 1 (IDO1) enzyme have demonstrated compelling anti-tumor properties in pre-clinical models and two such agents are currently being evaluated in clinical trials.1,2 The IDO1 enzyme is not a conventional cancer target in that it does not directly contribute to tumor cell growth or survival, but rather acts as an immune modulator involved in protecting tumors from immune-based destruction. Enzymatically, IDO1 catalyzes the initial and rate-limiting step in the catabolism of tryptophan to kynurenines.3 However, the principal role of IDO1 appears to be regulatory rather than metabolic, with a distinct hepatic enzyme tryptophan dioxygenase (TDO2) being responsible for maintaining normal tryptophan homeostasis.4 While IDO1 is not responsive to tryptophan levels, it was found to be elevated at sites of inflammation and immune privilege.4 The emerging concept of IDO1 as an immune response regulator came to the fore with a seminal study showing that treatment of pregnant female mice with the IDO pathway inhibitor 1-methyl-tryptophan (1MT) promoted T cell-mediated rejection of hemiallogeneic mouse concepti.5 The implications of this study were foundational to the development of current ideas about the physiologic ramifications of IDO1-mediated immune regulation, including the hypothesis that IDO1 might contribute to tumoral immune escape.
Genetic studies in mice have since demonstrated that IDO1 can indeed support spontaneous tumor and metastasis development.6,7 Both intact T cell immunity and targeting of IDO1 are required to achieve anti-tumor activity with IDO inhibitory molecules.8–12 IDO1 inhibitors have been found to act synergistically with select cytotoxic agents,11 suggesting that adjuvant treatment with IDO inhibitors could leverage clinical responses to conventional chemotherapy treatments. These findings are in line with the notion that tumor cell killing by cytotoxic agents can facilitate antigen presentation but that achieving an effective antitumor immune response also requires the concomitant disruption of dominant tumoral immune tolerance provided by IDO1 inhibition.13 In this scenario, compounds that integrate tumoricidal activity along with IDO1 inhibitory activity may produce substantially more robust single agent anti-tumor responses than IDO1 inhibitors that do not exert a cytotoxic effect. In screening for novel IDO1 inhibitors, we tested compounds with the ability to elicit this type of dual action in the mouse B16F10 melanoma tumor graft model. B16F10 cells form very aggressive, non-immunogenic tumors that are resistant to a wide variety of conventional cytotoxic agents as well as immunotherapeutics.14–16 Compounds from a brassinin-based series (brassinin, 5-Br-brassinin) and from a naphthoquinone-based series (menadione), exhibited robust IDO1-dependent, single agent anti-tumor activity,8,10 whereas the prototypical IDO1 inhibitor 1MT did not produce a significant B16F10 anti-tumor response unless combined with a cytotoxic agent.9
The preclinical demonstration of antitumor activity with these early IDO1 inhibitory lead structures led to a medicinal chemistry effort to develop pharmacologically viable clinical candidates. The structure-activity relationship (SAR)-based development of brassinin-based derivatives yielded only small increases in IDO1 inhibitory potency.17 Derivatization around the 1,4-naphthoquinone pharmacophore was more productive, yielding a series of pyranonaphthoquinone-based IDO1 inhibitors with Ki values in the 60–70 nM range.10 However, when evaluated against IDO1 expressed in cells, the inhibitory activity of the pyranonaphthoquinones was severely attenuated, with IC50 values on the order of 100–1000-fold greater than the Ki values,10 thus limiting their utility as potential therapeutic agents.
In addition to 1,4-naphthoquinone, our initial screening also identified the related phamacophore 1,2-naphthoquinone as exhibiting IDO1 inhibitory activity in the low micromolar range. In this study, we report that an anti-cancer agent currently in clinical trials, the 1,2-naphthoquinone derivative β-lapachone (3,4-dihydro-2,2-dimethyl-2H-naphthol[1,2-b]pyran-5,6-dione), is a nanomolar inhibitor of IDO1 enzymatic activity. Importantly, when evaluated in a cell-based assay, the IDO1 inhibitory activity of β-lapachone was not attenuated to nearly the same degree as with the pyranonaphthoquinones. This outcome suggests a new direction for medicinal chemistry efforts that can be used to produce more pharmacologically viable compounds. More immediately, the discovery that β-lapachone is a potent IDO1 inhibitor should be carefully considered in moving forward with clinical development plans for this compound in order to fully exploit this previously unrecognized anti-tumor mechanism of action.
Methods
Chemical compounds
L-Tryptophan, β-lapachone, and p-dimethylaminobenzaldehyde (Ehrlich’s reagent) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dicoumarol was purchased from MP Biomedicals (Santa Ana, CA, USA). Solutions were made using dimethylsulfoxide (DMSO) for cellular assays.
Docking computations
Small molecules were constructed in MOE V2011.1 (Chemical Computing Group, Inc., Montreal, Canada) and ionized and hydrogens added using MOE’s WashMDB function. The small molecule conformation was minimized to a gradient of 0.01 with the MMFF94x18,19 using a distance-dependent dielectric constant of 1. The crystal structure of IDO1 bound to 4-phenylimidizole was used for docking calculations.20 The 2-[N-cyclohexylamino]ethanesulfonic acid and 4-phenyl-1-imidazole ligands were removed from the active site docking, hydrogen atoms were added, and tautomeric states and orientations of Asn, Gln, and His residues were determined using Molprobity.21,22 Hydrogens were then added to crystallographic waters using MOE. The Amber9923 force field in MOE was used, and iron was parameterized in the Fe+3 state. Dioxygen was not added to the iron. All hydrogens were minimized to an rms gradient of 0.01, holding the remaining heavy atoms fixed. A stepwise minimization followed for all atoms using a quadratic force constant (100) to tether the atoms to their starting geometries; for each subsequent minimization, the force constant was reduced by a half until zero was reached. GOLD version 5.1 (Cambridge Crystallographic Data Centre, Cambridge, UK) was used with chemscore parameters adapted for scoring metal—ligand interactions (pachemscore.p450_csd.params).24 One-hundred genetic algorithm (GA) docking runs were performed with the initial_virtual_pt_match_max = 2.5, while all other parameters were set as defaults. Docking of 1,4-naphthquinone, 1,2-naphthquinone, 2,2-dimethyl-2H-benzo[g]chromene-5,10-dione (dehydro-α-lapachone) and 3,4-dihydro-2,2-dimethyl-2H-naphthol[1,2-b]pyran-5,6-dione (β-lapachone) produced a top-scoring binding pose with a ketone oxygen within coordination distance to the heme iron.
Biochemical assays
Purified human recombinant IDO1 (hrIDO1) enzyme was expressed in Escherichia coli and purified as previously described.25 IDO1 enzyme assays were performed using the potassium phosphate buffer system as previously published.25 Ehrlich’s reagent was used to detect kynurenine (Kyn) spectrophotometrically as described.25 Reagents including substrate and inhibitor were all mixed first, leaving the addition of the enyme for last to initiate the reaction at T = 0. To determine enzyme kinetics for the hrIDO1 preparation, enzyme assays were performed in 1 mL volumes with varying L-Trp concentrations (0–400 μM) and collection of 100 μL aliquots for Kyn analysis at multiple timepoints (0–90 minutes). The results confirmed that the hrIDO1 enzyme follows Michaelis-Menten kinetics as previously published26 with a Km of 110 μM and a Vmax of 5.9 μM/min (Supplemental Figure S1). Inhibitory activity of β-lapachone was subsequently evaluated in hrIDO1 enzyme reactions with varying concentrations of inhibitor (0–50 μM) at a fixed substrate concentration (100 μM L-Trp) for IC50 determination or varying concentrations of both inhibitor (0–800 nM) and substrate (0–400 μM) for Ki determination. Reactions were carried out in 100 μL volumes and were stopped at 15 minutes while enzyme activity was in the linear range. Data analysis and graphing were performed using Prism v.5.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Cell-based assays
HeLa cells (ATCC, Manassas, VA, USA) were seeded in a 24-well plate at a density of 50,000 cells per well in 500 μL DMEM (Mediatech, Manassas, VA, USA) + 10% fetal bovine serum (FBS; Mediatech) + 1% penicillin-streptomycin (PenStrep; Mediatech). IDO1 expression was induced for 24 hrs by the addition of IFNγ (Gibco BRL, Grand Island, NY, USA) to 100 ng/mL. The media was then discarded, the wells rinsed once, and 2-fold serial dilutions of β-lapachone (0–100 μM) in 200 μL DMEM + 10% FBS + 1% PenStrep were added in triplicate and incubated for 5 hr. The supernatant was transferred to a round-bottom 96-well plate and mixed with 40 μL 50% (w/v) trichloroacetic acid (TCA; LabChem Inc., Pittsburgh, PA, USA) to precipitate any proteins. The plate was incubated at 65 °C for 30 minutes followed by centrifugation at 1250 × g for 10 minutes. Next, 100 μL of clarified supernatant was transferred to a new flat-bottomed 96-well plate and mixed at equal volumes with 2% (w/v) p-dimethylamino-benzaldehyde (Ehrlich’s reagent) in acetic acid. The resulting reaction was measured at 490 nm using a Synergy HT microtiter plate reader (Bio-Tek, Winooski, VT, USA). Data were collected and analyzed using Excel (Microsoft Corp., Seattle, WA, USA) and Prism v.5.0 (GraphPad Software, Inc.).
Immediately after the supernatant was transferred for the previously described assay, the cells were fixed by adding 100 μL of media and 100 μL of 32% (w/v) TCA to each well and incubated for 1 hr at 4 °C. To assess cell viability, TCA-fixed cells were processed essentially as described.27 Fixed cells were washed four times in water, blotted, air-dried, and treated for 15 min at room temperature with 100 μL of 0.4% (w/v) sulfarhodamine B (SRB) (Sigma-Aldrich) in 1% acetic acid. Wells were then rinsed four times in 1% acetic acid, air-dried, and developed by adding 200 μL of 10 mM unbuffered Tris-HCl and incubating for 15 min at room temperature with gentle shaking. Staining intensity, proportional to cell number, was determined by reading the absorbance at 570 nm on a plate reader. Data were collected and analyzed using Excel (Microsoft Corp) and Prism v.5.0 (GraphPad Software, Inc.).
Western blot analysis
HeLa cells were seeded at 5 × 104 cells per well in a 24-well plate and treated with both 100 ng/mL of IFNγ and 5 μM β-lapachone. Whole cell lystates (WCL) were collected after 24 hr using RIPA buffer. Samples were prepared in 4 × NuPAGE sample buffer (Novex by Life Technologies, Carlsbad, CA, USA) using 20 μg of WCL and loaded onto a 10% Bis-Tris 12-well NuPAGE Mini gel (Novex by Life Technologies). Electrophoresis was followed by transfer to a nitrocellulose membrane (Millipore, Billerica, MA, USA). After blocking with 5% w/v non-fat dry milk in phosphate-buffered saline containing Tween-20 (PBST; Gibco) the membrane was probed using an in-house rabbit anti-IDO1 pAb (or rabbit anti-GAPDH pAB (Abcam, Cambridge, UK)) and detected using the horseradish peroxidase-conjugated anti-rabbit pAb (Cell Signaling, Danvers, MA, USA). The blot was treated with HyGlo ECL (Denville Scientific, South Plainfield, NJ, USA) and chemiluminescence was detected using a ChemiDoc XRS+ (Bio-Rad, Hercules, CA, USA).
Results
β-lapachone is a 1,2-naphthoquinone derivative with increased IDO1 inhibitory activity
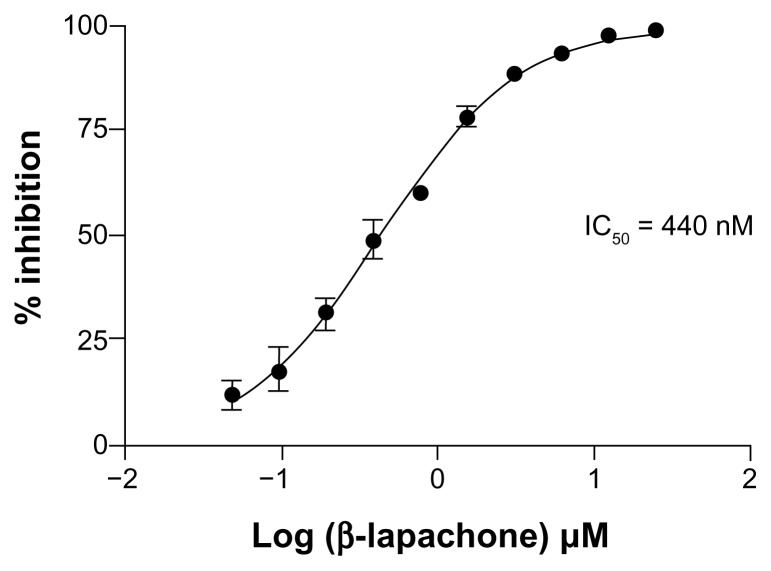
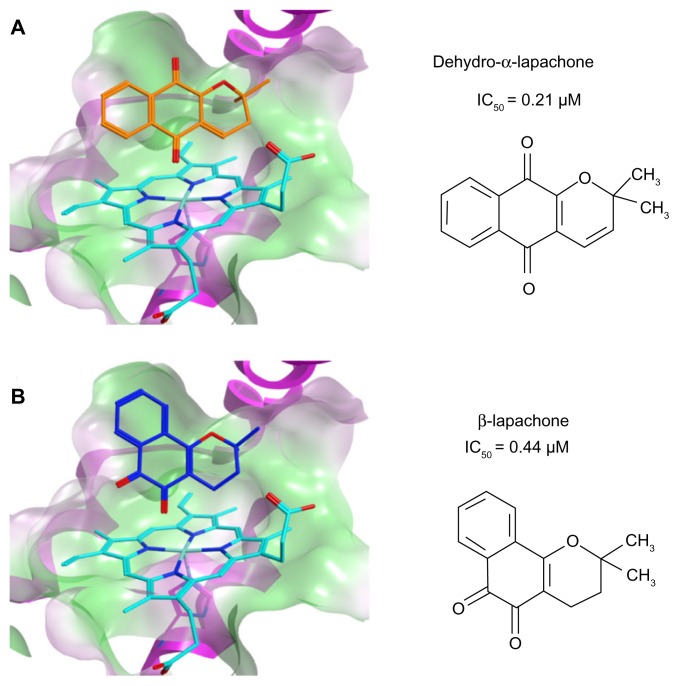
We previously reported results of a synthetic chemistry effort based on the identification of 1,4-naphthoquinone as a basic quinone pharmacophore with low micromolar inhibitory activity against the IDO1 enzyme (IC50 = 0.99 μM).10 The most potent inhibitors developed in this structural series were pyranonaphthoquinone compounds with Ki values in the 60–70 nM range. Menadione (IC50 = 1.1 μM), a 1,4-naphthoquinone derivative known to have anti-tumor properties, was selected at the outset of these studies to explore in vivo the relevant mechanism of action for this class of compounds. In an aggressive, non-immunogenic melanoma tumor graft model, menadione demonstrated robust anti-tumor activity that was dependent on the presence of both functional T cell immunity and intact IDO1.10 In this same report, we also identified the structurally analogous molecule 1,2-naphthoquinone as having IDO1 inhibitory activity (IC50 = 7.1 μM).10 Further chemical derivatization of this alternative pharmacophore was not pursued, but in the course of evaluating other commercially available compounds in this structural class, we identified β-lapachone as a submicromolar inhibitor of IDO1 (IC50 = 0.44 μM; Figure 1). As such, β-lapachone represents a >10-fold gain in potency over the basic 1,2-naphthoquinone pharmacophore. Previous computational docking studies utilizing the IDO1 crystal structure placed several naphthoquinones at the active site, with the quinone oxygen coordinated to the heme iron.10 An example of GOLD docking of the pyranonaphthoquinone-based inhibitor dehydro-α-lapachone (2,2-dimethyl-2H-benzo[g]chromene-5,10-dione; IC50 = 0.21 μM)10 is shown (Fig. 2A). Interestingly, GOLD docking of β-lapachone at the IDO1 active site (Fig. 2B) resulted in coordination of the quinone oxygen to the heme iron and positioning of the 2,2-dimethyloxane ring in a configuration that closely resembles the predicted orientation of dehydro-α-lapachone, thus providing a predictive SAR-based model for how these different molecules similarly inhibit IDO1.
Figure 1.
Inhibition of human IDO1 enzyme activity by β-lapachone. Dose-response assessment of increasing β-lapachone concentration on the activity of purified recombinant huIDO1. The assay was performed with 100 μM L-Trp substrate and the concentration of the product kynurenine was measured at 15 minutes while the enzyme was still active in the log phase. The data are plotted as percent inhibition of kynurenine production. The IC50 of 440 nM was determined by nonlinear curve fitting.
Figure 2.
β-Lapachone is predicted to bind in the IDO1 active site. Computational modeling at the IDO1 active site and IC50 values for inhibition of purified human recombinant IDO1 for (A) dehydro-α-lapachone (2,2-dimethyl-2H-benzo[g]chromene-5,10-dione) and (B) β-lapachone (3,4-dihydro-2,2-dimethyl-2H-naphthol[1,2-b]pyran-5,6-dione).
β-lapachone exhibits an uncompetitive IDO1 inhibition profile
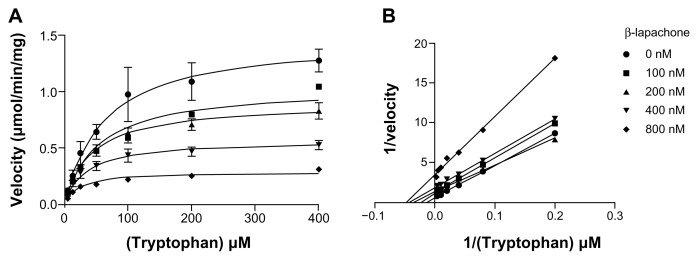
Inhibition of purified IDO1 enzyme by β-lapachone was examined in more detail, varying both inhibitor and substrate concentrations to determine its inhibition constant and mode of inhibition. Using nonlinear regression analysis, the inhibition constant for β-lapachone against purified recombinant human IDO1 enzyme was determined to be 450 nM (Fig. 3). Compared to current clinical candidates, β-lapachone is approximately 100-fold more potent than the prototypical IDO1 inhibitor 1MT11 and is only about 10-fold less potent than the level of inhibition achieved in an SAR-based, IDO1 inhibitor development program that has advanced into clinical trials.28 Nonlinear regression analysis of the IDO1 inhibition curves with β-lapachone (Fig. 3) best fit an uncompetitive inhibitor profile (global R2 value = 0.96), although a noncompetitive inhibitor profile was also a close fit so that a mode of inhibition that does not absolutely conform to either model, sometimes referred to as a mixed inhibitor, may be possible. This outcome is consistent with the data previously obtained with other naphthoquinone-based compounds10 and appears to be a common feature for this class of molecules. In terms of therapeutic utility, an uncompetitive or noncompetitive mode of action may be advantageous given that enzyme inhibition by a compound acting in this manner is not negatively impacted by high substrate concentration, which in the case of tryptophan, is upwards of 60 μM in circulation.
Figure 3.
β-Lapachone inhibits IDO1 activity through uncompetitive inhibition. Enzyme assays with purified recombinant huIDO1 were performed in the presence of varying concentrations of L-Trp and β-lapachone and the concentration of the product kynurenine was measured at 15 minutes. (A) Michaelis-Menton nonlinear regression plot of substrate-velocity curves at the five different inhibitor concentrations. These data best fit an uncompetitive inhibition model with a global (shared) R2 value of 0.96 and a Ki of 450 nM. (B) Corresponding Lineweaver-Burk plot. These plots show decreasing Km and Vmax as the inhibitor concentration is increased consistent with uncompetitive inhibition.
Inhibition of intracellular IDO1 activity by β-lapachone
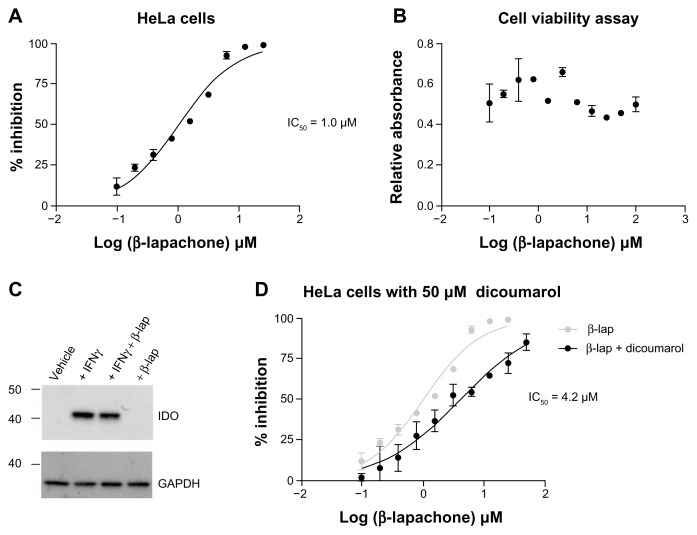
To assess intracellular activity, the ability of β-lapachone to inhibit IDO1 in IFNγ-induced HeLa cells was evaluated. In this cell-based assay, β-lapachone inhibited the release of kynurenine into the supernatant with an IC50 of 1.0 μM (Fig. 4A). No demonstrable impact of β-lapachone on viable cell numbers over the range of exposure was observed at the time of analysis. (Fig. 4B). Western blot analysis confirmed IDO1 protein induction in HeLa cells treated with IFNγ and demonstrated that IDO1 protein expression was unaffected by exposing cells to 5 μM β-lapachone (Fig. 4C), a concentration at which IDO1 activity is effectively blocked. In cells, β-lapachone can be reduced to the hydroquinone form by the enzyme NQO1 (NAD(P)H:quinone oxido-reductase 1). Treatment with the NQO1 inhibitor dicoumarol has been shown to render NQO1-expressing tumor-derived cell lines resistant to β-lapachone-mediated killing,29 consistent with the idea that NQO1-mediated biotransformation of β-lapachone is important for its cytotoxic mechanism of action. To investigate whether cellular metabolic processing of β-lapachone by NQO1 is also important for IDO1 inhibition, a parallel cell-based assay was carried out that included 50 μM dicoumarol to inhibit NQO1 activity. Addition of dicoumarol shifted the β-lapachone inhibition curve to the right, increasing the IC50 to 4.2 μM (Fig. 4D). This result suggests that when NQO1 activity is blocked, the quinone form of β-lapachone is still able to inhibit IDO1 in the cells at a low micromolar level, but that biotransformation of β-lapachone to the hydroquinone form by NQO1 may further enhance its inhibitory activity. This is consistent with our previous observation that the hydroquinone form of the 1,4-naphthoquinone menadione conjugated to glutathione is a more potent inhibitor of IDO1 in a purified enzyme assay than the quinone conjugated form.10
Figure 4.
Inhibition of intracellular IDO1 activity by β-lapachone. (A) Dose-response assessment of increasing β-lapachone concentration on IDO1 activity in HeLa cells following 24 hrs of induction with IFNγ (100 ng/mL). Data are plotted as percent inhibition of kynurenine production with an IC50 of 1.0 μM determined by nonlinear curve fitting. (B) SRB-based evaluation of viable cell numbers at the conclusion of the β-lapachone dose response assay shown in (A). (C) Western blot analysis of IDO1 protein in whole cell lysates from HeLa cells induced with IFNγ (100 ng/mL) and treated with β-lapachone (5 μM) as indicated. (D) β-lapachone dose-response assessment performed in parallel with (A) with the addition of the NQO1 inhibitor dicoumarol (50 μM). Data are plotted as percent inhibition of kynurenine production with an IC50 of 4.2 μM determined by nonlinear curve fitting.
Discussion
β-Lapachone was first identified as a pharmacologically active component of Pau d’Arco, a popular herbal supplement prepared from the bark of the Tabebuia avellanedae tree that has been promoted as a remedy for cancers and infections as well as allergies, arthritis, diabetes, flu, lupus, parasites, skin diseases, and ulcers. A variety of possible mechanisms of action have been proposed for the anti-tumor activity of β-lapachone, including DNA repair inhibition,30,31 poly(ADP-ribose) polymerase inhibition,32,33 altered calcium homeostasis,34 DNA topoisomerase inhibition,31,35 nuclear factor-κB inhibition,36 and release of mitochondrial cytochrome C.37,38 Recently, however, the preponderance of evidence primarily associates the pharmacological activity of β-lapachone with metabolic bioactivation by NQO1, which is expressed at elevated levels in a variety of human cancers.39–42 While NQO1 detoxifies many quinones, reduction of β-lapachone by NQO1 results in the production of reactive oxygen species that generate single-strand breaks in the DNA. This, in turn, induces hyperactivation of the DNA damage sensor poly(ADP-ribose) polymerase-1, which rapidly depletes NAD+ stores causing tumor cells to undergo a unique, caspase-independent programmed necrotic cell death.43,44
β-lapachone has been advanced as a clinical candidate based on its tumor-selective cytotoxic activity. In this study, we report that β-lapachone is also a potent inhibitor of the IDO1 enzyme. Biochemically, β-lapachone exhibited an uncompetitive inhibitory profile against IDO1. This mode of inhibition is consistent with other naphthoquinone-based derivatives,10 even though molecular docking predicts direct binding at the IDO1 active site. In this regard, there is an established precedent for active site heme iron binding by uncompetitive compounds such a 4-phenylimidazole20 and more sophisticated molecular interaction studies will be required to elucidate the precise mechanism of IDO1 inhibition by β-lapachone. With a Ki of 450 nM, β-lapachone is substantially more potent than the core 1,2-naphthoquinone structure on which it is based though not as potent as some of the pyranonaphthoquinone compounds that were developed through SAR-directed medicinal chemistry efforts.10 However, β-lapachone exhibited superior retention of intracellular IDO1 inhibitory activity with an IC50 of 1.0 μM, although this degree of inhibition may be partially dependent on biotransformation by NQO1. Despite not having been developed through an SAR-based medicinal chemistry effort, β-lapachone is among the more effective inhibitors against intracellular IDO1 reported to date. It is nearly 30-fold more potent in cells than menadione (IC50 = 29 μM in HeLa cells), which demonstrated IDO1-dependent, in vivo anti-tumor activity.10 As such, β-lapachone may have direct utility as an IDO1 inhibitor as well as presenting a promising molecular scaffold around which to focus future optimization efforts.
Necrotic cell death has been characterized as being pro-immunogenic,45 consistent with the possibility that the cytotoxic mechanism of action of β-lapachone may cooperatively potentiate the immune-based mechanism of anti-tumor activity associated with inhibition of IDO1. NQO1 activity in HeLa cells sensitizes them to β-lapachone induced killing with a reported LD50 of approximately 4 μM.46 Since the IC50 for IDO1 inhibition in cells is approximately 1 μM a therapeutic level of β-lapachone that elicits both effects should be achievable. Reciprocally, based on the biochemistry of tryptophan catabolism, IDO1 inhibition may also enhance the cytotoxic mechanism of action of β-lapachone. This is because the metabolic pathway initiated by IDO1-mediated catabolism of tryptophan is ultimately responsible for the de novo biosynthesis of NAD.47,48 Since the cytotoxicity of β-lapachone is exerted through depletion of NAD stores by hyperactivated poly(ADP-ribose) polymerase, interfering with de novo NAD biosynthesis could increase tumor cell sensitization. While an intriguing idea, it remains to be experimentally determined whether there are any tumor types for which this NAD biosynthesis pathway might provide a protective benefit.
Despite concerted efforts over the past decades to develop more effective agents, current cancer treatment regimens are too often inadequate, particularly for more advanced metastatic tumors. Eliminating cancer cells with cytotoxic agents and, more recently, with signal transduction inhibitors, can produce impressive short-term responses, however, the residual cancer often reemerges in a manner that is resistant to previously successful therapy. The focus on directly targeting cancer cells means that other aspects of the tumor milieu have been generally given short shrift. Tumors are not autonomous entities, however, but rather exist in an abnormal equilibrium with the host environment. Immunity, for instance, can benefit tumor outgrowth through factors associated with inflammation but can also effectively eliminate cancer when appropriately directed. Targeting tumors through different survival mechanisms, both endogenous and exogenous, may be required to achieve durable responses. Agents that can successfully incorporate multiple modes of action within a single molecule have inherent advantages over combinations of agents in terms of reducing the logistical complexity associated with clinical development. In this regard, β-lapachone would appear to be a particularly promising agent, with a classical tumor-directed cytotoxic mechanism of action integrated with the chemoimmunotherapeutic capability to inhibit IDO1. Recognition of β-lapachone’s IDO1 inhibitory activity should also stimulate new thinking regarding how it might be utilized clinically to achieve the greatest effect. For instance, the discovery that the tyrosine kinase inhibitor imatinib (ie, Gleevec) can interfere with IDO1 induction led to the formulation of a promising new strategy of combining it clinically with the αCTLA-4 antibody ipilimumab (ie, Yervoy) for the treatment of gastrointestinal stromal tumors.49 As agents targeting IDO1 are currently being evaluated in patients, the data emerging from these studies should be a valuable guide for determining the future clinical development strategy for β-lapachone.
Supplementary Figure
Enzyme kinetics for hrIDO1. Purified recombinant human IDO1 enzyme shows expected Michaelis-Menton kinetics with a calculated Vmax of 5.9 μM/min and a Km of 110 μM. The velocity is graphed directly from the assay readout in units of absorbance/minute. For determination of the Vmax and Km a standard curve was used to covert to kynurenine concentration-based units.
Acknowledgements
We thank James DuHadaway for helpful technical advice and Peter Curtis and Richard Metz for kindly providing purified IDO1 enzyme.
Footnotes
Author Contributions
Conceived and designed the experiments: AJM, HEF, JML, WPM. Analysed the data: HEF, JML, AJM. Wrote the first draft of the manuscript: HEF. Contributed to the writing of the manuscript: AJM. Agree with manuscript results and conclusions: HEF, JML, WPM, AJM. Jointly developed the structure and arguments for the paper: HEF, AJM. Made critical revisions and approved final version: HEF, JML, WPM, AJM. All authors reviewed and approved of the final manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
Funding
AJM is the recipient of a grant from Susan G. Komen for the Cure and additional funding support through NIH grant CA159337. AJM, JML and WPM receive funding support through NIH grant CA109542.
References
- 1.Liu X, Newton RC, Friedman SM, Scherle PA. Indoleamine 2,3-dioxygenase, an emerging target for anti-cancer therapy. Curr Cancer Drug Targets. 2009;9(8):938–52. doi: 10.2174/156800909790192374. [DOI] [PubMed] [Google Scholar]
- 2.Soliman H, Mediavilla-Varela M, Antonia S. Indoleamine 2,3-dioxygenase: is it an immune suppressor? Cancer J. 2010;16(4):354–9. doi: 10.1097/PPO.0b013e3181eb3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1(8):609–20. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- 4.Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5(11):2516–22. [PubMed] [Google Scholar]
- 5.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–3. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 6.Muller AJ, Sharma MD, Chandler PR, et al. Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proc Natl Acad Sci U S A. 2008;105(44):17073–8. doi: 10.1073/pnas.0806173105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith C, Chang MY, Parker KH, et al. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov. 2012;2(8):722–35. doi: 10.1158/2159-8290.CD-12-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee T, DuHadaway JB, Gaspari P, et al. A key in vivo antitumor mechanism of action of natural product-based brassinins is inhibition of indoleamine 2,3-dioxygenase. Oncogene. 2008;27(20):2851–7. doi: 10.1038/sj.onc.1210939. [DOI] [PubMed] [Google Scholar]
- 9.Hou DY, Muller AJ, Sharma MD, et al. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 2007;67(2):792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Malachowski WP, Duhadaway JB, et al. Indoleamine 2,3-dioxygenase is the anticancer target for a novel series of potent naphthoquinone-based inhibitors. J Med Chem. 2008;51(6):1706–18. doi: 10.1021/jm7014155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11(3):312–9. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 12.Muller AJ, DuHadaway JB, Jaller D, Curtis P, Metz R, Prendergast GC. Immunotherapeutic suppression of indoleamine 2,3-dioxygenase and tumor growth with ethyl pyruvate. Cancer Res. 2010;70(5):1845–53. doi: 10.1158/0008-5472.CAN-09-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muller AJ, Prendergast GC. Marrying immunotherapy with chemotherapy: why say IDO? Cancer Res. 2005;65(18):8065–8. doi: 10.1158/0008-5472.CAN-05-2213. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, McGowan P, Ashe S, et al. Tumor immunogenicity determines the effect of B7 costimulation on T cell-mediated tumor immunity. J Exp Med. 1994;179(2):523–32. doi: 10.1084/jem.179.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruczynski A, Hill BT. Classic in vivo cancer models: three examples of mouse models used in experimental therapeutics. Curr Protoc Pharmacol. 2002;Chapter 5(Unit 5):24. doi: 10.1002/0471141755.ph0524s15. [DOI] [PubMed] [Google Scholar]
- 16.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190(3):355–66. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaspari P, Banerjee T, Malachowski WP, et al. Structure-activity study of brassinin derivatives as indoleamine 2,3-dioxygenase inhibitors. J Med Chem. 2006;49(2):684–92. doi: 10.1021/jm0508888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halgren TA. MMFF VI. MMFF94 s option for energy minimization studies. J Comput Chem. 1999;20:720–9. doi: 10.1002/(SICI)1096-987X(199905)20:7<720::AID-JCC7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 19.Halgren TA. MMFF VII. Characterization of MMF94, MMF94 s, and other widely available force fields for conformational engergies and for intermolecular-interaction energies and geometries. J Comput Chem. 1999;20:740–74. doi: 10.1002/(SICI)1096-987X(199905)20:7<730::AID-JCC8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 20.Sugimoto H, Oda SI, Otsuki T, Hino T, Yoshida T, Shiro Y. Crystal structure of human indoleamine 2,3-dioxygenase: catalytic mechanism of O2 incorporation by a heme-containing dioxygenase. Proc Natl Acad Sci U S A. 2006;103(8):2611–6. doi: 10.1073/pnas.0508996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Word JM, Lovell SC, Richardson JS, Richardson DC. Asparagine and glutamine: using hydrogen atom contacts in the choice of side-chain amide orientation. J Mol Biol. 1999;285(4):1735–47. doi: 10.1006/jmbi.1998.2401. [DOI] [PubMed] [Google Scholar]
- 22.Lovell SC, Davis IW, Arendall WB, 3rd, et al. Structure validation by Calpha geometry: phi,psi and Cbeta deviation. Proteins. 2003;50(3):437–450. doi: 10.1002/prot.10286. [DOI] [PubMed] [Google Scholar]
- 23.Ponder JW, Case DA. Force fields for protein simulations. Adv Protein Chem. 2003;66:27–85. doi: 10.1016/s0065-3233(03)66002-x. [DOI] [PubMed] [Google Scholar]
- 24.Kirton SB, Murray CW, Verdonk ML, Taylor RD. Prediction of binding modes for ligands in the cytochromes P450 and other heme-containing proteins. Proteins. 2005;58(4):836–44. doi: 10.1002/prot.20389. [DOI] [PubMed] [Google Scholar]
- 25.Littlejohn TK, Takikawa O, Skylas D, Jamie JF, Walker MJ, Truscott RJW. Expression and purification of recombinant human indoleamine 2,3-dioxygenase. Prot Exp Purif. 2000;19(1):22–9. doi: 10.1006/prep.2000.1214. [DOI] [PubMed] [Google Scholar]
- 26.Sono M, Cady SG. Enzyme kinetic and spectroscopic studies of inhibitor and effector interactions with indoleamine 2,3-dioxygenase. 1. Norharman and 4-phenylimidazole binding to the enzyme as inhibitors and heme ligands. Biochemistry. 1989;28(13):5392–9. doi: 10.1021/bi00439a012. [DOI] [PubMed] [Google Scholar]
- 27.Skehan P, Storeng R, Scudiero D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82(13):1107–12. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 28.Yue EW, Douty B, Wayland B, et al. Discovery of potent competitive inhibitors of indoleamine 2,3-dioxygenase with in vivo pharmacodynamic activity and efficacy in a mouse melanoma model. J Med Chem. 2009;52(23):7364–7. doi: 10.1021/jm900518f. [DOI] [PubMed] [Google Scholar]
- 29.Pink JJ, Planchon SM, Tagliarino C, Varnes ME, Siegel D, Boothman DA. NAD(P)H:Quinone oxidoreductase activity is the principal determinant of beta-lapachone cytotoxicity. J Biol Chem. 2000;275(8):5416–24. doi: 10.1074/jbc.275.8.5416. [DOI] [PubMed] [Google Scholar]
- 30.Boothman DA, Pardee AB. Inhibition of radiation-induced neoplastic transformation by beta-lapachone. Proc Natl Acad Sci U S A. 1989;86(13):4963–7. doi: 10.1073/pnas.86.13.4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li CJ, Averboukh L, Pardee AB. beta-Lapachone, a novel DNA topoisomerase I inhibitor with a mode of action different from camptothecin. J Biol Chem. 1993;268(30):22463–8. [PubMed] [Google Scholar]
- 32.Vanni A, Fiore M, De Salvia R, et al. DNA damage and cytotoxicity induced by beta-lapachone: relation to poly(ADP-ribose) polymerase inhibition. Mutat Res. 1998;401(1–2):55–63. doi: 10.1016/s0027-5107(97)00273-x. [DOI] [PubMed] [Google Scholar]
- 33.Villamil SF, Podesta D, Molina Portela MD, Stoppani A. Characterization of poly(ADP-ribose)polymerase from Crithidia fasciculata: enzyme inhibition by beta-lapachone. Mol Biochem Parasitol. 2001;115(2):249–56. doi: 10.1016/s0166-6851(01)00291-2. [DOI] [PubMed] [Google Scholar]
- 34.Tagliarino C, Pink JJ, Dubyak GR, Nieminen AL, Boothman DA. Calcium is a key signaling molecule in beta-lapachone-mediated cell death. J Biol Chem. 2001;276(22):19150–9. doi: 10.1074/jbc.M100730200. [DOI] [PubMed] [Google Scholar]
- 35.Molina Portela MP, Stoppani AO. Redox cycling of beta-lapachone and related o-naphthoquinones in the presence of dihydrolipoamide and oxygen. Biochem Pharmacol. 1996;51(3):275–83. doi: 10.1016/0006-2952(95)02168-x. [DOI] [PubMed] [Google Scholar]
- 36.Manna SK, Gad YP, Mukhopadhyay A, Aggarwal BB. Suppression of tumor necrosis factor-activated nuclear transcription factor-kappaB, activator protein-1, c-Jun N-terminal kinase, and apoptosis by beta-lapachone. Biochem Pharmacol. 1999;57(7):763–74. doi: 10.1016/s0006-2952(98)00354-2. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Li CJ, Yu D, Pardee AB. Potent induction of apoptosis by beta-lapachone in human multiple myeloma cell lines and patient cells. Mol Med. 2000;6(12):1008–15. [PMC free article] [PubMed] [Google Scholar]
- 38.Li YZ, Li CJ, Pinto AV, Pardee AB. Release of mitochondrial cytochrome C in both apoptosis and necrosis induced by beta-lapachone in human carcinoma cells. Mol Med. 1999;5(4):232–9. [PMC free article] [PubMed] [Google Scholar]
- 39.Marin A, Lopez de Cerain A, Hamilton E, et al. DT-diaphorase and cytochrome B5 reductase in human lung and breast tumours. Br J Cancer. 1997;76(7):923–9. doi: 10.1038/bjc.1997.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malkinson AM, Siegel D, Forrest GL, et al. Elevated DT-diaphorase activity and messenger RNA content in human non-small cell lung carcinoma: relationship to the response of lung tumor xenografts to mitomycin Cl. Cancer Res. 1992;52(17):4752–7. [PubMed] [Google Scholar]
- 41.Belinsky M, Jaiswal AK. NAD(P)H:quinone oxidoreductase1 (DT-diaphorase) expression in normal and tumor tissues. Cancer Metastasis Rev. 1993;12(2):103–17. doi: 10.1007/BF00689804. [DOI] [PubMed] [Google Scholar]
- 42.Joseph P, Xie T, Xu Y, Jaiswal AK. NAD(P)H:quinone oxidoreductase1 (DT-diaphorase): expression, regulation, and role in cancer. Oncol Res. 1994;6(10–11):525–32. [PubMed] [Google Scholar]
- 43.Yu SW, Wang H, Poitras MF, et al. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297(5579):259–63. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 44.Bey EA, Bentle MS, Reinicke KE, et al. An NQO1- and PARP-1-mediated cell death pathway induced in non-small-cell lung cancer cells by beta-lapachone. Proc Natl Acad Sci U S A. 2007;104(28):11832–7. doi: 10.1073/pnas.0702176104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16(6):663–9. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 46.Sun X, Li Y, Li W, et al. Selective induction of necrotic cell death in cancer cells by beta-lapachone through activation of DNA damage response pathway. Cell Cycle. 2006;5(17):2029–35. doi: 10.4161/cc.5.17.3312. [DOI] [PubMed] [Google Scholar]
- 47.Braidy N, Guillemin GJ, Grant R. Effects of kynurenine pathway inhibition on NAD metabolism and cell viability in human primary astrocytes and neurons. Int J Tryptophan Res. 2011;4:29–37. doi: 10.4137/IJTR.S7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayaishi O. My life with tryptophan—never a dull moment. Protein Sci. 1993;2:472–5. doi: 10.1002/pro.5560020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balachandran VP, Cavnar MJ, Zeng S, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17(9):1094–100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Enzyme kinetics for hrIDO1. Purified recombinant human IDO1 enzyme shows expected Michaelis-Menton kinetics with a calculated Vmax of 5.9 μM/min and a Km of 110 μM. The velocity is graphed directly from the assay readout in units of absorbance/minute. For determination of the Vmax and Km a standard curve was used to covert to kynurenine concentration-based units.