Abstract
P-glycoprotein (P-gp), an efflux membrane transporter, is widely distributed throughout the body and is responsible for limiting cellular uptake and the distribution of xenobiotics and toxic substances. Hundreds of structurally diverse therapeutic agents are substrates to it and it impedes the absorption, permeability, and retention of the drugs, extruding them out of the cells. It is overexpressed in cancer cells and accountable for obstructing cell internalization of chemotherapeutic agents and for developing transporter mediated resistance by cancer cells during anti-tumor treatments. As it jeopardizes the success of drug delivery and cancer targeting, strategies are being developed to overcome P-gp mediated drug transport. This concise review represents a brief discussion on P-gp mediated drug transport and how it hinders the success of various therapies. Its main focus is on various strategies used to tackle this curb in the field of drug delivery and targeting.
Keywords: P-glycoprotein, drug efflux, bioavailability, drug delivery, drug resistance, P-glycoprotein inhibitor
Introduction
P-glycoprotein (P-gp) is one of the first members of the ATP-binding cassette (ABC) transporter which acts as a physiological barrier by extruding toxins and xenobiotics out of cells.1,2 It is being extensively studied and experimented upon recently and is gaining much importance in numerous researches. P-gp is primarily found in epithelial cells which have the excretory roles including apical surface of epithelial cells lining the colon, small intestine, pancreatic ductules, bile ductules, kidney proximal tubules, and the adrenal gland.3–5 It is also located in the endothelial cells of the blood brain barrier (BBB).6 The transporter is overexpressed on the surface of many neoplastic cells and restricts cell entry. The role of P-gp is likely to protect these susceptible organs from toxic compounds, preventing them to enter the cytosol and extrude them to the exterior.7 Thus it also enhances the secretion of metabolites and xenobiotics into bile, urine, and the lumen of gastrointestinal tract.1 P-gp in human forms a small gene family with two isoforms. The class I isoform (MDR1/ABCB1) is a drug transporter while the class II isoform (MDR2/3/ABCB4) carries out export of phosphatidylcholine into the bile.2,8 A single P-gp molecule can recognize and transport numerous drugs with a wide range of chemical structures, ranging from a molecular weight of 250 g/mol (cimetidine) to 1202 g/mol (cyclosporin).9
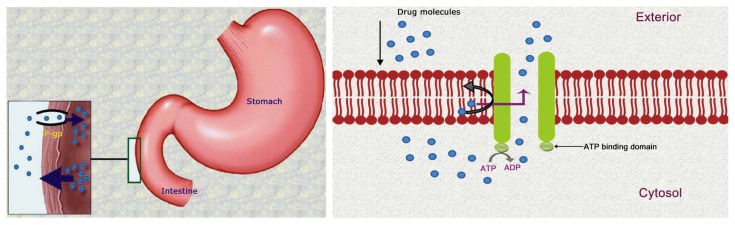
P-gp can extrude a wide range of structurally diverse compounds out of the cells. Hundreds of substrates (usually hydrophobic) interact with this ATP dependent transporter including anticancer agents, immunosuppressants, steroid hormones, calcium channel blockers, beta-adrenoreceptor blockers, cardiac glycosides, among others.2,10,11 Less permeable drugs (weak substrates) may also undergo a substantial extrusion. Thus it contributes greatly in the extrusion of many drugs from the blood into the intestinal lumen. P-gp is also responsible for enhancing the excretion of drugs out of hepatocytes and renal tubules into the adjacent luminal space. Therefore, P-gp can potentially reduce the absorption and oral bioavailability and decrease the retention time of a number of drugs.9,10 Additionally, it has a role in limiting cellular uptake of drugs from blood circulation into the brain while being present in the BBB.6 P-gp is overexpressed in cancer cells and is responsible for drug efflux in tumors. It prevents cell internalization of chemotherapeutic agents and makes the chemotherapy almost ineffective in many cases (Fig. 1). Hence, this protein is one of the main barriers in cancer treatment by chemotherapy.12,13 A variety of strategies are being developed to overcome the difficulties associated with P-gp in optimum drug delivery. Those include not only inhibition of P-gp, but also various techniques to bypass it.1,4,15,16 These promising approaches for optimizing drug delivery and targeting will be the focus of discussions in this review.
Figure 1.
Drug efflux by P-glycoprotein.
Inhibition of P-gp
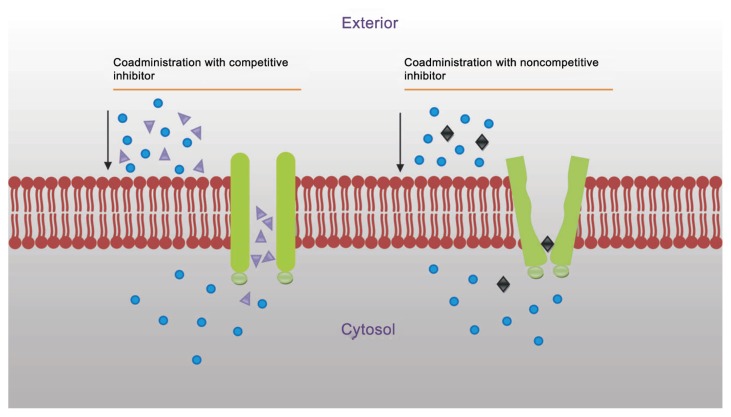
The inhibition of efflux pump is mainly done in order to improve the delivery of therapeutic agents. In general, P-gp can be inhibited by three mechanisms: (i) blocking drug binding site either competitively, non-competitively (Fig. 2) or allosterically; (ii) interfering with ATP hydrolysis; and (iii) altering integrity of cell membrane lipids.1,10,17–19 The goal is to achieve improved drug bioavailability, uptake of drug in the targeted organ, and more efficacious cancer chemotherapy through the ability to selectively block the action of P-gp. Inhibitors are as structurally diverse as substrates.19 Many inhibitors (verapamil, cyclosporin A, trans-flupenthixol, etc.) are themselves transported by P-gp.2
Figure 2.
Inhibition of P-glycoprotein to prevent drug efflux.
P-gp inhibitors are classified into three generations based on their specificity, affinity, and toxicity. First generation inhibitors are pharmacologically active substances which are clinically used for specific treatments but have the ability to inhibit P-gp. The usage of first generation inhibitors is limited due to their high serum concentrations (at the doses that are required to inhibit P-gp) and potential toxicity.20,21 They are substrate to other transporters and enzyme systems, resulting in unpredictable pharmacokinetic interactions. Second generation inhibitors lack the pharmacological activities and possess a greater P-gp affinity which include non-immunosuppressive analogues of cyclosporin A (PSC833) and D-isomer of verapamil (dexverapamil). However, second generation inhibitors inhibit the CYPA4 enzyme and other ABC transporters. Hence, metabolizing rate decreases and inhibition of two or more ABC transporters lead to complicated pharmacokinetic alterations. Third generation P-gp inhibitors are under clinical development, aiming to inhibit P-gp with higher specificity and lower toxicity (eg, tariquidar). They are developed by using structure activity relationships and many were found to be very specific and effective against P-gp, having minimal toxicity.22–24Table 1 illustrates the detailed classification of P-gp inhibitors.
Table 1.
| Generations | Examples | Specificity | Limitations |
|---|---|---|---|
| First generation | Verapamil, cyclosporin A, reserpine, quinidine, yohimbine, tamoxifen and toremifena | Non-selective and low binding affinities. | They are substrates to other transporters and enzyme systems. They are pharmacologically active. They themselves are transported by P-gp. |
| Second generation | Dexverapamil, dexniguldipine, valspodar (PSC 833), and Dofequidar fumarate (MS-209) | Higher specificity then first generation inhibitors but interact with other systems. | They are substrates to CYP 3A4 enzyme and other ABC transporters. |
| Third generation | Cyclopropyldibenzosuberane zosuquidar (LY335979), laniquidar (R101933), mitotane (NSC-38721), biricodar (VX-710), elacridar (GF120918/GG918), ONT-093, tariquidar (XR9576), and HM30181 | Highest specificity that specifically and potently inhibit P-gp function. | No limitations like the first and the second generation inhibitors. |
Monoclonal antibodies can be effectively used against P-gp in multidrug resistance (MDR) tumor cells. Many anti-P-gp monoclonal antibodies, such as MRK16 and MRK17, have been developed to overcome multidrug resistance. Conjugated monoclonal antibodies, such as bispecific antibody, immunotoxin, and radioisotope conjugates, have also been constructed to enhance the anti-tumor activity of monoclonal antibodies.28 The conformation-sensitive UIC2 monoclonal antibody can inhibit P-gp mediated substrate transport (calcein-AM, daunorubicin, and 99 mTc-hexakis-2-methoxybutylisonitrile). However, inhibition by UIC2 alone is usually partial because UIC2 binds only 10% to 40% of P-gp present in the cell membrane. This antibody recognizes and inhibits the rest of the P-gp molecules only in the presence of certain inhibitors, including vinblastine, cyclosporine A, and PSC 833 (valspodar). Therefore, simultaneous application of any of these inhibitors and UIC2 can enhance accumulation of certain substrates of P-gp by total inhibition of P-gp pump activity.29
Enhancement of Bioavailability and Transport
Even though P-gp decreases drug absorption of all of its substrates, it quantitatively does not have an equal significant impact on overall drug absorption for all of them. In the case of fast absorbing drugs having larger doses, efflux by P-gp poses less impact on drug absorption and therefore is not important in terms of bioavailability or pharmacokinetic properties. This is because the transport activity of P-gp becomes saturated by high concentrations of drug in the intestinal lumen. In the case of drugs requiring a very small dose for their pharmacological actions or the drugs that have very slow dissolution and diffusion rates, P-gp mediated drug efflux greatly interferes with their delivery. As it decreases drug absorption, those small amounts of drugs cannot reach the blood circulation in sufficient quantity and, at times, can be life threatening. Moreover, it can make the sustained release dosage forms of the substrates completely ineffective by limiting their absorptions.9
Usually, an inhibitor of P-gp is coadministered with the drug to enhance drug absorption.30,31 Methods are being developed in preparing clinically useful oral formulations of drugs having poor oral absorption, which are administered only by parenteral routes. When a drug and an inhibitor are coadministered, P-gp mediated transport mechanism remains the same for both the drug and the inhibitor, if the inhibitor is a substrate of it. In this process, drug and inhibitor must be discriminable by P-gp at molecular level as drug molecules should not be extruded. This is why a large difference between the rate of efflux of the substrate and the inhibitor is created. When the inhibitor molecules are effluxed, they rapidly bind to the P-gp binding sites again and thus the inhibition of P-gp by the modulators is cycled repeatedly, preventing efflux of drugs. This process depends on the hydrophobicity of the compounds.32,33
HM30181, a newly developed third generation P-gp inhibitor, showed promising results for increasing oral absorption of some drugs in recent studies. Kwak et al34 examined its pharmacologic characteristics and it showed the highest potency among several P-gp inhibitors, including cyclosporin A, XR9576, and GF120918. The inhibitory activity of HM30181 was highly selective to P-gp and it did not inhibit other ABC transporters. Its coadministration (10 mg/kg) greatly increased oral bioavailability of paclitaxel from 3.4% to 41.3% in rats. Importantly, oral coadministration of paclitaxel and HM30181showed a tumor inhibitory strength equal or superior to that of intravenous paclitaxel in the xenograft model in nude mice.34
P-gp inhibitors may have a great impact on altering pharmacokinetics of a drug.10 Since P-gp molecules are present in many organs like BBB, kidney proximal tubule, and bile ductule, their inhibition can potentially improve not only the absorption, but also the distribution, metabolism, and elimination of their substrates.10,35 Asperen et al36 observed a 10-fold increased oral bioavailability of paclitaxel in mice administered along with a P-gp blocker (valspodar). Sugie et al37 articulated that coadministration of azithromycin and cyclosporine resulted in decreased billiary excretion of azithromycin. BBB is considered as the main barrier to prevent drugs entering the central nervous system (CNS). Presence of P-gp in the BBB makes it additionally difficult, effluxing the drug molecules from the CNS.2,38 P-gp inhibition can prevent P-gp mediated drug efflux and assist the substrate molecules to enter the CNS. Kemper et al38 reported a 5-fold increase in brain uptake of paclitaxel by GF120918 (elacridar), a third generation P-gp inhibitor. A reduced clearance of paclitaxel was noticed, assuming the inhibition of P-gp in elimination pathway.38 P-gp inhibition can also increase the half-lives of the substrates as the inhibition may reduce billiary excretion and the clearance of the substrates in kidney proximal tubule, increasing renal reuptake. Orally coadministered doxorubicin and verapamil have shown to increase peak plasma level, prolong elimination of half-life, and increase volume of distribution of doxorubicin after oral administration.39 Both natural and synthetic inhibitors can be coadministered with drugs in oral dosage forms including polyethylene glycol (PEG) based surfactants, anionic gums, sodium alginate, poloxamers, thiomers, dendrimers, etc. Among these inhibitors, surfactants are considered to be the better choice as they were already approved for routine use in pharmaceutical formulations. These surfactants act by altering integrity of membrane lipids by changing the secondary and tertiary structure. Thus the function of P-gp is hampered due to disturbance in hydrophobic environment.10,40
Antimicrobial therapy
Drug efflux is a common mechanism of resistance in microorganisms, along the same lines as target modification or production of antibiotic inactivating enzymes. Efflux pumps are now recognized as one of the most important factors in accumulation of antimicrobials in all cell types, from prokaryotes to superior eukaryotes. MDR, mediated through efflux pumps, has been described for various organisms, including bacteria, fungi, and protozoa. All transporters, but the ABC family, function as secondary transporters. P-gp is one of the main ABC transporters that is greatly responsible for MDR in microorganisms.41–43 P-gp molecules prevent antimicrobial agents from entering the microorganisms. They reduce intracellular drug concentrations and thereby impede accessibility of drugs to their sites of action, ultimately leading to reduced susceptibility. This transport mechanism may result in treatment failure in many infectious diseases.44,45 MDR is a major problem in bacterial infections because it limits the option of antimicrobial agents. The treatment becomes troublesome in the case of the infections where P-gp substrates are the only choice. It poses several complications including increased costs, prolonged duration of hospital stay, and higher morbidity and mortality rates.46–48 The use of inhibitors may be a good strategy to overcome transporter mediated bacterial multidrug resistance. They can be used in conjunction with antibiotics and can extend the lifetime of the antibiotics, improving therapeutic efficacy. They can also suppress the emergence of resistant variants that may arise during the treatment. These inhibitors increase bacterial susceptibility towards antimicrobial agents.41,49–51
Seral et al52 examined the influence of inhibitors of P-gp (verapamil, cyclosporine, and GF120918) on the antimicrobial activity of macrolides (erythromycin, clarithromycin, roxithromycin, azithromycin, and telithromycin) in J774 murine macrophages. The result showed that P-gp enhanced the extrusion of azithromycin, erythromycin, telithromycin, and roxithromycin, resulting in suboptimal drug accumulation. Hence, inhibitors can enhance their accumulations inside the cells and increase antimicrobial actions.52
Leitner et al49 investigated the potency of tariquidar, a third-generation P-gp inhibitor, for overcoming bacterial resistance towards ciprofloxacin. Activity of tariquidar was evaluated by Staphylococcus aureus strains. The addition of tariquidar resulted in a 10-fold reduction in the minimum inhibitory concentration (MIC) of ciprofloxacin. The result suggested that tariquidar significantly increased susceptibility of S. aureus towards ciprofloxacin. Their high activity can be very promising for applications in infectious diseases. Their study also showed the improved susceptibility of Stenotrophomonas maltophilia towards elacridar.48
Cancer Chemotherapy
P-gp is overexpressed on the surface of cancer cells and prevents drug accumulation inside the tumor, acting as the efflux pump. It extrudes anticancer drugs before they can reach the intended target. Further, it often mediates the development of resistance of the cells to anticancer drugs. Therefore, the administered drugs remain ineffective or cannot bring the desired output. Several approaches have been taken to overcome P-gp mediated drug resistance.53,54 P-gp locates drugs which are localized in the plasma membrane only. Concurrent administration of cytotoxic drugs and inhibiting agents, like verapamil or cyclosporine, can restrain P-gp mediated extrusion and facilitate the drug in reaching the targeted area. Thus both chemotherapeutic agent and inhibiting agent are incorporated into the carrier system to overcome the difficulty associated with P-gp.55,56 Another strategy could be the involvement of anti-P-gp monoclonal antibody in refraining P-gp from extruding drugs. In this process, the antibody is conjugated to the drug loaded carrier system, which can sufficiently inhibit drug efflux.
Vincristine-loaded lipid nanoparticles conjugated to an anti-P-gp monoclonal antibody (MRK-16), showed greater cytotoxicity in resistant human myelogenous leukemia cell lines than non-targeted particles.14 Goda et al29 reported that combination therapy with UIC2 monoclonal antibody and cyclosporine A, a first generation P-gp inhibitor administered at the dose ineffective when applied alone, dramatically increased daunorubicin accumulation in xenotransplanted Pgp+ tumors. It established that combined application of UIC2 antibody and a class of modulators, used at low concentrations, can be a specific and effective way of blocking P-gp function in vivo.29 Danson et al57 developed SP1049C, a non-ionic block copolymer composed of a hydrophobic core and hydrophilic tail, which contained doxorubicin and was able to circumvent P-gp mediated drug resistance in a mouse model of leukaemia.57,58 Tidefelt et al59 found that by interacting with P-gp, valspodar can increase the cellular uptake of daunorubicin in leukemic blasts in vivo. In another study, folic acid, attached to PEG derivatized distearoyl-phosphatidylethanolamine in doxorubicin loaded liposomes, was used to target folate receptor overexpressing tumor cells. Folate receptor mediated cell uptake of targeted liposomal doxorubicin into a multidrug resistant subline of M109-HiFR cells (M109R-HiFR) was clearly unaffected by P-gp mediated drug efflux, in sharp contrast to uptake of free doxorubicin.15
Conclusion
P-gp is one of the main barriers for delivering drugs properly. A variety of approaches are being tested to develop P-gp inhibitors or mechanisms to bypass it. Proper inhibition will allow not only an increase in cellular uptake, transport, and half-lives of drugs, but also to predict their pharmacokinetics accurately and fine tune them for targeting specific region. These advances will result in cost effective therapy by saving the additional amount of drugs that was previously wasted by P-gp transport. Furthermore, it will shorten the treatment time with optimal drug delivery. Thus it can bring a great revolution in the field of drug delivery.
Footnotes
Author Contributions
Conceived and designed the experiments: MLA. Analyzed the data: MLA. Wrote the first draft of the manuscript: MLA. Contributed to the writing of the manuscript: MLA. Agree with manuscript results and conclusions: MLA. Jointly developed the structure and arguments for the paper: MLA. Made critical revisions and approved final version: MLA. Author reviewed and approved of the final manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication the author has provided signed confirmation of compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
Funding
Author(s) disclose no funding sources.
References
- 1.Srivalli KMR, Lakshmi PK. Overview of P-glycoprotein inhibitors: a rational outlook. Braz J Pharm Sci. 2012;48(3):353–67. [Google Scholar]
- 2.Sharom FJ. The P-glycoprotein multidrug transporter. Essays Biochem. 2011;50(1):161–78. doi: 10.1042/bse0500161. [DOI] [PubMed] [Google Scholar]
- 3.Edwards JE, Alcorn J, Savolainen J, Anderson BD, McNamara PJ. Role of P-glycoprotein in distribution of nelfinavir across the blood-mammary tissue barrier and blood-brain barrier. Antimicrob Agents Chemother. 2005;49(4):1626–8. doi: 10.1128/AAC.49.4.1626-1628.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melaine N, Liénard MO, Dorval I, Le Goascogne C, Lejeune H, Jégou B. Multidrug resistance genes and P-glycoprotein in the testis of the rat, mouse, guinea pig, and human. Biol Reprod. 2002;67(6):1699–707. doi: 10.1095/biolreprod.102.003558. [DOI] [PubMed] [Google Scholar]
- 5.Beaulieu E, Demeule M, Ghitescu L, Beliveau R. P-glycoprotein is strongly expressed in the luminal membranes of the endothelium of blood vessels in the brain. Biochem J. 1997;326(Pt 2):539–44. doi: 10.1042/bj3260539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma JD, Tsunoda SM, Bertino JS, Jr, Trivedi M, Beale KK, Nafziger AN. Evaluation of in vivo P-glycoprotein phenotyping probes: a need for validation. Clin Pharmacokinet. 2010;49(4):223–37. doi: 10.2165/11318000-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Fortuna A, Alves G, Falcao A. In vitro and in vivo relevance of the P-glycoprotein probe substrates in drug discovery and development: focus on rhodamine 123, digoxin and talinolol. J Bioequiv Availab. 2011:S2. [Google Scholar]
- 8.Ruetz S, Gros P. Phosphatidylcholine translocase: a physiological role for the mdr2 gene. Cell. 1994;77(7):1071–81. doi: 10.1016/0092-8674(94)90446-4. [DOI] [PubMed] [Google Scholar]
- 9.Lin JH, Yamazaki M. Role of P-glycoprotein in parmacokinetics: clinical implications. Clin Pharmacokinet. 2003;42(1):59–98. doi: 10.2165/00003088-200342010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Varma MVS, Ashokraj Y, Dey CS, Panchagnula R. P-glycoprotein inhibitors and their screening: a perspective from bioavailability enhancement. Pharmacol Res. 2003;48(4):347–59. doi: 10.1016/s1043-6618(03)00158-0. [DOI] [PubMed] [Google Scholar]
- 11.Sharom FJ, Liu R, Qu Q, Romsicki Y. Exploring the structure and function of the Pglycoprotein multidrug transporter using fluorescence spectroscopic tools. Seminars Cell Dev Biol. 2001;12(3):257–65. doi: 10.1006/scdb.2000.0251. [DOI] [PubMed] [Google Scholar]
- 12.Martins A, Vasas A, Schelz Z, et al. Constituents of Carpobrotus edulis inhibit P-glycoprotein of MDR1-transfected mouse lymphoma cells. Anticancer Res. 2010;30(3):829–35. [PubMed] [Google Scholar]
- 13.Bansal T, Jaggi M, Khar RK, Talegaonkar S. Emerging significance of flavonoids as P-glycoprotein inhibitors in cancer chemotherapy. J Pharm Pharm Sci. 2009;12(1):46–78. doi: 10.18433/j3rc77. [DOI] [PubMed] [Google Scholar]
- 14.Matsuo H, Wakasugi M, Takanaga H, et al. Possibility of the reversal of multidrug resistance and the avoidance of side effects by liposomes modified with MRK-16, a monoclonal antibody to P-glycoprotein. J Control Release. 2001;77(1–2):77–86. doi: 10.1016/s0168-3659(01)00460-6. [DOI] [PubMed] [Google Scholar]
- 15.Goren D, Horowitz AT, Tzemach D, Tarshish M, Zalipsky S, Gabizon A. Nuclear delivery of doxorubicin via folate-targeted liposomes with bypass of multidrug-resistance efflux pump. Clin Cancer Res. 2000;6(5):1949–57. [PubMed] [Google Scholar]
- 16.Mazel M, Clair P, Rousselle C, et al. Doxorubicin—peptide conjugates overcome multidrug resistance. Anticancer Drugs. 2001;12(2):107–16. doi: 10.1097/00001813-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro AB, Ling V. Effect of quercetin on Hoechst 33342 transport by purified and reconstituted P-glycoprotein. Biochem Pharmacol. 1997;53(4):587–96. doi: 10.1016/s0006-2952(96)00826-x. [DOI] [PubMed] [Google Scholar]
- 18.Drori S, Eytan GD, Assaraf YG. Potentiation of anticancer drug cytotoxicity by multidrug-resistance chemosensitizers involves alterations in membrane fluidity leading to increased membrane permeability. Eur J Biochem. 1995;228(3):1020–9. doi: 10.1111/j.1432-1033.1995.tb20352.x. [DOI] [PubMed] [Google Scholar]
- 19.Robert J, Jarry C. Multidrug resistance reversal agents. J Med Chem. 2003;46(23):4805–17. doi: 10.1021/jm030183a. [DOI] [PubMed] [Google Scholar]
- 20.Lomovskaya O, Bostian KA. Practical applications and feasibility of efflux pump inhibitors in the clinic—a vision for applied use. Biochem Pharmacol. 2006;71(7):910–8. doi: 10.1016/j.bcp.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Lomovskaya O, Zgurskaya HI, Totrov M, Watkins WJ. Waltzing transporters and ‘the dance macabre’ between humans and bacteria. Nat Rev Drug Discov. 2007;6(1):56–65. doi: 10.1038/nrd2200. [DOI] [PubMed] [Google Scholar]
- 22.Kuppens IE, Witteveen EO, Jewell RC, et al. A phase I, randomized, open-label, parallel-cohort, dose-finding study of elacridar (GF120918) and oral topotecan in cancer patients. Clin Cancer Res. 2007;13(11):3276–85. doi: 10.1158/1078-0432.CCR-06-2414. [DOI] [PubMed] [Google Scholar]
- 23.Pusztai L, Wagner P, Ibrahim N, et al. Phase II study of tariquidar, a selective P-glycoprotein inhibitor, in patients with chemotherapy-resistant, advanced breast carcinoma. Cancer. 2005;104(4):682–91. doi: 10.1002/cncr.21227. [DOI] [PubMed] [Google Scholar]
- 24.Malingré MM, Beijnen JH, Rosing H, et al. Co-administration of GF120918 significantly increases the systemic exposure to oral paclitaxel in cancer patients. Br J Cancer. 2001;84(1):42–7. doi: 10.1054/bjoc.2000.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas H, Coley HM. Overcoming multidrug resistance in cancer: an update on the clinical strategy of inhibiting p-glycoprotein. Cancer Control. 2003;10(2):159–65. doi: 10.1177/107327480301000207. [DOI] [PubMed] [Google Scholar]
- 26.Krishna R, Mayer LD. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci. 2000;11(4):265–83. doi: 10.1016/s0928-0987(00)00114-7. [DOI] [PubMed] [Google Scholar]
- 27.Newman MJ, Rodarte JC, Benbatoul KD, et al. Discovery and characterization of OC144–093, a novel inhibitor of P-glycoprotein-mediated multidrug resistance. Cancer Res. 2000;60(11):2964–72. [PubMed] [Google Scholar]
- 28.Nokihara H, Yano S, Sone S. Overcoming of multidrug resistance by anti-P-glycoprotein monoclonal antibody. Nippon Rinsho. 1997;55(5):1128–34. Japanese. [PubMed] [Google Scholar]
- 29.Goda K, Fenyvesi F, Bacsó Z, et al. Complete inhibition of P-glycoprotein by simultaneous treatment with a distinct class of modulators and the UIC2 monoclonal antibody. J Pharmacol Exp Ther. 2007;320(1):81–8. doi: 10.1124/jpet.106.110155. [DOI] [PubMed] [Google Scholar]
- 30.Woo JS, Lee CH, Shim CK, Hwang SJ. Enhanced oral bioavailability of paclitaxel by coadministration of the P-glycoprotein inhibitor KR30031. Pharm Res. 2003;20(1):24–30. doi: 10.1023/a:1022286422439. [DOI] [PubMed] [Google Scholar]
- 31.Banerjee SK, Jagannath C, Hunter RL, Dasgupta A. Bioavailability of tobramycin after oral delivery in FVB mice using CRL-1605 copolymer, an inhibitor of P-glycoprotein. Life Sci. 2000;67(16):2011–16. doi: 10.1016/s0024-3205(00)00786-4. [DOI] [PubMed] [Google Scholar]
- 32.Eytan GD, Regev R, Oren G, Assaraf YG. The role of passive transbilayer drug movement in multidrug resistance and its modulation. J Biol Chem. 1996;271(22):12897–902. doi: 10.1074/jbc.271.22.12897. [DOI] [PubMed] [Google Scholar]
- 33.Döppenschmitt S, Spahn-Langguth H, Regårdh CG, Langguth P. Role of P-glycoprotein-mediated secretion in absorptive drug permeability: An approach using passive membrane permeability and affinity to P-glycoprotein. J Pharm Sci. 1999;88(10):1067–72. doi: 10.1021/js980378j. [DOI] [PubMed] [Google Scholar]
- 34.Kwak JO, Lee SH, Lee GS, et al. Selective inhibition of MDR1 (ABCB1) by HM30181 increases oral bioavailability and therapeutic efficacy of paclitaxel. Eur J Pharmacol. 2010;627(1–3):92–8. doi: 10.1016/j.ejphar.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Batrakova EV, Miller DW, Li S, Alakhov VY, Kabanov AV, Elmquist WF. Pluronic P85 enhances the delivery of digoxin to the brain: in vitro and in vivo studies. J Pharmacol Exp Ther. 2001;296(2):551–7. [PubMed] [Google Scholar]
- 36.van Asperen J, van Tellingen O, Sparreboom A, et al. Enhanced oral bioavailability of paclitaxel in mice treated with the P-glycoprotein blocker SDZ PSC 833. Br J Cancer. 1997;76(9):1181–3. doi: 10.1038/bjc.1997.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugie M, Asakura E, Zhao YL, et al. Possible involvement of the drug transporters P glycoprotein and multidrug resistance-associated protein Mrp2 in disposition of azithromycin. Antimicrob Agents Chemother. 2004;48(3):809–14. doi: 10.1128/AAC.48.3.809-814.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kemper EM, van Zandbergen AE, Cleypool C, et al. Increased penetration of paclitaxel into the brain by inhibition of P-Glycoprotein. Clin Cancer Res. 2003;9(7):2849–55. [PubMed] [Google Scholar]
- 39.Kerr DJ, Graham J, Cummings J, et al. The effect of verapamil on the pharmacokinetics of adriamycin. Cancer Chemother Pharmacol. 1986;18(3):239–42. doi: 10.1007/BF00273394. [DOI] [PubMed] [Google Scholar]
- 40.Werle M. Natural and synthetic polymers as inhibitors of drug efflux pumps. Pharm Res. 2008;25(3):500–11. doi: 10.1007/s11095-007-9347-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gibbons S, Oluwatuyi M, Kaatz GW. A novel inhibitor of multidrug efflux pumps in Staphylococcus aureus. J Antimicrob Chemother. 2003;51(1):13–7. doi: 10.1093/jac/dkg044. [DOI] [PubMed] [Google Scholar]
- 42.Poole K. Efflux-mediated antimicrobial resistance. J Antimicrob Chemother. 2005;56(1):20–51. doi: 10.1093/jac/dki171. [DOI] [PubMed] [Google Scholar]
- 43.Normark BH, Normark S. Evolution and spread of antibiotic resistance. J Intern Med. 2002;252(2):91–106. doi: 10.1046/j.1365-2796.2002.01026.x. [DOI] [PubMed] [Google Scholar]
- 44.Poole K. Mechanisms of bacterial biocide and antibiotic resistance. J Appl Microbiol. 2002;92:55S–64. [PubMed] [Google Scholar]
- 45.Wright GD. Mechanisms of resistance to antibiotics. Curr Opin Chem Biol. 2003;7(5):563–9. doi: 10.1016/j.cbpa.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 46.Huet AA, Raygada JL, Mendiratta K, Seo SM, Kaatz GW. Multidrug efflux pump overexpression in Staphylococcus aureus after single and multiple in vitro exposures to biocides and dyes. Microbiology (Reading, Engl) 2008;154(Pt 10):3144–53. doi: 10.1099/mic.0.2008/021188-0. [DOI] [PubMed] [Google Scholar]
- 47.Escribano I, Rodríguez JC, Llorca B, García-Pachon E, Ruiz M, Royo G. Importance of the efflux pump systems in the resistance of Mycobacterium tuberculosis to fluoroquinolones and linezolid. Chemotherapy. 2007;53(6):397–401. doi: 10.1159/000109769. [DOI] [PubMed] [Google Scholar]
- 48.Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med. 2004;10(Suppl 12):S122–9. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 49.Leitner I, Nemeth J, Feurstein T, et al. The third-generation P-glycoprotein inhibitor tariquidar may overcome bacterial multidrug resistance by increasing intracellular drug concentration. J Antimicrob Chemother. 2011;66(4):834–9. doi: 10.1093/jac/dkq526. [DOI] [PubMed] [Google Scholar]
- 50.Van Bambeke F, Pagès JM, Lee VJ. Inhibitors of bacterial efflux pumps as adjuvants in antibiotic treatments and diagnostic tools for detection of resistance by efflux. Recent Pat Antiinfect Drug Discov. 2006;1(2):157–75. doi: 10.2174/157489106777452692. [DOI] [PubMed] [Google Scholar]
- 51.Renau TE, Léger R, Flamme EM, et al. Inhibitors of efflux pumps in Pseudomonas aeruginosa potentiate the activity of the fluoroquinolone antibacterial levofloxacin. J Med Chem. 1999;42(24):4928–31. doi: 10.1021/jm9904598. [DOI] [PubMed] [Google Scholar]
- 52.Seral C, Michot JM, Chanteux H, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F. Influence of P-glycoprotein inhibitors on accumulation of macrolides in J774 murine macrophages. Antimicrob Agents Chemother. 2003;47(3):1047–51. doi: 10.1128/AAC.47.3.1047-1051.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mazel M, Clair P, Rousselle C, et al. Doxorubicin-peptide conjugates overcome multidrug resistance. Anticancer Drugs. 2001;12(2):107–16. doi: 10.1097/00001813-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 54.Raub TJ. P-glycoprotein recognition of substrates and circumvention through rational drug design. Mol Pharm. 2005;3(1):3–25. doi: 10.1021/mp0500871. [DOI] [PubMed] [Google Scholar]
- 55.Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2002;54(5):631–51. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 56.Soma CE, Dubernet C, Bentolila D, Benita S, Couvreur P. Reversion of multidrug resistance by co-encapsulation of doxorubicin and cyclosporin A in polyalkylcyanoacrylate nanoparticles. Biomaterials. 2000;21(1):1–7. doi: 10.1016/s0142-9612(99)00125-8. [DOI] [PubMed] [Google Scholar]
- 57.Danson S, Ferry D, Alakhov V, et al. Phase I dose escalation and pharmacokinetic study of pluronic polymer-bound doxorubicin (SP1049C) in patients with advanced cancer. Br J Cancer. 2004;90(11):2085–91. doi: 10.1038/sj.bjc.6601856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Batrakova EV, Dorodnych TY, Klinskii EY, et al. Anthracycline antibiotics non-covalently incorporated into the block copolymer micelles: in vivo evaluation of anti-cancer activity. Br J Cancer. 1996;74(10):1545–52. doi: 10.1038/bjc.1996.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tidefelt U, Liliemark J, Gruber A, et al. P-Glycoprotein inhibitor valspodar (PSC 833) increases the intracellular concentrations of daunorubicin in vivo in patients with P-glycoprotein-positive acute myeloid leukemia. J Clin Oncol. 2000;18(9):1837–44. doi: 10.1200/JCO.2000.18.9.1837. [DOI] [PubMed] [Google Scholar]