Arabidopsis root response to salinity requires the calcium regulatory protein annexin1.
Abstract
Salinity (NaCl) stress impairs plant growth and inflicts severe crop losses. In roots, increasing extracellular NaCl causes Ca2+ influx to elevate cytosolic free Ca2+ ([Ca2+]cyt) as a second messenger for adaptive signaling. Amplification of the signal involves plasma membrane reduced nicotinamide adenine dinucleotide phosphate oxidase activation, with the resultant reactive oxygen species triggering Ca2+ influx. The genetic identities of the Ca2+-permeable channels involved in generating the [Ca2+]cyt signal are unknown. Potential candidates in the model plant Arabidopsis (Arabidopsis thaliana) include annexin1 (AtANN1). Here, luminescent detection of [Ca2+]cyt showed that AtANN1 responds to high extracellular NaCl by mediating reactive oxygen species-activated Ca2+ influx across the plasma membrane of root epidermal protoplasts. Electrophysiological analysis revealed that root epidermal plasma membrane Ca2+ influx currents activated by NaCl are absent from the Atann1 loss-of-function mutant. Both adaptive signaling and salt-responsive production of secondary roots are impaired in the loss-of-function mutant, thus identifying AtANN1 as a key component of root cell adaptation to salinity.
Soil salinity is an increasing threat to global crop productivity, with up to one-third of agricultural land affected (Munns and Tester, 2008; Kronzucker and Britto, 2011). Salinity (NaCl) stress impairs plant growth and inflicts severe crop losses (Munns and Tester, 2008). Raised concentrations in soil solution or irrigation water perturb osmotic relations, making it difficult for roots to take up water. Uptake of Na+ deleteriously affects the cellular K+:Na+ ratio and may lead to cell death. In roots, high extracellular NaCl causes Ca2+ influx to elevate cytosolic free Ca2+ ([Ca2+]cyt) as a second messenger for adaptive signaling (Lynch et al., 1989; Kiegle et al., 2000; Shi et al., 2000; Tracy et al., 2008). Exposure to salinity activates the Salt Overly Sensitive (SOS) pathway, leading to Ca2+-dependent increased activity of SOS1, a plasma membrane Na+-H+ antiporter that enables adaptation through Na+ efflux (Shi et al., 2000; Chung et al., 2008). Salinity also increases SOS1 expression in Arabidopsis (Arabidopsis thaliana), and this requires activation of the AtSOS3/calcineurin B-like4 Ca2+ sensor (Shi et al., 2000). Reactive oxygen species (ROS), sourced from plasma membrane NADPH oxidase activity, help stabilize AtSOS1 transcripts (Chung et al., 2008). Growth of better-adapted secondary roots is impaired in Atsos1 (Huh et al., 2002) and involves superoxide anion production, possibly by NADPH oxidases (Roach and Kranner, 2011). These enzymes are now known to play a role in xylem loading of Na+ (Jiang et al., 2012).
The channels involved in transiently elevating [Ca2+]cyt in response to increasing extracellular NaCl have not been identified at the genetic level. Manipulation of membrane voltage by varying external concentrations of K+ and Ca2+ has indicated that both hyperpolarization- and depolarization-activated plasma membrane Ca2+-permeable channels can operate in generating a NaCl-induced [Ca2+]cyt increase (Tracy et al., 2008). The Arabidopsis genome contains two families of channel subunit genes that may contribute to NaCl-induced signaling, the Cyclic Nucleotide-Gated Channels (CNGC) and the Glu Receptors (Dodd et al., 2010) Members of both groups have been shown to be competent in plasma membrane Ca2+ flux (Ali et al., 2007; Vincill et al., 2012), but none have been shown to function in NaCl-induced [Ca2+]cyt elevation.
Plant annexins have been shown to form Ca2+-permeable channels in planar lipid bilayers (Laohavisit et al., 2009, 2010, 2012). These soluble proteins are capable of membrane binding and insertion (for review, see Laohavisit and Davies, 2011). The most abundant annexin in Arabidopsis, AtANN1, can exist as a plasma membrane protein (Lee et al., 2004) and is responsible for the root epidermal plasma membrane Ca2+- and K+-permeable conductance that is activated by extracellular hydroxyl radicals (OH•), the most reactive of the ROS (Laohavisit et al., 2012). In this study, we have tested for the involvement of AtANN1 in the generation of root and root epidermal NaCl-induced [Ca2+]cyt elevation. In most cases, high concentrations of NaCl were tested, as these are known to promote extracellular OH• formation (Demidchik et al., 2010), cause accumulation of AtANN1 in membranes (Lee et al., 2004), and promote secondary root formation (Huh et al., 2002). Results show that AtANN1 does not contribute to root Na+ uptake but is a component of the [Ca2+]cyt signal, particularly that generated at the extracellular [Ca2+] of saline soils and by production of ROS. The impairment in [Ca2+]cyt signaling is reflected in the poor ability of Atann1 roots to up-regulate NaCl-responsive transcripts and generate secondary roots when grown in saline conditions.
RESULTS
AtANN1 Restricts Root Epidermal Net Na+ Influx and Mediates NaCl-Induced [Ca2+]cyt Elevation
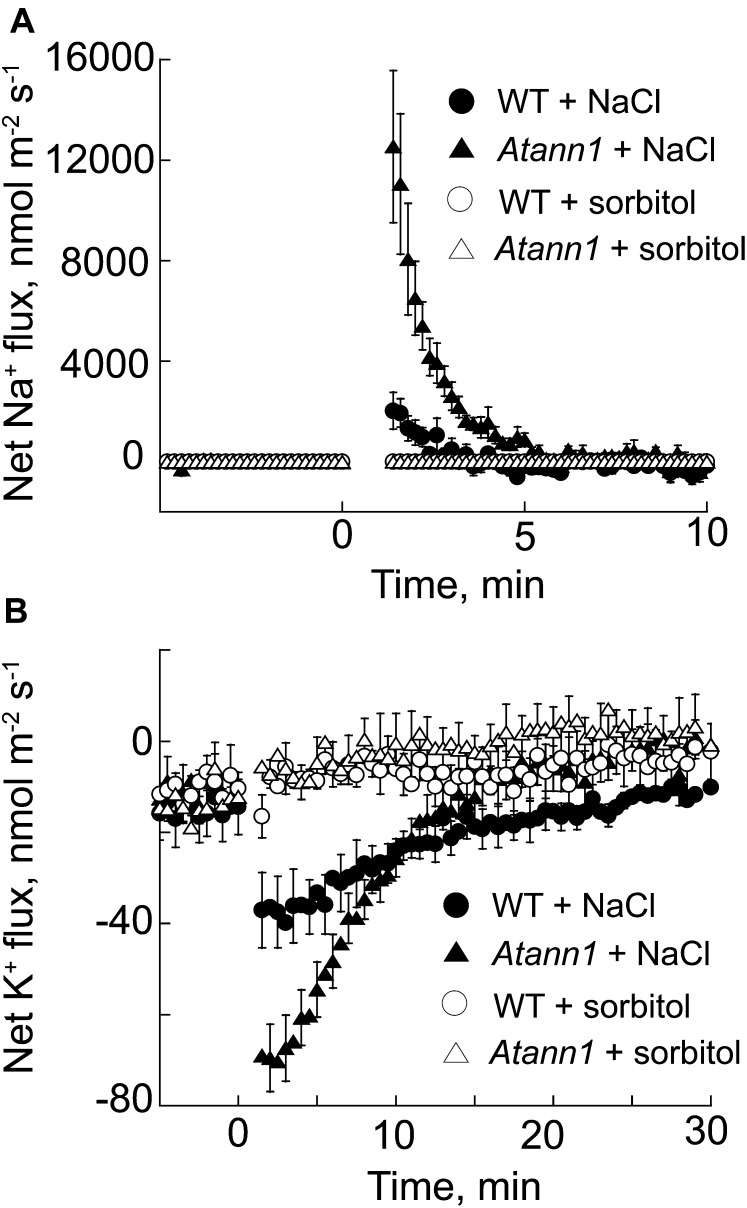
Na+ entry into root cells is mediated by plasma membrane nonselective cation channels (Demidchik and Tester, 2002; Gobert et al., 2006; Guo et al., 2008; Kronzucker and Britto, 2011). As AtANN1 was found previously to have plasma membrane cation transport activity (Laohavisit et al., 2012), we first tested for AtANN1’s possible participation in Na+ entry by measuring net fluxes at root epidermal cells using a vibrating ion-selective microelectrode (Shabala et al., 2006). Wild-type cells sustained a maximum mean net Na+ influx of 2,023 ± (se) 732 nmol m–2 s–1 when challenged with 50 mm NaCl (1 mm extracellular Ca2+), followed by a recovery phase (Fig. 1A; n = 4). Maximum mean net Na+ influx for the Atann1 loss-of-function mutant (Lee et al., 2004; Laohavisit et al., 2012) was significantly higher than the wild type (12,538 ± 3,032 nmol m–2 s–1, P = 0.02, Student’s t test; n = 5; Fig. 1A).
Figure 1.
NaCl causes greater net Na+ influx and K+ efflux at root epidermal cells of Atann1 than of the wild type (WT). Net fluxes in response to 50 mm NaCl were measured using a vibrating ion-selective microelectrode; bathing solution was 1 mm CaCl2, 0.1 mm KCl, and 2 mm MES/Tris, pH 6. Measurements in the first 60 s after test addition were discarded to allow for establishment of diffusion gradients. The sign convention is “influx positive.” A, Mean ± se net Na+ fluxes of the wild type (circle) and Atann1 (triangle) in response to addition of NaCl (black symbol) or the d-sorbitol osmotic equivalent (white symbol) as indicated by the arrow (n = 4–7 roots). B, Net K+ fluxes in response to NaCl or the d-sorbitol osmotic equivalent (n = 4–7 roots).
High extracellular salinity increases net K+ efflux from roots (Shabala et al., 2006). Net K+ efflux from Atann1 (n = 5) was also significantly (2-fold) higher than the wild type (n = 4; P = 0.04; Fig. 1B), commensurate with the mutant’s greater net Na+ influx. Isoosmotic controls using d-sorbitol showed that responses were due to NaCl rather than osmotic stress (n = 5; Fig. 1, A and B). Resolving net Na+ fluxes at higher NaCl concentrations was not possible due to methodological limitations, but increasing NaCl stress to 220 mm also caused significantly greater net K+ release from Atann1 compared with the wild type (wild-type peak efflux, –737 ± 108 nmol m–2 s–1, n = 4; Atann1, –1,270 ± 22 m–2 s–1, n = 7; P = 0.003). These results show that AtANN1 is effectively a negative regulator of net Na+ influx. As AtANN1 is a plasma membrane cation channel that can mediate K+ efflux (Gorecka et al., 2007; Laohavisit et al., 2012), we anticipated that Atann1 should have lower Na+-induced K+ loss than the wild type. That K+ efflux was greater in the mutant was not consistent with simply a loss of K+ efflux function.
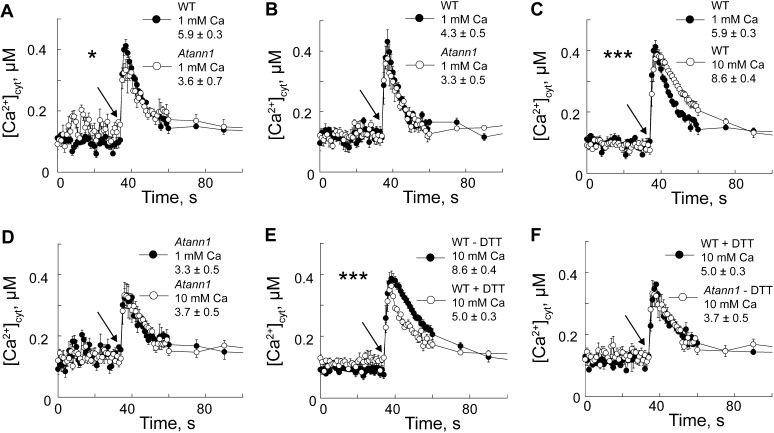
We reasoned that greater epidermal Na+ influx could elicit a larger [Ca2+]cyt signal in Atann1 than the wild type. To investigate this, root epidermal protoplasts were isolated from plants cytosolically expressing aequorin as a [Ca2+]cyt indicator (Laohavisit et al., 2012). With extracellular Ca2+ at 1 mm (as in flux experiments), NaCl (220 mm) evoked a rapid, monophasic, and transient [Ca2+]cyt increase (Fig. 2A) that was significantly lower in Atann1 than the wild type, both in terms of initial increment (P = 0.023; n = 4 for Atann1 and 8 for the wild type) and total [Ca2+]cyt mobilized (P = 0.025); no significant differences between genotypes were observed for the osmotic control (Fig. 2B; initial increment, P = 0.30; total [Ca2+]cyt mobilized, P = 0.185, n = 4 for Atann1 and 8 for the wild type). There was no significant difference between NaCl- and osmotically induced [Ca2+]cyt elevation by the wild type, but these data show that AtANN1 is required for a significant NaCl-specific increase in [Ca2+]cyt at 1 mm Ca2+.
Figure 2.
[Ca2+]cyt signaling is impaired in Atann1 root epidermal protoplasts. A, Mean ± se [Ca2+]cyt response of wild-type (WT; black circle) and Atann1 (white circle) protoplasts (70 per μL; 0.1 mm KCl and 2 mm Tris/MES, pH 5.8) in response to 220 mm NaCl added at 35 s indicated by arrow) with 1 mm extracellular Ca2+ (n = 4; inset, mean ± se total [Ca2+]cyt mobilized). B, Same as A but with isotonic d-sorbitol (n = 4). C, Increasing extracellular Ca2+ from 1 mm (black circle) to 10 mm (white circle) increased wild-type response to 220 mm NaCl (n = 8). D, Atann1 response to 220 mm NaCl with 10 mm Ca2+ (black circle) did not differ from that with 1 mm Ca2+ (white circle; n = 4). E, At 10 mm Ca2+, DTT (1 mm; black circle) lowers wild-type [Ca2+]cyt response to 220 mm NaCl (n = 4). F, At 10 mm Ca2+, Atann1 (white circle) without DTT phenocopies the wild-type response to 220 mm NaCl with DTT (black circle; n = 8). Asterisk denotes level of significance difference.
AtANN1 Mediates NaCl-Induced Ca2+ Influx at the High Extracellular Ca2+ Found in Saline Soils
Extracellular Ca2+ was increased to 10 mm to mimic saline soils (Kronzucker and Britto, 2011). Under these conditions, there were no statistically significant differences in the “touch” control responses between the wild type and Atann1 (Supplemental Fig. S1A). Elevation of extracellular Ca2+ to 10 mm significantly increased the total [Ca2+]cyt mobilized by wild-type root epidermal protoplasts in response to 220 mm NaCl (Fig. 2C; P = 0.001, n = 8), and the response was not significantly different from the osmotic control. Elevations of [Ca2+]cyt in the wild type were significantly inhibited by the Ca2+ channel blocker Gd3+, which also blocks AtANN1 channel activity (Laohavisit et al., 2012), demonstrating reliance on Ca2+ influx (Supplemental Fig. S1B; total [Ca2+]cyt mobilized, P = 0.0003, n = 3). In the same test on the Atann1 mutant, an irregular [Ca2+]cyt response to NaCl was observed, indicating residual Gd3+-sensitive and -insensitive components, but overall no significant differences were found (Supplemental Fig. S1C; n = 3). Atann1 protoplasts were unresponsive to increased extracellular Ca2+ under both NaCl (Fig. 2D; P = 0.86, n = 8) and isoosmotic challenge (Supplemental Fig. S1D; total [Ca2+]cyt mobilized, P = 0.877; although kinetics appear altered, there was no significant difference in time taken to reach peak response, P = 0.87; n = 8). The [Ca2+]cyt responses to 150 mm NaCl were also significantly lower in Atann1 compared with the wild type at 10 mm extracellular Ca2+ (Supplemental Fig. S1E; initial [Ca2+]cyt increment, P = 0.038; total [Ca2+]cyt mobilized, P = 0.001, n = 6). At whole-root level (Supplemental Fig. S2, A–D), there were no statistically significant differences in total [Ca2+]cyt increase between Atann1 and the wild type challenged with 50 to 250 mm NaCl or its osmotic equivalent (Supplemental Fig. S2E; n = 3). This is perhaps to be expected, given that cell-specific changes in [Ca2+]cyt measured with aequorin may not manifest at the whole-organ level (Dodd et al., 2006; Laohavisit et al., 2012). At the highest [NaCl], secondary peak increases in [Ca2+]cyt were more pronounced in Atann1 than in the wild type (Supplemental Fig. S2, A and C), suggesting a role for AtANN1 in regulating the response.
AtANN1 Is Required for ROS-Induced [Ca2+]cyt Elevation on NaCl Exposure
On exposure to extracellular NaCl, plasma membrane NADPH oxidases are activated to produce extracellular superoxide anion (Yang et al., 2007; Chung et al., 2008; Kaye et al., 2011; Xie et al., 2011; Jiang et al., 2012; Liu et al., 2012; Ma et al., 2012). This drives production of extracellular OH•, with up to a 5-fold increase in [OH•] detected in NaCl-stressed Arabidopsis roots (Demidchik et al., 2010). AtANN1 forms the root epidermal plasma membrane Ca2+-permeable channel conductance that is activated by extracellular OH• (Laohavisit et al., 2012), suggesting that it could contribute to the plasma membrane Ca2+ influx pathway acting downstream of NADPH oxidase activity in salt stress (Chung et al., 2008; Ma et al., 2012). Reducing conditions were imposed (with 1 mm dithiothreitol [DTT]) on wild-type root epidermal protoplasts (expressing aequorin) to oppose ROS activation of plasma membrane Ca2+ channels (Demidchik et al., 2009) in response to NaCl. There was no significant effect of DTT on touch responses (Supplemental Fig. S1; n = 4). The initial [Ca2+]cyt increase of wild-type protoplasts in response to 220 mm NaCl (in 10 mm Ca2+) was not significantly lowered by reducing conditions (Fig. 2E; P = 0.26), but the total [Ca2+]cyt mobilized was significantly lowered (P = 0.0001, n = 4 for + DTT, n = 8 for – DTT), consistent with NaCl stress activating a later and ROS-dependent [Ca2+]cyt response. Atann1 without DTT (n = 8) phenocopied the wild type’s response to NaCl with DTT (n = 4; Fig. 1F; no significant difference), clearly showing that AtANN1 was responsible for the ROS-dependent (i.e. DTT-inhibited) component of the [Ca2+]cyt response.
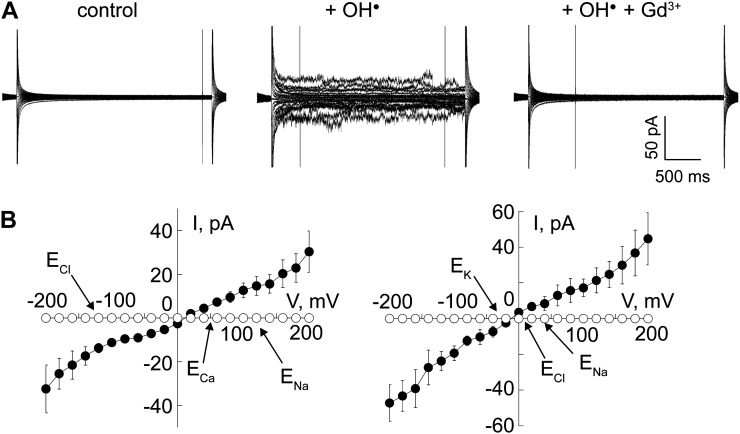
We tested the ability of AtANN1 to function in this process by assaying for Ca2+, K+, and Na+ permeability of the OH•-activated AtANN1 ionic conductance. Recombinant AtANN1 was incorporated from the equivalent of the cytosol (cis compartment) into artificial planar lipid bilayers as mimetics of the plant plasma membrane. Na+ and Ca2+ gradients across the bilayer qualitatively simulated in vivo gradients under salinity stress and activation by OH• (at the equivalent of the extracellular face of the plasma membrane, trans compartment) was achieved by addition of 1 mm Cu2+ and 1 mm ascorbate, as described previously (Laohavisit et al., 2012). An OH•-activated current was evident that was blocked by 50 µm Gd3+ at the trans face, indicating that AtANN1 mediated the current across the bilayer (Fig. 3A; Supplemental Fig. S3). Boiled AtANN1 did not generate a conductance (Supplemental Fig. S3; n = 3). The AtANN1-mediated OH•-activated conductance was Ca2+ permeable, with the reversal voltage ([Erev], at which net current is 0; +10 ± 6 mV; n = 6) lying close to the equilibrium potential for Ca2+ rather than Na+ (Fig. 3B). Erev of the AtANN1-mediated currents were used to estimate permeabilities. A Na+-to-Cl– permeability ratio of 3 was estimated (Supplemental Fig. S3; n = 6). The Ca2+-to-Na+ permeability ratio was 11 (n = 6; Fig. 3B), two orders of magnitude higher than the previously determined Ca2+-to-K+ permeability ratio of 0.64 under identical recording conditions (Laohavisit et al., 2012), thus showing that AtANN1 would be competent to amplify the [Ca2+]cyt signal while resisting further Na+ ingress. Na+ permeated poorly relative to K+ with a permeability ratio of 18 (Fig. 3C; n = 6).
Figure 3.
Recombinant AtANN1 forms an OH-activated Ca2+-permeable conductance that is weakly permeable to Na+. AtANN1 (3 μg) was incorporated from the cis chamber into a planar lipid bilayer and exposed to OH generated at the opposite (trans) membrane face. A, Macroscopic currents recorded from a representative trial in response to voltage steps of 20-mV increments from –200 to +200 mV, applied from a 0-mV baseline with cis 200 mm CaCl2 and 200 mm NaCl (pH 6), and trans 1 mm CaCl2 and 1 mm NaCl (pH 6). The pH was set with 10 mm Tris/Bis-Tris propane. Left, control; middle, maximum current in response to trans OH; and right, with trans OH and Gd3+. Magnified traces of control and OH-activated currents are shown in Supplemental Figure S3. B, Mean ± se current-voltage relationships from six individual trials using conditions described in A; OH-activated conductance (black circles) and OH-activated conductance blocked by Gd3+ (white circles). Equilibrium potentials (E) are marked by arrows. Current flowing below the v axis is cation flow from trans to cis or anion flow from cis to trans. C, Mean ± se current-voltage relationships of the OH-activated conductance (black circles) with cis 200 mm KCl and 50 mm NaCl (pH 6) and trans 50 mm KCl and 200 mm NaCl (pH 6); mean ± se Erev was –10.7 ± 2.4 mV (n = 4).
AtANN1 Is Required for NaCl-Induced Ca2+ Influx Currents across Root Epidermal Plasma Membrane
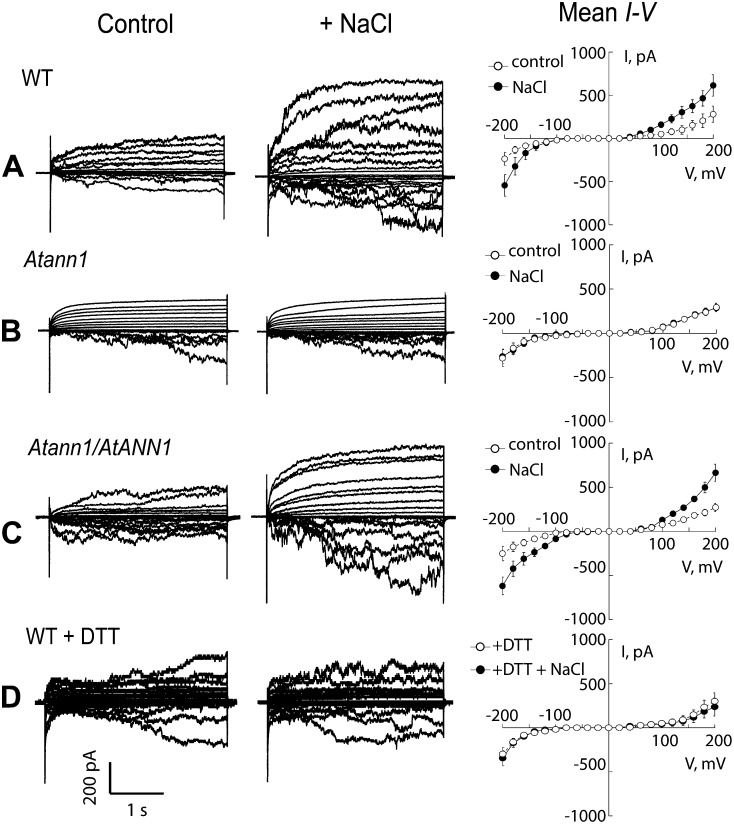
Patch clamp electrophysiology experiments on wild-type Arabidopsis root protoplasts have shown that 50 mm NaCl causes a plasma membrane hyperpolarization-activated Ca2+ influx current to form after 15 min and that this relies on NADPH oxidase activity to generate ROS (Ma et al., 2012). Here, in patch clamp trials on root epidermal protoplasts (no aequorin), 50 mm NaCl evoked a significant increase in wild-type plasma membrane hyperpolarization-activated Ca2+ influx current after 20 min exposure (Fig. 4; n = 6; P < 0.05), while Atann1 did not respond (Fig. 4; n = 6). The efflux currents evoked in the wild type at depolarized voltages (carrying Ca2+ and K+) were also absent from Atann1. Both influx and efflux currents were restored by complementation of the Atann1 mutant (Fig. 4; n = 6). The presence of DTT in the bath and pipette solutions prevented current activation by NaCl in the wild type (Fig. 4; n = 6), demonstrating the need for ROS production. This phenocopying of the Atann1 mutant (without DTT) supports AtANN1’s mediating a ROS-activated conductance. Higher concentrations of NaCl were not tested, as they destabilize the seal between the membrane and patch clamp electrode (Shabala et al., 2006). Overall, the aequorin and electrophysiology data show that oxidation shapes the [Ca2+]cyt response to NaCl at high extracellular Ca2+, with AtANN1 mediating an oxidation-activated Ca2+ influx component.
Figure 4.
NaCl-activated root epidermal plasma membrane currents require AtANN1. Currents from A, the wild type; B, Atann1; C, AtANN1/Atann1; and D, the wild type with 1mm DTT (in bath and pipette solutions) were sampled using the patch clamp “whole-cell” mode under control conditions (white circle) and after exposure to 50 mm NaCl (black circles). There were no significant differences in control currents between genotypes. Data are mean ± se, and maximal NaCl-activated currents are shown (n = 6). Current flowing below the voltage axis is net positive charge entering the protoplast. Ca2+ influx occurs in the physiological voltage range. At voltages more negative than the equilibrium potential for K+ (–156 mV), K+ influx is a possible component of the inward current. Bathing solution for epidermal protoplasts comprised 20 mm CaCl2, 0.1 mm KCl, 20 μm NaCl, and 5 mm MES-Tris, pH 5.6, adjusted to 270 mOsm with d-sorbitol. Pipette solution comprised 40 mm K-gluconate, 10 mm KCl, 0.4 mm CaCl2, 1 mm bis(2-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid, and 2 mm MES-Tris, pH 7.2, adjusted to 270 mOsm with d-sorbitol.
NaCl-Induced Transcription and Adaptive Root Growth Are Impaired in Atann1
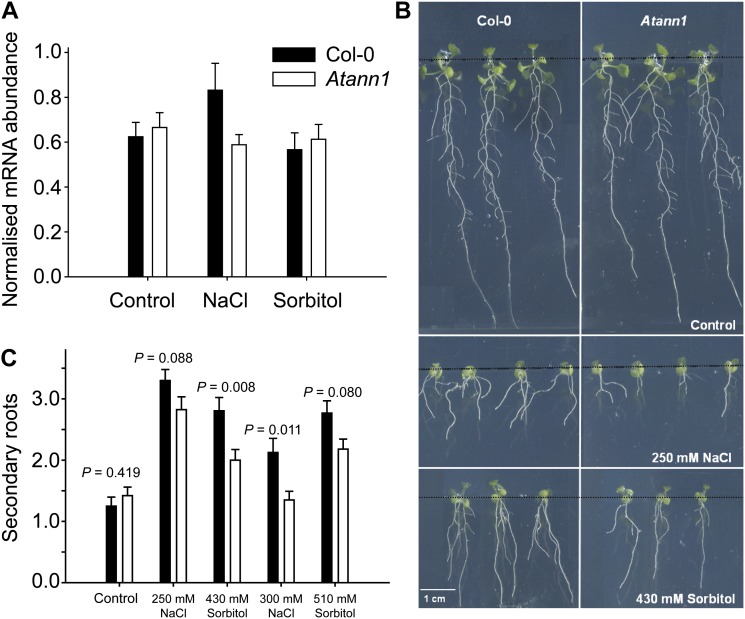
Transcripts of the salinity and osmotic stress-responsive genes Responsive to Dessication29A (AtRD29A), Dehydration-Responsive Element Binding2A (AtDREB2A), and AtDREB2B (Dinneny et al., 2008; Nakashima et al., 2009; Kaye et al., 2011) were all significantly up-regulated in the wild type and Atann1 roots at 24 h of NaCl exposure, with extracellular Ca2+ at 1.5 mm (Supplemental Fig. S4). Up-regulation of AtRD29A was significantly lower in Atann1 compared with the wild type (two-way ANOVA; P = 0.05; Supplemental Fig. S4). AtSOS1 was up-regulated in wild-type roots to levels comparable with a previous study on chronic salinity stress (Dinneny et al., 2008) but was not up-regulated in Atann1, consistent with AtANN1’s operation in [Ca2+]cyt-driven signaling (Fig. 5A; P < 0.05, n = 3). Impaired [Ca2+]cyt signaling under salinity stress should be deleterious to adaptive root growth. Secondary root production under salinity stress at an extracellular [Ca2+] of 1.5 mm was significantly impaired in Atann1, confirming this annexin’s involvement in adaptive root growth (Fig. 5, B and C; n = 4). When extracellular [Ca2+] was increased to 10 mm, wild-type secondary root production was not changed; it was still impaired in Atann1, but this was not significantly different to the wild type (300 mm NaCl, mean ± se: wild type, 2 ± 0.7; Atann1, 1.6 ± 0.2, n = 3).
Figure 5.
Root adaptation to salinity requires AtANN1. A, AtSOS1 transcript abundance increases under chronic salinity exposure (150 mm NaCl, 24 h) of wild-type (black bar) but not Atann1 (white bar) roots. The isoosmotic control was 275 mm sorbitol. Mean ± se transcript abundance (assayed by quantitative PCR) from three trials with 40 roots per genotype and test. B, Atann1 development of secondary roots in response to growth on 250 NaCl or isoosmotic sorbitol was impaired. Plants are shown 9 d after transfer to control or test plates. Black, horizontal lines demarcate the junction between control medium (top) and test (bottom). C, Mean ± se secondary roots, 9 d after transfer to control or test plates. Data are from four trials using 36 to 40 plants per genotype per treatment with seed from two separate harvests. Black bar, the wild type and white bar, Atann1. Significance was tested with the Student’s t test or Mann-Whitney U rank sum test, if data were not normally distributed.
DISCUSSION
AtANN1 Restricts Na+ Influx
Root epidermal cells are initial sensing points of soil conditions and are where root expression of AtANN1 is highest (Dinneny et al., 2008). The low permeability of AtANN1 to Na+ and the greater net epidermal Na+ influx of the Atann1 mutant suggest that this annexin does not contribute to Na+ uptake. Rather, it suggests that AtANN1 is a negative regulator of Na+ influx that may be influencing activity of Na+ uptake routes such as AtCNGC3 and AtCNGC10 (Gobert et al., 2006; Guo et al., 2008), posttranslationally or even at transcriptional level. It was found previously that the mutant’s root epidermal plasma membrane voltage was not significantly different to the wild type (Laohavisit et al., 2012), indicating that greater Na+ influx is not simply the effect of a more negative voltage. The greater NaCl-induced K+ efflux from the mutant could simply be the consequence of greater Na+ influx but could be indicative of a greater driving force for K+ efflux, given the loss of AtANN1 as a plasma membrane K+ efflux pathway and hence potential accumulation of K+ as a consequence. NaCl-induced K+ efflux from roots is mediated in part by extracellular OH• activating the plasma membrane K+ channel Guard Cell Outwardly Rectifying K (GORK; Demidchik et al., 2010). GORK responds normally to extracellular OH• in Atann1 (Laohavisit et al., 2012) and should be functional in the studies here. AtANN1 has peroxidase activity (Konopka-Postupolska et al., 2009) and can be extracellular (Laohavisit and Davies, 2011). Therefore, the NaCl-stressed Atann1 mutant may have greater capacity for extracellular OH• production (resulting from increased hydrogen peroxide concentration), leading to greater GORK activation and K+ efflux. However, AtANN1 peroxidase activity is very weak, and because it is a copper-binding protein that could catalyze OH• production (Kung et al., 2006), its absence could just as readily lead to less GORK activation. The mechanisms underlying the perturbed Na+ and K+ fluxes now require elucidation.
AtANN1-Dependent Mobilization of Ca2+ Depends on Extracellular Ca2+ and ROS
The ionic component of NaCl stress might be expected to cause a greater [Ca2+]cyt elevation than the hyperosmotic component, but intriguingly, there were no significant differences between the wild type’s [Ca2+]cyt elevation in response to NaCl and isoosmotic sorbitol. This does not, however, preclude subtle differences that could not be resolved by aequorin or differences in the mechanisms of [Ca2+]cyt elevation. Results here show AtANN1’s involvement in mobilizing Ca2+ under salinity exposure, with its effect on epidermal [Ca2+]cyt dependent on the concentration of extracellular Ca2+ and the production of ROS. At 1 mm extracellular Ca2+, AtANN1 contributes to the initial [Ca2+]cyt increase and total [Ca2+]cyt mobilized in response to a NaCl-specific component of the response to salinity. How AtANN1 works at this extracellular [Ca2+] now needs to be determined. As Arabidopsis roots produce extracellular OH• in response to NaCl even at 0.1 mm Ca2+ (Demidchik et al., 2010), it remains feasible that AtANN1 mediates OH•-activated Ca2+ influx at 1 mm extracellular Ca2+. Clearly, other transport pathways are operating at the hyperpolarizing voltage favored by the low [K+] assay solution (representing typical soil solution K+), and these now need to be identified.
In the presence of 220 mm NaCl, AtANN1 is required for the additional epidermal [Ca2+]cyt increase caused by raising external Ca2+ to the 10 mm Ca2+ found in saline soils. With the degree of resolution afforded by aequorin, this does not appear to be NaCl specific but an osmotic response. At this external Ca2+ concentration, AtANN1 accounts for the ROS-sensitive component of salinity-induced [Ca2+]cyt generation. This is consistent with our finding (using patch clamping) that AtANN1 is a necessary component of the epidermal plasma membrane Ca2+ influx conductance activated by NaCl and known from a previous study to require NADPH oxidase activity (Ma et al., 2012). We envisage that as NADPH oxidase activity and extracellular OH• production by roots increase under high salinity stress (Yang et al., 2007; Demidchik et al., 2010; Kaye et al., 2011; Xie et al., 2011; Liu et al., 2012; Ma et al., 2012), AtANN1 fulfills its previously defined role of mediating the root epidermal plasma membrane Ca2+ influx conductance that is activated by extracellular OH• (Laohavisit et al., 2012). The current-voltage relationship of recombinant AtANN1 in vitro suggests that AtANN1’s contribution to Ca2+ influx across the plasma membrane would decline if membrane voltage depolarized, for example through increased soil [K+]. Although the mechanism through which AtANN1 forms a transbilayer conductance is unclear, its ability shown in this study to form an OH•-activated Ca2+-permeable conductance that can discriminate against Na+ agrees well with a role in Ca2+ signaling during salinity stress.
AtANN1-Dependent Transcription and Root Growth
ROS levels in Arabidopsis roots are elevated for the first 24 h of exposure to NaCl (Xie et al., 2011), and we found significant up-regulation of salinity and osmotic stress-responsive genes at this time point. Salt stress induction of AtRD29A requires NADPH oxidase activity (Kaye et al., 2011), and the failure of the Atann1 mutant to show normal induction (with NaCl but not the osmotic equivalent) agrees with a role for AtANN1 in mediating a NaCl-specific ROS-dependent component of Ca2+ signaling, at low (1.5 mm) external Ca2+. A role for AtRD29A in production of secondary roots during salt stress has yet to be determined, but it is interesting that its transcription under osmotic stress was not significantly affected in Atann1 (agreeing with protoplast [Ca2+]cyt responses), but secondary root production was. This shows that AtANN1 can act in secondary root formation induced by the osmotic component of salinity stress at low external Ca2+ independently of epidermal [Ca2+]cyt signaling. NaCl-induced secondary root production does involve AtSOS1 (Huh et al., 2002). Transcriptional up-regulation of AtSOS1 in the wild type is known to be small (Dinneny et al., 2008). In wild-type roots (at 1.5 mm external Ca2+), NaCl caused greater AtSOS1 up-regulation than the isoosmotic control, showing that the effect was specific to the ionic component of the treatment. This up-regulation failed in Atann1 (at 1.5 mm external Ca2+), and although a causal link between epidermal signaling and root growth cannot be made, this is in agreement with the loss of a NaCl-specific component of salinity-induced [Ca2+]cyt elevation at low external Ca2+. This lesion in AtSOS1 regulation may have contributed to the mutant’s poor germination under saline conditions (Lee et al., 2004), and it is expected that cytosolic [Na+] content of Atann1 would be higher than the wild type. Stability of the AtSOS1 transcript specifically requires the Respiratory Burst Oxidase Homolog C (AtRBOHC) NAPDH oxidase and extracellular OH• production (Chung et al., 2008). Although other NADPH oxidases, such as AtRBOHJ (Kaye et al., 2011), are involved in adaptation to salinity, there appears to be an absolute requirement for AtRBOHC in salt-induced AtSOS1 transcript stability (Chung et al., 2008). As AtANN1 mediates the extracellular OH•-activated Ca2+ influx pathway in the epidermis (Laohavisit et al., 2012) and it is likely that it relies on AtRBOHC for ROS generation (Foreman et al., 2003), we postulate that AtANN1 lies downstream of AtRBOHC in stabilization of AtSOS1 (Fig. 6). Ca2+ influx via AtANN1 could form a positive feedback, causing Ca2+-dependent activation of AtRBOHC (Takeda et al., 2008) to maintain AtSOS1 stability. Secondary root growth involves superoxide anion production, possibly by NADPH oxidases (Roach and Kranner, 2011). The involvement of these enzymes may help explain why the Atann1 mutant was compromised in its ability to generate secondary roots under salinity stress. As an ROS-activated Ca2+ transport pathway, AtANN1 is expected to lie downstream of NADPH oxidases. The impaired up-regulation of AtSOS1 in the Atann1 mutant may have contributed to impaired secondary root formation (Huh et al., 2002) when external Ca2+ was 1.5 mm. Although AtANN1 accounted for the high Ca2+-dependent component of the NaCl-induced [Ca2+]cyt response of epidermal protoplasts, secondary root formation by the Atann1 mutant was not significantly lower than that of the wild type at high external Ca2+. The pathway from AtANN1-mediated and -independent [Ca2+]cyt increase to secondary root production now needs to be explored in greater detail and would be aided by single cell type studies (Kiegle et al., 2000; Dinneny et al., 2008).
Figure 6.
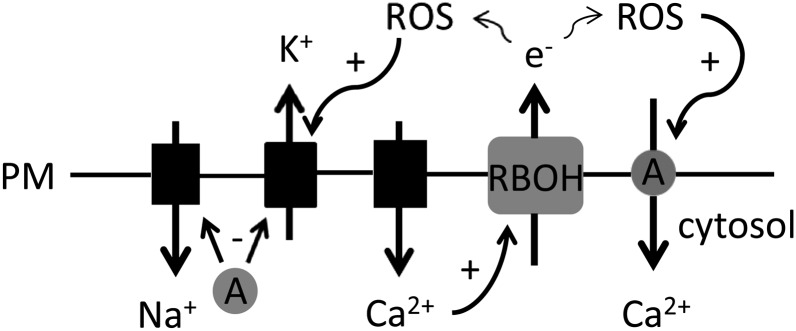
Schematic summary of possible events at the epidermal plasma membrane (PM) under salinity exposure. AtANN1 (A) is a negative regulator of Na+ influx and K+ efflux during salinity exposure, by unknown mechanism. AtANN1 is depicted as cytosolic but could be acting as a membrane integral or peripheral protein. K+ efflux could be mediated by the OH-activated GORK (Demidchik et al., 2010). NaCl or osmotic effect causes elevation of [Ca2+]cyt by unknown mechanism; the participation of plasma membrane Ca2+-permeable channels is shown. Elevation of [Ca2+]cyt results in stimulation of NADPH oxidase (RBOH) activity (Takeda et al., 2008) and the resultant production of extracellular OH stimulates Ca2+ entry through AtANN1. At low external Ca2+, AtRBOHC and AtANN1 are involved in AtSOS1 stabilization (not shown).
Annexin up-regulation in salt-stressed crops such as chickpea (Cicer arietinum) and tomato (Solanum lycopersicum) has now been recorded (Manaa et al., 2011; Molina et al., 2011), pointing to positive roles in adaptation. Previously, AtANN1 was found to be important for drought tolerance (Konopka-Postupolska et al., 2009; Huh et al., 2010) and germination under salinity stress (Lee et al., 2004). This study has revealed AtANN1 to be a significant component of [Ca2+]cyt signaling and adaptive root growth under salinity stress. The impaired ability of Atann1 to form secondary roots under osmotic stress may help explain its drought sensitivity. In addition to mediating [Ca2+]cyt signals, AtANN1 inhibits root Na+ influx and K+ efflux, pointing to annexins as tools to identify the underlying proteins in those critical transport routes and as future target proteins in their own right for consideration in the generation of crops with greater resilience to salinity stress.
CONCLUSION
Salinity-induced [Ca2+]cyt increase in Arabidopsis roots requires AtANN1, especially for the component generated by oxidation. Results to date suggest that AtANN1 is acting directly as a Ca2+ transport route in the epidermal plasma membrane. How AtANN1 forms this pathway requires further examination. The Atann1 mutant could be a useful tool in both the identification of Na+ influx pathways and the elucidation of processes governing secondary root formation in adaptive growth.
MATERIALS AND METHODS
Plant Material and Growth Analysis
Arabidopsis (Arabidopsis thaliana ecotype Columbia), its Atann1 homozygous transfer DNA loss-of-function mutant (single transfer DNA insert in the third exon of At1g35720; GenBank accession no. AF083913), the 35S-complemented mutant, and Atann1 constitutively expressing cytosolic apoaequorin (35S promoter) were as described previously (Lee et al., 2004; Laohavisit et al., 2012). Plants were grown vertically on one-half-strength Murashige and Skoog medium with 1% (w/v) Suc and 0.8% (w/v) bactogar, pH 5.7. After a 2-d stratification, growth was at 25°C in a 16-h day at 100 μmol m–2 s–1 irradiance. In experiments on secondary roots, seedlings were transferred from control plates at 3 d to split medium plates in which the top 4 cm of medium was always control and the remainder was control or test. The hypocotyls bridged the interface between the two media so that the cotyledons were not exposed to the lower medium. d-Sorbitol (ultrapure) was used to generate equivalent osmolarity to NaCl tests, determined with a vapor pressure osmometer. Secondary roots were counted after 9 d and scanned at 300 dots per inch.
Flux Analysis
Net fluxes were measured from root epidermis of 6-d-old plants, using Na+- and K+-selective extracellular vibrating microelectrodes as described previously but with a 4-tert-butylcalix[4]arene-tetra acetic acid tetraethyl ester sensor for Na+ (Shabala et al., 2006; Jayakannan et al., 2011). Measurements were taken from developmentally equivalent mature epidermis, 2 to 3 mm from the apex of immobilized root apical segments (8–10 mm). Analysis was as described previously (Shabala et al., 2006).
[Ca2+]cyt Determination
Excised roots from 6- to 7-d-old seedlings were incubated in coelentrazine solution (10 μm coelentrazine [Lux Biotechnology], 10 mm CaCl2, 0.1 mm KCl, and 2 mm Tris/MES, pH 5.8) for 4 h in the dark. Single roots (washed with coelentrazine-free buffer) were placed into wells of a white 96-well plate (Greiner Bio-One) with 100 µL buffer and recovered in darkness for 30 min. Root epidermal protoplasts were prepared for luminometry, and luminescence was recorded in a plate reader luminometer as described previously (Laohavisit et al., 2012). Occasionally, resting [Ca2+]cyt was higher in the mutant than the wild type. Calibration to convert luminescent values to [Ca2+]cyt was performed as described by Knight et al. (1997).
Planar Lipid Bilayers and Patch Clamp Electrophysiology
AtANN1 was expressed in Saccharomyces cerevisiae, purified by lipid affinity, and verified by immunoblotting as described previously (Laohavisit et al., 2012). Experiments were performed with protein from three separate purifications. Planar lipid bilayers were formed from 25 mg mL–1 (1-palmitoyl 2-oleoyl phosphatidylethanolamine, cholesterol, and 1-palmitoyl 2-oleoyl phosphatidyl-Ser in a 5:3:2 ratio, respectively, at room temperature [20°C–24°C] as described by Laohavisit et al. [2012]). Annexin (3 µg) protein was added to the cis chamber. The bilayer was held at –150 mV (cis negative) to aid insertion. OH• were generated in the trans by 1 mm CuCl2 and ascorbate (Laohavisit et al., 2012). GdCl3 was also added to trans. For permeability estimates, the K+-to-Cl– permeability ratio of 53 was used, determined previously under identical recording conditions to those used here (Laohavisit et al., 2012). Patch clamp recordings on root epidermal protoplasts were as described by Laohavisit et al. (2012). Standard patch clamp procedures were applied (Véry and Davies, 2000).
Transcript Analysis
Salinity stress treatment and harvest were adapted from Dinneny et al. (2008). In each trial, a total of 35 to 40 7-d seedlings grown on solid control medium (1.5 mm Ca2+) were transferred to untreated plates or plates containing 150 mm NaCl or 275 mm d-sorbitol for 24 h. Total RNA was extracted from excised roots using RNeasy Plant Mini Kit (Qiagen) according to manufacturer’s protocol with a DNase digestion step (Qiagen). Prior to reverse transcription, RNA integrity was assessed on a 1% (w/v) agarose MOPS gel. RNA (500 ng) was reverse transcribed with QuantiTect Reverse Transcription Kit (Qiagen) according to manufacturer’s instructions. Primers were as follows: AtSOS1 (At2G01980), 5′-3′ CGGAAATTCACATATCAGCAAGG and 3′-5′ GAAGAAGGCGTAGAACAAATTGG; AtRD29A (At5G52310) 5′-3′ AACCACCACTCAACACACAC and 3′-5′ TCTTAGCTCTAGCCTTTACTTTCC; AtDREB2A (At5G05410) 5′-3′ TCGAGGTAGCAGGCTTTGGCT and 3′-5′ TCAGACGCATCAGACCGAGGGA; and AtDREB2B (At3G11020) 5′-3′ TCTTGTGGAACCAGGCCGGACA and 3′-5′ TGGCCCCAATACTGCTGCTCAA. Control primers were from the geNorm Arabidopsis reference gene kit (Primer Design; http://www.primerdesign.co.uk). This contains primer pairs for six endogenous control genes. All six primer pairs were tested on complementary DNA from each treatment/genotype, encompassing a selection from each of the three biological replicates. Expression values were calculated from the cycle threshold (Ct) values as described in the geNorm handbook and put through the geNorm software. This determined how many internal controls were required for accurate normalization (in this case, two) and which primer pairs were the most stable across the treatments.
Specificity of primers was validated by sequencing amplicon and melt curve analysis. Quantitative PCR was performed with a Rotor-Gene 3000 thermocycler using Rotor-Gene SYBR Green PCR Kit (Qiagen; two technical replicates per reaction) following manufacturer’s guidelines with 6 ng complementary DNA and 0.50 µm final primer concentration. Absence of genomic DNA in RNA was confirmed with a no reverse transcription control. Six endogenous control primers from the Arabidopsis geNorm kit were tested for stability across all treatment groups (control, NaCl, and sorbitol) using geNorm software (Vandesompele et al., 2002) according to manufacturer’s guidelines. The mean reaction efficiencies (within ± 5% of the median efficiency) of each primer pair were quantified using LinReg PCR software (Ramakers et al., 2003). Quantification of targets was calculated based on the Pfaffl model (Pfaffl, 2001): reaction efficiency (RE)–Ct target normalized by the geometric mean of RE–Ct endogenous controls. All data are from three independent trials. Significance was tested by two-way ANOVA (genotype and treatment as the two factors) with Holm-Sidak post hoc analyses.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AF083913, Atann1, At1g35720; AB007791, AtDREB2A, At5G05410; AB016571, AtDREB2B, At3g11020; AB056455; AtRD29A, At5g52310; AF256224, AtSOS1, and At2g01980.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. [Ca2+]cyt responses of root epidermal protoplasts.
Supplemental Figure S2. [Ca2+]cyt elevation is impaired in single, excised Atann1 roots.
Supplemental Figure S3. AtANN1 forms a cation conductance in artificial planar lipid bilayers.
Supplemental Figure S4. Root adaptation to salinity requires AtANN1.
Glossary
- [Ca2+]cyt
cytosolic free Ca2+
- ROS
reactive oxygen species
- OH•
hydroxyl radicals
- DTT
dithiothreitol
- Erev
reversal voltage
References
- Ali R, Ma W, Lemtiri-Chlieh F, Tsaltas D, Leng Q, von Bodman S, Berkowitz GA. (2007) Death don’t have no mercy and neither does calcium: Arabidopsis CYCLIC NUCLEOTIDE GATED CHANNEL2 and innate immunity. Plant Cell 19: 1081–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JS, Zhu JK, Bressan RA, Hasegawa PM, Shi HZ. (2008) Reactive oxygen species mediate Na+-induced SOS1 mRNA stability in Arabidopsis. Plant J 53: 554–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Cuin TA, Svistunenko D, Smith SJ, Miller AJ, Shabala S, Sokolik A, Yurin V. (2010) Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced cell death. J Cell Sci 123: 1468–1479 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Shang Z, Shin R, Thompson E, Rubio L, Laohavisit A, Mortimer JC, Chivasa S, Slabas AR, Glover BJ, et al. (2009) Plant extracellular ATP signalling by plasma membrane NADPH oxidase and Ca2+ channels. Plant J 58: 903–913 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Tester M. (2002) Sodium fluxes through nonselective cation channels in the plasma membrane of protoplasts from Arabidopsis roots. Plant Physiol 128: 379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinneny JR, Long TA, Wang JY, Jung JW, Mace D, Pointer S, Barron C, Brady SM, Schiefelbein J, Benfey PN. (2008) Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science 320: 942–945 [DOI] [PubMed] [Google Scholar]
- Dodd AN, Jakobsen MK, Baker AJ, Telzerow A, Hou SW, Laplaze L, Barrot L, Poethig RS, Haseloff JP, Webb AAR. (2006) Time of day modulates low-temperature Ca signals in Arabidopsis. Plant J 48: 962–973 [DOI] [PubMed] [Google Scholar]
- Dodd AN, Kudla J, Sanders D. (2010) The language of calcium signaling. Annu Rev Plant Biol 61: 593–620 [DOI] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDG, et al. (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446 [DOI] [PubMed] [Google Scholar]
- Gobert A, Park G, Amtmann A, Sanders D, Maathuis FJM. (2006) Arabidopsis thaliana cyclic nucleotide gated channel 3 forms a non-selective ion transporter involved in germination and cation transport. J Exp Bot 57: 791–800 [DOI] [PubMed] [Google Scholar]
- Gorecka KM, Thouverey C, Buchet R, Pikula S. (2007) Potential role of annexin AnnAt1 from Arabidopsis thaliana in pH-mediated cellular response to environmental stimuli. Plant Cell Physiol 48: 792–803 [DOI] [PubMed] [Google Scholar]
- Guo KM, Babourina O, Christopher DA, Borsics T, Rengel Z. (2008) The cyclic nucleotide-gated channel, AtCNGC10, influences salt tolerance in Arabidopsis. Physiol Plant 134: 499–507 [DOI] [PubMed] [Google Scholar]
- Huh GH, Damsz B, Matsumoto TK, Reddy MP, Rus AM, Ibeas JI, Narasimhan ML, Bressan RA, Hasegawa PM. (2002) Salt causes ion disequilibrium-induced programmed cell death in yeast and plants. Plant J 29: 649–659 [DOI] [PubMed] [Google Scholar]
- Huh SM, Noh EK, Kim HG, Jeon BW, Bae K, Hu HC, Kwak JM, Park OK. (2010) Arabidopsis annexins AnnAt1 and AnnAt4 interact with each other and regulate drought and salt stress responses. Plant Cell Physiol 51: 1499–1514 [DOI] [PubMed] [Google Scholar]
- Jayakannan M, Babourina O, Rengel Z. (2011) Improved measurements of Na+ fluxes in plants using calixarene-based microelectrodes. J Plant Physiol 168: 1045–1051 [DOI] [PubMed] [Google Scholar]
- Jiang CF, Belfield EJ, Mithani A, Visscher A, Ragoussis J, Mott R, Smith JAC, Harberd NP. (2012) ROS-mediated vascular homeostatic control of root-to-shoot soil Na delivery in Arabidopsis. EMBO J 31: 4359–4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye Y, Golani Y, Singer Y, Leshem Y, Cohen G, Ercetin M, Gillaspy G, Levine A. (2011) Inositol polyphosphate 5-phosphatase7 regulates the production of reactive oxygen species and salt tolerance in Arabidopsis. Plant Physiol 157: 229–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiegle E, Moore CA, Haseloff J, Tester M, Knight MR. (2000) Cell-type specific calcium responses to drought, NaCl and cold in Arabidopsis root: a role for endodermis and pericycle in stress signal transduction. Plant J 23: 267–278 [DOI] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR. (1997) Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J 12: 1067–1078 [DOI] [PubMed] [Google Scholar]
- Konopka-Postupolska D, Clark G, Goch G, Debski J, Floras K, Cantero A, Fijolek B, Roux S, Hennig J. (2009) The role of annexin 1 in drought stress in Arabidopsis. Plant Physiol 150: 1394–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Britto DT. (2011) Sodium transport in plants: a critical review. New Phytol 189: 54–81 [DOI] [PubMed] [Google Scholar]
- Kung C-CS, Huang W-N, Huang Y-C, Yeh K-C. (2006) Proteomic survey of copper-binding proteins in Arabidopsis roots by immobilized metal affinity chromatography and mass spectrometry. Proteomics 6: 2746–2758 [DOI] [PubMed] [Google Scholar]
- Laohavisit A, Brown AT, Cicuta P, Davies JM. (2010) Annexins: components of the calcium and reactive oxygen signaling network. Plant Physiol 152: 1824–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laohavisit A, Davies JM. (2011) Annexins. New Phytol 189: 40–53 [DOI] [PubMed] [Google Scholar]
- Laohavisit A, Mortimer JC, Demidchik V, Coxon KM, Stancombe MA, Macpherson N, Brownlee C, Hofmann A, Webb AAR, Miedema H, et al. (2009) Zea mays annexins modulate cytosolic free Ca2+, form a Ca2+-permeable conductance and have peroxidase activity. Plant Cell 21: 479–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laohavisit A, Shang ZL, Rubio L, Cuin TA, Véry AA, Wang AH, Mortimer JC, Macpherson N, Coxon KM, Battey NH, et al. (2012) Arabidopsis annexin1 mediates the radical-activated plasma membrane Ca²+- and K+-permeable conductance in root cells. Plant Cell 24: 1522–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Lee EJ, Yang EJ, Lee JE, Park AR, Song WH, Park OK. (2004) Proteomic identification of annexins, calcium-dependent membrane binding proteins that mediate osmotic stress and abscisic acid signal transduction in Arabidopsis. Plant Cell 16: 1378–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SG, Zhu DZ, Chen GH, Gao XQ, Zhang XS. (2012) Disrupted actin dynamics trigger an increment in the reactive oxygen species levels in the Arabidopsis root under salt stress. Plant Cell Rep 31: 1219–1226 [DOI] [PubMed] [Google Scholar]
- Lynch J, Polito VS, Läuchli A. (1989) Salinity stress increases cytoplasmic ca activity in maize root protoplasts. Plant Physiol 90: 1271–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma LY, Zhang H, Sun LR, Jiao YH, Zhang GZ, Miao C, Hao FS. (2012) NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na+/K+ homeostasis in Arabidopsis under salt stress. J Expt Bot 63: 305–317 [DOI] [PubMed] [Google Scholar]
- Manaa A, Ben Ahmed H, Valot B, Bouchet JP, Aschi-Smiti S, Causse M, Faurobert M. (2011) Salt and genotype impact on plant physiology and root proteome variations in tomato. J Exp Bot 62: 2797–2813 [DOI] [PubMed] [Google Scholar]
- Molina C, Zaman-Allah M, Khan F, Fatnassi N, Horres R, Rotter B, Steinhauer D, Amenc L, Drevon JJ, Winter P, et al. (2011) The salt-responsive transcriptome of chickpea roots and nodules via deepSuperSAGE. BMC Plant Biol 11: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R, Tester M. (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59: 651–681 [DOI] [PubMed] [Google Scholar]
- Nakashima K, Ito Y, Yamaguchi-Shinozaki K. (2009) Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiol 149: 88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers C, Ruijter JM, Deprez RHL, Moorman AFM. (2003) Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett 339: 62–66 [DOI] [PubMed] [Google Scholar]
- Roach T, Kranner I. (2011) Extracellular superoxide production associated with secondary root growth following desiccation of Pisum sativum seedlings. J Plant Physiol 168: 1870–1873 [DOI] [PubMed] [Google Scholar]
- Shabala S, Demidchik V, Shabala L, Cuin TA, Smith SJ, Miller AJ, Davies JM, Newman IA. (2006) Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+ -permeable channels. Plant Physiol 141: 1653–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Ishitani M, Kim C, Zhu JK. (2000) The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci USA 97: 6896–6901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L. (2008) Local positive feedback regulation determines cell shape in root hair cells. Science 319: 1241–1244 [DOI] [PubMed] [Google Scholar]
- Tracy FE, Gilliham M, Dodd AN, Webb AAR, Tester M. (2008) NaCl-induced changes in cytosolic free Ca2+ in Arabidopsis thaliana are heterogeneous and modified by external ionic composition. Plant Cell Environ 31: 1063–1073 [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology 3: research0034.1–research0034.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véry A-A, Davies JM. (2000) Hyperpolarization-activated calcium channels at the tip of Arabidopsis root hairs. Proc Natl Acad Sci USA 97: 9801–9806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincill ED, Bieck AM, Spalding EP. (2012) Ca2+ conduction by an amino acid-gated ion channel related to glutamate receptors. Plant Physiol 159: 40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie YJ, Xu S, Han B, Wu MZ, Yuan XX, Han Y, Gu QA, Xu DK, Yang Q, Shen WB. (2011) Evidence of Arabidopsis salt acclimation induced by up-regulation of HY1 and the regulatory role of RbohD-derived reactive oxygen species synthesis. Plant J 66: 280–292 [DOI] [PubMed] [Google Scholar]
- Yang YL, Xu SJ, An LZ, Chen NL. (2007) NADPH oxidase-dependent hydrogen peroxide production, induced by salinity stress, may be involved in the regulation of total calcium in roots of wheat. J Plant Physiol 164: 1429–1435 [DOI] [PubMed] [Google Scholar]