Abstract
The mechanism and biological effects of comparing recombinant human epidermal growth factor (rhEGF) with recombinant human basic fibroblast growth factor (rhβFGF) were evaluated on the model of incised wounds on ventral side of rabbit’s ears in order to direct their medication in clinical. Total of 72 incised wounds on ventral side of 24 New Zealand rabbits’ ears were divided randomly into two therapeutic groups (rhEGF group with 10 μg/cm2 and rhβFGF group with 100 AU/cm2) and a control group (1% silver sulfadiazine cream, SD-Ag). The observation of wounds, the measurements of healing wound area, the calculation of wound closing index, the histopathological examination, the electrical microscopic examination, and the expression of examining integrin β1 mRNA of samples of three groups by in situ hybridization technique were used to evaluate the results of wound healing. The results showed that the wound healing was accelerated in all wounds treated with rhEGF and rhβFGF, and the quality of healing wounds of two therapeutic groups was better than that of the control group. In rhβFGF group, new granulation tissue was more than that of rhEGF group in earlier period and metaphase of healing wound, however, the velocity of re-epithelialization in rhEGF group was faster than that of rhβFGF group in metaphase and late period. The results indicate that rhEGF and rhβFGF can improve wound healing, but the detailed mechanisms and the biological effects were different. rhβFGF may be used to promote the growth of granulation tissue in earlier period and metaphase of healing wound, however, rhEGF may be used to accelerate the velocity of re-epithelialization in metaphase and late period. That rhβFGF and rhEGF were utilized in sequence can not only accelerate the velocity of healing wound and promote the quality of healing wound but also obtain the best ratio of effects versus value.
Keywords: Epidermal growth factor, basic fibroblast growth factor, wound healing
Introduction
The cellular growth factor is a special kind of factors which can stimulate the proliferation of target cells, increase the synthesis of extracellular matrix and promote the wound healing [1-4]. It’s an important bioactive polypeptide that participates in wound healing of trauma and large-area burn [5,6]. With the development of modern genetic engineering technology, the growth factor has been batch to obtained and used widely in wound healing. It may not only improve the survival ratio and quality of life, but also reduce the medical costs. The epidermal growth factor (EGF) and basic fibroblast growth factor (βFGF) are the focus of growth factors [7-11]. Because of neglecting the differences of their sources and structures, the differences mechanisms of promoting wound healing, thus impacting on their applications such as the indications and timing. So we would like to study the effective phase and the mechanism of middling dosage rhEGF and rhβFGF on improving the quality of wound healing with relevant references [12,13], through comparing rhEGF with rhβFGF improving the wound healing on the model of incised wounds on ventral side of rabbit’s ears, in order to direct their medication in clinical.
Materials and methods
Animal model
Total of 12 New Zealand rabbits (provided by the department of animal experiments, Sun Yet-Sen University), weight of 2300-3000 gram, male and female unlimited, feeding a week in single cage before the experiment. 8% sodium sulfide is used for removing the hair of ventral side of rabbit’s ears on the day before experiment. We use ether for inhaled anesthesia and rabbit frame for fixed. Three rounds in 1.5 cm diameter are marked with the template along the long axis on the ventral side of each rabbit’s ear, distributing equally. After disinfection, 72 circular wounds of the full thickness defect, deepening to the perichondrium are completed by scalpel along the mark totally. All of the wounds are stochastically divided into two therapeutic groups (rhEGF group and rhβFGF group) and a control group (1% silver sulfadiazine cream, SD-Ag).
Growth factors and application methods
Recombinant human epidermal growth factor (rhEGF) and recombinant human basic fibroblast growth factor (rhβFGF) are provided by Zhuhai YiSheng biological pharmaceutical Co. LTD. The moderate doses of rhEGF (10 μg/cm2) and rhβFGF (100 AU/cm2) are respectively used to the wounds of the therapeutic groups while 1% silver sulfadiazine cream are used to that of the control group. Each wound needs sterilization every day until healing. After cleaning each wound by normal saline and drying by sterile gauze, we use the rhEGF 10 μg/cm2 to cover the wounds and the rhβFGF 100 AU/cm2 to spray the wounds, then bandage the wounds after 5 minutes. While the wounds are covered by 1% silver sulfadiazine cream and bandaged in the control group.
General observation
We observed granulation tissue growth and wound healing time, and described the area of wound healing with the wound photography and transparent graph paper. The wound area was measured by the transparent graph paper, through the computer image analysis technique. The wound closing index was calculated according to the following formula: wound closing index = (1 - post-treatment wound area) / original wound area × 100%.
Histopathological examination
The wound organization were taken on 3rd day, 7th day, 14th day, 21th day, 28th day, 60th day and 90th day. The specimen were stained by HE staining (hematoxylin-eosin staining, HE staining) after fixed by the 10% formalin liquid. We observed the granulation tissue growth and epithelization time. We calculated randomly the number of the fibroblasts and capillaries in the five visions of 10 × 20 times, then take the average value. We detected the collagen and the quality of the wound healing by VG staining (Van - Gerson staining, VG staining). All the differences would be compared between the groups.
Electrical microscopic examination
The specimen were taken on 3rd day, 7th day, 14th day, 21th day, 28th day, 60th day and 90th day, fixed by electron fixed liquid. We observed the ultra-micro structure, including the cellular and sub-cellular structures, the cellular relationship and the capillaries, the collagen, etc. Then we could compare the similarities and differences of ultra-micro structure between the groups.
Integrin β1 mRNA in situ hybridization
The specimen were taken on 3rd day, 7th day, 14th day, 21th day, 28th day, 60th day and 90th day, fixed 4-6 hours by 4% polyformaldehyde (dispensing by 0.1 MPBS contained 0.1% of the DEPC). We cut it into slices of 5-7 μm thickness, then completed the situ hybridization according to the instructions of integrin β1 mRNA kit (Wuhan Boshide biological technology Co. LTD). We examined and compared the expression of integrin β1 mRNA between the groups.
Statistical analysis
We used the SPSS11.0 statistics software package for analysis. The data was described as x̅ ± s. The differences between groups were compared by the chi-square test (P < 0.05 for statistical significance).
Results
General observation
Before the healing, the wounds of two treatment groups were dry. The granulation tissue was soft, rosy, easy to bleed and grew actively. While exudation, swelling, paleness, toughness and hard to bleed could be seen on the wound of control group. From 3rd to 14th day, the granulation tissue of the rhβFGF group grew faster than that in the rhEGF group. The granulation tissue of the rhβFGF group was big granular and higher than the original wound, while that of the rhEGF group was small granular. The epithelial tissues of the rhβFGF group is not obvious at the wound edge, on the contrary, that of the rhEGF group is obvious. From 14th to 21th day, the marginal tissue of the rhEGF group crawled obviously. Epithelization would be seen at the same time. At the rhEGF group, the crawling velocity of the epithelium would increase from 21th to 24th day, and the wound was fully covered with epithelium and healing after 24th day, while the granulation tissue of the rhβFGF group grew slowly and the wound gradually shrink. The wound of the two therapeutic groups would heal completely for 28th day. The healing wound surface was brightly red and the epithelization was almost obvious in the two therapeutic groups. While the complete wound healing of the control group needed 30 days, and the healing wound surface was dark red and higher than the normal skin. Compared with the two control group, the hypertrophic scar of the two therapeutic groups was less apparent, the difference of epithelial color was less obviously and a few hair growth could be seen on the healing surface on the 60-90 days. The wound closing index of the two therapeutic groups was significantly different from the control group (P < 0.05). Meanwhile, the difference of the wound closing index was also significant between the two therapeutic groups (P < 0.05) (Table 1).
Table 1.
Comparison of wound closing index (%, ± s) at different time phages in rhEGF group, rhβFGF group and control group (χ2 test)
| 7 days (n = 24) | 14 days (n = 22) | 21 days (n = 18) | 28 days (n = 14) | |
|---|---|---|---|---|
| rhβFGF group (100 AU/cm2) | 17.30 ± 3.23 | 37.43 ± 5.31 | 71.73 ± 7.12 | 98.15 ± 2.45 |
| rhEGF group (10 μg/cm2) | 13.97 ± 1.48 | 31.22 ± 4.53 | 87.23 ± 5.41 | 99.37 ± 5.39 |
| control group (SD-Ag) | 8.75 ± 2.31 | 15.34 ± 3.17 | 30.17 ± 4.92 | 79.86 ± 4.62 |
Histopathological examination
Among the specimens of the three groups stained by HE, we could find that the number of fibroblast and capillary in the rhβFGF group was significantly more than that in the rhEGF group, and that of the two therapeutic groups was also significantly more than that of the control group in the earlier period (3~7 days). In the metaphase (7~14 days), the number of fibroblast and capillary was no significant difference by comparing the rhβFGF group with the rhEGF group, but that was significant difference by comparing the two therapeutic groups with the control group (P < 0.05). On the 28th day, the number of the epithelial layers in the rhEGF group was more than that in the rhβFGF group, but the wound of the control group did not healed completely. Compared to the control group, the fibroblast and capillaries of the two therapeutic groups were significantly less on the 60th-90th day. We could find that the collagen was slim and arranged regularly and the basic membrane was complete and the dermal papillary was visible in the two therapeutic groups, on the contrary, the collagen was crassitude and arranged disorderly and the basic membrane was incomplete and the dermal papillary was invisible in the control group. The number of collagen stained by VG in the two therapeutic groups was significantly more than that in the control group, and there was no significant difference by comparing the rhβFGF group with the rhEGF group.
Electrical microscopic examination
Compared to the control group on the 3rd~14th day, the specimens of the two therapeutic groups had more fibroblasts, 2-3 nucleoli, expansion endoplasmic reticulum and hypertrophic mitochondria. Desmosome junction could be seen among the fibroblasts, macrophages and mastocytes. The number of capillary increased and the cavity filled with red blood cells expanded. The number of fibroblast and capillary in the rhβFGF group was more than that in the rhEGF group on the 3rd-14th day (Figure 1); In the late period (60th-90th day), compared to the control group, less fibroblast of the therapeutic groups could be seen microscopically. The nuclear chromatin concentrated at the edge. The expansion of endoplasmic reticulum was not apparent. The lysosome in macrophages increased with cell fragments. The mastocyte degranulated into vacuolation. The collagen fiber became slim with regular arrangement. The capillary decreased with closed lumen, but there was hypertrophic scar ultrastructure in the control group.
Figure 1.
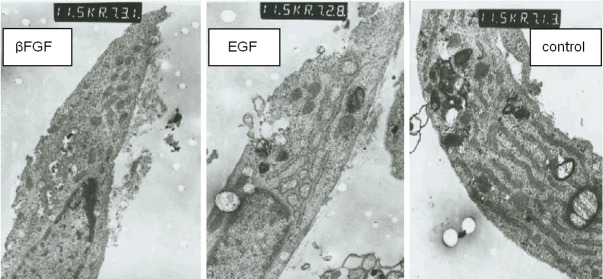
Compared to the control group on the 14th day, the fibroblast of the two treatment groups had expansion endoplasmic reticulum and hypertrophic mitochondria and more mitochondria.
Integrin β1 mRNA in situ hybridization
The results showed that the fibroblast cytoplasm of the control group was not stained, while that in the therapeutic groups showed a positive result. The positive cells were distributed not only in the papillary and subdermic, but also in some parts of epidermal layer of the healing wound. There was no significant difference between the two therapeutic groups, which indicated that the effect of promoting wound healing of rhβFGF and rhEGF was closely related with the expression of integrin β1. On the 28th day, the epithelial layers in the rhEGF group were more than that in the rhβFGF group, which was the same as the result observed by the light microscope with HE staining (Figure 2).
Figure 2.
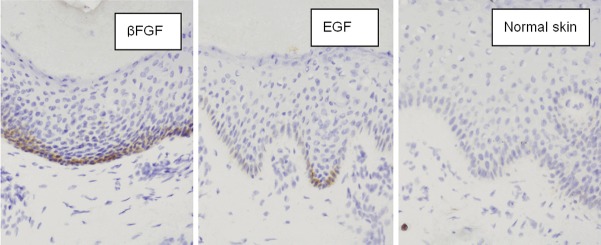
On the 28th day, the fibroblast cytoplasm of the control group was not stained, while that in the two therapeutic groups showed a positive result. The positive cells were distributed not only in the papillary and subdermis, but also in some parts of epidermal layer of the healing wound. The epithelial layers in the rhEGF group were more than that in the rhβFGF group; the base membrane and the papillae of corium were evident at 28 days after treatment of rhEGF, that of rhβFGF group were blurring. (in situ hybridization × 400).
Discussion
Growth factor existed widely in living organism. It combined with the specific receptor of the target cells. It had the biological effects through intracellular transition, tyrosine phosphorylation or induction by cAMP (Cyclic adenosine monophosphate) and cGMP (Cyclic guanosine monophosphate). The effects for promoting wound healing included as followed: chemotaxis and induction of the inflammatory cells and fibroblasts into the wound, stimulation of proliferation and differentiation, promotion of vascularization of the wound, adjustment the secretion of extracellular matrix such as collagen and induction of the cell factor synthesis of the contiguous cell [14-17]. By the research of general observation, calculation of the wound closure index, histopathological examination, and electrical microscopic examination, the results showed that both the wound healing time and curative effect of the therapeutic groups were much better than that of the control group. It meant both rhβFGF and rhEGF could promote the wound healing [18], but distinction existed in the mechanism, timing and effects. The βFGF realizes the wound healing mainly through promoting the proliferation and steroids activities in the wounds and burns, furthermore, βFGF can promote the scarless wound healing upon the induction of apoptosis of myofibroblasts and reduce scar formation [19]. Our experiment also showed the strengthen performance of wound healing in the rhβFGF group as followed: high activity of proliferation in the fibroblast, increased secretion of the collagen, capillary increased and the lumen expansion. These effects of rhβFGF mainly ruled in the earlier period or the metaphase, whereas the mechanism of promoting wound healing by EGF was different. Firstly, EGF combined with the EGF-receptor on the basic membrane near the wound [20]. Then it would promote the basic membrane cells separation and transition. The wound epithelization would be accelerated by mitosis, which formed stratified epithelium cells from monolayer cell. Therefore, the main role of the EGF was promoting the re-epithelization for healing wound in the metaphase or late period [21], though it could promote the fibroblast proliferation, collagen secretion and wound vascularization in the early period and metaphase. We could find that the epithelial time in the rhEGF group was significantly shorter than that in the rhβFGF group. What’s more, the epithelial layers in the rhEGF group were more than that in the rhβFGF group or the control group in the same phase. So, rhEGF played an important role in closing wound, preventing infection and reducing scar. Compared to the control group, the epithelial regeneration completely would be detected in the rhEGF group. A complete layer of granulation and cuticle emerged in the metaphase or late period.
Integrin that is a kind of receptor among the laminin has a close relationship with rhEGF and rhβFGF [22,23]. The largest family of integrin was composed of β1 sub-unit. As the transmembrane protein, integrin β1 worked as not only a signal communication pathway, but also the important connection bridge between cells and matrix. Integrin β1 was still one of the main landmarks of the epithelial stem cells. After integrin β1 combined with the extracellular matrix, the main biological effects included: induction fibroblast for transition and proliferation directly, or stimulation other immunocompetent cells for transformation and movement indirectly. Then more collagen, angiogenic factors or fibroblast growth factors would be produced, leading to the growth of capillary and the contraction of connective tissue [24,25]. On the other hand, our results showed that positive cells of the integrin β1 expression mainly distributed around the basic membrane, where concentrated the epidermal stem cell. Whether the epidermal stem cell participated the wound healing induced by EGF and βFGF [26]? Did it mean that EGF and βFGF could induce the proliferation of epidermal stem cell through increasing the expression of integrin β1 in the therapeutic groups? All of them needed further studies.
The wound healing of any serious wounds and burns included the granulation formation and re-epithelization, it was participated and regulated by a variety of cell growth factors. While different kinds of cell growth factors had different biological effect. Generally, the effective concentration of growth factor was limited in the wound. Wound healing would be accelerated obviously by adding exogenous growth factor. If it combined with the cell growth factors, the performance of promoting wound healing and epithelization would be better. The experiment showed that, although rhEGF and rhβFGF could promote wound healing, but the emphasis of healing were different. rhβFGF focused on the growth of granulation tissue in the early period and metaphase, while rhEGF focused on the re-epithelization in the metaphase and late period. This difference had clinical significance for rational use of them. When granulation was urgent needed to fill with the wound in the serious injuries, the wounds and burns in early or metaphase, βFGF would be better. If epithelization was priority in the moderate injuries or the late period of wound healing, EGF was recommended. In view of the different wound and different period of wound healing, the application of different cell growth factors should be considerable. It was helpful to fulfill the biological effects of cell growth factors and improve the patients’ quality of life. It could also avoid the side effects of cell growth factors and improve the cost performance of them as far as possible. That is, this experiment provided primary evidence, but more accurate evidences needed for further studies.
Disclosure of conflict of interest
None.
References
- 1.Grazul-Bilska AT, Johnson ML, Bilski JJ, Redmer DA, Reynolds LP, Abdullah A, Abdullah KM. Wound healing: the role of growth factors. Drugs Today (Barc) 2003;39:787–800. doi: 10.1358/dot.2003.39.10.799472. [DOI] [PubMed] [Google Scholar]
- 2.Yang Y, Xia T, Zhi W, Wei L, Weng J, Zhang C, Li X. Promotion of skin regeneration in diabetic rats by electrospun core-sheath fibers loaded with basic fibroblast growth factor. Biomaterials. 2011;32:4243–54. doi: 10.1016/j.biomaterials.2011.02.042. [DOI] [PubMed] [Google Scholar]
- 3.Sheng J, Zeng B, Jiang P, Fan C. Effect of local basic fibroblast growth factor and 5-fluorouracil on accelerating healing and preventing tendon adhesion after flexor tendon repair. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011;25:711–7. [PubMed] [Google Scholar]
- 4.Kondo S, Kuroyanagi Y. Development of a wound dressing composed of hyaluronic acid and collagen sponge with epidermal growth factor. J Biomater Sci Polym Ed. 2012;23:629–43. doi: 10.1163/092050611X555687. [DOI] [PubMed] [Google Scholar]
- 5.Steenfos HH. Growth factors and wound healing. Scand J Plast Reconstr Surg Hand Surg. 1994;28:95–105. doi: 10.3109/02844319409071186. [DOI] [PubMed] [Google Scholar]
- 6.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 7.Yu A, Matsuda Y, Takeda A, Uchinuma E, Kuroyanagi Y. Effect of EGF and βFGF on Fibroblast Proliferation and angiogenic cytokine production from cultured dermal substitutes. J Biomater Sci Polym Ed. 2011 doi: 10.1163/092050611X580463. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Guo X, Zhang W, Hou C, Wei C, Liu S. Experimental study of the effect of chitosan/alginate dressings on wound immersed in seawater. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23:708–12. [PubMed] [Google Scholar]
- 9.Fujisawa K, Miyamoto Y, Nagayama M. Basic fibroblast growth factor and epidermal growth factor reverse impaired ulcer healing of the rabbit oral mucosa. J Oral Pathol Med. 2003;32:358–66. doi: 10.1034/j.1600-0714.2003.t01-1-00111.x. [DOI] [PubMed] [Google Scholar]
- 10.Xiang Q, Xiao J, Zhang H, Zhang X, Lu M, Zhang H, Su Z, Zhao W, Lin C, Huang Y, Li X. Preparation and characterization of βFGF- encapsulated liposomes and evaluation of wound-healing activities in the rat. Burns. 2011;37:886–95. doi: 10.1016/j.burns.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 11.Fernández-Montequín JI, Valenzuela-Silva CM, Díaz OG, Savigne W, Sancho-Soutelo N, Rivero-Fernández F, Sánchez-Penton P, Morejón-Vega L, Artaza-Sanz H, García-Herrera A, González-Benavides C, Hernández-Cañete CM, Vázquez-Proenza A, Berlanga-Acosta J, López-Saura PA Cuban Diabetic Foot Study Group. Intra-lesional injections of recombinant human epidermal growth factor promote granulation and healing in advanced diabetic foot ulcers: multicenter, randomized, placebo-controlled, double-blind study. Int Wound J. 2009;6:432–43. doi: 10.1111/j.1742-481X.2009.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demidova-Rice TN, Hamblin MR, Herman IM. Acute and Impaired Wound Healing: Pathophysiology and Current Methods for Drug Delivery, Part 2: Role of Growth Factors in Normal and Pathological Wound Healing: Therapeutic Potential and Methods of Delivery. Adv Skin Wound Care. 2012;25:349–370. doi: 10.1097/01.ASW.0000418541.31366.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanz Garcia S, Santos Heredero X, Izquierdo Hernandez A, Pascual Peña E, Bilbao de Aledo G, Hamann C. Experimental model for local application of growth factors in skin re-epithelialisation. Scand J Plast Reconstr Surg Hand Surg. 2000;34:199–206. doi: 10.1080/02844310050159765. [DOI] [PubMed] [Google Scholar]
- 14.Ayvazyan A, Morimoto N, Kanda N, Takemoto S, Kawai K, Sakamoto Y, Taira T, Suzuki S. Collagen-gelatin scaffold impregnated with βFGF accelerates palatal wound healing of palatal mucosa in dogs. J Surg Res. 2011;171:e247–57. doi: 10.1016/j.jss.2011.06.059. [DOI] [PubMed] [Google Scholar]
- 15.Kim JM, Bak EJ, Chang JY, Kim ST, Park WS, Yoo YJ, Cha JH. Effects of HB-EGF and epiregulin on wound healing of gingival cells in vitro. Oral Dis. 2011;17:785–93. doi: 10.1111/j.1601-0825.2011.01836.x. [DOI] [PubMed] [Google Scholar]
- 16.Kanazawa S, Fujiwara T, Matsuzaki S, Shingaki K, Taniguchi M, Miyata S, Tohyama M, Sakai Y, Yano K, Hosokawa K, Kubo T. βFGF regulates PI3-kinase-Rac1-JNK pathway and promotes fibroblast migration in wound healing. PLoS One. 2010;5:e12228. doi: 10.1371/journal.pone.0012228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaiana CA, Leonard MK, Drummy LF, Singh KM, Bubulya A, Vaia RA, Naik RR, Kadakia MP. Epidermal growth factor: layered silicate nanocomposites for tissue regeneration. Biomacromolecules. 2011;12:3139–46. doi: 10.1021/bm200616v. [DOI] [PubMed] [Google Scholar]
- 18.Kondo S, Kuroyanagi Y. Development of a wound dressing composed of hyaluronic acid and collagen sponge with epidermal growth factor. J Biomater Sci Polym Ed. 2012;23:629–43. doi: 10.1163/092050611X555687. [DOI] [PubMed] [Google Scholar]
- 19.Abe M, Yokoyama Y, Ishikawa O. A possible mechanism of basic fibroblast growth factor-promoted scarless wound healing: the induction of myofibroblast apoptosis. Eur J Dermatol. 2012;22:46–53. doi: 10.1684/ejd.2011.1582. [DOI] [PubMed] [Google Scholar]
- 20.Zong S, Liang Z, Ou B. Effect of topical external administration of recombinant human epidermal growth factor on expression of epidermal growth factor receptor and its mRNA in scald wound of diabetes mellitus rat. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2010;24:150–5. [PubMed] [Google Scholar]
- 21.Değim Z, Celebi N, Alemdaroğlu C, Deveci M, Öztürk S, Özoğul C. Evaluation of chitosan gel containing liposome-loaded epidermal growth factor on burn wound healing. Int Wound J. 2011;8:343–54. doi: 10.1111/j.1742-481X.2011.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki Y, Yanagisawa M, Yagi H, Nakatani Y, Yu RK. Involvement of beta1-integrin up-regulation in basic fibroblast growth factor- and epidermal growth factor-induced proliferation of mouse neuroepithelial cells. J Biol Chem. 2010;285:18443–51. doi: 10.1074/jbc.M110.114645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho E, Dagnino L. Epidermal growth factor induction of front-rear polarity and migration in keratinocytes is mediated by integrin-linked kinase and ELMO2. Mol Biol Cell. 2012;23:492–502. doi: 10.1091/mbc.E11-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285–98. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- 25.Goodman SL, Picard M. Integrins as therapeutic targets. Trends Pharmacol Sci. 2012;33:405–12. doi: 10.1016/j.tips.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Sun X, Fu X, Han W, Zhao Y, Liu H, Sheng Z. Dedifferentiation of human terminally differentiating keratinocytes into their precursor cells induced by basic fibroblast growth factor. Biol Pharm Bull. 2011;34:1037–45. doi: 10.1248/bpb.34.1037. [DOI] [PubMed] [Google Scholar]


