Light alters the physiological and transcriptional responses of tobacco plants to the fungal elicitor cryptogein through modified antioxidant availability and increased photooxidative damage caused by singlet oxygen.
Abstract
The fungal elicitor cryptogein triggers a light-dependent hypersensitive response in tobacco (Nicotiana tabacum). To assess the effect of light on this nonhost resistance in more detail, we studied various aspects of the response under dark and light conditions using the tobacco-cryptogein experimental system. Here, we show that light drastically alters the plant’s transcriptional response to cryptogein, notably by dampening the induction of genes involved in multiple processes, such as ethylene biosynthesis, secondary metabolism, and glutathione turnover. Furthermore, chlorophyll fluorescence measurements demonstrated that quantum yield and functioning of the light-harvesting antennae decreased simultaneously, indicating that photoinhibition underlies the observed decreased photosynthesis and that photooxidative damage might be involved in the establishment of the altered response. Analysis of the isomer distribution of hydroxy fatty acids illustrated that, in the light, lipid peroxidation was predominantly due to the production of singlet oxygen. Differences in (reduced) glutathione concentrations and the rapid development of symptoms in the light when cryptogein was coinfiltrated with glutathione biosynthesis inhibitors suggest that glutathione might become a limiting factor during the cryptogein-induced hypersensitive response in the dark and that this response might be modified by an increased antioxidant availability in the light.
Cryptogein belongs to the class of elicitins (i.e. small 10-kD Cys-rich proteins secreted by most Phytophthora species) that possess sterol and lipid transfer properties and are able to trigger a pathogen resistance response in tobacco (Nicotiana tabacum), including the expression of defense-related genes and systemic acquired resistance (SAR; Ponchet et al., 1999; Osman et al., 2001; Blein et al., 2002; Buhot et al., 2004; Garcia-Brugger et al., 2006). Cryptogein is initially recognized by a plasma membrane receptor (Wendehenne et al., 1995; Buhot et al., 2001), followed by the activation of various protein kinases, such as the wound-induced protein kinase, the salicylic acid-induced protein kinase, and the tobacco mitogen-activated protein kinase (MAPK) Ntf4 (Lebrun-Garcia et al., 1998; Ren et al., 2006; Dahan et al., 2009). In turn, these kinases trigger calcium influxes that subsequently induce calcium-dependent transcriptional changes (Amelot et al., 2012) and activate a wide variety of cellular responses, including an NADPH oxidase-dependent oxidative burst (Simon-Plas et al., 2002; Amelot et al., 2011).
Light is not only the primary energy source that, on a daily basis, fuels plants for their growth and development. Emerging evidence indicates that light also plays a defining role in the establishment of plant immunity to both bacterial and fungal infections (Karpinski et al., 2003; Bechtold et al., 2005; Roberts and Paul, 2006; Kangasjärvi et al., 2012) by regulating defense responses directly via light-responsive signaling pathways. Mutants perturbed in light perception or light signal transduction may display strongly altered resistance responses (Genoud et al., 2002). Recently, resistance gene-mediated defense has been found to be under circadian control (Wang et al., 2011). Alternatively, light has an evident effect on the generation of reactive oxygen species (ROS) via photosynthesis and photorespiration (Queval et al., 2007; Foyer and Noctor, 2009). As ROS and ROS-derived electrophiles are well-known players in plant-pathogen interactions (Apel and Hirt, 2004), light-induced redox perturbations are likely to contribute to ROS-regulated defenses.
Infection of tobacco leaves with avirulent strains of Ralstonia solanacearum (Cacas et al., 2005) or Pseudomonas syringae pv syringae and treatment with cryptogein (Montillet et al., 2005) result in a typical hypersensitive response (HR) correlated with the appearance of necrotic lesions, increased expression of defense-related genes, and SAR (Garcia-Brugger et al., 2006). Previously, we have shown that cryptogein infiltration markedly induces the 9-lipoxygenase (LOX) pathway, linked with a coordinated increase in 9-LOX and lipid acyl hydrolase (patatin) gene expression (Cacas et al., 2005). The subsequent production of fatty acid hydroperoxides provokes HR cell death (Rustérucci et al., 1999; Cacas et al., 2005; Montillet et al., 2005).
Although cryptogein treatments are usually performed in the dark, cryptogein-driven HR symptoms have been found to be strongly delayed in the light. In the light, lipid peroxidation events were mainly mediated by the direct action of ROS instead of through 9-LOX enzymatic activity in the dark (Cacas et al., 2005; Montillet et al., 2005). Consequently, we identified conditions under which light appeared not to be required for the plant’s resistance to infection but actually repressed or delayed the development of HR, prompting us to assess the effect of light on the nonhost resistance mechanisms within the tobacco-cryptogein interaction as a model system and special emphasis on the early changes in redox status and gene expression. Here, we show that under light conditions, tobacco leaves reduce the elicitation process and defense gene expression by maintaining photosynthetic activity and by keeping the cellular redox balance in a reduced state.
RESULTS AND DISCUSSION
Light Inhibits the Development of Cryptogein-Induced HR Symptoms
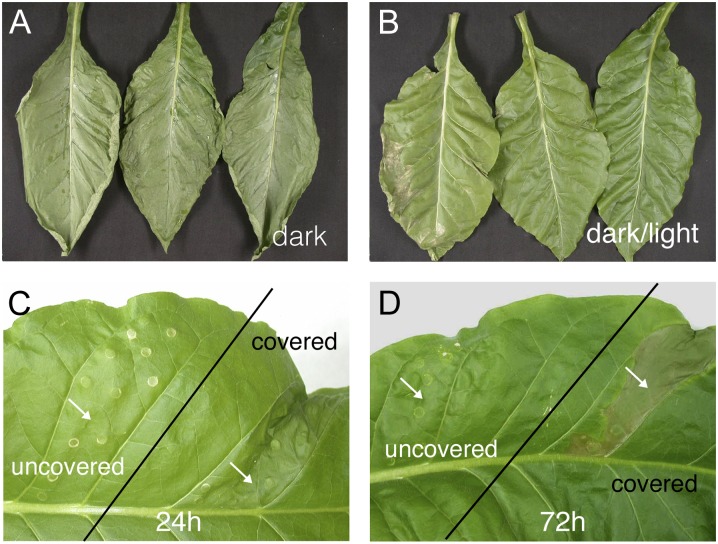
Cryptogein-induced HR symptoms can be observed either on detached tobacco leaves after application of the elicitor to the petiole, thereby provoking symptoms on the entire leaf surface, or by infiltrating leaf segments still attached to the plant. In the latter case, symptoms are contained within the infiltrated parts. HR symptoms are fully visible within 24 h when treated leaves or plants are kept in the dark (Rustérucci et al., 1999), whereas in the light, HR symptoms are inhibited (Cacas et al., 2005). When compared with dark-incubated leaves (Fig. 1A), leaves kept in day/night regimes (Fig. 1B) or continuous light (data not shown) displayed altered HR symptoms. In both continuous light and day/night regimes, small cell death lesions could be observed in the youngest leaves. The number and size of these necrotic areas increased with leaf age (Montillet et al., 2005). To investigate the effect of light in more detail, two different parts of young fully developed leaves were infiltrated with cryptogein between two leaf veins at the beginning of the light period. One part was covered with aluminum foil and, hence, kept constantly in the dark (Fig. 1, C and D). The plant was subsequently submitted to successive 12-h/12-h light/dark cycles at 350 µmol m−2 s−1 light intensity. Within 24 h, the infiltrated part of the leaf kept in the dark developed necrosis, whereas the part exposed to the light remained unaffected (Fig. 1C). This protection remained effective after three light/dark cycles (Fig. 1D; 72 h of incubation). When a plant with elicited leaves was transferred to the dark after one light/dark cycle, necrosis developed on the initially light-exposed parts within 24 h. A similar transfer after two or three light/dark periods delayed the appearance of cell death symptoms by 48 h. This memory effect was abolished after six light/dark periods (data not shown), suggesting that light could fully reverse the elicitation process. Alternatively, light might suppress the cryptogein response until cryptogein degradation causes a gradual loss in its effectiveness. In similar experiments done under low light intensities (100 µmol m−2 s−1), HR symptoms developed in 70% to 80% of the infiltrated leaf areas, pointing toward a reduced degree of protection (data not shown). Finally, we observed that the protection by light was also effective when cryptogein was infiltrated at the beginning of the dark period or under continuous light conditions (data not shown).
Figure 1.
Inhibition of cryptogein-induced HR by light. A, Symptom development of leaves incubated for 39 h in the dark. B, Symptom development of excised leaves incubated for 39 h in a 12-h/12-h dark/light cycle. At a light intensity of 350 µmol m−2 s−1, cryptogein (10 µL at 20 µm) was applied to the petiole of the leaves at the end of the night period. C and D, Cryptogein (1 µg mL−1) infiltrated twice between two veins (indicated by the arrows) in a tobacco leaf. The infiltrated part in the top region of the leaf was kept in the dark, covered with aluminum foil. The plant was exposed at 350 µmol m−2 s−1 with a 12-h/12-h day/night regime. Infiltration was carried out 2 h after starting the daylight period, and photographs were taken 24 h (C) and 72 h (D) later. When the bottom part of the leaf was kept in the dark and the top part was exposed to light, symptoms developed similarly in the covered part (data not shown). Two of the four independent experiments are shown.
Light Alters the Transcriptional Response of Tobacco to Cryptogein
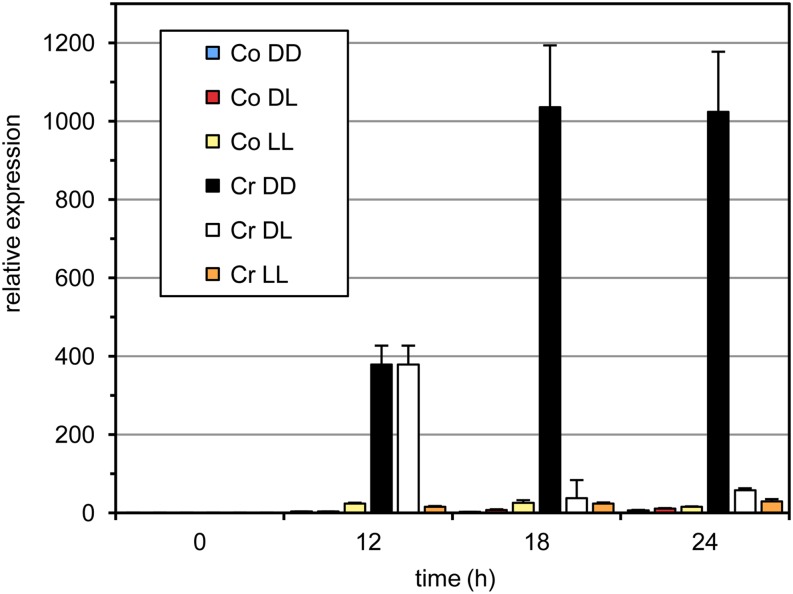
To identify the transcriptional changes associated with the dark-dependent cryptogein-induced HR, we first established an appropriate experimental setup. We monitored LOX1 transcript levels under different dark/light regimes as a marker transcript for HR induction (Cacas et al., 2005). In the dark, LOX1 transcript levels strongly increased 12 h after inoculation, reaching maximum levels after 18 to 24 h. Under dark/light (12 h/12 h) conditions, transcript levels decreased sharply 18 h after inoculation, whereas under continuous light, no induction was observed (Fig. 2). These results prompted us to sample leaf material 12 h after inoculation for a comprehensive transcriptome analysis to compare the effects of cryptogein inoculation under dark and light conditions. To this end, we used complementary DNA (cDNA)-amplified fragment length polymorphism (AFLP), an efficient tool for quantitative transcript profiling (Breyne et al., 2003; Vandenabeele et al., 2003). Tobacco leaves from two independent experiments were harvested before and 12 h after infiltration with water (control) or cryptogein both under continuous dark and light conditions. In total, 64 primer combinations were used to monitor 4,523 nonredundant cDNA-AFLP transcript fragments, of which 526 fragments were differentially expressed between the four conditions (for details, see “Materials and Methods”).
Figure 2.
Accumulation of LOX1 transcripts in cryptogein-treated tobacco leaves under different dark and light regimes determined by semiquantitative real-time PCR. Excised leaves were treated with either water (control [Co]) or cryptogein (Cr) and subsequently kept for 24 h in the dark (DD), in the light (LL), or 12 h in the dark and then 12 h in the light (DL). PCRs were done in triplicate on material from three biological repeats. One representative experiment is shown; error bars represent the sd of the technical variation. [See online article for color version of this figure.]
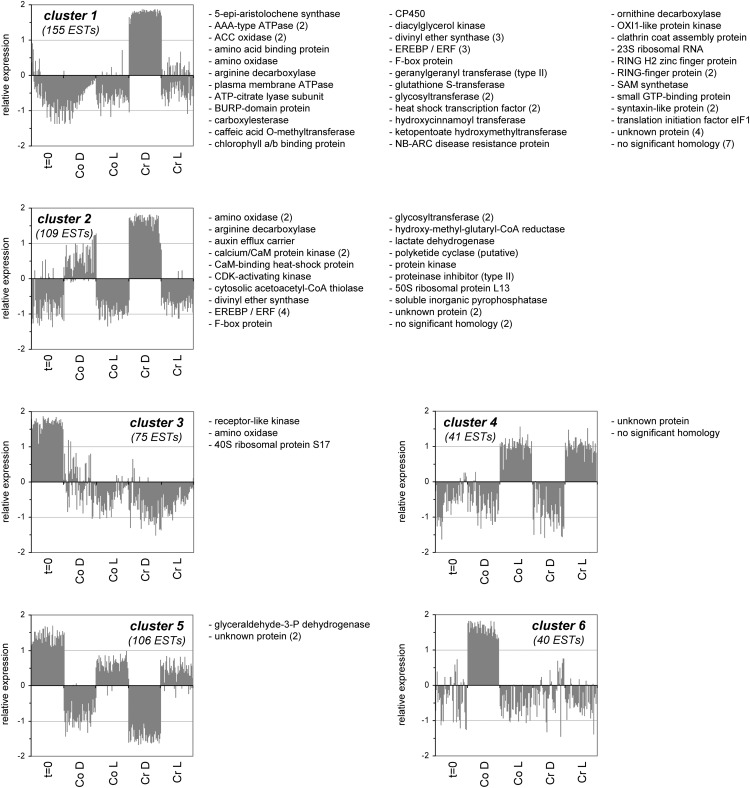
Analysis of the obtained expression profiles of the differentially expressed transcript fragments with the K-means clustering algorithm, revealed six main gene expression clusters (Fig. 3). Cluster 1 contained 155 fragments that accumulated specifically during treatment of tobacco leaves with cryptogein in the dark. Cluster 2 held 109 fragments that, besides being strongly up-regulated by cryptogein in the dark, were also induced by darkness alone, albeit to a lesser degree. Cluster 3 grouped 75 expressed sequence tags (ESTs) that were down-regulated over time, irrespective of the treatment. Cluster 4 contained 41 fragments induced by exposure to light, regardless of cryptogein treatment. Furthermore, cluster 5 comprised 106 ESTs specifically down-regulated in the dark, whereas cluster 6 contained 40 fragments strongly up-regulated in the dark, but only in the absence of cryptogein treatment. Fragments specifically induced by cryptogein, either in the dark or in the light, were not found.
Figure 3.
Clusters obtained by the K-means clustering algorithm, including annotation, of ESTs differentially regulated in cryptogein-treated tobacco leaves under different light regimes, obtained by cDNA-AFLP. Excised leaves were treated with either water (control [Co]) or cryptogein (Cr) and subsequently kept in the dark (D) or in the light (L) for 12 h. The numbers in parentheses indicate how many ESTs were identified corresponding to the same gene. ACC, aminocyclopropane-1-carboxylic acid; BURP, acronym for BNM2 (Brassica napus microspore-derived embryo), USP (unknown seed protein), RD22 (drought-induced protein), and PG1β (β-subunit of polygalacturonase isozyme1); CaM, calmodulin; CDK, cyclin-dependent kinase; elF, elongation factor; EREBP/ERF, ethylene response element binding protein/ethylene response factor; NB-ARC, nucleotide-binding adaptor shared by APAF-1 (apoptotic peptidase activating factor1), R proteins, and CED-4 (Caenorhabditis elegans cell death gene); OXI1, oxidative signal-inducible1; RING, really interesting new gene; SAM, S-adenosyl-l-Met.
Sequence information was obtained mainly from ESTs up-regulated by cryptogein treatment in the dark, present in clusters 1 and 2. From cluster 1, 55 transcript fragments were effectively sequenced. From cluster 2, 28 fragments were sequenced that displayed an induction by cryptogein in the dark at least 2-fold larger than that in the control treatment. In total, sequence information for 90 fragments was obtained (Fig. 3; Supplemental Table S1). As BLASTN searches revealed that most cDNA-AFLP fragments were highly similar to larger tobacco cDNA clones, the annotation process was greatly facilitated.
To consolidate the expression data obtained by cDNA-AFLP and to gain additional insights into the kinetics of the observed gene expression changes, two cDNA-AFLP primer combinations were used on an expanded set of samples from a third independent experiment. As a result, more detailed expression patterns for three ESTs, representing three different genes encoding a RING-finger protein, a hydroxycinnamoyl transferase, and a divinyl ether synthase (DES), were obtained (Supplemental Fig. S1). Although minor differences between experiments were found, the obtained expression patterns corroborated the previously observed profiles. In addition, in all three cases, gene induction occurred also in the light (albeit to a significantly lower level), and differences in expression levels between the different treatments were most prominent after 12 to 24 h.
Cryptogein activates two distinct tobacco MAPKs (Ren et al., 2006; Dahan et al., 2009). This posttranslational activation was not accompanied by any changes in transcript levels. Hence, the differences in response between dark and light could not be linked to differential transcriptional activation of these MAPKs (Supplemental Fig. S2). Nevertheless, our gene expression data revealed several putative signaling components and transcription factors. Among the ESTs present in clusters 1 and 2 (Fig. 3), seven sequences were homologous to protein kinases. The protein kinase identified in cluster 1 shares similarity with the Arabidopsis (Arabidopsis thaliana) OXIDATIVE SIGNAL-INDUCIBLE1, a Ser/Thr kinase that is required for full activation of a MAPK signaling cascade after treatment with ROS or elicitors (Rentel et al., 2004). Cluster 1 also contains a putative diacylglycerol kinase that generates phosphatidic acid, an important secondary messenger involved in both biotic and abiotic stress responses (Arisz et al., 2009). In addition, a putative heat shock transcription factor and two small GTP-binding proteins, one of which is a type II or Rab geranylgeranyl transferase implicated in protein prenylation (Thompson and Okuyama, 2000), were up-regulated by cryptogein in the dark. In addition, 13 different fragments were found that showed homology to proteins of unknown function or shared no significant homology with any protein present in public databases.
Cryptogein induced two transcripts involved in ethylene biosynthesis: 1-aminocyclopropane-1-carboxylate oxidase and an S-adenosyl-l-Met synthetase. Together with the induction of seven transcripts encoding putative APETALA2-type ethylene-responsive element-binding proteins, probably reflects the previously reported cryptogein-induced ethylene production in cultured tobacco cells (Blein et al., 1991). A possible increase in the production of polyamines is implied by the induction of polyamine biosynthesis genes (Orn and Arg decarboxylases). Polyamines, also derived from S-adenosyl-l-Met synthetase, are important substrates for (poly)amine oxidases, generating hydrogen peroxide (H2O2) during the oxidative burst in plant-pathogen interactions (Cona et al., 2006). An apoplastic polyamine oxidase has been shown to be a key contributor to the oxidative burst induced by cryptogein in tobacco cells (Yoda et al., 2006). Clusters 1 and 2 consistently revealed the induction of a putative polyamine oxidase. In addition, polyamines can be acceptor substrates of hydroxycinnamoyl transferase, an enzyme involved in lignin biosynthesis (see below). Three different transcript fragments present in clusters 1 or 2 were linked to terpene biosynthesis and the prenylation of proteins, probably corresponding to the reported induction of sesquiterpene phytoalexin biosynthesis by elicitors and pathogens (Yin et al., 1997). Cytosolic acetoacetyl-CoA thiolase and 3-hydroxy-3-methylglutaryl-CoA reductase are both enzymes involved in the biosynthesis of mevalonate, a precursor for terpenoids, whereas geranylgeranyl transferase is an enzyme that catalyzes the transfer of a terpenoid moiety to specific target proteins, thereby changing its properties.
Dark-Induced HR Is Accompanied by the Induction of Oxylipin Biosynthesis Pathways
Two DES-encoding transcripts involved in oxylipin biosynthesis were also up-regulated (Fig. 3). Oxylipins are a group of biologically active compounds, including jasmonates, that are induced upon wounding, pathogen attack, and abiotic stresses, possess antifungal and antimicrobial properties, and have signaling functions during disease resistance and HR. Plant oxylipin biosynthesis is initiated by the activation of diverse LOXs. Subsequently, hydroperoxy products of LOXs are metabolized to a diverse range of oxylipins by DESs, closely related members of the cytochrome P450 family (Rustérucci et al., 1999; Howe and Schilmiller, 2002; Montillet et al., 2002; Farmer et al., 2003; Göbel et al., 2003). One of the DESs identified here acts together with LOX1 to form oxylipins (Fammartino et al., 2007). Consequently, induction of the 9-LOX pathway results in the induction of cell death-promoting hydroperoxides. Therefore, we also assessed transcript levels of α-DIOXYGENASE1 (α-DOX1), because oxylipins generated via α-DOX have been suggested to antagonistically affect cell death (Hamberg et al., 2003). However, direct comparison between LOX1 and α-DOX1 transcript accumulation upon cryptogein treatment revealed that, in both cases, their induction in the dark was impaired in the light (Fig. 2; Supplemental Fig. S3). This result does not support the hypothesis that anti-cell-death properties of α-DOX1-generated oxylipins play a role in the altered cryptogein-induced phenotype in the light. In summary, we showed previously that the cryptogein-induced HR is characterized by increased 9-LOX-mediated hydroxyoctadecatrienoic acid (HOTE) levels, LOX enzyme activity, and LOX1 transcript levels (Cacas et al., 2005). The gene expression results presented here provide further indications that, in the dark, oxylipin biosynthesis is essential for HR and cell death, whereas this pathway is inhibited in the light.
Involvement of Putative Glycosyl Transferase and Glutathione-S-Transferases
Among the genes up-regulated by cryptogein in the dark, four glycosyl transferases were identified. Although glycosyl transferases have been implicated in oxidative stress responses in Arabidopsis, it remains unclear whether their transcriptional regulation directly involves redox status (Lim et al., 2008; Tognetti et al., 2010). One glycosyl transferase has been shown to glucosylate salicylic acid and other simple phenolics and to be implicated in tobacco-pathogen interactions (Lee and Raskin, 1999). Two additional tobacco transferases could be distinguished among the genes specifically induced by cryptogein in the dark. Both caffeic acid O-methyltransferase and hydroxycinnamoyl transferase are probably involved in lignin biosynthesis (Boerjan et al., 2003).
One EST identified was derived from a gene encoding a glutathione-S-transferase (GST). This fragment was specifically produced after cryptogein treatment in the dark (cluster 1) and strongly resembled a tomato (Solanum lycopersicum) τ-class GST with antioxidative stress and antiapoptotic properties in yeast cells (Kilili et al., 2004).
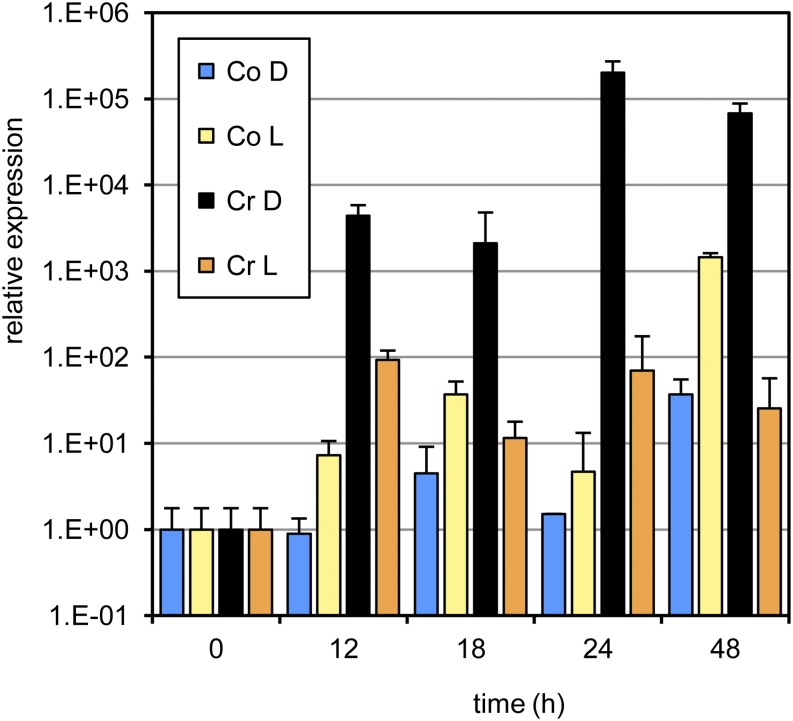
As experiments done in parallel (see below) suggested a differential role for glutathione (GSH) in cryptogein-treated leaves, we investigated the accumulation of transcripts of three tobacco genes encoding previously described plant GSTs. Expression levels of the gene encoding the probable GST or auxin-regulated protein A (ParA/STR246C) were strongly induced by cryptogein in the dark, whereas this up-regulation was virtually absent in the light. Transfer to the light of plants first kept in the dark for 12 h significantly diminished the powerful induction seen in continuous darkness (Fig. 4). ParA/STR246C is regulated by auxin and is localized in the nucleus, instead of in the cytosol. The functional significance of this nuclear targeting is unknown (Takahashi et al., 1995). The same ParA/STR246C fragment had also been identified as strongly up-regulated in jasmonate-elicited Bright Yellow-2 tobacco cells and in catalase-deficient tobacco plants (Goossens et al., 2003; Vandenabeele et al., 2003), suggesting a possible involvement in jasmonic acid and/or redox signaling. We previously described the accumulation of two additional tobacco GSTs in response to cryptogein in the dark (Davoine et al., 2006). Similar to ParA/STR246C, their transcript accumulation was significantly suppressed in the light (Supplemental Fig. S4).
Figure 4.
Accumulation of ParA/STR246C transcripts in cryptogein-treated tobacco leaves under different dark and light regimes determined by semiquantitative real-time PCR. Excised leaves were treated with either water (control [Co]) or cryptogein (Cr) and subsequently kept in the dark (D) or in the light (L) for 48 h. PCRs were done in triplicate on material from three biological repeats. One representative experiment is shown; error bars represent the sd of the technical variation. [See online article for color version of this figure.]
Considering all the obtained gene expression data, and although the functional significance of these observations remains to be characterized in detail, we could link several molecular players to a number of biological processes already associated with cryptogein responses in tobacco. In addition, it has become clear that the expression of numerous genes induced by cryptogein in the dark is inhibited in the light. Relating these expression patterns to the differential responses to cryptogein in the dark or the light is an important first step in the further elucidation of the underlying molecular processes and biochemical pathways. Interestingly, more than 30 of the cryptogein dark-induced transcript tags were also up-regulated by H2O2 in high-light-treated catalase-deficient plants (Vandenabeele et al., 2003). A similar comparison with a set of jasmonate-responsive tobacco genes (Goossens et al., 2003) resulted in seven ESTs induced both by jasmonate and cryptogein in the dark. These comparisons suggest that the response to cryptogein in the dark involves both redox and jasmonate signaling.
Singlet Oxygen Is an Important Component of the Altered Response to Cryptogein in the Light
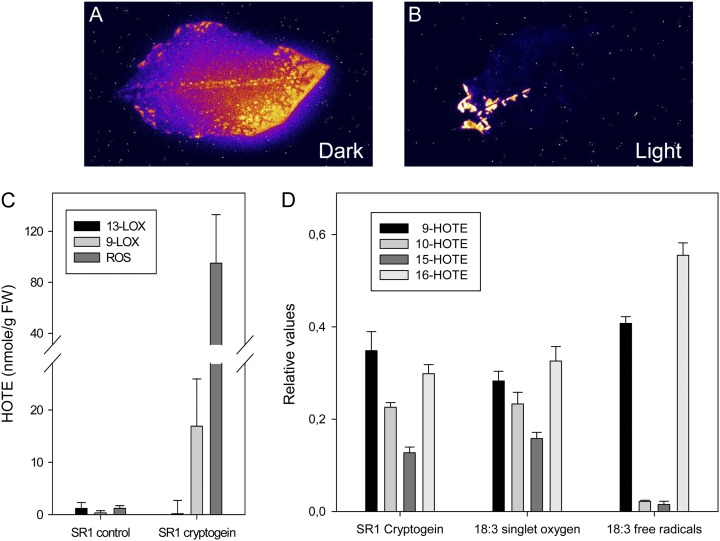
Oxidative reactions, such as lipid peroxidation (LPO), generate light-emitting molecules, including triplet carbonyls and singlet oxygen, that cause spontaneous luminescence that can be imaged through highly sensitive cameras (Havaux et al., 2006). Such autoluminescence imaging was used to visualize cryptogein-induced LPO. The autoluminescence intensities observed were much higher after incubation in the dark than in plants exposed to dark/light cycles (12 h/12 h; Fig. 5, A and B), in agreement with the increased HOTE levels described (Cacas et al., 2005). We observed previously that, in the light, necrotic areas are characterized by ROS-mediated LPO (Montillet et al., 2005). Using catalase-deficient tobacco plants that accumulate photorespiratory H2O2 under high-light conditions, we established that H2O2 played an important role in the execution of cryptogein-triggered cell death in the light and proposed free radical-mediated LPO to explain this process (Montillet et al., 2005). However, it was shown later that carbon fixation is inhibited in chloroplasts of tobacco mosaic virus-infected cells (Liu et al., 2007), creating excess excitation energy in plants under illumination. Hence, it is plausible that, in the light, singlet oxygen is produced by the light-harvesting antennae and/or PSII and contributes to the LPO process. As it is possible to discriminate between LPO mediated by free radicals or singlet oxygen by analyzing the isomer distribution of hydroxy fatty acids (Triantaphylidès et al., 2008), we examined the ROS-mediated cryptogein-induced LPO in tobacco leaves in the light. Total LPO was confirmed to be mainly due to the production of ROS (Fig. 5C), and the hydroxy fatty acid distribution resulting from ROS-mediated LPO predominantly displayed a singlet oxygen signature (Fig. 5D). Taken together, these data indicate that, in the light, the chloroplastic production of singlet oxygen is a major component of the altered response to cryptogein. Although H2O2 accumulates and plays an important role during the response to cryptogein in the light, LPO seems to be primarily triggered by singlet oxygen.
Figure 5.
The main LPO processes in cryptogein-treated tobacco leaves in the light are caused by singlet oxygen. A and B, Autoluminescence imaging of detached leaves (treated as in Fig. 1, A and B). The necrotic areas show an emission of light attributed to lipid peroxidation processes. Photographs from one of two independent experiments are shown. C and D, Excised tobacco leaves treated with cryptogein and incubated for 48 h in continuous light (350 µmol m−2 s−1). Leaves developing HR symptoms were analyzed for lipid peroxidation processes. C, Quantification of lipid peroxidation mediated by 13-LOX, 9-LOX, or ROS and expressed as HOTE levels measured by HPLC-UV (Montillet et al., 2005). FW, Fresh weight. D, Specific HOTE distribution in ROS-mediated lipid peroxidation of elicited SR1 leaves, as measured by HPLC-tandem mass spectrometry (Triantaphylidès et al., 2008). As a reference, the distribution of the 18:3 oxidation by methylene blue in the light (singlet oxygen) and by tert-butyl hydroperoxide (free radicals) is also shown. Results are means ± sd of three analyses carried out on material from three leaves.
Quantum Yield and Functioning of the Light-Harvesting Antennae Decrease Simultaneously in the Light
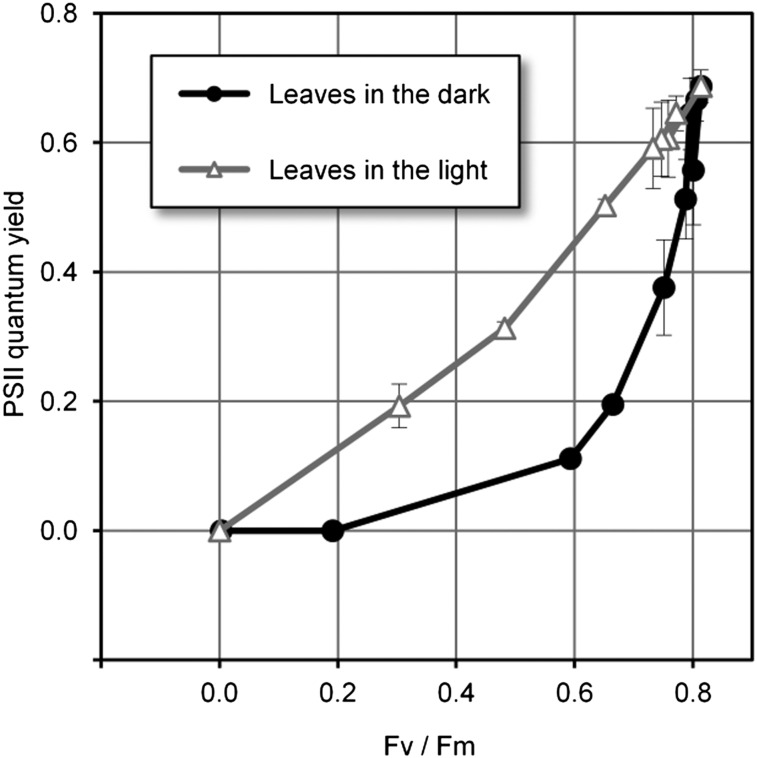
Alterations to the photosynthetic apparatus can be examined by chlorophyll fluorescence measurements (Genty et al., 1989). The effect of cryptogein on (dark-adapted) tobacco leaves incubated in the dark had been investigated previously by analyzing both functioning of the light-harvesting antennae (Fv/Fm) and quantum yield of PSII (ΔF/Fm; Stallaert et al., 1995). Both parameters decreased with time after inoculation (up to 24 h) when compared with control leaves. During the experiments described here, ΔF/Fm decreased before Fv/Fm did (Fig. 6). This observation can be explained by assuming that, in the dark, the 9-LOX product accumulation initiates cell death. Thus, photosynthetic electron flux may be disrupted before antennae and PSII disorganization.
Figure 6.
Correlation between ΔF/Fm and Fv/Fm. Cryptogein-treated leaves were incubated either in the dark or in the light until the HR symptoms appeared. Fluorescence parameters were determined after 24 and 48 h at different spots on the same leaf, corresponding to different degrees of HR symptoms. Values are means ± sd of ΔF/Fm corresponding to the Fv/Fm ratio from six to eight measurements carried out on similar areas from three different leaves.
In (dark-adapted) leaves elicited in the light and displaying altered HR symptoms, after 48 h of incubation, we observed that both Fv/Fm and ΔF/Fm (Fv/Fm = 0.733 ± 0.038 and ΔF/Fm = 0.591 ± 0.062) were very comparable to initial values (Fv/Fm = 0.814 ± 0.007 and ΔF/Fm = 0.687 ± 0.025) and to values obtained with untreated leaves (Fv/Fm = 0.772 ± 0.018 and ΔF/Fm = 0.645 ± 0.027). These data indicate that photosynthetic activity is fully maintained under these conditions. Additionally, our chlorophyll fluorescence measurements demonstrated that, in leaves elicited in the light accompanied by a partial inhibition of HR symptoms, both parameters decreased simultaneously (Fig. 6).
The latter correlation implies that photoinhibition lies at the origin of the decreased photosynthesis once the necrotic process is engaged. Together with the above-mentioned observations on singlet oxygen production involved in LPO during HR cell death in the light, these results suggest that photooxidative damage could be directly involved in the establishment of HR. This hypothesis is supported by evidence showing that manipulation of the chlorophyll catabolism in Arabidopsis alters HR cell death kinetics (Mur et al., 2010).
High-light treatment of catalase-deficient Arabidopsis plants has been shown to result in the accumulation of photorespiratory H2O2 and the induction of H2O2 marker genes, combined with LPO that displayed a singlet oxygen signature (Triantaphylidès et al., 2008). High CO2 levels inhibited the observed up-regulation of both H2O2 and singlet oxygen marker genes (Triantaphylidès et al., 2008). In other words, overproduction of peroxisomal H2O2 might lead to plastidic singlet oxygen formation and singlet oxygen-dependent LPO and, subsequently, to cell death. In the current context, this mechanism might play a predominant role in HR development when cryptogein-treated plants are incubated in the light. As suggested by our previous studies in both tobacco and Arabidopsis (Montillet et al., 2005; Triantaphylidès et al., 2008), H2O2 might lie at the basis of the photoinhibition, eventually giving rise to singlet oxygen accumulation, either by direct interaction or via the activation of specific signal transduction pathways (Kim et al., 2008).
Effect of Light on GSH Levels and Redox Balance in Cryptogein-Elicited Leaves
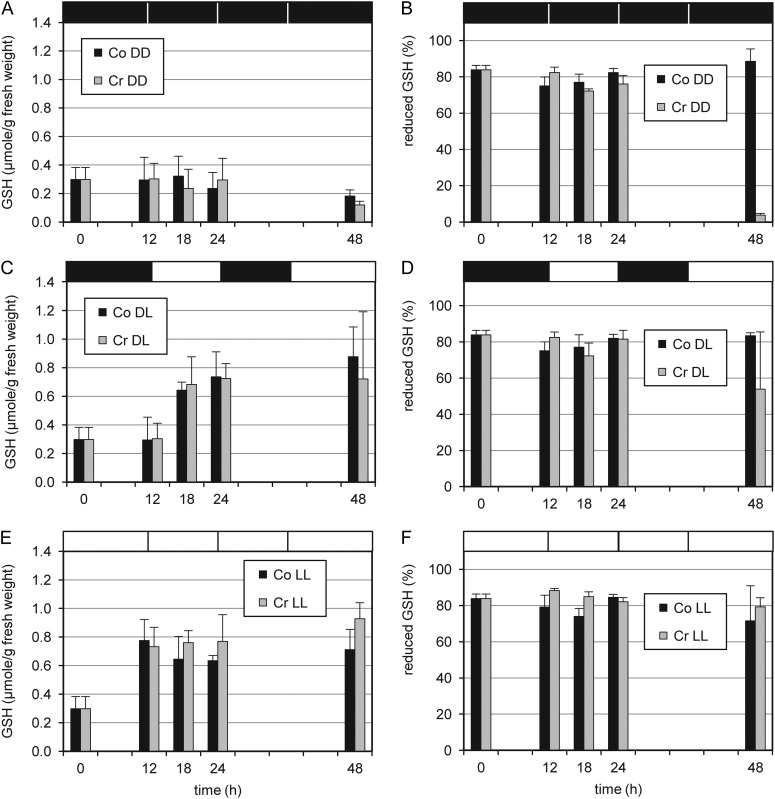
Total GSH levels differed importantly in tobacco leaves treated with cryptogein in the dark or in the light. Whereas cryptogein treatment did not induce total GSH levels as such, the presence of light approximately doubled them (Fig. 7). GSH has been detected virtually in all cell compartments and is the major source of nonprotein thiols in most plant cells. Both total GSH levels and the reduced-to-oxidized GSH ratio, indicative of the cellular redox balance, have been suggested to be involved in ROS perception (Foyer and Noctor, 2009). Furthermore, reduced GSH acts as an antioxidant and has been implicated directly in the reduction of most ROS generated in response to abiotic or biotic stresses (Mullineaux and Rausch, 2005; Foyer and Noctor, 2009). The observed rather stable total GSH concentrations in leaves treated with cryptogein in the dark were combined with a drastically diminished proportion of reduced GSH (Fig. 7). Although it cannot be excluded that this decrease in the dark merely reflects cellular breakdown, these results suggest that (reduced) GSH could become a limiting factor for redox reactions during the cryptogein-induced HR in the dark. On the contrary, in the light, our data point toward a rapid increase in the antioxidant pool of GSH, thereby altering the HR occurring after cryptogein treatment in the dark. In cryptogein-elicited tobacco leaves, various reactive electrophile oxylipins have been found to conjugate to GSH. These conjugation reactions happened spontaneously but could also be catalyzed by GSTs, contributing to the net consumption of GSH (Davoine et al., 2006). The observed up-regulation of a newly identified τ-class GST (Fig. 4) and two previously identified GST genes (Davoine et al., 2006; Supplemental Fig. S4) might reflect the increasing demand for GSH, reinforcing our hypothesis that (reduced) GSH may become a limiting factor upon cryptogein treatment in the dark.
Figure 7.
Changes in GSH levels of excised leaves in response to light and to cryptogein treatment. Comparisons of leaves incubated in the dark (A and B), in a dark/light cycle (C and D), and under continuous light (E and F) are shown. A, C, and E, Total GSH levels. B, D, and F, Proportion of reduced GSH. Co, Control; Cr, cryptogein; DD, dark; DL, 12 h/12 h of dark/light cycle; LL, light. Measurements were done with eight leaf discs (four leaves from four different plants, two discs per leaf).
We explored the possibility of inhibiting HR symptoms in the dark (i.e. complete dehydration of the infiltrated leaf segments; Montillet et al., 2005) by coinfiltrating leaves with cryptogein and GSH (1 or 2 mm). However, HR symptoms developed similarly as in cryptogein-treated leaves (Fig. 8B), indicating that the effects of the fatty acid hydroperoxides generated by 9-LOX could not be inhibited by increased GSH supply. In other words, even though GSH levels are reduced by cryptogein in the dark, this is not causative for cryptogein-induced HR. Next, we investigated the effects of GSH biosynthesis inhibitors on the development of the altered HR symptoms in the light. Leaf segments coinfiltrated with cryptogein plus buthionine sulfoximine (BSO) or methionine sulfoximine (MSO) rapidly developed an exaggerated cell death phenotype, reminiscent of the phenotype induced by oxidative stress (Fig. 8E; Supplemental Fig. S5; Montillet et al., 2005; Triantaphylidès et al., 2008). GSH, BSO, or MSO infiltration in control leaves had no effect (Fig. 8, C and F). This result demonstrates that GSH plays an important role in the execution of the altered HR symptoms in the light.
Figure 8.
Effect of the coinfiltration of cryptogein, GSH, and BSO under dark or light conditions. A to C, Cryptogein plus GSH in the dark in tobacco leaves after 48 h. D to F, Cryptogein plus BSO, an inhibitor of GSH biosynthesis, in the light in tobacco leaves after 48 h. Leaves were infiltrated with 0.1 μm cryptogein (A), 0.1 μm cryptogein plus 1 mm GSH (B), 1 mm GSH (C), 0.1 μm cryptogein (D), 0.1 μm cryptogein plus 0.5 mm BSO (E), and 0.5 mm BSO (F).
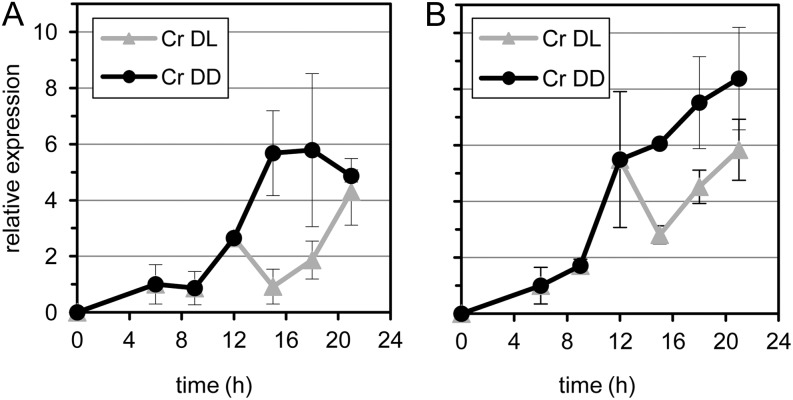
In plant-pathogen interactions, a well-known effect of the cellular redox balance is the regulation of the activity of transcription factors, such as NONEXPRESSOR OF PATHOGEN-RESISTANT1 (PR-1; Fobert and Després, 2005), that, in its reduced form, activates the expression of SAR genes. Therefore, we investigated the expression of two such pathogen-induced tobacco defense genes, PR-1a and PR-1b/PR-Q (Rivière et al., 2008). The application of light after 12 h of cryptogein treatment in the dark significantly delayed the cryptogein-induced increase in gene expression in the dark (Fig. 9). Taken together, incubation with cryptogein in the light was associated with increased levels of total GSH and delayed PR gene expression when compared with dark conditions. These results suggest that light decreases defense gene expression and HR symptoms by the increased availability of GSH, in favor of normal metabolism and tissue recovery.
Figure 9.
Transcript accumulation of two tobacco defense-related genes. A and B, PR-1a and PR-1b/PR-Q in cryptogein-treated tobacco leaves under different dark and light regimes, respectively, as determined by semiquantitative real-time PCR. Excised leaves were treated with either water (control [Co]) or cryptogein (Cr) and subsequently incubated in the dark (DD) or in a 12-h/12-h dark/light cycle (DL) for 21 h. Error bars represent the sd of the technical variation within one representative experiment.
Conclusions on the Light Effect in Plant-Pathogen Interactions
In the Arabidopsis plant model, genetic evidence has been provided for the role of light perception in the response to the avirulent pathogen P. syringae (Genoud et al., 2002). Both SAR and HR are compromised in the phytochrome A or phytochrome B mutant. Light is also involved in pathogen-induced accumulation of salicylic acid and in PR-1 gene expression (Genoud et al., 2002; Zeier et al., 2004; Griebel and Zeier, 2008). In this context, Arabidopsis resistance to Turnip crinkle virus has been shown to be light dependent (Chandra-Shekara et al., 2006). In addition, comparison between the immunity to infection and the acclimation to high light also revealed some common features (Karpinski et al., 2003; Bechtold et al., 2005). In both cases, ROS are produced and play a role in signaling, whereas salicylic acid is involved in resistance and acclimation (Mateo et al., 2006). Moreover, high light is capable of producing HR-like lesions in leaves, and the spreading of this cell death process is under the control of LESION-SIMULATING DISEASE1 (Mateo et al., 2004). Thus, in Arabidopsis, light appears to be a conditional factor for the development of the plant’s resistance to infection.
In tobacco, incubation of cryptogein-treated leaves in the dark is accompanied specifically by increases in 9-LOX and patatin transcript levels that lead to cell death. In the light, however, this up-regulation of gene expression and HR cell death are strongly inhibited (Cacas et al., 2005). Here, a cDNA-AFLP analysis yielded an inventory of 264 additional transcript fragments that are associated specifically with the response to cryptogein in the dark (Fig. 3, clusters 1 and 2). These transcripts represent at least 56 different genes. Based on their homology with previously identified genes, we can conclude that they are involved in a wide variety of cellular processes. No clusters of transcripts specifically induced by cryptogein in the light were identified, suggesting that the synergy between cryptogein and light does not result in particular changes in gene expression. Hence, light appeared primarily to inhibit gene expression observed in the dark. In addition, (reduced) GSH levels were maintained, whereas GSH synthesis inhibitors provoked exaggerated cell death during cryptogein treatment in the light. This suggests that the occurrence of photosynthetic activity protects, at least to some extent, against cell death initiation by maintaining or increasing GSH levels. Furthermore, based on the perceived LPO signatures, we postulate that once the cell death process has been initiated, progressive disorganization of the photosynthetic apparatus in the light will cause massive production of singlet oxygen, leading to subsequent peroxidation of membranes and other biomolecules.
Thus, our findings on the tobacco-cryptogein model, combined with comparable decreases in HR symptom development triggered by light during interactions between tobacco and the avirulent pathogen P. syringae (Montillet et al., 2005) or the bacterial pathogen R. solanacearum (Cacas et al., 2005), lead to the hypothesis that light can have a dual effect on the defense processes against pathogen attack as provoked by elicitins. On the one hand, light appears to slow down the onset of the defense response and protect leaf tissues from cell death initiation; on the other hand, light, via the production of singlet oxygen, can also be considered as an important determinant in the execution of cell death.
MATERIALS AND METHODS
Plant Material, Growing Conditions, Cryptogein Treatment, and Sampling
Tobacco (Nicotiana tabacum ‘Petit Havana SR1’) plants were grown at 100 µmol m−2 s−1 light radiance with a light/dark cycle (14 h/10 h at 25°C/20°C) and 70% relative humidity in three independent replicate experiments. Mature 6-week-old plants were used for all experiments. Cryptogein was applied either by infiltration (0.5–1 mL of 0.1 µm cryptogein in water) between the secondary veins of fully developed attached leaves or to the petiole (10 μL of 20 μm cryptogein in water followed by three times 10 µL of water to ensure full absorption) of excised leaves selected from the middle stem (four leaves per plant). Leaves were detached just before the start of the light cycle. Control leaves were treated identically, but with water only. Leaves were subsequently placed horizontally with their petioles dipped in water in controlled 1-m3 growth chambers at 360 µL L−1 CO2 (corresponding to ambient atmosphere) under three different lighting conditions: continuous darkness (24 h at 5 µE m−2 s−1 and 20°C), continuous light (24 h at 350 µE m−2 s−1 and 25°C), and dark/light regime (12 h/12 h at 5 µE m−2 s−1/350 µE m−2 s−1 and 20°C/25°C).
For simultaneous treatments with cryptogein and GSH, tobacco leaves were infiltrated in the dark with cryptogein (10 μL of 20 μm) through the petiole. After 4 h of incubation, GSH (0.5–1 mL of 1 or 2 mm) was infiltrated between the veins. Alternatively, leaves were coinfiltrated with cryptogein (0.1 µm) plus GSH (1 or 2 mm) between veins BSO and MSO, two GSH synthesis inhibitors, were coinfiltrated with cryptogein at concentrations of 0.5 or 1 mm. All images in Figure 8 are from coinfiltration experiments. Leaf discs of 2.5 cm diameter (approximately 0.13 g) were sampled from four leaves from four different plants (two discs per leaf) and frozen immediately in liquid nitrogen. The zero time point (not yet elicited with cryptogein) contained 16 leaf discs from eight leaves from eight plants. Different leaf discs from the same leaves were harvested for the GSH analysis.
cDNA-AFLP
Total RNA was extracted from material sampled from two independent experiments using TRIzol reagent (Invitrogen). Templates for cDNA-AFLP were prepared as described (Breyne et al., 2003). Sixty-four randomly chosen primer combinations were used (BstC1-Mse11 to BstC1-Mse14, BstC1-Mse21 to BstC1-Mse24, BstC1-Mse31 to BstC1-Mse34, BstC1-Mse41 to BstC1-Mse44, BstC2-Mse11 to BstC2-Mse14, BstC2-Mse21 to BstC2-Mse24, BstC2-Mse31 to BstC2-Mse34, BstC2-Mse41 to BstC2-Mse44, BstC3-Mse11 to BstC3-Mse14, BstC3-Mse21 to BstC3-Mse24, BstC3-Mse31 to BstC3-Mse34, BstC3-Mse41 to BstC3-Mse44, BstC4-Mse11 to BstC4-Mse14, BstC4-Mse21 to BstC4-Mse24, BstC4-Mse31 to BstC4-Mse34, and BstC4-Mse41 to BstC4-Mse44; with 1, 2, 3, and 4 representing the selective nucleotides A, C, G, and T, respectively), and amplification products were separated on polyacrylamide gels. Gels were dried on 3MM Whatman paper, exposed to Kodak BioMax MS films, and scanned in a PhosphorImager 445 SI (GE Healthcare). The gel images were processed with AFLP QuantarPRO (Keygene Products), a software application that allows accurate quantification of band intensities in DNA fingerprints. The intensity of individual bands was determined and normalized semiautomatically for variations, such as running or loading differences and image artifacts. The obtained raw expression data were further processed via the ARRAY-AN application as described (Vandenabeele et al., 2003). Statistically significant differentially expressed fragments were selected from the resulting normalized signal intensities by ANOVA with The Institute for Genomic Research (TIGR) Multiexperiment Viewer 4.0 (P < 0.02). In addition, only fragments with a minimal normalized average signal intensity (more than 1,000) in at least one out of five conditions and a coefficient of variation greater than 0.7 (calculated as the sd on all averaged values across the time course divided by the average expression over the time course) were considered for further analysis. Differentially expressed transcript tags were excised from the gel, reamplified by PCR, and directly sequenced by dideoxy sequencing.
Sequencing and BLAST Analysis
Sequences from 90 transcript tags were compared with those in public databases (National Center for Biotechnology Information). When no significant homology (e > 10e−3) was found, the FASTA algorithm was used to find a corresponding gene index from TIGR to retrieve additional homologous sequence information. With this TIGR gene index, the database was searched again, finally resulting in 71 fragments similar to genes with a known function, nine sequences without any assigned function, and 10 not homologous to any sequence in public databases.
GSH, LPO, and Chlorophyll Fluorescence Measurements
Leaf GSH levels were determined by HPLC fluorescence detection of the monobromobimane derivatives as described (Davoine et al., 2005). Spontaneous photon emission (autoluminescence) caused by LPO was measured from whole tobacco leaves after 2 h of dark adaptation with a liquid N2-cooled CCD camera as described (Havaux et al., 2006). Integration time was 20 min. ROS- and LOX-mediated LPO was evaluated by HPLC-UV analysis of HOTE, and singlet oxygen-mediated lipid peroxidation by HOTE isomer distribution determined by HPLC-electrospray ionization-tandem mass spectroscopy analysis, as described (Triantaphylidès et al., 2008). Fv/Fm and ΔF/Fm were calculated according to Genty et al. (1989). Measurements were done after 0, 6, 24, and 48 h (control in the dark); 24 and 48 h (cryptogein in the dark); 0, 24, and 48 h (control in the light); and 24 and 48 h (cryptogein in the light). Previous to each Fv/Fm determination, leaves were dark adapted for 30 min. ΔF/Fm was measured at a light intensity of 130 µmol m−2 s−1 after the leaves had adapted to this irradiation level for 15 min.
Quantitative Reverse Transcription-PCR
Total RNA was extracted with TRIzol Reagent (Invitrogen). First-strand cDNA was prepared using 2.5 μg of total RNA, SuperScript II Reverse Transcriptase (Invitrogen), and oligo(dT)15 primer. Five microliters of 10-fold-diluted first-strand cDNA was used as a template in the subsequent PCR, run on an iCycler (Bio-Rad) with 200 nm primers and Platinum Supermix-UDG (Invitrogen) supplemented with fluorescein dye in a final volume of 25 μL per reaction, according to the manufacturer’s instructions. All PCRs were done in triplicate on material from three biological repeats with similar results. One representative experiment is shown. The following primer combinations were used for real-time semiquantitative PCR analysis: LOX1 (GenBank accession no. X84040), 5′-TTGGAGTTCCAGGAGCATTC-3′ and 5′-CGCAATGTGTCTGGTGTTTC-3′; ParA (X80829), 5′-AGCACAAGCTTGGTCTTGC-3′ and 5′-AAATTTATAGACAGAGTAGC-3′; PR-1b (X03465), 5′-GCAGACTGCAACCTCGTACA-3′ and 5′-CCTAGCACATCCAACACGAA-3′; and PR-Q (X54456), 5′-GCCATATCTGGTGCTGGTCT-3′ and 5′-ACGAGCCACTAGCCTTTTCA-3′. All obtained expression values were normalized using either glyceraldehyde-3-phosphate dehydrogenase, amplified with degenerate primers (Winge et al., 1997), or the elongation factor 1α (D63396), amplified with the primers 5′-CTCCAAGGCTAGGTATGATG-3′ and 5′-CTTCGTGGTTGCATCTCAAC-3′, as a control.
Sequence data from this article can be found in the GenBank/EMBL libraries under accession numbers JZ470866 to JZ470953.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Verification and kinetics of gene expression patterns in cryptogein-treated tobacco leaves under different dark and light regimes as determined by cDNA-AFLP.
Supplemental Figure S2. Transcript accumulation of two tobacco MAPK genes.
Supplemental Figure S3. Transcript accumulation of the tobacco α-DOX1 gene in cryptogein-treated leaves under different dark and light regimes as determined by semiquantitative real-time PCR.
Supplemental Figure S4. Transcript accumulation of two tobacco GST genes.
Supplemental Figure S5. The effect of coinfiltration of cryptogein, GSH, and MSO under dark or light conditions.
Supplemental Table S1. GenBank accession numbers, functional annotation, transcript levels, and sequences of identified ESTs.
Acknowledgments
We thank Prof. Martin J. Mueller (University of Wuerzburg) for the HPLC-tandem mass spectrometry analyses of fatty acid hydroxylases, Dr. Michel Havaux (Commissariat à l’Energie Atomique) for the lipid peroxidation imaging and helpful discussion, Dr. Michel Ponchet (Institut National de la Recherche Agronomique) and Christian Brière (Laboratoire de Recherche en Sciences Végétales) for the kind gift of cryptogein, and Dr. Martine De Cock (VIB-Ghent University) for help in preparing the manuscript.
Glossary
- SAR
systemic acquired resistance
- MAPK
mitogen-activated protein kinase
- ROS
reactive oxygen species
- HR
hypersensitive response
- LOX
lipoxygenase
- cDNA
complementary DNA
- AFLP
amplified fragment length polymorphism
- DES
divinyl ether synthase
- H2O2
hydrogen peroxide
- HOTE
hydroxyoctadecatrienoic acid
- GST
glutathione-S-transferase
- GSH
glutathione
- LPO
lipid peroxidation
- Fv/Fm
functioning of the light-harvesting antennae
- ΔF/Fm
quantum yield of PSII
- BSO
buthionine sulfoximine
- MSO
methionine sulfoximine
- TIGR
The Institute for Genomic Research
References
- Amelot N, Carrouche A, Danoun S, Bourque S, Haiech J, Pugin A, Ranjeva R, Grima-Pettenati J, Mazars C, Brière C. (2011) Cryptogein, a fungal elicitor, remodels the phenylpropanoid metabolism of tobacco cell suspension cultures in a calcium-dependent manner. Plant Cell Environ 34: 149–161 [DOI] [PubMed] [Google Scholar]
- Amelot N, Dorlhac de Borne F, San Clemente H, Mazars C, Grima-Pettenati J, Brière C. (2012) Transcriptome analysis of tobacco BY-2 cells elicited by cryptogein reveals new potential actors of calcium-dependent and calcium-independent plant defense pathways. Cell Calcium 51: 117–130 [DOI] [PubMed] [Google Scholar]
- Apel K, Hirt H. (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373–399 [DOI] [PubMed] [Google Scholar]
- Arisz SA, Testerink C, Munnik T. (2009) Plant PA signaling via diacylglycerol kinase. Biochim Biophys Acta 1791: 869–875 [DOI] [PubMed] [Google Scholar]
- Bechtold U, Karpinski S, Mullineaux PM. (2005) The influence of the light environment and photosynthesis on oxidative signalling responses in plant-biotrophic pathogen interactions. Plant Cell Environ 28: 1046–1055 [Google Scholar]
- Blein J-P, Coutos-Thévenot P, Marion D, Ponchet M. (2002) From elicitins to lipid-transfer proteins: a new insight in cell signalling involved in plant defence mechanisms. Trends Plant Sci 7: 293–296 [DOI] [PubMed] [Google Scholar]
- Blein J-P, Milat M-L, Ricci P. (1991) Responses of cultured tobacco cells to cryptogein, a proteinaceous elicitor from Phytophthora cryptogea: possible plasmalemma involvement. Plant Physiol 95: 486–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerjan W, Ralph J, Baucher M. (2003) Lignin biosynthesis. Annu Rev Plant Biol 54: 519–546 [DOI] [PubMed] [Google Scholar]
- Breyne P, Dreesen R, Cannoot B, Rombaut D, Vandepoele K, Rombauts S, Vanderhaeghen R, Inzé D, Zabeau M. (2003) Quantitative cDNA-AFLP analysis for genome-wide expression studies. Mol Genet Genomics 269: 173–179 [DOI] [PubMed] [Google Scholar]
- Buhot N, Douliez J-P, Jacquemard A, Marion D, Tran V, Maume BF, Milat M-L, Ponchet M, Mikès V, Kader J-C, et al. (2001) A lipid transfer protein binds to a receptor involved in the control of plant defence responses. FEBS Lett 509: 27–30 [DOI] [PubMed] [Google Scholar]
- Buhot N, Gomès E, Milat M-L, Ponchet M, Marion D, Lequeu J, Delrot S, Coutos-Thévenot P, Blein J-P. (2004) Modulation of the biological activity of a tobacco LTP1 by lipid complexation. Mol Biol Cell 15: 5047–5052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacas J-L, Vailleau F, Davoine C, Ennar N, Agnel J-P, Tronchet M, Ponchet M, Blein J-P, Roby D, Triantaphylidès C, et al. (2005) The combined action of 9 lipoxygenase and galactolipase is sufficient to bring about programmed cell death during tobacco hypersensitive response. Plant Cell Environ 28: 1367–1378 [Google Scholar]
- Chandra-Shekara AC, Gupte M, Navarre D, Raina S, Raina R, Klessig D, Kachroo P. (2006) Light-dependent hypersensitive response and resistance signaling against Turnip Crinkle Virus in Arabidopsis. Plant J 45: 320–334 [DOI] [PubMed] [Google Scholar]
- Cona A, Rea G, Angelini R, Federico R, Tavladoraki P. (2006) Functions of amine oxidases in plant development and defence. Trends Plant Sci 11: 80–88 [DOI] [PubMed] [Google Scholar]
- Dahan J, Pichereaux C, Rossignol M, Blanc S, Wendehenne D, Pugin A, Bourque S. (2009) Activation of a nuclear-localized SIPK in tobacco cells challenged by cryptogein, an elicitor of plant defence reactions. Biochem J 418: 191–200 [DOI] [PubMed] [Google Scholar]
- Davoine C, Douki T, Iacazio G, Montillet J-L, Triantaphylidès C. (2005) Conjugation of keto fatty acids to glutathione in plant tissues: characterization and quantification by HPLC-tandem mass spectrometry. Anal Chem 77: 7366–7372 [DOI] [PubMed] [Google Scholar]
- Davoine C, Falletti O, Douki T, Iacazio G, Ennar N, Montillet J-L, Triantaphylidès C. (2006) Adducts of oxylipin electrophiles to glutathione reflect a 13 specificity of the downstream lipoxygenase pathway in the tobacco hypersensitive response. Plant Physiol 140: 1484–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fammartino A, Cardinale F, Göbel C, Mène-Saffrané L, Fournier J, Feussner I, Esquerré-Tugayé M-T. (2007) Characterization of a divinyl ether biosynthetic pathway specifically associated with pathogenesis in tobacco. Plant Physiol 143: 378–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer EE, Alméras E, Krishnamurthy V. (2003) Jasmonates and related oxylipins in plant responses to pathogenesis and herbivory. Curr Opin Plant Biol 6: 372–378 [DOI] [PubMed] [Google Scholar]
- Fobert PR, Després C. (2005) Redox control of systemic acquired resistance. Curr Opin Plant Biol 8: 378–382 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. (2009) Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid Redox Signal 11: 861–905 [DOI] [PubMed] [Google Scholar]
- Garcia-Brugger A, Lamotte O, Vandelle E, Bourque S, Lecourieux D, Poinssot B, Wendehenne D, Pugin A. (2006) Early signaling events induced by elicitors of plant defenses. Mol Plant Microbe Interact 19: 711–724 [DOI] [PubMed] [Google Scholar]
- Genoud T, Buchala AJ, Chua N-H, Métraux J-P. (2002) Phytochrome signalling modulates the SA-perceptive pathway in Arabidopsis. Plant J 31: 87–95 [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais J-M, Baker NR. (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87–92 [Google Scholar]
- Göbel C, Feussner I, Rosahl S. (2003) Lipid peroxidation during the hypersensitive response in potato in the absence of 9-lipoxygenases. J Biol Chem 278: 52834–52840 [DOI] [PubMed] [Google Scholar]
- Goossens A, Häkkinen ST, Laakso I, Seppänen-Laakso T, Biondi S, De Sutter V, Lammertyn F, Nuutila AM, Söderlund H, Zabeau M, et al. (2003) A functional genomics approach toward the understanding of secondary metabolism in plant cells. Proc Natl Acad Sci USA 100: 8595–8600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel T, Zeier J. (2008) Light regulation and daytime dependency of inducible plant defenses in Arabidopsis: phytochrome signaling controls systemic acquired resistance rather than local defense. Plant Physiol 147: 790–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M, Sanz A, Rodriguez MJ, Calvo AP, Castresana C. (2003) Activation of the fatty acid α-dioxygenase pathway during bacterial infection of tobacco leaves: formation of oxylipins protecting against cell death. J Biol Chem 278: 51796–51805 [DOI] [PubMed] [Google Scholar]
- Havaux M, Triantaphylidès C, Genty B. (2006) Autoluminescence imaging: a non-invasive tool for mapping oxidative stress. Trends Plant Sci 11: 480–484 [DOI] [PubMed] [Google Scholar]
- Howe GA, Schilmiller AL. (2002) Oxylipin metabolism in response to stress. Curr Opin Plant Biol 5: 230–236 [DOI] [PubMed] [Google Scholar]
- Kangasjärvi S, Neukermans J, Li S, Aro E-M, Noctor G. (2012) Photosynthesis, photorespiration, and light signalling in defence responses. J Exp Bot 63: 1619–1636 [DOI] [PubMed] [Google Scholar]
- Karpinski S, Gabrys H, Mateo A, Karpinska B, Mullineaux PM. (2003) Light perception in plant disease defence signalling. Curr Opin Plant Biol 6: 390–396 [DOI] [PubMed] [Google Scholar]
- Kilili KG, Atanassova N, Vardanyan A, Clatot N, Al-Sabarna K, Kanellopoulos PN, Makris AM, Kampranis SC. (2004) Differential roles of Tau class glutathione S-transferases in oxidative stress. J Biol Chem 279: 24540–24551 [DOI] [PubMed] [Google Scholar]
- Kim C, Meskauskiene R, Apel K, Laloi C. (2008) No single way to understand singlet oxygen signalling in plants. EMBO Rep 9: 435–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun-Garcia A, Ouaked F, Chiltz A, Pugin A. (1998) Activation of MAPK homologues by elicitors in tobacco cells. Plant J 15: 773–781 [DOI] [PubMed] [Google Scholar]
- Lee H-i, Raskin I. (1999) Purification, cloning, and expression of a pathogen inducible UDP-glucose:salicylic acid glucosyltransferase from tobacco. J Biol Chem 274: 36637–36642 [DOI] [PubMed] [Google Scholar]
- Lim CE, Choi JN, Kim IA, Lee SA, Hwang Y-S, Lee C-H, Lim J. (2008) Improved resistance to oxidative stress by a loss-of-function mutation in the Arabidopsis UGT71C1 gene. Mol Cells 25: 368–375 [PubMed] [Google Scholar]
- Liu Y, Ren D, Pike S, Pallardy S, Gassmann W, Zhang S. (2007) Chloroplast-generated reactive oxygen species are involved in hypersensitive response-like cell death mediated by a mitogen-activated protein kinase cascade. Plant J 51: 941–954 [DOI] [PubMed] [Google Scholar]
- Mateo A, Funck D, Mühlenbock P, Kular B, Mullineaux PM, Karpinski S. (2006) Controlled levels of salicylic acid are required for optimal photosynthesis and redox homeostasis. J Exp Bot 57: 1795–1807 [DOI] [PubMed] [Google Scholar]
- Mateo A, Mühlenbock P, Rustérucci C, Chang CC-C, Miszalski Z, Karpinska B, Parker JE, Mullineaux PM, Karpinski S. (2004) LESION SIMULATING DISEASE 1 is required for acclimation to conditions that promote excess excitation energy. Plant Physiol 136: 2818–2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montillet J-L, Agnel J-P, Ponchet M, Vailleau F, Roby D, Triantaphylidès C. (2002) Lipoxygenase-mediated production of fatty acid hydroperoxides is a specific signature of the hypersensitive reaction in plants. Plant Physiol Biochem 40: 633–639 [Google Scholar]
- Montillet J-L, Chamnongpol S, Rustérucci C, Dat J, van de Cotte B, Agnel J-P, Battesti C, Inzé D, Van Breusegem F, Triantaphylidès C. (2005) Fatty acid hydroperoxides and H2O2 in the execution of hypersensitive cell death in tobacco leaves. Plant Physiol 138: 1516–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullineaux PM, Rausch T. (2005) Glutathione, photosynthesis and the redox regulation of stress-responsive gene expression. Photosynth Res 86: 459–474 [DOI] [PubMed] [Google Scholar]
- Mur LAJ, Aubry S, Mondhe M, Kingston-Smith A, Gallagher J, Timms-Taravella E, James C, Papp I, Hörtensteiner S, Thomas H, et al. (2010) Accumulation of chlorophyll catabolites photosensitizes the hypersensitive response elicited by Pseudomonas syringae in Arabidopsis. New Phytol 188: 161–174 [DOI] [PubMed] [Google Scholar]
- Osman H, Mikes V, Milat M-L, Ponchet M, Marion D, Prangé T, Maume BF, Vauthrin S, Blein J-P. (2001) Fatty acids bind to the fungal elicitor cryptogein and compete with sterols. FEBS Lett 489: 55–58 [DOI] [PubMed] [Google Scholar]
- Ponchet M, Panabières F, Milat M-L, Mikes V, Montillet J-L, Suty L, Triantaphylidès C, Tirilly Y, Blein J-P, (1999) Are elicitins cryptograms in plant-Oomycete communications? Cell Mol Life Sci 56: 1020–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queval G, Issakidis-Bourguet E, Hoeberichts FA, Vandorpe M, Gakière B, Vanacker H, Miginiac-Maslow M, Van Breusegem F, Noctor G. (2007) Conditional oxidative stress responses in the Arabidopsis photorespiratory mutant cat2 demonstrate that redox state is a key modulator of daylength-dependent gene expression, and define photoperiod as a crucial factor in the regulation of H2O2-induced cell death. Plant J 52: 640–657 [DOI] [PubMed] [Google Scholar]
- Ren D, Yang K-Y, Li G-J, Liu Y, Zhang S. (2006) Activation of Ntf4, a tobacco mitogen-activated protein kinase, during plant defense response and its involvement in hypersensitive response-like cell death. Plant Physiol 141: 1482–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentel MC, Lecourieux D, Ouaked F, Usher SL, Petersen L, Okamoto H, Knight H, Peck SC, Grierson CS, Hirt H, et al. (2004) OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature 427: 858–861 [DOI] [PubMed] [Google Scholar]
- Rivière M-P, Marais A, Ponchet M, Willats W, Galiana E. (2008) Silencing of acidic pathogenesis-related PR-1 genes increases extracellular β-(1→3)-glucanase activity at the onset of tobacco defence reactions. J Exp Bot 59: 1225–1239 [DOI] [PubMed] [Google Scholar]
- Roberts MR, Paul ND. (2006) Seduced by the dark side: integrating molecular and ecological perspectives on the influence of light on plant defence against pests and pathogens. New Phytol 170: 677–699 [DOI] [PubMed] [Google Scholar]
- Rustérucci C, Montillet J-L, Agnel J-P, Battesti C, Alonso B, Knoll A, Bessoule J-J, Etienne P, Suty L, Blein J-P, et al. (1999) Involvement of lipoxygenase-dependent production of fatty acid hydroperoxides in the development of the hypersensitive cell death induced by cryptogein on tobacco leaves. J Biol Chem 274: 36446–36455 [DOI] [PubMed] [Google Scholar]
- Simon-Plas F, Elmayan T, Blein J-P. (2002) The plasma membrane oxidase NtrbohD is responsible for AOS production in elicited tobacco cells. Plant J 31: 137–147 [DOI] [PubMed] [Google Scholar]
- Stallaert VM, Ducruet J-M, Tavernier E, Blein J-P. (1995) Lipid peroxidation in tobacco leaves treated with the elicitor cryptogein: evaluation of high-temperature thermoluminescence emission and chlorophyll fluorescence. Biochim Biophys Acta 1229: 290–295 [Google Scholar]
- Takahashi Y, Sakai T, Ishida S, Nagata T. (1995) Identification of auxin-responsive elements of parB and their expression in apices of shoot and root. Proc Natl Acad Sci USA 92: 6359–6363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GA, Jr, Okuyama H. (2000) Lipid-linked proteins of plants. Prog Lipid Res 39: 19–39 [DOI] [PubMed] [Google Scholar]
- Tognetti VB, Van Aken O, Morreel K, Vandenbroucke K, van de Cotte B, De Clercq I, Chiwocha S, Fenske R, Prinsen E, Boerjan W, et al. (2010) Perturbation of indole-3-butyric acid homeostasis by the UDP-glucosyltransferase UGT74E2 modulates Arabidopsis architecture and water stress tolerance. Plant Cell 22: 2660–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantaphylidès C, Krischke M, Hoeberichts FA, Ksas B, Gresser G, Havaux M, Van Breusegem F, Mueller MJ. (2008) Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol 148: 960–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenabeele S, Van Der Kelen K, Dat J, Gadjev I, Boonefaes T, Morsa S, Rottiers P, Slooten L, Van Montagu M, Zabeau M, et al. (2003) A comprehensive analysis of hydrogen peroxide-induced gene expression in tobacco. Proc Natl Acad Sci USA 100: 16113–16118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Barnaby JY, Tada Y, Li H, Tör M, Caldelari D, Lee D-u, Fu X-D, Dong X. (2011) Timing of plant immune responses by a central circadian regulator. Nature 470: 110–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendehenne D, Binet M-N, Blein J-P, Ricci P, Pugin A. (1995) Evidence for specific, high-affinity binding sites for a proteinaceous elicitor in tobacco plasma membrane. FEBS Lett 374: 203–207 [DOI] [PubMed] [Google Scholar]
- Winge P, Brembu T, Bones AM. (1997) Cloning and characterization of rac-like cDNAs from Arabidopsis thaliana. Plant Mol Biol 35: 483–495 [DOI] [PubMed] [Google Scholar]
- Yin S, Mei L, Newman J, Back K, Chappell J. (1997) Regulation of sesquiterpene cyclase gene expression: characterization of an elicitor- and pathogen-inducible promoter. Plant Physiol 115: 437–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoda H, Hiroi Y, Sano H. (2006) Polyamine oxidase is one of the key elements for oxidative burst to induce programmed cell death in tobacco cultured cells. Plant Physiol 142: 193–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeier J, Pink B, Mueller MJ, Berger S. (2004) Light conditions influence specific defence responses in incompatible plant-pathogen interactions: uncoupling systemic resistance from salicylic acid and PR-1 accumulation. Planta 219: 673–683 [DOI] [PubMed] [Google Scholar]