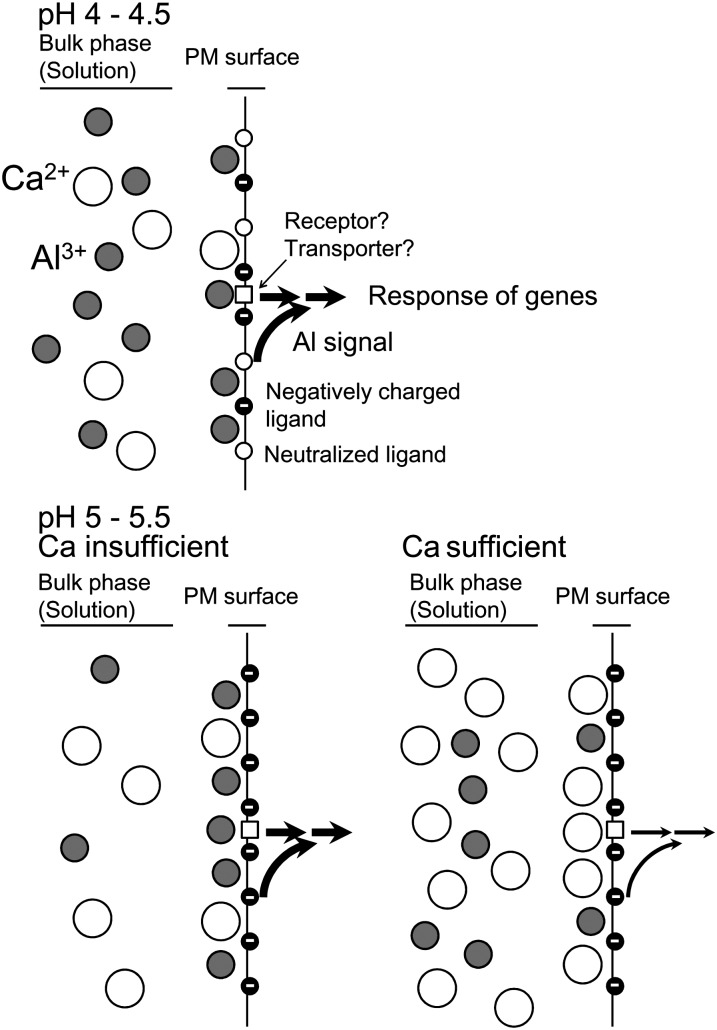
Rhizotoxicities of Al3+ and H+ occur at moderately acidic soil conditions (pH [water] = 5–5.5), especially under conditions of low Ca supply.
Abstract
Al3+ and H+ toxicities predicted to occur at moderately acidic conditions (pH [water] = 5–5.5) in low-Ca soils were characterized by the combined approaches of computational modeling of electrostatic interactions of ions at the root plasma membrane (PM) surface and molecular/physiological analyses in Arabidopsis (Arabidopsis thaliana). Root growth inhibition in known hypersensitive mutants was correlated with computed {Al3+} at the PM surface ({Al3+}PM); inhibition was alleviated by increased Ca, which also reduced {Al3+}PM and correlated with cellular Al responses based on expression analysis of genes that are markers for Al stress. The Al-inducible Al tolerance genes ALUMINUM-ACTIVATED MALATE TRANSPORTER1 and ALUMINUM SENSITIVE3 were induced by levels of {Al3+}PM too low to inhibit root growth in tolerant genotypes, indicating that protective responses are triggered when {Al3+}PM was below levels that can initiate injury. Modeling of the H+ sensitivity of the SENSITIVE TO PROTON RHIZOTOXICITY1 knockout mutant identified a Ca alleviation mechanism of H+ rhizotoxicity, possibly involving stabilization of the cell wall. The phosphatidate phosphohydrolase1 (pah1) pah2 double mutant showed enhanced Al susceptibility under low-P conditions, where greater levels of negatively charged phospholipids in the PM occur, which increases {Al3+}PM through increased PM surface negativity compared with wild-type plants. Finally, we found that the nonalkalinizing Ca fertilizer gypsum improved the tolerance of the sensitive genotypes in moderately acidic soils. These findings fit our modeling predictions that root toxicity to Al3+ and H+ in moderately acidic soils involves interactions between both toxic ions in relation to Ca alleviation.
Aluminum (Al), principally in the form of Al3+ released from soil clay minerals, is one of the most important rhizotoxic ions in acidic soils and is abundant in soil solutions at pH (water) of less than 5.0. Many forest and grass land species naturally adapted to acid soils are very tolerant of H+ and Al3+ and thrive in soils where the pH is less than 4.0. However, most crop plants used for agriculture show inhibitory growth effects, even when the soil pH is neutralized by liming to moderately acidic pH values in the range of pH 5 to 5.5. For example, crops sensitive to Al3+ and H+ such as turnip (Brassica rapa; Kinraide and Parker, 1990) and alfalfa (Medicago sativa; Yokota and Ojima, 1995) show growth inhibition at these soil pH values. Field research and soil experiments have shown that inhibitory effects of moderately acidic soils (pH > 5) can be ameliorated by the application of Ca fertilizers, even if they are nonalkalinizing, such as gypsum, and this leads to improvement in crop productivity (Carvalho and Van Raij, 1997; Mora et al., 1999). This indicates that the soil Ca status is an important factor in determining crop yield at moderately low soil pH values with regard to Al3+ and H+ rhizotoxicity occurring in these soils. An understanding of the complex situation of acid soil stress in soil pH in the range of pH (water) 5 to 5.5 is important for designing efficient soil acidity management and breeding programs for resistant crop use in low-input agricultural systems.
The complex rhizotoxicities at moderately acidic conditions that can be alleviated by Ca have been predicted by modeling studies in wheat (Triticum aestivum; Kinraide, 2003). The model first computes the activity of the rhizotoxicants and alleviants at the plasma membrane (PM) surface, for example {Al3+}PM and {H+}PM, and {Ca2+}PM, using a speciation-based Gouy-Chapman-Stern electrostatic (SGCS) model (Kinraide and Wang, 2010). The mechanisms of toxicity and alleviation are then modeled by regression analyses for root growth inhibition fitted to the nonlinear equations (Kinraide, 2003; Kinraide et al., 2004). The modeling studies well describe the complicated events in the Al-toxic solutions near the PM surface under moderately acidic conditions. For example, the elevation of pH from 4.5 to between 5 and 5.5 decreases the activity of the most rhizotoxic Al species, Al3+ in the solution ({Al3+}bulk), while it increases the negativity of the PM surface because of dissociation of H+ from potentially negative ligands such as phospholipids. As a result, {Al3+}PM remains at moderately high levels at the PM surface at pH greater than 5 due to the attraction to negative charges on the PM surface, but it can be alleviated by coexisting cations such as Ca2+ and even by another rhizotoxic cation, H+. These modeling studies have proposed different mechanisms of Ca alleviation in this complex situation (Kinraide, 1998; Kinraide et al., 2004). Mechanisms I (the electrostatic displacement of toxicant at the PM surface) and II (the restoration at the PM surface of Ca2+ electrostatically displaced by the toxicant) are events at the PM surface, but mechanism III, which explains the remaining portion of the Ca alleviation, may involve other physiological responses, including unknown mechanisms. These predictions, derived from the modeling study, likely explain the complex nature of moderately acidic soils but may require further validation because they were developed using root growth as the sole criterion for rhizotoxicity.
Although these types of modeling approaches have not been performed using Arabidopsis (Arabidopsis thaliana) plants, clear symptoms of Al3+ and H+ rhizotoxicity at moderately acidic conditions (pH ≥ 5) has been identified in Arabidopsis (Kobayashi and Koyama, 2002; Iuchi et al., 2007). A quantitative trait locus study of Al tolerance at moderately acidic conditions (4 μm Al, pH 5; Kobayashi and Koyama, 2002) identified a very similar genetic architecture of Al tolerance to that derived from a study that employed a lower pH value but with a greater level of Al (50 μm Al, pH 4.2; Hoekenga et al., 2003). The former conditions employed a lower Ca concentration (200 μm) than the latter (3 mm), which accounted for the predictions of {Al3+}PM in relation to {Ca2+}PM by electrostatics. On the other hand, several Al3+- and H+-sensitive transfer DNA insertion knockout (KO) mutant genotypes have been identified using the lower ionic-strength moderately acidic media (Sawaki et al., 2009). These lines exhibit different degrees of hypersensitivity to moderately acidic conditions because of the dysfunction of different tolerance genes, suggesting the involvement of different mechanisms. In Arabidopsis, ALUMINUM-ACTIVATED MALATE TRANSPORTER1 (AtALMT1) regulates Al-activated root malate excretion that protects the root tip from acute Al toxicity by Al exclusion (Hoekenga et al., 2006), and ALUMINUM SENSITIVE3 (ALS3) regulates internal Al sequestration involved in long term Al tolerance (Larsen et al., 1997, 2005). The KO mutants for these genes display Al hypersensitivity. In addition, SENSITIVE TO PROTON RHIZOTOXICITY1 (STOP1)-KO, a suppressor of multiple genes for Al and H+ tolerance, shows sensitivity to Al3+ and H+ (Iuchi et al., 2007; Sawaki et al., 2009). These sensitive genotypes are useful tools for evaluating Al3+ and H+ toxicity in the pH range 5 to 5.5. On the other hand, several cellular responses, such as the induction of gene expression, have been identified in Arabidopsis that could be useful in the estimation of the attraction of {Al3+} to the PM, which is computed by our electrostatic-based model. Therefore, Arabidopsis appears to be a useful model system for the validation of modeling based on the SGCS model and to further our understanding of Al3+ and H+ rhizotoxicities at moderately acidic conditions in relation to Ca2+ alleviation.
Computation of {Al3+}PM requires accurate speciation of Al and other solutes in the bulk solution. The original SGCS program is suitable for relatively simple solutions (Kinraide and Wang, 2010). However, the rooting medium used for the Arabidopsis assays exceeds the number of solutes that can be accurately assessed by the SGCS program (Kobayashi et al., 2007). Consequently, we updated the modeling methodology using the speciation program GEOCHEM-EZ, which is suitable for complex media (Famoso et al., 2010; Shaff et al., 2010). This improved model, used in conjunction with molecular biological assays such as biomarker analysis of Al-inducible gene expression, has allowed us in this study to validate the predicted {Al3+}PM rhizotoxicity in relation to {Ca2+}PM alleviation from the wheat modeling studies. The updated modeling of Ca alleviation in mutants uncovered one of the mechanisms of Ca alleviation in the H+-sensitive mutant and identified an Al-sensitive double mutant genotype, phosphatidate phosphohydrolase1 (pah1) and pah2 (Nakamura et al., 2009), that fitted previous predictions. Finally, we demonstrate the ability of gypsum to ameliorate the sensitive phenotype of selected genotypes, when they were grown in moderately acidic soil culture. Taken together, we present here experimental validation of the SGCS-based modeling, and its combination with molecular physiology provides a deeper understanding of plant Al3+ and H+ toxicity in relation to Ca2+ alleviation at pΗ of at least 5.0.
RESULTS
Al and H+ toxicity in Al-Hypersensitive Mutants at Relatively High pH in Complex Solutions, and Computation of {Al3+}PM in Complex Mediums Using SGCS and GEOCHEM-EZ
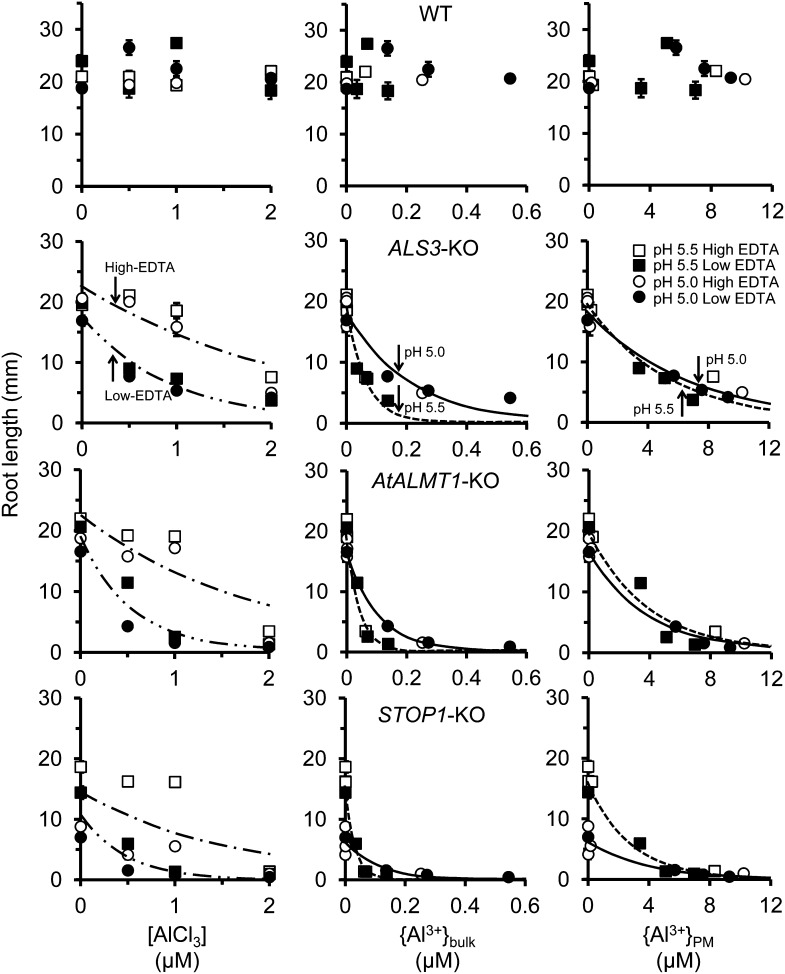
To validate the predictions obtained from the SGCS modeling studies in wheat, where {Al3+}PM determines Al rhizotoxicity at moderately acidic conditions, we were required to use a sensitive phenotype at pH greater than 5 that could provide sufficient data sets. Ecotype Columbia (Col-0) is one of the most Al-tolerant natural accessions, and it did not show Al sensitivity in moderately acidic conditions (Fig. 1). The Al-hypersensitive mutants derived from Col-0, AtALMT1-KO, and ALS3-KO and the Al3+- and H+-hypersensitive mutant STOP1-KO showed sensitivity in the moderately acidic conditions (pH 5–5.5, up to 2 μm Al; Fig. 1). Thus, these mutants were deemed adequate to evaluate rhizotoxicities at pH greater than 5 and for obtaining sufficient data sets for growth modeling in relation to Ca presented subsequently in this study.
Figure 1.
Al inhibition of root growth of Arabidopsis seedlings in hydroponic culture with different concentrations of EDTA. Seedlings were grown for 7 d in basal media containing different concentrations of AlCl3 (0, 0.5, 1, or 2 μm) at pH 5.0 or 5.5. Root lengths were plotted against AlCl3 concentration (left), Al3+ activity in bulk phase media (middle), and Al3+ activity at the PM surface (right) were calculated using GEOCHEM-EZ and SGCS. Values are means ± se (n = 10; se bars are not shown if they overlap the symbols). Curves were fitted according to the nonlinear regression model RL = a + bexp(c[AlCl3] or {Al3+}bulk or {Al3+}PM). [AlCl3], Concentration of [AlCl3]; {Al3+}bulk, activity of Al3+ in solution; {Al3+}PM, activity of Al3+ at the plasma membrane surface. Note the high sensitivity of STOP1-KO to pH 5.0 at zero Al.
In preliminary experiments, we found that accurate speciation is critical to simulate Al rhizotoxicity in the complex nutrient solution used to grow Arabidopsis (Supplemental Fig. S1), whereas the previous speciation program in the SGCS program was based on more simple nutrient solutions (e.g. CaCl2 plus AlCl3). Consequently, we updated the computational modeling of electrostatic ion interactions by SGCS using the comprehensive speciation program GEOCHEM-EZ to compute values for {Al3+}bulk. Figure 1 shows differences in root growth inhibition in relation to different indices of Al in the solutions (AlCl3 concentration in the bulk solution, Al3+ activity in the bulk solution, and Al3+ activity at the PM surface). Root growth inhibition of the hypersensitive mutants (AtALMT1-KO, ALS3-KO, and STOP1-KO) was affected by the concentration of the chelator EDTA, used here to reduce the Al concentration in solution, as seen in the plots of root length (RL) versus the concentration of total Al (Fig. 1, left). When RL was plotted against {Al3+}bulk determined by speciation of the EDTA-containing media using GEOCHEM-EZ (Fig. 1, center), the apparent effect of EDTA disappeared (observe the positions of the white and black symbols, and note that the drawn curves now pertain to pH). The effect of EDTA was to bind the rhizotoxic Al3+ and reduce its free concentration in the medium. All three Al-hypersensitive mutants showed steeper declines in root growth at pH 5.5 than at pH of 5.0 for RL versus {Al3+}bulk (Fig. 1, center). This greater sensitivity at pH 5.5 occurs because incremental increases in {Al3+}bulk at pH 5.5 cause greater increases in {Al3+}PM than do similar increases in {Al3+}bulk at pH 5.0. Finally, responses to Al3+ coalesced into a single function for the Al-sensitive mutants (AtALMT1-KO and ALS3-KO) when RL was plotted against {Al3+}PM computed by the SGCS program (Fig. 1, right). This indicates that the model correctly predicts that {Al3+}PM, rather than {Al3+}bulk, is the determinant of Al toxicity in Arabidopsis growing in complex media (the same was found for wheat grown in a simple CaCl2 solution). In addition, we found a distinct pattern in the H+-hypersensitive STOP1-KO mutant (Fig. 1, bottom right), where H+ toxicity in moderately acidic conditions was observed in the sensitive genotype (pH 5–5.5). These results presented in Figure 1 indicated the improved model was sufficient to model H+ and Al3+ toxicity in relation to Ca2+ alleviation at moderate acidity.
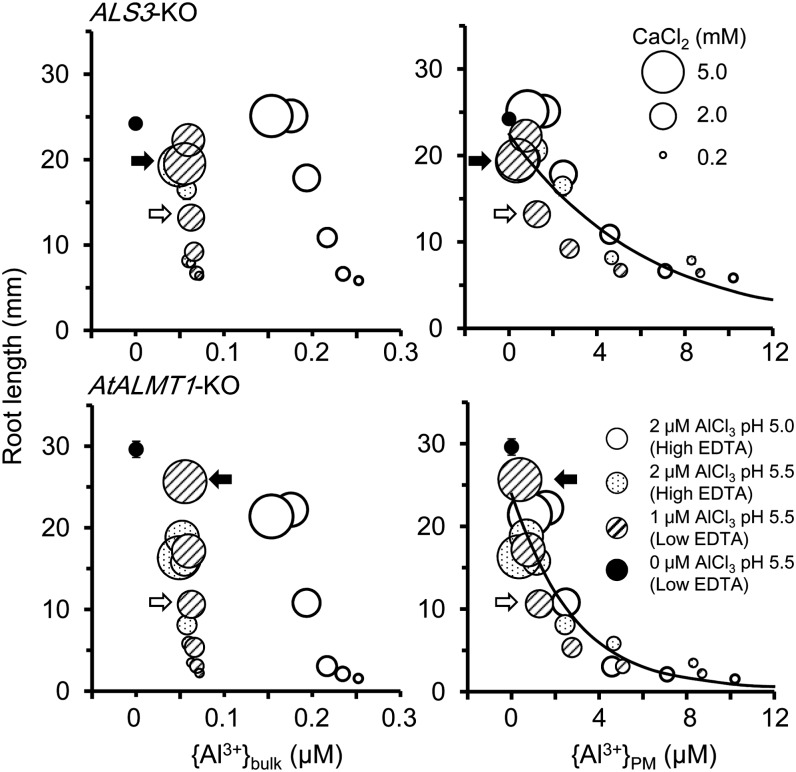
Ca2+ Alleviation of Al Toxicity in Al-Hypersensitive Mutants at pH Greater Than 5 in Relation to {Al3+}PM
To evaluate Ca2+ alleviation of Al toxicity at pH greater than 5, AtALMT1-KO and ALS3-KO were grown in solutions containing 1 or 2 μm Al at pH 5.0 and 5.5 with varying concentrations of Ca2+. Figure 2 shows that root elongation increased with increasing Ca2+. Furthermore, Ca2+ induced large reductions in {Al3+}PM but only small reductions in {Al3+}bulk. In 1 μm Al solutions at pH 5.5, {Al3+}PM decreased from 8.70 to 0.38 μm (22.9-fold decrease) as the concentration of Ca2+ increased from 0.2 to 5 mm, whereas the {Al3+}bulk only decreased from 0.07 to 0.06 μm. Under those conditions, plots of RL versus {Al3+}bulk yielded two separate regression curves for each genotype, but the plots fit a single nonlinear curve for each genotype for RL versus {Al3+}PM (Fig. 2). These findings indicate that Ca2+ alleviates Al toxicity at pH greater than 5 by mainly reducing {Al3+}PM, and it also supports the concept that {Al3+}PM determines Al rhizotoxicity, as predicted by previous wheat modeling studies. This pattern of a single curve fit to {Al3+}PM that is not affected by {Ca2+}PM suggested that {Ca2+}PM alleviates {Al3+}PM toxicity mainly by electrostatic displacement (mechanism I of Ca alleviation) and/or restoration of {Ca2+}PM (mechanism II of Ca alleviation).
Figure 2.
Ca alleviation of Al toxicity in ALS3-KO and AtALMT1-KO under weakly acidic conditions. Seedlings were grown in Al-toxic solutions containing various levels of CaCl2, AlCl3, EDTA, and pH as shown. Circle size represents CaCl2 concentration. Values are means ± se (n = 10; se bars are not shown if they overlap the symbols). White arrows and black arrows indicate points at which 2 mm and 5 mm CaCl2 (in 1 μm AlCl3 at pH 5.5) induced 50% to 70% root growth inhibition or nearly no inhibition, respectively. The drawn curves were fitted to the nonlinear regression model RL = a + bexp(c{Al3+}PM).
Expression of Al-Inducible Genes in Response to {Al3+}PM
The above results suggested that {Al3+}PM is the determinant of Al toxicity in Arabidopsis growing at moderately low pH and that it is alleviated by {Ca2+}PM. However, this is based on root elongation being the single criteria of toxicity, which was similar to that of the previous modeling studies in wheat. To further evaluate {Al3+}PM toxicity in relation to Ca2+ alleviation at pH greater than 5, the expression levels of Al-inducible genes not having an Al tolerance function, but associated with Al3+ damage, were quantified for use as Al biomarkers to evaluate Al3+ attraction to the PM surface. Al-inducible genes with the most enhanced expression levels under severe conditions (25 μm; Zhao et al., 2009) compared with milder toxicity conditions (10 μm; Sawaki et al., 2009) were selected from previously obtained microarray data (Supplemental Tables S1 and S2). Levels of {Al3+}PM toxic to hypersensitive mutants were expected to trigger the expression of these sensitive biomarker genes, even if in the tolerant genotype Col-0 showed no root growth inhibition.
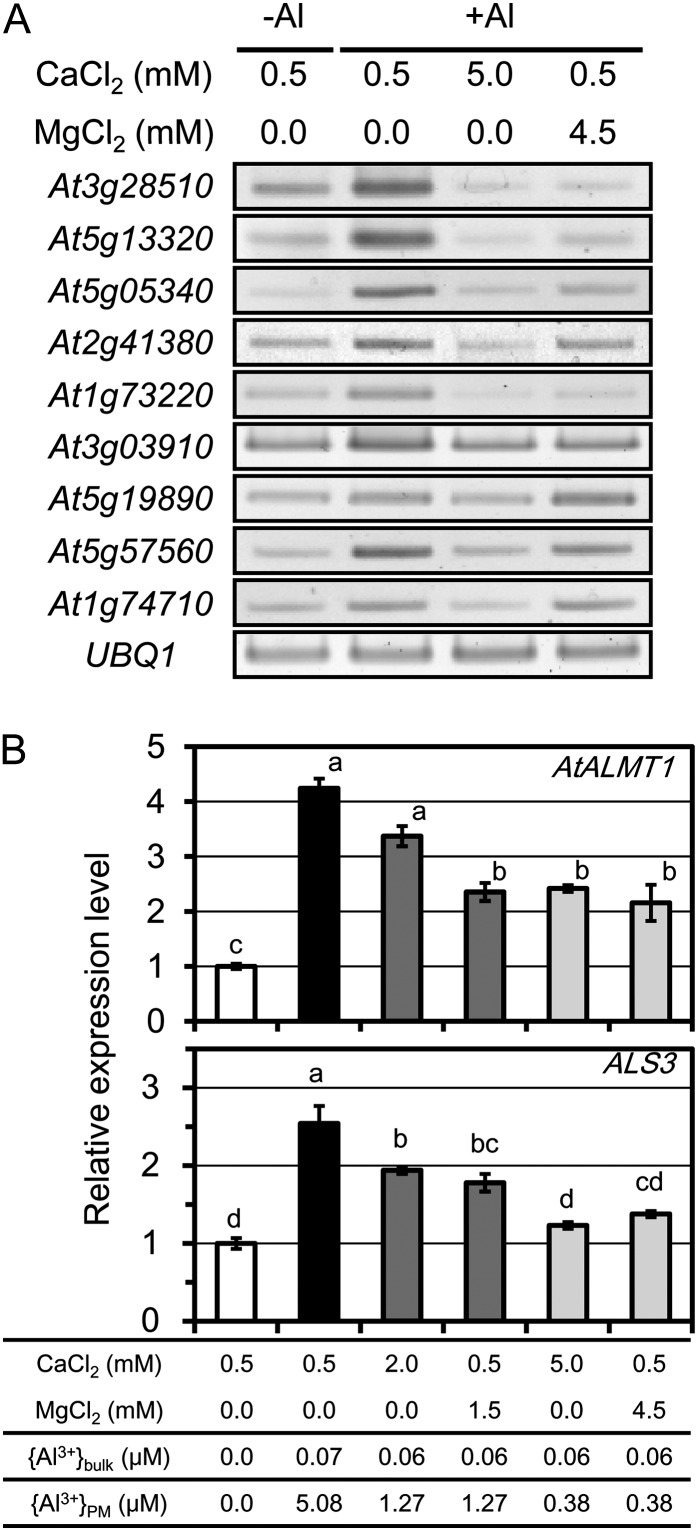
When Col-0 seedlings were exposed to solutions containing 1 μm Al at pH 5.5 (nontoxic levels to this genotype), the expression levels of the Al biomarker genes were sharply induced in the presence of 0.5 mm Ca2+, with {Al3+}PM reaching 5.08 μm (Fig. 3A). At this level of {Al3+}PM, root growth of both Al-hypersensitive KO lines (AtALMT1-KO and ALS3-KO) was completely inhibited, indicating that the solution contained toxic levels of Al3+ to these mutants. Elevation of Ca2+ to 5 mm reduced expression levels of the biomarker genes in Col-0 to levels similar to those seen in zero-Al solutions at 0.5 mm Ca2+ (Fig. 3A), where growth of the KO mutants was not inhibited. Clearly, the expression of biomarker genes was well correlated with growth inhibition of hypersensitive mutants by {Al3+}PM as controlled by Ca2+. According to the SGCS model, Mg2+ can reduce {Al3+}PM similarly to Ca2+. In fact, for most of the tested genes, repression by the combination of 0.5 mm Ca2+ and 4.5 mm Mg2+ was nearly equal to that observed at 5 mm Ca2+. This indicates that {Al3+}PM determines growth responses in relation to Ca2+ alleviation and validates the {Al3+}PM rhizotoxicity computed from the SGCS electrostatics model.
Figure 3.
Expression levels of Al biomarker genes in Al-toxic solutions with different levels of CaCl2 and MgCl2. Roots of wild-type Col-0 seedlings, grown for 10 d in control solutions, were incubated for 6 h in 1 μm Al at pH 5.5. A, Al-responsive genes that are sharply up-regulated by Al stress in a dose-dependent manner (i.e. greater in 25 μm than in 10 μm) and are highly up-regulated in Al compared with other stressors in agreement with the above selection criterion (Supplemental Tables S1 and S2). Transcripts were determined by semiquantitative RT-PCR. UBQ1 transcript was used as the internal control. B, Means ± se of expression levels of AtALMT1 and ALS3 relative to those in plants in zero Al (n = 3). Different letters indicate significant difference (Tukey’s test, P < 0.05).
Expression of Al-Inducible Al Tolerance Genes AtALMT1 and ALS3
Expression patterns of the sensitive biomarker genes indicated that {Al3+}PM was correlated to the cellular response of gene expression to Al. This suggested that expression of bona fide Al tolerance genes would also be regulated by {Al3+}PM, as this fitted the computed {Al3+}PM by the SGCS electrostatic model. To examine this possibility, we analyzed the expression levels of AtALMT1 and ALS3 in the same plant samples used for the previous biomarker analysis. The expression levels of AtALMT1 and ALS3 for plants grown on 1 μm Al were 4.2 and 2.5 times greater, respectively, than those grown on zero Al (Fig. 3B). These genes were gradually repressed when {Al3+}PM toxicity was alleviated by {Ca2+}PM in the KO mutants for each gene. In the presence of 2.0 mm CaCl2, {Al3+}PM reached 1.27 μm, and this induced 70% growth inhibition in the AtALMT1-KO and 50% growth inhibition in the ALS3-KO (Fig. 2, white arrows). Under these conditions, the expression levels of AtALMT1 and ALS3 were 3.4 and 1.9 times greater, respectively, than those of the control plants (Fig. 3B). This suggests that these tolerance genes are induced by {Al3+}PM in the tolerant genotype, Col-0 (no growth inhibition), at concentrations that initiate growth inhibition in the KO mutant for each gene. At 5 mm CaCl2, where growth of neither KO mutant was inhibited (Fig. 2, black arrows), AtALMT1 expression was maintained at a 2.4-fold higher rate than measured in the control (zero Al), while ALS3 expression was similar to that of the control (Fig. 3B). This may be due to the different functions of these genes; the former is critical in acute Al toxicity conditions for protecting the root tip via Al exclusion (Hoekenga et al., 2006), whereas the latter protects the root by Al sequestration for long-term Al tolerance (Larsen et al., 1997, 2005). These results strongly suggest that these Al-tolerant genes are induced when {Al3+}PM is too low to initiate injury in the wild type.
Effect of Ca2+ on the Hypersensitivity of AtALMT1-KO, ALS3-KO, and STOP1-KO; Modeling of the Ca Alleviation Mechanism
Three mechanisms of Ca alleviation of Al3+ and H+ toxicities were proposed in previous modeling studies (Kinraide, 1998). Applying the same concepts to this study can help to identify the Ca alleviation mechanism at moderately acidic conditions, and it may identify the genotypic differences for the Ca alleviation response among the mutants. This can be estimated from coefficient in the equations that describe the Ca toxicants interactions (Eq. 1). Equation 1 can be further expanded to Equation 2 (where a = RL at initial transfer [set to zero because ungerminated seeds were used in this research] and b = maximal RL predicted for the nontoxicants = 24.3] and used for the analysis.
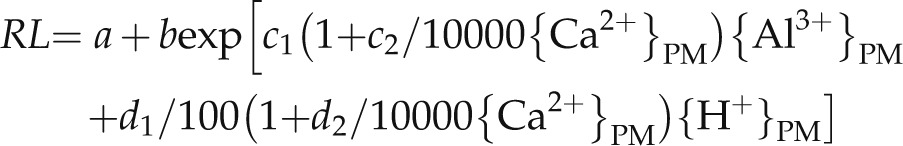 |
(Eq. 1) |
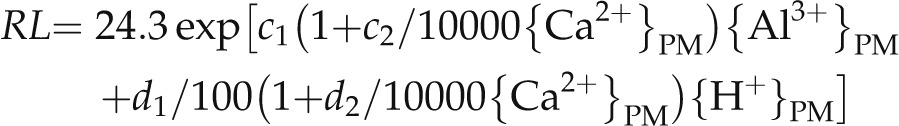 |
(Eq. 2) |
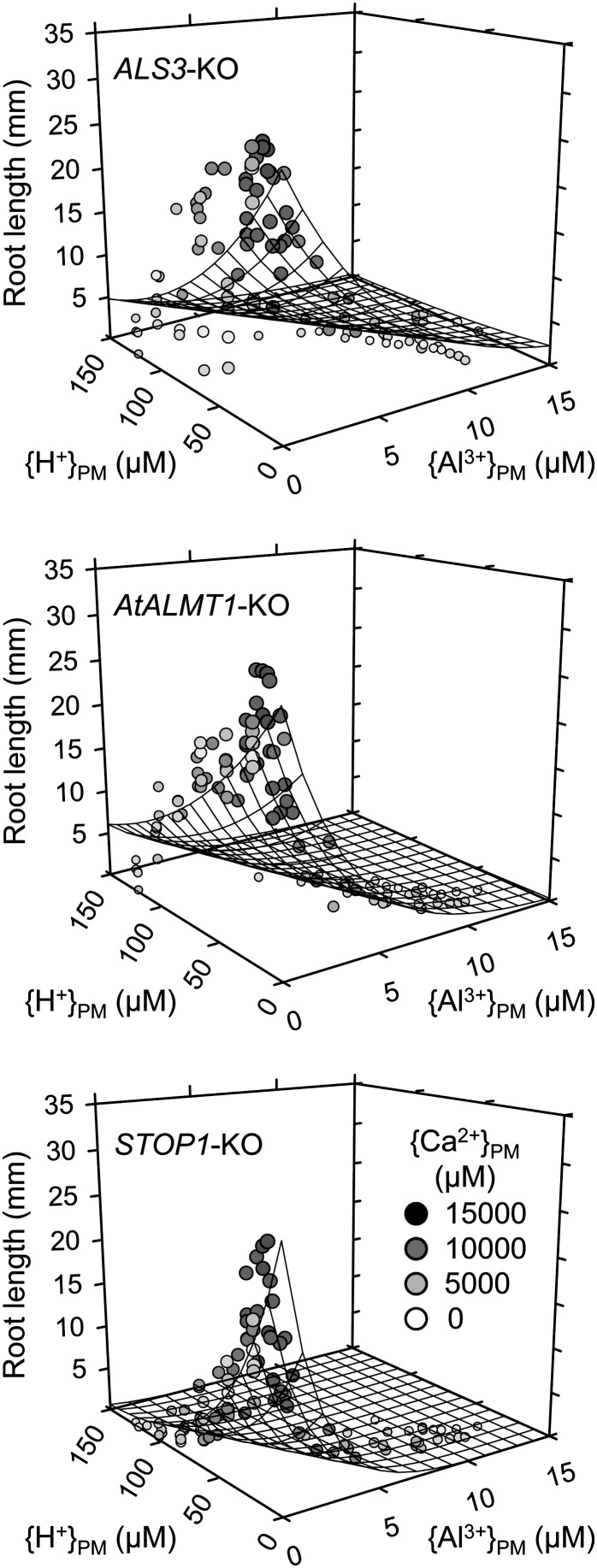
The growth data showed multidimensional exponential patterns when plotted against ionic activities computed by the SGCS model (Fig. 4; see Kinraide, 2003 and Kinraide et al., 2004 for further discussion of nonlinear equations). The coefficients in Equation 2 were derived by the conditions that identify the largest r2 by the regression analysis. The coefficients c1 and d1 describe tolerance to the toxicants (c1 for Al tolerance and d1 for H+ tolerance), while c2 and d2 show the degree of Ca alleviation. Because we removed all data when Ca2+ was less than 1 mm to avoid growth inhibition by Ca deficiency at the PM surface, c2 and d2 describe mechanism III for Ca alleviation.
Figure 4.
Three-dimensional scatterplots of RLs of AL3S-KO, AtALMT1-KO, and STOP1-KO in various Al3+- and H+-rhizotoxic solutions containing various concentration of CaCl2. RL was plotted against computed {Al3+}PM and {H+}PM combinations at different {Ca2+}PM.
Negative values of coefficients c1 and d1 indicate the exponential decline of root growth to {Al3+}PM and {H+}PM, respectively. The largest negative value of d1 occurred in STOP1-KO, indicating that H+ sensitivity appeared in various conditions at moderately acidic conditions (Table I). Negative values for c2 or d2 would reduce the negative values of the extended toxicant strength coefficients c1(1 + c2/10,000{Ca2+}PM) or d1(1 + d2/10,000{Ca2+}PM). Thus, negative values for c2 or d2 would signify the reduction of toxicities by {Ca2+}PM. The coefficient c2 was not significantly different from zero for any of the mutants, indicating that mechanism III did not contribute to the alleviation of {Al3+}PM toxicity. By contrast, d2 was significantly negative for each of the KO mutants, indicating that {Ca2+}PM contributed to the alleviation of {H+}PM toxicity by mechanism III. The smaller negative value for d2 for STOP1-KO suggested that Ca2+ alleviation by mechanism III made a smaller contribution to alleviation in this genotype.
Table I. Summary of statistics from regression analysis according to Equation 2.
ns, Coefficient in all genotypes were not significantly different from zero at the 5% level.
Ca2+ Alleviation of H+-Induced Root Tip Damage in the STOP1 Mutant
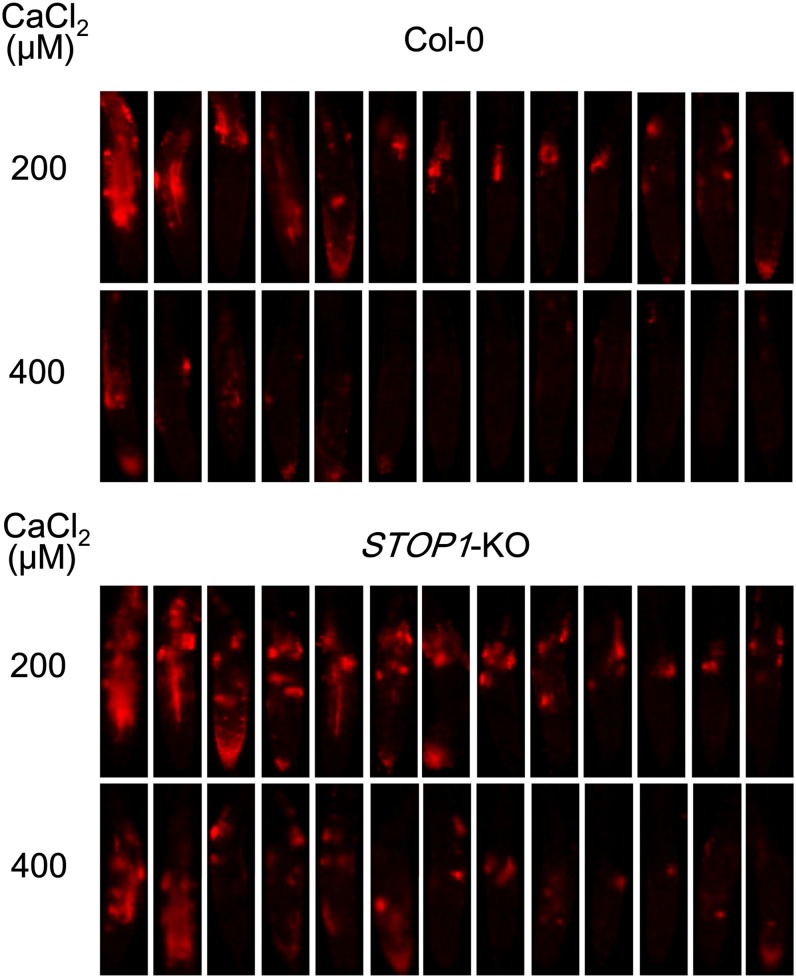
In low-Ca2+ solutions, H+ toxicity induces irreversible damage in the growing root tips of Arabidopsis. Ca2+ (and borate) will alleviate this damage, perhaps reflecting stabilization of pectic polysaccharides in cell walls (Koyama et al., 2001). This alleviation, based on stabilizing the cell wall, is an example of Ca2+ alleviation by mechanism III in the STOP1-KO mutant. To test this possibility, we compared root tip damage in STOP1-KO and Col-0. Both genotypes exhibited root tip damage within 90 min of exposure to a pH 4.7 solution containing 200 μm CaCl2; the damaged cells were stained by propidium iodide that penetrated the damaged PM and monitored by red fluorescence (Fig. 5). Most of the root tips from both genotypes showed damage in 200 μm CaCl2, but the degree of damage in the STOP1-KO was greater than in Col-0. In addition, alleviation of the damage in the STOP1-KO in 400 μm CaCl2 was much less than in Col-0. This indicates that STOP1-KO is likely to be defective in the mechanism of proton tolerance that alleviated proton stress in Col-0 by mechanism III, which involves Ca alleviation of the proton toxicity in the cell wall, but not at the PM surface.
Figure 5.
Ca2+ alleviation of H+ damage in root tips of wild-type Col-0 and STOP1-KO. Seedlings grown for 5 d in a control solution (pH 5.5) were soaked in a solution containing 200 or 400 μm CaCl2 (pH 4.7) for 90 min and then stained with propidium iodide. Damaged cells show red fluorescence.
Al3+ Sensitivity of a Mutant with a More Negatively Charged PM
The above experiments validate the reliability of the SGCS electrostatic model to simulate Al toxicity in moderately acidic conditions. It suggested that we could use this model to find new genotypes with altered Al sensitivity. Based on the SGCS electrostatics model, a greater intrinsic negative charge at the PM surface should enhance Al3+ sensitivity; this proposal has been previously supported experimentally (Wagatsuma and Akiba, 1989; Yermiyahu et al., 1997; Khan et al., 2009). To evaluate this hypothesis further, we compared the Al tolerance of Col-0 with the Al tolerance of a mutant with a more negatively charged PM. The pah1pah2 double mutant has a defective phosphatidate phosphohydrolase and has been reported to maintain PM phospholipids (the major negatively charged ligand in the PM) under phosphate (Pi) starvation conditions (Nakamura et al., 2009). By contrast, the Pi-starved Col-0 ecotype replaces phospholipids with other, less negatively charged lipids, thereby recycling the now-limiting Pi. This would make the pah1pah2 double mutant sensitive to Al because of enhanced Al accumulation caused by increasing PM surface negativity.
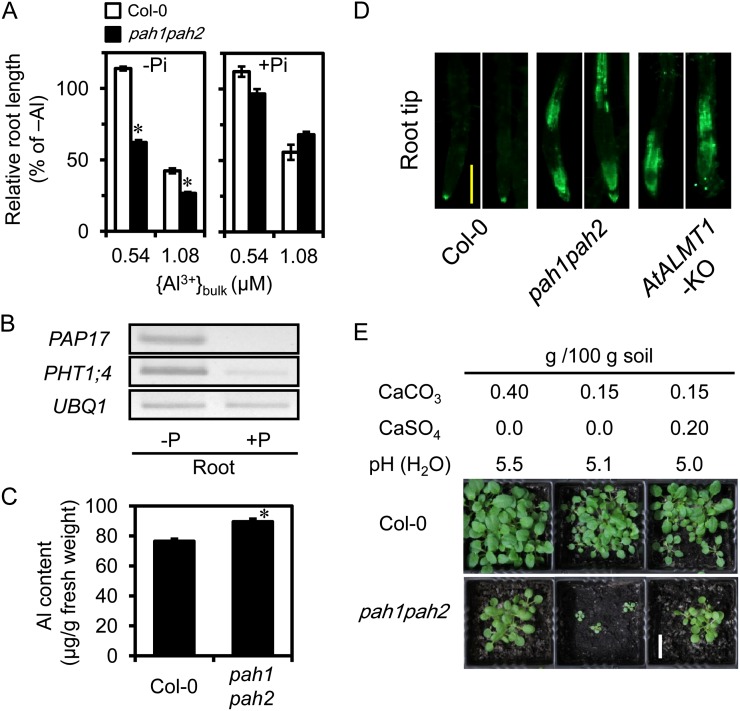
Col-0 and the pah1pah2 mutants were grown in solution containing the same level of {Al3+}bulk with different levels of Pi. The pah1pah2 was more sensitive to Al3+ than Col-0 in the absence of Pi (Fig. 6A). By contrast, the two genotypes showed no significant differences in Al tolerance when they were grown in a solution containing 35 μm Pi, in which the PM surface negativity and {Al3+}PM would be equal for the two genotypes (Fig. 6A). These conditions (+P and –P) produced differences in the P nutrient status, as judged by the induction of typical Pi deprivation-inducible biomarker genes (Fig. 6B). Additionally, root Al accumulation determined via inductively coupled plasma mass spectrometry (ICP-MS) was greater in pah1pah2 than in Col-0 in zero-Pi conditions (Fig. 6C). After incubation in Al-rhizotoxic conditions, the root tips of pah1pah2 showed brighter fluorescence than that of Col-0 when stained with morin (an Al-detecting fluorescent probe), while the pattern of Al accumulation was different than that seen in the AtALMT1-KO that lacks the malate exudation-based Al exclusion mechanism protecting the root apex (Fig. 6D). The pah1pah2 mutant should retain Al exclusion capacity depending on AtALMT1, while it exhibited enhanced Al accumulation in some parts of root tip. These characteristics fit well to the expected phenotype of the pah1pah2 mutant.
Figure 6.
Al resistance of Col-0 and pah1pah2 double mutant in Al-toxic solution in the presence or absence of Pi. A, Seedlings were grown for 7 d in Al-toxic solutions ({Al3+}bulk, 0.54 or 1.08 μm) in zero (total Al added, 2 or 4 μm) or 35 μm Pi (total Al added, 5 and 9.7 μm) at pH 5.0. Means of relative RL ± se are shown (n = 10). Asterisks indicate the significance of difference from Col-0 by Student’s t test (P < 0.05). B, Expression of Pi starvation-responsive genes in roots of Col-0 grown for 7 d in solution in the presence or absence of Pi, as used in root growth experiments. Transcripts were determined by semiquantitative RT-PCR. UBQ1 transcript was used as the internal control. C, Al content of roots of Col-0 and pah1pah2 with Al stress treatment. Seedlings were grown for 10 d in Al-toxic solutions ({Al3+}bulk of 0.54 μm, total added Al, 2 μm) with zero Pi at pH 5.0. Al concentration in the roots was quantified using ICP-MS. Means ± se (n = 4) are shown. Asterisks indicate the significance of difference from Col-0 by Student’s t test (P < 0.05). D, Morin staining of Col-0 and pah1pah2 after incubation in Al-toxic solution for 24 h. Bar = 20 μm. E, Seedlings of Col-0 and pah1pah2 were grown for 4 weeks on acidic andosol fertilized with CaCO3 and a combination of CaCO3 and CaSO4. Soil pH was determined. Bar = 1 cm.
In addition, the pah1pah2 mutant showed Al3+ sensitivity that could be alleviated by nonalkalinizing Ca2+ in moderately acidic soil (Fig. 6E). It also showed evidence for the involvement of Al rhizotoxicity under moderate acidity, which could be determined by {Al3+}PM and was alleviated by Ca. Taken together, these results support the claim that {Al3+}PM is the determinant for Al3+ rhizotoxicity at the moderate acidity, which was predicted by the calculations conducted using the SGCS model and the molecular/physiological experiments demonstrating that the sensitive phenotype caused by increased PM surface negativity appears in Ca-insufficient conditions.
Ameliorative Effect of Ca2+ in H+-Sensitive Accessions of Arabidopsis Grown in Moderately Acidic Soil
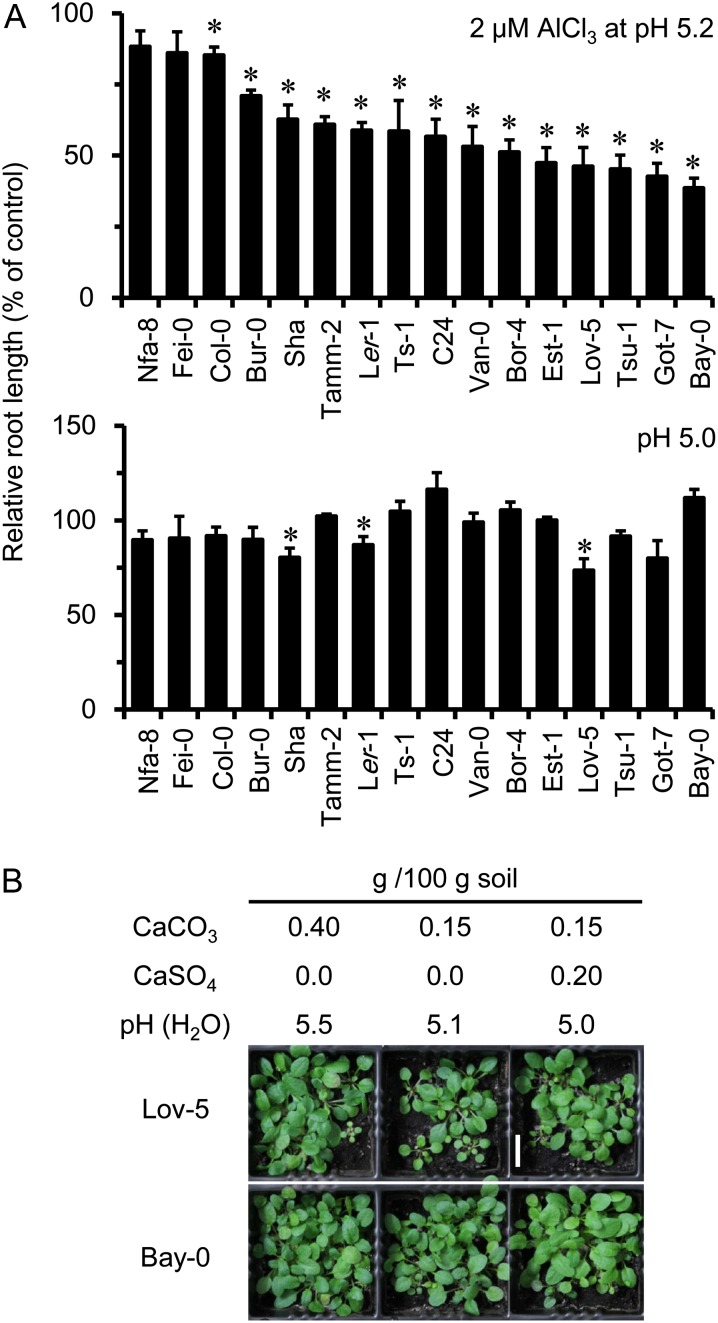
Although sensitivity to Al and H+ toxicities was observed in the sensitive mutant genotypes described above, we felt it would be informative to survey the natural variation for Al and H+ sensitivity. Also, it is known that many of the problems on acidic soils in agriculture arise from the introduction of sensitive species (and varieties) to acidic soil environments. This can be mimicked by testing the response of sensitive Arabidopsis accessions adapted to nonacidic soil environments. Therefore, we compared Al3+ and H+ sensitivity among 16 Arabidopsis accessions from a group of 20 accessions for which full genomic sequences have been determined (Clark et al., 2007) to examine the occurrence of sensitivity when they are introduced to the moderately acidic soil conditions used in this study. We first screened these accessions on nutrient solution and identified accessions sensitive to 2 μm Al at pH 5.2 (relative to 0 μm Al at pH 5.2; Fig. 7A, top) and accessions sensitive to zero Al at pH 5.0 (relative to zero Al at pH 5.5; Fig. 7A, bottom). These findings suggest that some of the accessions carry sensitivity to Al and H+ levels expected in moderately acidic soils. The two most sensitive accessions, Lowkin-5 (Lov-5) and Bayreuth-0 (Bay-0), were then grown on moderately acidic soil (Fig. 7B). These H+-sensitive accessions showed growth suppression in soil culture at moderate acidity (0.15 g CaCO3 per 100 g soil; pH 5.1). The accessions Lov-5 and Bay-0 were comparable in Al sensitivity, but Lov-5 was much more sensitive to low pH. When both accessions were grown on soil of moderate acidity, growth of Lov-5 was more suppressed than Bay-0. This H+ sensitivity of Lov-5 was mostly alleviated by the addition of 0.20 g CaSO4 (soil pH 5.0; Fig. 7B); the Al sensitivity of Bay-0 (Al-sensitive accession) was also alleviated. This indicates that soil at pH 5.1 was intoxicating to sensitive genotypes because of the toxic levels of both Al3+ and H+ in the soil and that Ca fertilizer in the form of gypsum alleviated both stresses in soils without elevating pH. Similar events are expected to occur in sensitive crop genotypes if they are introduced into moderately acidic soils with low Ca levels.
Figure 7.
Comparison of sensitivity of Arabidopsis accessions to Al3+ and H+ rhizotoxicities at pH of at least 5.0. A, Seedlings were grown hydroponically for 7 d in slightly H+-toxic (pH 5.0) and Al3+-toxic (2 μm AlCl3 at pH 5.2) solutions and were compared with seedlings grown in the control solutions (Al control: pH 5.2 and zero Al; pH control: pH 5.5 and zero Al). Means of relative values ± se (n = 6) are indicated. Asterisks denote a significant difference between the RL of seedlings grown in the toxic solutions compared with those grown in the control solutions (*P < 0.05, Student’s t test). B, Lov-5 and Bay-0 accessions grown for 4 weeks on acidic andosol fertilized with CaCO3 or combinations of CaCO3 and CaSO4. Soil pH (water) was also indicated. Bar = 1 cm.
DISCUSSION
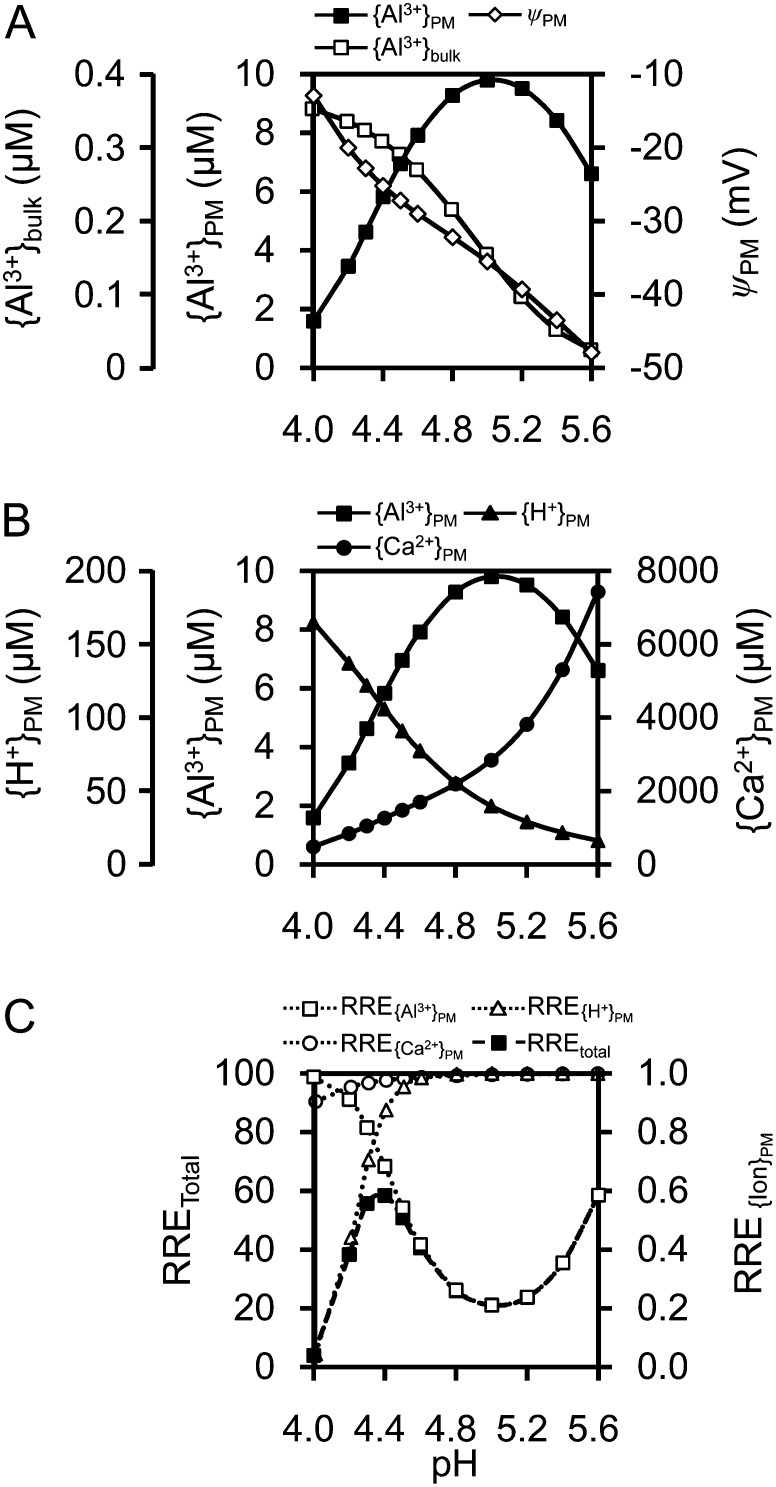
Prior to discussing these results, we will briefly explain why Al and H+ rhizotoxicities of moderately acidic soils were predicted by the SGCS modeling in wheat (Kinraide et al., 2004) and particularly in low-Ca soils. The pH adjustment of soils or Al-containing solution culture media has multiple chemical and physiological effects. Figure 8 illustrates the multiple effects of pH adjustment in solutions containing constant CaCl2 (200 μm) and AlCl3 (0.5 μm). Elevating pH decreases {Al3+}bulk (Al3+ activity in solution) but increases the negativity of PM surface electrical potential (ψPM; Fig. 8A). This combination produced {Al3+}PM at pH greater than 5 comparable to that seen at a lower pH. The attraction of {Al3+}PM is reduced by {Ca2+}PM (Fig. 8B), which induces an alleviating effect of Ca; this means that low Ca levels increase toxicity. Growth inhibition is regulated by these factors (Fig. 8C). Reactions of H+ are more complicated (as it can alleviate Al toxicity), but we can simulate root growth using our new model (Fig. 8C) that includes the growth suppression in moderately acid pH values (pH > 5). These Al and H+ toxicities are enhanced in Ca-insufficient conditions.
Figure 8.
Computed wheat seedling responses to pH adjustment of a growth medium composed of 200 μm CaCl2 and 0.5 μm AlCl3. Published models (Kinraide et al., 2004; Kinraide and Wang, 2010) were used to compute solution species, PM surface electrical potential (ψPM), PM surface ion activities ({Al3+}PM, {Ca2+}PM, and {H+}PM), and growth responses. Total relative root elongation (RRE; on a scale of 0 to 100) was computed as 100 times the product of partial relative root elongations (on a scale of 0 to 1) in response to individual ions [RRETotal = 100(RRE{Ca2+}PM × RRE{H+}PM × RRE{Al3+}PM]. RRE{Ca2+}PM, RRE{H+}PM, and RRE{Al3+}PM indicate relative root elongation in response to {Ca2+}PM, {H+}PM and {Al3+}PM. The H+-induced reduction in the negativity of ψPM reduced the attraction of cations. Ca2+ activity in the solution was nearly constant at 178 ± 1 μm, but at lower pH values, {Ca2+}PM became marginally insufficient. Despite the decline in {Al3+}bulk as pH increased, the increasing negativity of ψPM caused an increase in {Al3+}PM until it reached a maximum at pH 5.0.
As expected from the above analyses, we clearly found Al and H+ toxicities in dilute (low Ca levels) nutrient solutions at higher pH values (pH ≥ 5.0). All of the sensitive KO mutant genotypes showed clear sensitivity to Al and H+ at pH greater than 5 at low Ca levels, despite being derived from Col-0, one of the most Al-tolerant natural accessions (Kobayashi et al., 2007; Fig. 7). In addition, by updating the SGCS modeling method using the GEOCHEM-EZ speciation software, we could successfully predict the {Al3+}PM of Arabidopsis plants grown in medium containing multiple nutrients. This allowed for the validation of predictions (i.e. Al3+ and H+ toxicities at pH > 5) derived from the wheat modeling studies, which were generated using root growth inhibition as a sole criterion of toxicity. The inhibition by Al in the sensitive genotypes at pH greater than 5 was correlated with the attraction of {Al3+} to the PM calculated by the SGCS electrostatics model (Fig. 1) and was not affected by the {Al3+} in bulk solution. This attraction was well correlated with the induction of the Al-inducible biomarker genes, which again supports the concept that the attraction of Al3+ to the PM surface and {Al3+} at the PM surface are key aspects of Al toxicity (Fig. 3). The induction of these genes was suppressed by increasing {Ca2+}PM (reducing damage and {Al3+} attraction to the PM) and was well correlated with growth recovery in sensitive genotypes (Figs. 2 and 3). On the other hand, H+ sensitivity appeared in H+-hypersensitive genotypes at pH greater than 5 in low-Ca systems (Figs. 1 and 7). These results validated the prediction of the modeling method, which can explain Al and H+ toxicity of moderately acidic soils in relation to Ca alleviation.
Toxicity at moderately acidic conditions occurred in some of the naturally occurring Arabidopsis accessions, which are naturally adapted to different environments. The sensitive accessions displayed growth inhibition in both hydroponic growth conditions and moderately acidic soil (Fig. 7). This indicates that moderate soil acidity could be a serious stress factor for sensitive crop genotypes such as the introduction of accessions from nonacidic soils into acid soil agriculture. The observed sensitivity of these Arabidopsis accessions was explained by {Al3+}PM, which remained at levels sufficiently high to be intoxicating at pH greater than 5 (Figs. 7 and 8). This can explain why a pH of 5.5 is commonly a target for the pH amendment of acidic soils: pH 5.5 will eliminate acid soil toxicity for all but a few hypersensitive cultivars. This toxicity can be removed by increasing the Ca2+ concentration in soil solutions. This accounts for the effectiveness of the application of a nonalkalinizing Ca fertilizer, such as gypsum, which is more soluble and more penetrating than limestone, even though it does not increase soil pH.
Modeling approaches help to uncover the mechanisms of Ca alleviation (Fig. 4; Table I), which are different between toxicants or genotypes. We found that Al toxicity can be alleviated by Ca2+ through electrostatic displacement of Al3+ and restoration of Ca-required ligands, as both of these processes occur at the PM surface (Fig. 2; mechanisms I and II for Ca2+ alleviation; Kinraide, 1998). Mechanism III alleviation (other unknown mechanisms) did not seem to alleviate Al toxicity in any of the KO mutants, which accounts for the previous modeling in wheat (Kinraide, 2003). By contrast, mechanism III appeared to alleviate H+ toxicity, but the effectiveness appears to be small in the case of STOP1-KO (Table I). This finding suggests that the hypersensitivity of STOP1-KO reflects the absence of a strong mechanism III alleviation of H+ toxicity in this genotype. Where it does occur, mechanism III alleviation of H+ may include Ca2+ stabilization of the cell wall (Koyama et al., 2001), which involves stabilizing pectic residues in the rhamnogalacturonan II (O’Neill et al., 2004) and polygalacturonic acid (Carpita and Gibeaut, 1993) structures that require Ca2+ for cross linking. These cross-linking processes are pH dependent, and the Ca2+ demand increases greatly at pH less than 5.0 (Koyama et al., 2001). The stop1 mutant shows repressed expression of several genes that encode proteins related to stabilization of pectic polysaccharides such as polygalacturonase inhibitor protein1 that stabilize polygalacturonic acid (Spadoni et al., 2006) and borate transporter II that is critical for borate mediating pectin stabilization (Sawaki et al., 2009). This suggests that the STOP1-KO possesses a lower capacity for mechanisms of cell wall stabilization that reduce damage in the root tips under H+ toxicity (Fig. 5), which fits with the mechanism III of Ca alleviation of proton toxicity (Kinraide, 1998). These results were successfully predicted by our electrostatic modeling and regression analysis. Similar analyses may be useful for the quantitative characterization of additional genotypes with different types of Al3+ and H+ sensitivities.
Recent findings on Al sensitivity suggest that other endogenous organic cations might play a similar role to Ca in ameliorating Al toxicity. Nezames et al. (2012) recently cloned an Al-sensitive Arabidopsis mutant that has a defect in Al exclusion from the root and found the gene encodes a putative nuclear localized ribosomal biogenesis factor. The relevance of this finding is that this Al-sensitive mutant has reduced levels of polyamines and exogenous application of a cationic polyamine, spermidine, restored Arabidopsis Al tolerance. Hence, it is possible that at least in Arabidopsis, cationic polyamines synthesized in the symplasm and released into the cell wall could also play a similar role to Ca2+ in electrostatically reducing {Al3+}PM.
We identified a new Al-sensitive genotype that was predicted by SGCS modeling of Al toxicity. Based on the SGCS electrostatic model, we could expect enhanced susceptibility in genotypes exhibiting increased negativity at the PM surface. This occurs in the pah1pah2 double mutant, which has greater amounts of phospholipids in cell membranes under P-deficient conditions; the mole percentage of phosphatidylcholine in pah1pah2 is 23%, whereas it is 6% in Col-0 (Nakamura et al., 2009). Phosphatidate phosphohydrolases release Pi from PM phospholipids as one of the adaptive strategies of plants to P deficiency. The lack of this capacity in the mutant increased the negative charge at the PM surface under P deficiency and, in turn, increased sensitivity to increased {Al3+}PM. Similar Al sensitivity was identified in rice (Oryza sativa) containing greater amounts of phospholipid in the PM (Khan et al., 2009). Although PM lipid composition may affect Al tolerance via other mechanisms such as Al permeability (Wagatsuma et al., 2005; Ryan et al., 2007), PM surface negativity associated with P recycling is a factor that influences Al tolerance in some plant species and varieties.
Use of the SGCS model in conjunction with molecular physiology approaches has been shown here to be useful for improving our knowledge of Al stress responses (tolerance and injury) in plants. For example, {Al3+}PM-inducible gene expression was reduced by Ca2+ and was comparably reduced by Mg2+, which mimics the electrostatic functions of Ca2+ (Fig. 3; Kinraide and Wang, 2010). This suggests that PM ligands specific to Al (possibly Al3+ sensors at the PM or Al3+-permeable ligands that transport Al to the internal sensing molecule) may be involved in the processes required for gene induction. This sensing system for the activation of tolerance genes is likely sensitive enough to protect the sensitive root tips at levels of {Al3+}PM too low to initiate injury. In fact, at pH of at least 5.0, 2 μm Al did not inhibit root growth of Col-0, but two major genes for Al tolerance were induced by values of {Al3+}PM (Fig. 3) that were comparable to, or lower than, those causing growth inhibition in the KO mutant of each gene (Fig. 2). Differential response of the tolerance genes (AtALMT1 and ALS3) to {Al3+}PM suggests that different pathways of Al3+ activation trigger these genes. AtALMT1 is part of the Al exclusion mechanisms that protect the roots from acute Al toxicity (Hoekenga et al., 2006; Kobayashi et al., 2007), whereas ALS3 is involved in the process of Al sequestration, contributing to long-term Al tolerance (Larsen et al., 1997, 2005). Further research that is integrated with other factors, such as quantification of cellular Al concentrations, would be useful to clarify this possibility.
In conclusion, we present here experimental evidence that reveals the existence of Al3+ and H+ toxicities in moderately acid soils at pH of at least 5 and mechanisms for Ca2+ alleviation of these toxicities. This was originally predicted by a modeling approach using root growth inhibition as the sole criteria of toxicity but is now supported by additional data obtained by molecular physiological analysis of these toxicities, which include the use of Al-responsive biomarker genes. Our results indicate that Al toxicity depends on ion activities at the PM surface (Fig. 9). At pH greater than 5, {Al3+}bulk is very small, but Al3+ is concentrated at the PM surface because of attraction of the trivalent Al cation by the negative charges of ligands such as phospholipids. {Al3+}PM is the primary factor determining Al rhizotoxicity, but it also regulates expression of Al-responsive genes, thus suggesting that some receptor proteins, and/or Al-permeable transporters that deliver Al to the cytosol and localize at the PM surface, play roles in Al sensing. Both Al3+ and H+ toxicities can be alleviated by Ca2+, but there would likely be insufficient Ca2+ in many low-fertility acid soils for effective alleviation (<200 μm Ca2+ at pH 5.05–5.14 and <500 μm Ca2+ at pH 4.6; Adams and Moore, 1983; Wright and Wright, 1987). Based on these findings, we suggest that the application of gypsum, which is much more soluble and more penetrating than limestone, would have a positive, low-cost impact on crop productivity in infertile, low-Ca2+ acid soils. This fertilizer also can alleviate Al toxicity by forming the Al(SO4)+ complex that should be considerably less toxic than Al3+ (Kinraide, 1997). In addition, breeding programs of acid soil-tolerant crops need to consider that tolerant phenotypes may be affected by the Ca status in the soils.
Figure 9.
Model for Al rhizotoxicity and Ca2+ alleviation in weakly acidic conditions. Increased pH causes increased PM surface negativity because of dissociation of H+ from acidic ligands such as phospholipids. These effects increase {Al3+}PM despite reduced {Al3+}bulk. Ca2+ additions have little effect upon {Al3+}bulk but have large effects upon {Al3+}PM because of Ca-induced reductions of PM negativity.
MATERIALS AND METHODS
Plant Materials
Arabidopsis (Arabidopsis thaliana) accession Col-0 (JA58) was obtained from the RIKEN BioResource Center. The following 16 accessions were obtained from the Arabidopsis Biological Resource Center: Bay-0 (CS22676), Borky-4 (Bor-4; CS22677), Burren-0 (Bur-0; CS22678), C24 (CS22680), Col-0 (CS22681), Estland-1 (Est-1; CS22683), St. Maria d. Feria-0 (Fei-0; CS22684), Goettingen-7 (Got-7; CS22685), Landsberg erecta-1 (Ler-1; CS22686), Lov-5 (CS22695), Nfa-8 (CS22687), Shakdara (Sha; CS22690), Tammisari-2 (Tamm-2; CS22691), Tossa de Mar-1 (Ts-1; CS22692), Tsushima-1 (Tsu-1; CS22693), and Vancouver-0 (Van-0; CS22694). The SALK Arabidopsis transfer DNA insertion mutants of AtALMT1-KO (SALK_009629), ALS3-KO (SALK_061074), and STOP1-KO (SALK_114108) were also obtained from the Arabidopsis Biological Resource Center. AtALMT1-KO and ALS3-KO were Al-sensitive mutants, while STOP1-KO was Al and H+ sensitive. All gene KO lines were homozygous and identical to those used in the study by Sawaki et al. (2009). The pah1pah2 double mutant, which shows increased phospholipid content in PMs because of dysfunction of AtPAH1 and AtPAH2 under P-deficient conditions, was identical to that used in Nakamura et al. (2009).
Growth Tests in Hydroponic and Soil Cultures
Root growth tests in hydroponic culture were carried out as described by Kobayashi et al. (2007). The medium was adjusted according to each experiment. The basic test solution contained one-fiftieth-strength MGRL nutrients without P (Fujiwara et al., 1992), while the CaCl2 concentration was increased to 200 μm. The medium contained 1.34 μm Na-EDTA and 0.172 μm FeSO4⋅7H2O. For the low-EDTA medium, Na-EDTA was reduced to 0.172 μm, but FeSO4⋅7H2O remained at 0.172 μm. Al- and H+-rhizotoxic solutions were prepared by adding 10 mm AlCl3⋅6H2O to give final concentrations of 0 to 6 μm Al at pH values 4.5, 4.7, 5.0, 5.2, or 5.5 adjusted with 0.1 n HCl and 0.1 n NaOH. In experiments on Ca2+ and Mg2+ alleviation of Al and H+ toxicities, CaCl2 and MgCl2 were added to the solution to give appropriate concentrations. For comparing growth response of Col-0 and pah1pah2 double mutant in relation to Pi, both seedlings were grown for 7 d in the Al-toxic solutions containing same levels of {Al3+}bulk (0.54 or 1.08 μm), which were calculated using GEOCHEM-EZ in the presence of 35 μm Pi (total Al added, 5 and 9.7 μm) or absence of Pi (total Al added, 2 or 4 μm) at pH 5.0. In all growth tests, solutions were renewed every 2 d, and seedlings were grown for 7 d at 24°C ± 2°C under a 12-h-light/12-h-dark photoperiod, with light supplied at a photosynthetic photon flux density of 37 μmol m–2 s–1. Fifteen seedlings of each line (the wild type and mutants) were grown in each pot. We used the 10 longest roots from 15 seedlings for data analysis (this eliminated seedlings from the experiment that failed to grow normally because of late germination). RL measurements were used for analysis of phenotypes and nonlinear regression analysis.
For soil culture, seedlings were grown for 4 weeks on an acidic andosol containing large amounts of organic matter and phytotoxic Al; this was obtained from the Kawatabi experimental farm at Tohoku University. The properties of these soils are well characterized and are frequently used in physiological experiments of Al and proton toxicities (Ikka et al., 2007). The soil was fertilized with both macro- and micronutrients as described by Kobayashi et al. (2005). CaCO3 was added at 0.15 and 0.4 g per 100 g dry soil, and CaSO4 was added at 0.20 g per 100 g dry soil. The soil pH (water) was measured as described by Ikka et al. (2007).
Computation of Al3+ and H+ Rhizotoxicities
The free Al3+ concentration in complex culture solutions was calculated using GEOCHEM-EZ (Shaff et al., 2010). Free ions in the bulk solution and at the PM surface were calculated by a SGCS electrostatic model (Kinraide and Wang, 2010). Growth data of the wild type and mutants (AtALMT1-KO, ALS3-KO, and STOP1-KO) were obtained from experiments using more than 30 different combinations of Al, pH, and Ca. Data (obtained from the five longest seedlings from 15 seedlings) were analyzed by nonlinear regression analysis using MYSTAT12 (SYSTAT) to obtain coefficients for the equations (Eqs. 1 and 2) used to fit curves to the data. The estimated value of a coefficient was considered significant if the 95% confidence interval did not encompass zero.
Expression Analysis of Al-Responsive Genes
Arabidopsis Col-0 was grown for 10 d in basal medium in the presence of 35 μm Pi as described previously (Kobayashi et al., 2007). The roots were immersed in low-EDTA test solutions with or without 1 μm AlCl3 at pH 5.5 containing various final concentrations of CaCl2 and MgCl2. After 6-h incubation, roots were collected and total RNA was extracted as described by Suzuki et al. (2003). Total RNA was reverse transcribed to complementary DNA using ReverTra Ace (Toyobo) with oligo(dT) primer, and then the expression levels of genes were determined by real-time PCR. Quantitative real-time PCR was performed with Power SYBR Green PCR master mix and an ABI PRISM 7000 instrument (Applied Biosystems). Data were analyzed using the GeneAmp 7000 Sequence Detection System version 1.2 (Applied Biosystems) according to the manufacturer’s protocol. Expression levels of each gene were normalized to the expression level of UBIQUITIN1 (UBQ1). The expressions of major Al-inducible Al tolerance genes, namely AtALMT1 and ALS3, were quantified using specific primer pairs as follows: AtALMT1, 5′-CACAGTTTTACATGACGTTGATAATGAT-3′ (forward) and 5′-TCTTCATGTTTTTCATGGTTTGAGTT-3′ (reverse); ALS3, 5′-TATCGATCCTTGCCGGGACTTCA-3′ (forward) and 5′-GCTTGTCTTGGCGTTGCTCCTA-3′ (reverse); and UBQ1, 5′-TCGTAAGTACAATCAGGATAAGATG-3′ (forward) and 5′-CACTGAAACAAGAAAAACAAACCCT-3′ (reverse). Transcript levels of Al-responsive genes were analyzed by semiquantitative reverse transcription (RT)-PCR using gene-specific primers (Supplemental Table S3). The amplified fragments from the early exponential phase were stained with SYBR Green I (Molecular Probes) and visualized using a Typhoon 9410 imager (Amersham Biosciences).
Morin Staining
Morin staining was performed following the method described by Tice et al. (1992) using morin (Sigma). Briefly, Col-0, AtALMT1-KO, and pah1pah2 seedlings that were grown for 5 d in basal media in the absence of Pi after 5 d of pregrowing in 35 μm Pi-containing media were incubated in Al-toxic solution ({Al3+}bulk = 1.08 μm) for 24 h. The roots were stained with 100 μm morin for 15 min, rinsed by distilled water, and then observed with a fluorescence microscope (Olympus BX51).
Quantification of Root Al Concentrations sing ICP-MS
Three sets of both Col-0 and pah1pah2 double mutants seedlings (about 150 seedlings each) were grown for 7 d under –P and +Al conditions (–P, 2 μm Al, pH 5.0), and then the roots were excised and rinsed in distilled water. The root samples were digested with concentrated HNO3 (2 mL; electronic chemical grade; Kanto Chemical) and concentrated H2O2 (0.5 mL; semiconductor grade; Santoku Chemical), and then digestate was dried using a DigiPREP digestion apparatus (SCP Science). The samples were then solubilized in 3 mL of 2% (v/v) HNO3 in ultrapure water. The root Al concentrations were analyzed using ICP-MS according to manufacturer’s manual (ELAN DRC-e; Perkin Elmer).
Root Tip Viability
The viability of growing root tips after exposure to H+ stress was analyzed as described by Koyama et al. (2001). Briefly, seedlings of STOP1-KO and wild-type Col-0 were grown in stress-free culture solution (pH 5.5) for 5 d. The roots of seedlings were then immersed in solutions with low pH (pH 4.7) containing 200 or 400 μm CaCl2. After 90 min of treatment, roots were stained with propidium iodide (3 μg mL–1) for 15 s and then observed under a fluorescent microscope (IMT-2-21-RFL; Olympus) using a dichroic mirror unit (IMT-2-DG; Olympus) and a density reduction filter (ND6; Olympus).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Al inhibition of root growth of Arabidopsis seedlings in hydroponic culture with different concentrations of EDTA.
Supplemental Table S1. List of Al-responsive genes expressing greater induction in Col-0 treated with 25 μm Al than with 10 μm Al.
Supplemental Table S2. List of genes identified as being uniquely induced by Al using a comparative microarray (Zhao et al., 2009) in which induction in the wild-type lines treated with 25 μm Al was greater than in wild-type lines treated with 10 μm Al.
Supplemental Table S3. Sequences of primers used for semiquantitative RT-PCR.
Acknowledgments
We thank the BioResource Center of RIKEN, the Arabidopsis Biological Resource Center, and the Nottingham Arabidopsis Stock Center for providing Arabidopsis seeds.
Glossary
- PM
plasma membrane
- {Al3+}PM
{Al3+} at the plasma membrane surface
- {H+}PM
{H+} at the plasma membrane surface
- {Ca2+}PM
{Ca2+} at the plasma membrane surface
- SGCS
speciation-based Gouy-Chapman-Stern electrostatic
- {Al3+}bulk
Al3+
- KO
knockout
- Col-0
ecotype Columbia
- RL
root length
- Pi
phosphate
- ICP-MS
inductively coupled plasma mass spectrometry
- Lov-5
Lowkin-5
- Bay-0
Bayreuth-0
- ψPM
plasma membrane surface electrical potential
- RT
reverse transcription
References
- Adams F, Moore BL. (1983) Chemical factors affecting root growth in subsoil horizons of coastal plain soils. Soil Sci Soc Am J 47: 99–102 [Google Scholar]
- Carpita NC, Gibeaut DM. (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 3: 1–30 [DOI] [PubMed] [Google Scholar]
- Carvalho MCS, Van Raij B. (1997) Calcium sulphate, phosphogypsum and calcium carbonate in the amelioration of acid subsoils for root growth. Plant Soil 192: 37–48 [Google Scholar]
- Clark RM, Schweikert G, Toomajian C, Ossowski S, Zeller G, Shinn P, Warthmann N, Hu TT, Fu G, Hinds DA, et al. (2007) Common sequence polymorphisms shaping genetic diversity in Arabidopsis thaliana. Science 317: 338–342 [DOI] [PubMed] [Google Scholar]
- Famoso AN, Clark RT, Shaff JE, Craft E, McCouch SR, Kochian LV. (2010) Development of a novel aluminum tolerance phenotyping platform used for comparisons of cereal aluminum tolerance and investigations into rice aluminum tolerance mechanisms. Plant Physiol 153: 1678–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T, Hirai MY, Chino M, Komeda Y, Naito S. (1992) Effects of sulfur nutrition on expression of the soybean seed storage protein genes in transgenic petunia. Plant Physiol 99: 263–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekenga OA, Maron LG, Piñeros MA, Cançado GM, Shaff J, Kobayashi Y, Ryan PR, Dong B, Delhaize E, Sasaki T, et al. (2006) AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc Natl Acad Sci USA 103: 9738–9743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekenga OA, Vision TJ, Shaff JE, Monforte AJ, Lee GP, Howell SH, Kochian LV. (2003) Identification and characterization of aluminum tolerance loci in Arabidopsis (Landsberg erecta × Columbia) by quantitative trait locus mapping. A physiologically simple but genetically complex trait. Plant Physiol 132: 936–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikka T, Kobayashi Y, Iuchi S, Sakurai N, Shibata D, Kobayashi M, Koyama H. (2007) Natural variation of Arabidopsis thaliana reveals that aluminum resistance and proton resistance are controlled by different genetic factors. Theor Appl Genet 115: 709–719 [DOI] [PubMed] [Google Scholar]
- Iuchi S, Koyama H, Iuchi A, Kobayashi Y, Kitabayashi S, Kobayashi Y, Ikka T, Hirayama T, Shinozaki K, Kobayashi M. (2007) Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance. Proc Natl Acad Sci USA 104: 9900–9905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MSH, Tawaraya K, Sekimoto H, Koyama H, Kobayashi Y, Murayama T, Chuba M, Kambayashi M, Shiono Y, Uemura M, et al. (2009) Relative abundance of Δ5-sterols in plasma membrane lipids of root-tip cells correlates with aluminum tolerance of rice. Physiol Plant 135: 73–83 [DOI] [PubMed] [Google Scholar]
- Kinraide TB. (1997) Reconsidering the rhizotoxicity of hydroxyl, sulphate, and fluoride complexes of aluminum. J Exp Bot 48: 1115–1124 [Google Scholar]
- Kinraide TB. (1998) Three mechanisms for the calcium alleviation of mineral toxicities. Plant Physiol 118: 513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide TB. (2003) Toxicity factors in acidic forest soils: attempts to evaluate separately the toxic effects of excessive Al3+ and H+ and insufficient Ca2+ and Mg2+ upon root elongation. Eur J Soil Sci 54: 323–333 [Google Scholar]
- Kinraide TB, Parker DR. (1990) Apparent phytotoxicity of mononuclear hydroxy-aluminum to four dicotyledonous species. Physiol Plant 79: 283–288 [Google Scholar]
- Kinraide TB, Pedler JF, Parker DR. (2004) Relative effectiveness of calcium and magnesium in the alleviation of rhizotoxicity in wheat induced by copper, zinc, aluminum, sodium, and low pH. Plant Soil 259: 201–208 [Google Scholar]
- Kinraide TB, Wang P. (2010) The surface charge density of plant cell membranes (σ): an attempt to resolve conflicting values for intrinsic σ. J Exp Bot 61: 2507–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Furuta Y, Ohno T, Hara T, Koyama H. (2005) Quantitative trait loci controlling aluminium tolerance in two accessions of Arabidopsis thaliana (Landsberg erecta and Cape Verde Islands). Plant Cell Environ 28: 1516–1524 [Google Scholar]
- Kobayashi Y, Hoekenga OA, Itoh H, Nakashima M, Saito S, Shaff JE, Maron LG, Piñeros MA, Kochian LV, Koyama H. (2007) Characterization of AtALMT1 expression in aluminum-inducible malate release and its role for rhizotoxic stress tolerance in Arabidopsis. Plant Physiol 145: 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Koyama H. (2002) QTL analysis of Al tolerance in recombinant inbred lines of Arabidopsis thaliana. Plant Cell Physiol 43: 1526–1533 [DOI] [PubMed] [Google Scholar]
- Koyama H, Toda T, Hara T. (2001) Brief exposure to low-pH stress causes irreversible damage to the growing root in Arabidopsis thaliana: pectin-Ca interaction may play an important role in proton rhizotoxicity. J Exp Bot 52: 361–368 [PubMed] [Google Scholar]
- Larsen PB, Geisler MJ, Jones CA, Williams KM, Cancel JD. (2005) ALS3 encodes a phloem-localized ABC transporter-like protein that is required for aluminum tolerance in Arabidopsis. Plant J 41: 353–363 [DOI] [PubMed] [Google Scholar]
- Larsen PB, Kochian LV, Howell SH. (1997) Al inhibits both shoot development and root growth in als3, an Al-sensitive Arabidopsis mutant. Plant Physiol 114: 1207–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora ML, Schnettler B, Demanet R. (1999) Effect of liming and gypsum on soil chemistry, yield, and mineral composition of ryegrass grown in an acidic Andisol. Commun Soil Sci Plant Anal 30: 1251–1266 [Google Scholar]
- Nakamura Y, Koizumi R, Shui G, Shimojima M, Wenk MR, Ito T, Ohta H. (2009) Arabidopsis lipins mediate eukaryotic pathway of lipid metabolism and cope critically with phosphate starvation. Proc Natl Acad Sci USA 106: 20978–20983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezames CD, Ochoa V, Larsen PB. (2012) Mutational loss of Arabidopsis SLOW WALKER2 results in reduced endogenous spermine concomitant with increased aluminum sensitivity. Funct Plant Biol 40: 67–78 [DOI] [PubMed] [Google Scholar]
- O’Neill MA, Ishii T, Albersheim P, Darvill AG. (2004) Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall pectic polysaccharide. Annu Rev Plant Biol 55: 109–139 [DOI] [PubMed] [Google Scholar]
- Ryan PR, Liu Q, Sperling P, Dong B, Franke S, Delhaize E. (2007) A higher plant Δ8 sphingolipid desaturase with a preference for (Z)-isomer formation confers aluminum tolerance to yeast and plants. Plant Physiol 144: 1968–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaki Y, Iuchi S, Kobayashi Y, Kobayashi Y, Ikka T, Sakurai N, Fujita M, Shinozaki K, Shibata D, Kobayashi M, et al. (2009) STOP1 regulates multiple genes that protect Arabidopsis from proton and aluminum toxicities. Plant Physiol 150: 281–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaff JE, Schultz BA, Craft EJ, Clark RT, Kochian LV. (2010) GEOCHEM-EZ: a chemical speciation program with greater power and flexibility. Plant Soil 330: 207–214 [Google Scholar]
- Spadoni S, Zabotina O, Di Matteo A, Mikkelsen JD, Cervone F, De Lorenzo G, Mattei B, Bellincampi D. (2006) Polygalacturonase-inhibiting protein interacts with pectin through a binding site formed by four clustered residues of arginine and lysine. Plant Physiol 141: 557–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Hibino T, Kawazu T, Wada T, Kihara T, Koyama H. (2003) Extraction of total RNA from leaves of Eucalyptus and other woody and herbaceous plants using sodium isoascorbate. Biotechniques 34: 988–990, 992–993 [DOI] [PubMed] [Google Scholar]
- Tice KR, Parker DR, Demason DA. (1992) Operationally defined apoplastic and symplastic aluminum fractions in root tips of aluminum-intoxicated wheat. Plant Physiol 100: 309–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagatsuma T, Akiba R. (1989) Low surface negativity of root protoplasts from aluminum-tolerant plant species. Soil Sci Plant Nutr 35: 443–452 [Google Scholar]
- Wagatsuma T, Khan MSH, Rao IM, Wenzl P, Tawaraya K, Yamamoto T, Kawamura T, Hosogoe K, Ishikawa S. (2005) Methylene blue stainability of root-tip protoplasts as an indicator of aluminum tolerance in a wide range of plant species, cultivars and lines. Soil Sci Plant Nutr 51: 991–998 [Google Scholar]
- Wright RJ, Wright SF. (1987) Effects of aluminum and calcium on the growth of subterranean clover in Appalachian soils. Soil Sci 143: 341–348 [Google Scholar]
- Yermiyahu U, Brauer DK, Kinraide TB. (1997) Sorption of aluminum to plasma membrane vesicles isolated from roots of Scout 66 and Atlas 66 cultivars of wheat. Plant Physiol 115: 1119–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Ojima K. (1995) Physiological response of root tip of alfalfa to low pH and aluminum stress in water culture. Plant Soil 171: 163–165 [Google Scholar]
- Zhao CR, Ikka T, Sawaki Y, Kobayashi Y, Suzuki Y, Hibino T, Sato S, Sakurai N, Shibata D, Koyama H. (2009) Comparative transcriptomic characterization of aluminum, sodium chloride, cadmium and copper rhizotoxicities in Arabidopsis thaliana. BMC Plant Biol 9: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]