Metabolite profiling and quantitative genetics analyses uncover a strawberry peroxidase gene as an important factor controlling the flux to soluble (flavonoids) and insoluble (lignin) polyphenols in fruits.
Abstract
Plant phenolics have drawn increasing attention due to their potential nutritional benefits. Although the basic reactions of the phenolics biosynthetic pathways in plants have been intensively analyzed, the regulation of their accumulation and flux through the pathway is not that well established. The aim of this study was to use a strawberry (Fragaria × ananassa) microarray to investigate gene expression patterns associated with the accumulation of phenylpropanoids, flavonoids, and anthocyanins in strawberry fruit. An examination of the transcriptome, coupled with metabolite profiling data from different commercial varieties, was undertaken to identify genes whose expression correlated with altered phenolics composition. Seventeen comparative microarray analyses revealed 15 genes that were differentially (more than 200-fold) expressed in phenolics-rich versus phenolics-poor varieties. The results were validated by heterologous expression of the peroxidase FaPRX27 gene, which showed the highest altered expression level (more than 900-fold). The encoded protein was functionally characterized and is assumed to be involved in lignin formation during strawberry fruit ripening. Quantitative trait locus analysis indicated that the genomic region of FaPRX27 is associated with the fruit color trait. Down-regulation of the CHALCONE SYNTHASE gene and concomitant induction of FaPRX27 expression diverted the flux from anthocyanins to lignin. The results highlight the competition of the different phenolics pathways for their common precursors. The list of the 15 candidates provides new genes that are likely to impact polyphenol accumulation in strawberry fruit and could be used to develop molecular markers to select phenolics-rich germplasm.
Anthocyanins, flavonoids (flavan-3-ols and flavonols), and phenylpropanoids are among the major phenolics that accumulate in ripe strawberry (Fragaria × ananassa) fruit (Fig. 1; Aaby et al., 2007; Fait et al., 2008; Tulipani et al., 2008). Anthocyanins give rise to the red color of strawberry fruit, which attracts frugivores that help to disperse seeds (Willson and Whelan, 1990). Flavonols are thought to function as protective chemicals against UV-B light in fruit skin (Solovchenko and Schmitz-Eiberger, 2003), whereas proanthocyanidins such as condensed (epi)catechin and afzelechin dimers (flavan-3-ols) contribute to defense and stress resistance (Panjehkeh et al., 2010). The 1-O-acyl-Glc esters of cinnamic, 4-coumaric, and caffeic acids (phenylpropanoids) may serve as energy-rich substrates in plant secondary metabolism (Lunkenbein et al., 2006a; Vogt, 2010), and ellagic acid may play a role in protection from predation and in plant growth regulation (Vattem and Shetty, 2005).
Figure 1.
Scheme of the shikimate-phenylpropanoid-flavonoid-anthocyanidin-lignin pathway. Names (in boldface) and structures of metabolites quantified by LC-MS analysis are shown.
In plants, phenolics arise from the shikimate, phenylpropanoid, flavonoid, anthocyanin, and lignin pathways (Vogt, 2010). The shikimate pathway is required for the biosynthesis of the aromatic amino acids (Fig. 1). In most plants, the biosynthesis of phenolics starts with phenylpropanoid formation (e.g. coumaric acid) from the primary metabolite Phe. The phenylpropanoids can be further modified in many ways, including the conversion of monomeric phenylpropanoids to lignin (insoluble phenolics) and the elongation and cyclization of phenylpropanoids by the sequential addition of three molecules of malonyl-CoA to form the flavonoids and anthocyanidins. Some of the genes and enzymes involved in phenolics biosynthesis were discovered and characterized in the model plants Arabidopsis (Arabidopsis thaliana), maize (Zea mays), and petunia (Petunia hybrida) and have been analyzed recently in Fragaria spp. (Lunkenbein et al., 2006a, 2006b; Almeida et al., 2007; Griesser et al., 2008a, 2008b; Schwab et al., 2011). The basic biosynthetic pathway leading to anthocyanins has been almost completely elucidated (Ververidis et al., 2007). Considerable progress has also been made in understanding the regulation of this pathway, and transcription factors that control the expression of the structural genes have been characterized, but it is not yet known how this pathway is compartmentalized (Aharoni et al., 2001; Boudet, 2007; Allan et al., 2008; Gonzalez et al., 2008). Since phenolics are ubiquitous in all plant organs, they are an integral part of the human diet and contribute to the nutritional quality of foods.
Identification of the genetic determinants governing both fruit quality and agronomical traits is essential for the sustained improvement of crops (Capocasa et al., 2008). The strawberry fruit is highly appreciated for its tasty flavor and nutritional value. However, fruit quality attributes have been reduced or lost because breeding of modern cultivars has mainly focused on agronomical traits such as fruit size and yield. Thus, improvement of strawberry flavor, shelf life, and nutritional quality has become an important factor in current breeding programs, even when these quality attributes are controlled by a complex genetic background and are frequently associated with negative agronomic characters (Zorrilla-Fontanesi et al., 2011). The challenge for breeders, who want to produce berry fruit with high nutritional value while maintaining an outstanding fruit quality, is not only the knowledge of the single trait but also what is affecting the variation and how different traits are correlated together. Since many different fruit traits have been successfully modified with breeding strategies, breeding to increase one or more beneficial phytochemicals in fruit is likely to be achievable (Aharoni et al., 2004).
The 600 strawberry varieties found today stem from five or six original wild species and are members of the Rosaceae family (Hancock, 1999). The common cultivated strawberry has an octoploid genome (2n = 8x = 56) that resulted from an accidental hybridization between two related species, the scarlet strawberry (Fragaria virginiana) that originated in North America and the South American domesticated Fragaria chiloensis (Horvath et al., 2011). The origin of strawberry and the early breeding practices reduced the initial genetic variation. During the 200 years of strawberry breeding, the initial diversity increased due to the introgression of wild strawberry germplasm or using unrelated progenitors, but these introgressions did not compensate for the loss of diversity observed in modern strawberry cultivars (Gil-Ariza et al., 2009). Although genetic variation is low, the total phenolics content and composition of phenolic compounds strongly differ between strawberry genotypes (Rekika et al., 2005; Hernanz et al., 2007; Tulipani et al., 2008; Pincemail et al., 2012). Thus, cultivated strawberry varieties appear to be a good experimental model to elucidate new factors regulating flux through the phenolics pathway, especially because the genome sequence of Fragaria vesca (2n = 2x = 14), one of the progenitors of strawberry, can be used as a genomic reference for the genus (Shulaev et al., 2011). Besides, the first microarray analysis of plant tissue was successfully performed on RNA isolated from fruits of the cultivated strawberry cv Elsanta, indicating that polyploidy is not a hindrance for this type of analysis (Aharoni et al., 2000).
In this study, an examination of the transcriptome, coupled with metabolite profiling analysis of different strawberry genotypes, was undertaken to reveal genes whose expression levels correlated with altered phenolics composition. To exploit the impact of strawberry genetic diversity on phenolics, 16 strawberry varieties were used as a source of defined natural variation and as a resource for the identification of candidate regulatory genes. The validity of the approach was strengthened by the functional characterization of a peroxidase gene that showed the highest transcript level difference in phenolics-rich versus phenolics-poor genotypes. In addition, the role of the peroxidase gene in color and total polyphenols production was suggested by the identification of quantitative trait loci (QTLs) linked to color in the region where the peroxidase gene is located and in two segregation populations. Analysis of transgenic plants confirmed the metabolic interaction between anthocyanin and lignin biosynthesis. Our results expand the knowledge of mechanisms associated with lignin and phenolics biosynthesis and facilitate the development of novel strawberry cultivars with nutritionally superior properties.
RESULTS
Metabolite Profiling Analysis
The metabolite profiles of fully ripe fruits of 16 phenotypically different strawberry varieties (Fig. 2) were evaluated for their variation in phenolic compounds by liquid chromatography (LC)-mass spectrometry (MS; Fig. 1). The identity of the metabolites was confirmed by reference to authentic metabolites run under identical conditions and to literature data (Fossen et al., 2004; Lunkenbein et al., 2006a, 2006b; Griesser et al., 2008a, 2008b; Hanhineva et al., 2008). The major known phenolic metabolites were quantified in the positive and negative MS mode by the internal standard method and were expressed as per mil equivalent of dry weight, assuming a response factor of 1. This is a good method of obtaining robust relative quantification rapidly (Fig. 3). Metabolites, whose structures could not be unambiguously elucidated, were not taken into account. The heat map (Fig. 3A), which displays the relative levels of 16 phenolic metabolites of the varieties, showed that each variety accumulated a unique concentration pattern of metabolites (Fig. 2). The maximum and minimum levels of these metabolites in the studied genotypes are shown in parentheses (Fig. 3A). Some varieties, such as 49, accumulated only relatively low levels of various soluble phenolics in the fruits, whereas 3 contained relatively high amounts of many metabolites, as can be seen by the number of blue and red squares, respectively. On the other hand, some metabolites, such as pelargonidin glucoside, occurred in high levels in numerous varieties, whereas its malonylated derivative was only observed in a few genotypes, such as 42. The concentration values of the individual compounds were used to calculate the cumulative levels of phenylpropanoids, including ellagic acid, flavonoids, anthocyanins, and total phenolics (Fig. 3B). Varieties 5 and 3 were superior genotypes with regard to the total phenolics content due to their high amounts of anthocyanins and phenylpropanoids. In contrast, genotypes 49 and 21 produced only low levels of soluble phenolics.
Figure 2.
Fruit phenotypes of strawberry varieties that were analyzed in the study and their identifier codes (ID). Some varieties are shown twice to visualize the variations in size, shape, and color and to show cross sections of the fruits.
Figure 3.
Quantification of strawberry fruit phenolics and calculation of phenylpropanoid, flavonoid, anthocyanin, and total phenolics levels. Quantification of metabolites was performed by LC-MS analysis as per mil equivalents of the dry weight with the help of the internal standard biochanin A (relative concentration). A, Heat map presentation of the metabolite levels in different varieties. Varieties with the highest and lowest levels of individual metabolites are shown in red and blue, respectively. Minimum and maximum levels of individual metabolites are shown in parentheses. B, Deduced levels (relative concentration as per mil equivalent of the internal standard) of phenylpropanoids, flavonoids, anthocyanins, and total phenolics with sd of five biological replicates.
Microarray Analysis
To couple the metabolite profiling analysis with an overall examination of the transcriptome, we next performed microarray analyses using selected strawberry genotypes. In total, 17 unique comparative microarray analyses were conducted on RNA isolated from receptacles of 12 different varieties that strongly differed in the level of one of 20 soluble phenolic metabolites, certain phenolic subgroups, and total phenolics, as determined by the metabolite profiling analysis (pairs of genotypes and the strategy of the analysis are detailed in Figure 3A and Supplemental Table S1). Oligonucleotide-based microarrays were manufactured from a Fasta archive containing 18,152 uniESTs from a collection of strawberry sequences (Bombarely et al., 2010). The 17 pairwise comparisons of transcript levels between genotypes with contrasting metabolites revealed, in total, 26,416 differentially expressed ESTs (more than 4-fold, P < 0.01; Fig. 4A). Most of the mRNA expression levels differed by less than 50-fold, but some transcripts exceeded 200-fold and reached up to 925-fold expression ratios. The latter were thus selected as top candidate genes (Fig. 4B). For the most promising transcripts, protein-coding genes corresponding to the ESTs could be deduced from the genome sequence of F. vesca (Table I; Shulaev et al., 2011). The F. vesca database did not contain protein-coding genes for five expressed mRNAs (FRA1441, FRA192B, FRA2718, FRA2456, and FRA3519). But FRA1441 clearly mapped on scaffold 0513150 (http://www.rosaceae.org/gb/gbrowse/fragaria_vesca_v1.0/), whereas the other sequences yielded ambiguous results and were not further considered. Besides the high differential expression levels of the candidates, they also appeared as differentially expressed (more than 4-fold) in most of the 17 comparative EST expression analyses, as indicated by the number of circles (Fig. 4B). Due to EST redundancy, gene 19544, which has been annotated as a putative heme-containing PRX27 gene, showed up five times among the most highly differentially expressed genes, whereas gene 10776 and gene 19724 appeared two times. This confirms the good reproducibility of the microarray platform. The role of gene 19544 was analyzed in detail, as its expression might affect the quality and quantity of phenolics.
Figure 4.
Selection of top candidate genes. A, Total ESTs that showed differential expression (more than 4-fold, P < 0.01) in 17 microarray analyses. Microarray analyses were performed on different strawberry varieties that accumulated opposite levels of phenolics, as determined by metabolite profiling analysis (Fig. 3). B, ESTs showing the highest levels of differential expression. Protein-coding genes corresponding to the ESTs were deduced from the genome sequence of F. vesca (Shulaev et al., 2011). Gene 19544 (boldface) appeared five times as highly differentially expressed due to EST redundancy.
Table I. Candidate genes putatively correlated with phenolics accumulation in strawberry fruit.
| Gene No. | Putative Annotation |
|---|---|
| Gene 19544 | Peroxidase27 like |
| Gene 23392 | Cucumisin like |
| FRA1441 | Maps on scf0513150 |
| FRA192B | No homology |
| FRA2718 | No homology |
| FRA2456 | No homology |
| Gene 21343 | Expansin A8 like |
| Gene 23054 | Hothead like |
| Gene 35152 | Spidroin1 like |
| Gene 10776 | SRG1 like |
| Gene 03515 | Tasselseed2 like |
| FRA3519 | No homology |
| Gene 27098 | GDSL esterase/lipase like |
| Gene 19724 | Heat shock protein like |
| Gene 22502 | Acyl-CoA-binding domain-containing protein |
| Gene 33865 | Ephrin A1 (LERK-1) like |
| Gene 30399 | Homeobox-Leu zipper ATHB15 like |
| Gene 03472 | Ubiquitin C-terminal hydrolase12 like |
| Gene 00897 | Defensin like |
FaPRX27 Is Expressed in Root and Red Fruit
Gene 19544 of the F. vesca genome encodes a putative FaPRX27 peroxidase. Peroxidases catalyze the reduction of hydrogen peroxide (H2O2) by taking electrons from various donor molecules. Class III plant peroxidases oxidize donors such as phenolics, lignin precursors, or secondary metabolites. The F. vesca genome harbors 84 genes encoding putative heme-dependent peroxidases (http://supfam.org/SUPERFAMILY/). It is assumed that peroxidases are implicated in different physiological processes, such as lignification, suberization, cross linking of cell wall proteins, auxin catabolism, salt tolerance, defense against pathogen attack, and oxidative stress (Valério et al., 2004; Mathé et al., 2010; Lüthje et al., 2011). Therefore, it is important to determine the expression pattern of PRX27 to understand the physiological roles and characteristics. FaPRX27 gene expression was analyzed by quantitative PCR in vegetative tissues, flowers, and fruits of different developmental stages of cv Elsanta strawberry plants (Fig. 5). Significant mRNA levels were only detected in roots and red, ripe fruits, consistent with the results of the microarray analyses. FaPRX27 transcript levels were negligible in other tissues.
Figure 5.
Relative FaPRX27 gene expression determined by quantitative PCR in fruit of different developmental stages, vegetative tissues, and flower of strawberry cv Elsanta. SG, Small green fruit; GW, green/white fruit; W, white fruit; T, turning fruit; R, red ripe fruit. An interspacer gene was used as an internal control for normalization, and white fruit was set at 1 as a reference (means ± se of five to six replicates with two sets of cDNAs). Relative changes are shown.
FaPRX27 Might Be Involved in Lignin Biosynthesis
The five ESTs contained on the microarray covered major regions of gene 19544, but alignments with known peroxidase sequences as well as BLAST searches in the National Center for Biotechnology Information (NCBI) and EMBL yielded a different intron-exon structure, as proposed by the F. vesca genome database (Shulaev et al., 2011). Detailed analysis showed that gene 19544 presumably consists of two different open reading frames erroneously fused due to a misannotation of an intron. The intron removed a stop codon, eventually attaching the peroxidase sequence to a second gene that codes for a Hyp-rich glycoprotein (Supplemental Figs. S1–S3). The 3′ and 5′ untranslated regions contained in the ESTs confirmed the assumption. Primers were designed that amplified a 990-bp nucleotide sequence (FaPRX27) corresponding to a 35.1-kD protein consisting of 329 amino acids with a calculated pI of 8.47 (Fig. 6; Supplemental Fig. S2). Eukaryotic linear motif search predicted a signal peptide for the secretory pathway, spanning from amino acids 1 to 26, and a transmembrane region from amino acids 7 to 29 (http://elm.eu.org/search/; Dinkel et al., 2012). The full-length complementary DNA (cDNA) of FaPRX27 was cloned by SmaI and XhoI substitution into the pGEX vector and eventually introduced into Escherichia coli Rosetta(DE3)pLysS. Cell extracts after induction of the recombinant protein production contained an additional 61-kD fusion protein (FaPRX27-GST [for glutathione S-transferase]), as shown by SDS-PAGE analysis (Supplemental Fig. S4). However, most induced protein was found as insoluble, suggesting that the GST-tagged FaPRX27 was mainly present in inclusion bodies. The expression of FaPRX27 without the GST tag was not tested.
Figure 6.
Protein sequence alignment of class III peroxidases. FaPRX27, Arabidopsis AtPRX27 (gi|15232058|ref|NP_186768.1), potato (Solanum tuberosum) SlTPX1 (gi|678547|gb|AAA65637.1), and horseradish (Armoracia rusticana) ArPrxC (gi|14603|emb|CAA00083.1) are shown. The alignment was performed by ClustalX with Geneious (http://www.geneious.com/web/geneious/).
Soluble protein fractions were used in enzyme assays with ferulic acid, caffeic acid, and coniferyl alcohol in the presence of H2O2, since the involvement of FaPRX27 in lignin biosynthesis was assumed due to the observed interference with the anthocyanin/flavonoid pathway. After addition of the substrates, a clear and rapid decrease of absorption was observed, indicating the consumption of the phenylpropanoids (Fig. 7, A–C). Besides, the solutions turned yellow only when FaPRX27 was added. A guaiacol-containing solution quickly turned purple when FaPRX27 was added but remained colorless when an empty vector control extract was used (Fig. 7D). The enzymatic activity was 0.02 µmol min−1 mg−1 total proteins with guaiacol as substrate (Fig. 7E). Absorption did not change when cinnamic acid, 4-coumaric acid, vanillin, cinnamaldehyde, 4-hydroxybenzaldehyde, and 3,4-dihydroxybenzaldehyde served as substrates. It is assumed that in these cases, self-absorption of the protein extract and similar absorption coefficients of substrates and products interfere with the photometric determination of activity. Therefore, enzymatic assays were additionally subjected to LC-MS analysis to detect potential products (Supplemental Figs S5–S14). The starting material was almost completely consumed (less than 5% of the starting material remained unreacted) when ferulic acid and coniferyl alcohol were incubated with FaPRX27 (Fig. 8A; Supplemental Fig. S11). Caffeic acid, guaiacol, vanillin, and 3,4-dihydroxybenzaldehyde were also transformed, albeit the bulk of the substrates remained untouched (Supplemental Figs. S7, S9, S10, and S14). Although exact identification of the enzymatic products was not achieved, the interpretation of their mass spectra allowed the conclusion that dimeric structures were formed (Fig. 8). Compound 1 (386 g mol−1; Fig. 8) appears to be a dehydrodimer of ferulic acid, whereas structures 2, 3, and 5 (342 g mol−1) are probably formed by decarboxylation of a dehydrodimer precursor (Ward et al., 2001). These data point to a function of FaPRX27 in lignin biosynthesis, as dehydrodimers are the major products of the initial steps of ferulic acid polymerization by lignin peroxidase (Ward et al., 2001). Finally, total lignin content was determined in fruits of genotypes that accumulated extremely high and low levels of total phenolics (Fig. 9B). Varieties 3 and 5 contained significantly lower levels of the cell wall polymer than 21 and 25 (Fig. 9A), consistent with the hypothesis that FaPRX27 produced lignin at the expense of soluble phenolic compounds. The results clearly support the hypothesis that FaPRX27 codes for a functional peroxidase putatively involved in the lignification in root and strawberry fruit.
Figure 7.
Functional characterization of the FaPRX27 protein. A to C, Extracts from E. coli strain Rosetta(DE3)pLysS expressing FaPRX27 were incubated with 140 mm H2O2 and 18 mm ferulic acid (A), caffeic acid (B), and coniferyl alcohol (C), while the absorbance was monitored at 310, 285, and 262 nm, respectively. Decreasing values indicate consumption of the substrates. E. coli extracts expressing an empty vector served as a control. D, The reaction mixture with FaPRX27 and the substrate guaiacol quickly turned purple, but it remained colorless when the empty vector control was added. E, FaPRX27 activity was determined photometrically by measuring the increase in A470 due to oxidation products of guaiacol (see “Materials and Methods”). F, Consumption of substrates by FaPRX27 was also quantified by LC-MS. Names and chemical structures of screened substrates and percentage of converted substrate (in parentheses) after 5 min of incubation are shown.
Figure 8.
LC-MS analysis of products formed by FaPRX27 from ferulic acid. A, The UV trace at 280 nm shows the formation of novel compounds (1–7) by FaPRX27. B and C, E. coli extracts expressing an empty vector (B) and assays without the addition of enzyme (C) served as controls. mAU, Milliabsorbance units. D, Mass spectra (-MS) and product ion spectra (-tandem MS of the indicated ion) of products 1 to 7 as shown in A.
Figure 9.
A, Lignin content of strawberry genotypes with high levels (black bars) and low levels (white bars) of soluble phenolics. B, Content of total soluble phenolics. C, Relative expression levels of FaPRX27 in different genotypes calculated from transcript levels determined by microarray analysis. FaPRX27 levels in genotypes 3 and 5 did not differ and were set as 1. Transcript levels in 21 and 25 were calculated from microarray data obtained from the comparisons of genotypes 5/21, 3/25, 5/25, and 21/25. D, Simplified scheme to show the proposed effect of FaPRX27 expression on the total amount of soluble phenolics.
A QTL Linked to Color Is Detected in the Region of FaPRX27
To further investigate the potential role of the FaPRX27 gene for the accumulation of phenolic compounds and agronomic traits such as color, we developed a QTL approach using two segregating populations of the cultivated strawberry. FaPRX27 is localized in the upper region of the linkage group 3 (LG3) chromosome of F. vesca (Shulaev et al., 2011). Gene information from the diploid genome was used on the cultivated strawberry due to synteny and colinearity between diploid and octoploid Fragaria spp. genomes (Rousseau-Gueutin et al., 2008). Consequently, we considered the four linkage groups, arbitrary named LG3a, LG3b, LG3c, and LG3d, holding the FaPRX27 gene. These four linkage groups belong to the homology group 3, which is homologous to LG3 of the diploid F. vesca. For mapping the gene in the two segregating populations, we developed polymorphic markers using simple sequence repeat (SSR) sequences localized close to the peroxidase gene. These markers were SSRCL317CGfB2 and SSR-PER for the Spanish F2 population Dover × Camarosa and the French F1 population Capitola × CF1116, respectively. The detected polymorphisms were mapped in populations, and markers were localized at the top of one of the four homologous linkage groups of the populations, LG3-D and LG3-A for the Spanish and French strawberry octoploid map, respectively (Rousseau-Gueutin et al., 2008).
QTLs were detected for all quantitative traits using composite interval mapping for either Capitola or CF1116 for a French strawberry population and Camarosa or Dover for a Spanish population. Subsequently, we considered only QTLs significantly superior to the log of the odds threshold (3.0 and 3.1 for the Spanish and French segregating populations, respectively) that were colocalized with FaPRX27 (gene 19544) and therefore with the SSR markers developed near the peroxidase gene (Table II). In the French segregating population, in the region of the peroxidase gene, we identified QTLs linked to total polyphenol and total flavonoids on the male map. In the Spanish population, the peroxidase gene 19544 was colocalized with QTLs for epiafzelechin-pelargonidin glucoside and total flavonoids. In both populations, in the region of the peroxidase gene, we identified a QTL linked to visual fruit color decrease (Fig. 10).
Table II. MapQTL summary for color 2009 in LG3-D of the Spanish segregating population.
| Map | LOD | Percentage Expl | Add | Dom | Locus |
|---|---|---|---|---|---|
| 0 | 5.5 | 13.4 | −0.27 | 0.22 | CL317CGfB2 |
| 7.8 | 0.2 | 7.3 | −0.16 | −0.08 | g676CGdD1 |
| 26.7 | 3.8 | 20.8 | −0.11 | −0.32 | g235eD |
| 36.7 | 3.9 | 19.3 | −0.01 | −0.39 | BFACT43A3+ |
| 53.3 | 1.9 | 11.6 | −0.28 | 0.14 | CFACT74B2 |
| 59.8 | 1.9 | 12.7 | −0.49 | 0.01 | CFACT37CD3+ |
| 74.1 | 18.0 | 75.5 | −0.46 | 0.54 | CFACT42B |
Add, Additive effects; Dom, dominance effects; Expl, explained.
Figure 10.
QTL analyses of a Spanish and a French segregating population of strawberry. A, Linkage group 3 (LG3-D) of a Spanish population map and color QTL detection. B, Male LG3-A of a French population and QTL linked to color, total flavonoids, and total polyphenols. Markers developed to localize FaPRX27 are boxed. cM, Centimorgan; LOD, log of the odds.
Competition of the Anthocyanin/Flavonoid and Lignin Pathways for Common Precursors
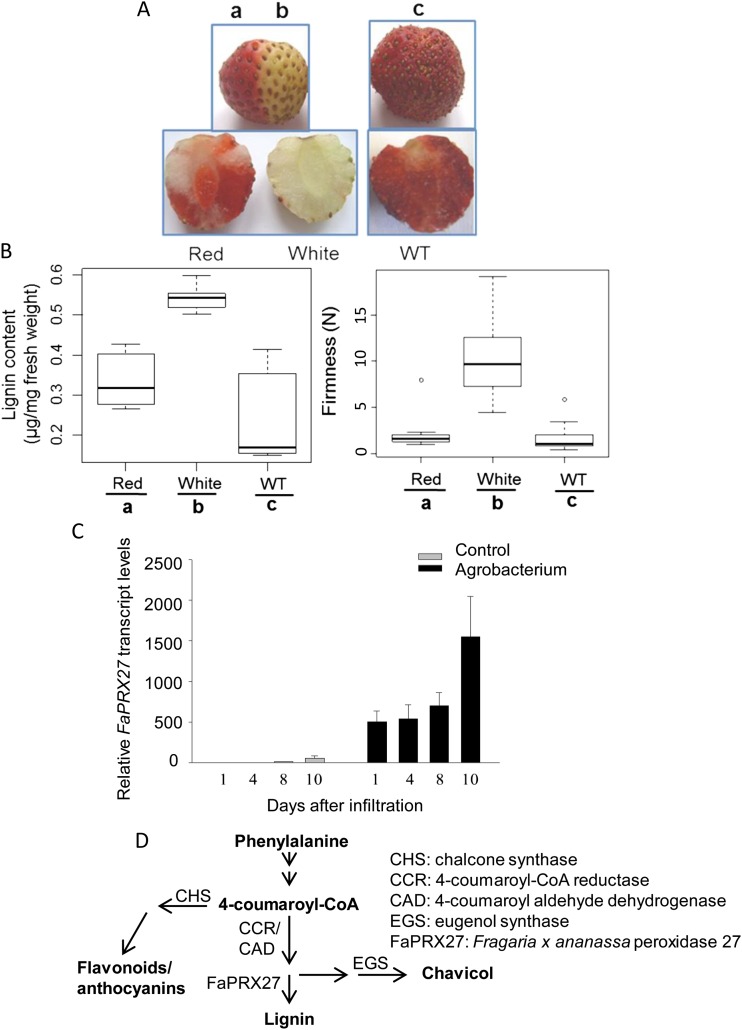
Recently, we have shown that stably transformed antisense-silenced CHALCONE SYNTHASE (CHS) strawberry plants produced significantly reduced levels of anthocyanins in the fruits when compared with their wild-type counterparts but instead accumulated 4-coumaroyl-CoA-derived metabolites such as 4-coumaryl alcohol and 4-coumaryl acetate (Lunkenbein et al., 2006b). These metabolites are precursors of aroma chemicals such as chavicol, the reaction being catalyzed by EUGENOL SYNTHASE (Fig. 11D; Hoffmann et al., 2011). Transient RNA interference (RNAi)-mediated down-regulation of CHS and simultaneous overexpression of EUGENOL SYNTHASE efficiently redirected the anthocyanin/flavonoid pathway to the phenolic volatiles (Hoffmann et al., 2006, 2011). To confirm the competition of the anthocyanin/flavonoid biosynthesis and lignin pathways for common substrates, we down-regulated CHS expression during strawberry fruit ripening by agroinfiltration of an inverted hairpin RNAi construct and analyzed the effect on lignin content. One-half of a fruit was agroinfiltrated, whereas the other half remained untreated (Fig. 11A). Silencing of CHS, as evidenced by the loss of pigmentation, is accompanied by a significant increase in lignin content, which correlates with enhanced firmness of the fruits (Fig. 11B). Transcript analyses affirmed the reduced levels of CHS transcripts due to RNAi-mediated gene silencing (Hoffmann et al., 2006) and revealed the strong induction of FaPRX27 expression upon agroinfiltration (Fig. 11C), whereas CINNAMOYL COENZYME A REDUCTASE (CCR) and CINNAMYL ALCOHOL DEHYDROGENASE (CAD) expression remained unaffected (data not shown). However, fruits of stable CHS-deficient transgenic strawberry lines showed wild-type lignin levels and FaPRX27 expression levels (Lunkenbein et al., 2006b). RNAi-mediated down-regulation of FaPRX27 was unsuccessful, probably because the massive induction of FaPRX27 transcription preceded RNAi. It can be concluded that reduced flux through the anthocyanin/flavonoid pathway along with increased FaPRX27 transcript levels significantly increased lignin content and associated firmness at the expense of anthocyanins in strawberry fruit (Fig. 11D).
Figure 11.
Competition of the anthocyanin and lignin pathways for common substrates in fruits of cv Elsanta. A, Phenotypes of a fruit whose left half remained untreated (a; red) whereas the right half was injected with A. tumefaciens containing an FaCHS-inverted hairpin RNA construct (b; white) and of an untreated wild-type control fruit (WT; c) 14 d after injection. B, Lignin content and fruit firmness were measured 14 d after infiltration. The box plots show data from different groups with the following biological replicates: firmness of red (n = 8), white (n = 8), and wild-type (n = 10) fruit and lignin content of each group (n = 6). N, Newton. C, Relative expression of FaPRX27 in response to agroinfiltration. Fruits were infiltrated with A. tumefaciens cells in the turning ripening stage and harvested 1, 4, 8, and 10 d after injection. Wild-type (untreated) fruits were used as controls. Expression levels in control (gray columns) and agroinfiltrated fruits (black columns) were monitored by quantitative real time-PCR using specific primers for FaPRX27 and the interspacer gene. The latter was used as an internal control for normalization. The control fruit (1 d) was used as the reference (set to 1). Values are means ± se of six replicates from two independent fruits and are shown as relative changes. D, Phenolics pathway showing the relationship of flavonoids/anthocyanins, lignin, and chavicol formation in strawberry fruit.
DISCUSSION
Metabolite Profiling Analysis
As evidence accumulates about the positive health benefits of consuming a diet rich in phenolics, so does our requirement for understanding the accumulation of these compounds in staple foods. To correlate gene expression profiles with phenolic compound accumulation in strawberry fruit, fruit phenolics were isolated from diverse genotypes and major structurally identified compounds were quantified by LC-MS (Fig. 3). Total phenolics content in the studied varieties showed a variation of a factor of less than 2, whereas the ranges of the levels of phenolic subgroups and in particular of individual compounds (shown in parentheses in Fig. 3A) were much larger. For example, pelargonidin glucoside malonate was detected in one genotype in a concentration of 10 mg g−1 equivalent of the dry weight but fell under the limit of detection (0.05 mg g−1 equivalent dry weight) in most of the other varieties. Therefore, it seems that manipulation of the concentration of single compounds will be easier to achieve for breeders than alterations in the total amount of certain groups of secondary metabolites. There is probably more flexibility for the plant to divert biosynthetic pathways than to increase the total biomass. Shifting biomass from lignin biosynthesis to polysaccharide production has been achieved in aspen (Populus tremuloides). Down-regulation of 4-coumarate-CoA ligase resulted in a 45% decrease in lignin content and a concomitant 15% increase in cellulose content (Hu et al., 1999). These figures were further increased to a 52% reduction in lignin content and a 30% increase in cellulose content when coniferaldehyde 5-hydroxylase was also down-regulated (Li et al., 2003). Down-regulation of CCR in transgenic tobacco (Nicotiana tabacum) resulted in a decrease in lignin content and a concomitant increase in Xyl and Glc associated with the cell wall (Chabannes et al., 2001). Similarly, the redirection of different plant carbon resources to the production of soluble phenolics in fruit should be achievable (Griesser et al., 2008a). However, it is crucial that pathway manipulations do not interfere with fruit quality characteristics such as taste and firmness as well as defense against pathogens and insects.
Microarray Analysis
Combining metabolite profiling data with gene expression analyses has provided unique and comprehensive overviews of the transcriptional regulation of metabolic shifts, responses to plant hormones, as well as adaption of stress tolerance mechanisms (Yonekura-Sakakibara et al., 2008; Janz et al., 2010; Kogel et al., 2010; Stushnoff et al., 2010; Rohrmann et al., 2011; Zifkin et al., 2012). In this study, a custom-made oligonucleotide-based microarray platform (Roche-NimbleGen) was employed for the comprehensive investigation of differential gene expression in strawberry varieties that were selected by metabolite profiling analysis due to their opposite levels of phenolics (Fig. 3; Supplemental Table S1). Seventeen unique comparative microarray analyses yielded 25 cDNAs that exceeded the 200-fold expression ratio, representing 19 genes. The first microarray analysis performed with cDNA from strawberry fruit dates from the year 2000 and led to the identification of a gene involved in fruit flavor formation (Aharoni et al., 2000). Later, differential gene expression by microarray was determined in strawberry achene versus receptacle tissue and in fruit compromised by oxidative stress (Aharoni and O’Connell, 2002; Aharoni et al., 2002). In these studies, similar high changes in expression levels (up to 300-fold) were observed for genes in different tissues of the same genotype. Detailed inspection of the most highly differentially expressed ESTs in our study and alignment with the F. vesca genome revealed redundancy of the ESTs represented on the array but confirmed the reliability of the analysis. The top candidates (Supplemental Fig. S15) appeared in a number of comparative analyses as highly differentially expressed genes, which demonstrates their importance in phenolics metabolism. Gene 19544, annotated as putative FaPRX27, was remarkable, as it showed up five times among the top candidates and displayed the highest difference in expression level, but it has not been described in previous studies. Thus, FaPRX27 was investigated further, since peroxidases are assumed to function in lignin biosynthesis, which is connected with the pathway of soluble phenolics via coumaroyl-CoA (Vassão et al., 2008).
FaPRX27
Peroxidases (EC 1.11.1.X) are oxidoreductases that catalyze the oxidation of various substrates by reducing H2O2 to water. Phenolic compounds (e.g. lignin precursors and secondary metabolites) can serve as electron donor molecules. The subcategory of heme peroxidases carry a protoporphyrin IX complexed with Fe(III) (the ferric form in the ground state) in their active sites and share a very similar three-dimensional structure (Mathé et al., 2010). Recently, a separate, additional hydroxylic cycle of peroxidases, which leads to the formation of various reactive oxygen species, has been proposed (Cosio and Dunand, 2009).
Land plants contain a large number of class III peroxidases (EC 1.11.1.7). The Arabidopsis, rice (Oryza sativa), and F. vesca genomes harbor 73, 138, and 84 genes encoding peroxidases, respectively (Passardi et al., 2004; Valério et al., 2004; http://supfam.org/SUPERFAMILY/). The large number of PRX genes in rice resulted from various duplication events that were tentatively traced back (Passardi et al., 2004). Comparison with the predicted number of heme-containing peroxidase genes in sequenced plant genomes showed that F. vesca and Arabidopsis contain the lowest number of PRX genes (http://supfam.org/SUPERFAMILY/). As a consequence of the large number of genes and the two possible catalytic cycles, it is assumed that class III plant peroxidases are involved in a broad range of physiological processes like cell elongation, cell wall differentiation, or defense. They function in cell wall metabolism (Marjamaa et al., 2009), wound healing, auxin catabolism, removal of H2O2, oxidation of toxic reductants, defense against pathogen and insect attack, as well as symbiosis and normal cell growth (Cosio and Dunand, 2009; Lüthje et al., 2011). Their roles in the modification of cell wall structures may include suberin polymerization, cross linking of the structural nonenzymatic proteins such as expansins, catalyzing the formation of diferulic acid linkages between polysaccharide-bound lignin or ferulic acid residues in polysaccharides, and the production of hydroxyl radicals with the ability to cleave cell wall polymers (Marjamaa et al., 2009). Few peroxidase genes exhibit an organ-dependent expression, while others are active in the whole plant or expressed in lignifying tissue (Valério et al., 2004). FaPRX27 transcripts were exclusively found in root and fruit, consistent with the expression pattern of its closest homolog from the Arabidopsis genome, AtPRX27 (Valério et al., 2004; Cosio and Dunand, 2009).
Lignification is one of the functions classically attributed to class III peroxidases (Quiroga et al., 2000; Marjamaa et al., 2009). It is a cell wall-fortifying process that occurs in xylem tissue in a scheduled manner during tissue differentiation. A number of studies have implicated different peroxidase genes from Arabidopsis (AtPRX13, AtPRX17, AtPRX30, AtPRX42, AtPRX53, AtPRX55, AtPRX64, AtPRX66, and AtPRX71) in the lignification of specific tissues, but AtPRX27 is not among them (Cosio and Dunand, 2009). Besides, the participation of class III plant PRXs in lignin formation has been studied in many other plant species and cell culture systems, most importantly tobacco, Zinnia elegans, tomato (Solanum lycopersicum), and both gymnosperm and angiosperm tree species (Marjamaa et al., 2009). Most of these studies are based on finding PRX enzymes with suitable catalyzing properties and localization of proteins or gene expression in lignifying xylem. However, there are only a few examples of transgenic plants or mutant lines with modified PRX expression and, consequently, altered lignification patterns probably due to the large number and possible functional redundancy of PRX enzymes (Mansouri et al., 1999).
FaPRX27 represents a classical class III plant peroxidase (Veitch, 2004). The basic protein (calculated pI of 8.47) harbors a predicted signal peptide for the secretory pathway (amino acids 1–26), a predicted transmembrane region (amino acids 7–29), six predicted N-glycosylation sites (amino acids 82–87, 95–100, 151–156, 168–173, 209–214, and 216–221), and assumed phosphorylation sites (http://elm.eu.org/search/; Dinkel et al., 2012). Most of the putative membrane-bound PRXs were predicted as secretory proteins (Lüthje et al., 2011). Besides cleavable N-terminal signal peptides, transmembrane domains have been calculated by several prediction programs for a number of class III peroxidases. Glycans could stabilize the proteins and affect substrate access in peroxidases because of their large size (Mathé et al., 2010). Key features of the central active site that have been identified in the horseradish peroxidase (ArPrxC) three-dimensional structure are completely conserved in the amino acid sequence of FaPRX27, namely Arg-65, Phe-68, His-69, Asn-96, His-194, and Pro-164, that accepts a hydrogen bond from reducing substrates and determines peroxidase substrate specificity (Fig. 5; Veitch, 2004). The proximal (Thr-195 and Asp-247) and distal (Asp-70, Gly-75, Asp-77, and Ser-79) Ca2+-binding sites are also maintained. Residues 70 to 97 are important for activity in plant peroxidases, as Asn-96 in this loop is hydrogen bonded to the active-site distal His-69, thereby orienting the hydrogen-bonding network in the distal cavity and regulating the acid dissociation constant of this amino acid. A conserved Glu-93 participates in the same hydrogen-bonding network, which also involves the distal Ca2+ (Cosio and Dunand, 2009). Among the nine Cys residues found in FaPRX27, eight occur in conserved positions consistent with four potential disulfide bridges (Mathé et al., 2010).
Although there are large variations of intron number and length among PRX class III paralogs and orthologs, intron positions and phases are remarkably conserved (Mathé et al., 2010). This suggests that intron loss, or marginally intron gain, probably occurred during the expansion of the gene family in some plant lineages. The coding sequence of FaPRX27 is disrupted by three introns at perfectly conserved positions according to the classical pattern of peroxidase genes (Supplemental Fig. S3).
Enzymatic assays confirmed the role of FaPRX27 in lignin biosynthesis, as precursors such as coniferyl alcohol, ferulic acid, and caffeic acid were transformed into dehydrodimers in the presence of H2O2. Ferulic acid dehydrodimers produced in vitro and in vivo by plant PRXs have been extensively characterized (Ward et al., 2001). Different regioisomers resulting from radical coupling are found in plant cell walls, where they cross link polymers, providing strength and rigidity. The units resulting from the monolignols 4-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol, when incorporated into the lignin polymer, are called H, G, and S units, respectively (Vanholme et al., 2010). Lignin extracted from strawberry fruit is mainly composed of G (65%–80%), S (8%–21%), and traces of H (1%–3%) units, as can be expected for angiosperm dicot lignin (Vanholme et al., 2010) and was confirmed by lignin extracted from fruits (Bunzel et al., 2005). Thus, the high proportion of guaiacol (G) groups in strawberry fruit lignin correlates well with the substrate specificity of FaPRX27.
Lignin formation in fruit may contribute to their firmness, as microarray analyses have shown that genes involved in the degradation of pectin and cellulose, two structure-defining and stabilizing polymer components of fruits, were not differentially expressed in three strawberry varieties producing fruits with firm (cv Holiday), medium (cv Elsanta), and soft (cv Gorella) structures. Interestingly, two genes encoding proteins catalyzing successive reactions in lignin metabolism (CCR and CAD) showed the highest differences in expression levels (Salentijn et al., 2003). These results were confirmed by a second group in other varieties (Carbone et al., 2006). Similarly, during postharvest ripening of loquat (Eriobotrya japonica) fruit, firmness increased and showed a positive correlation with the accumulation of lignin in the flesh and increased activities of CAD and PRX (Cai et al., 2006).
Furthermore, QTL analysis showed that FaPRX27 is linked to a region implicated in fruit color decrease. This experimental approach is functionally relevant and confirms FaPRX27 as a functional lignin peroxidase putatively involved in the polymerization of soluble phenolics in planta. The gene product competes with enzymes of the flavonoid and anthocyanin pathway for substrates (Fig. 9D) and thus determines the intensity of the red fruit color. Coumaroyl-CoA represents a common intermediate, as it can be converted to PRX substrates (ferulic acid and coniferyl alcohol) and serves as a precursor of flavonoids and anthocyanins. Our study showed that varieties expressing FaPRX27 to high levels accumulated low amounts of soluble phenolics but contained significantly higher concentrations of lignin than genotypes showing extremely low transcript levels. Similarly, overexpression of a basic peroxidase (SlTPX1) in tomato yielded a 40% to 220% increase of lignin content in the leaf in transgenic plants (Mansouri et al., 1999). In contrast, in Arabidopsis, silencing of hydroxycinnamoyl-CoA shikimate/quinate hydroxycinnamoyl transferase resulted in the repression of lignin synthesis and led to the redirection of the metabolic flux into flavonoids through chalcone synthase activity (Besseau et al., 2007). Hypolignified stems of Arabidopsis resulting from the concurrent down-regulation of CADc, CADd, and CCR1 accumulated higher levels of flavonol glycosides, which suggests a redirection of the phenolic pathway (Thévenin et al., 2011). The maize R2R3-MYB factor ZmMYB31 repressed lignin biosynthesis in transgenic Arabidopsis and redirected the phenylpropanoid carbon flux toward the biosynthesis of anthocyanins (Fornalé et al., 2010). Finally, we showed by RNAi-mediated down-regulation of CHS, which is accompanied by a significant increase of FaPRX27 transcript levels (more than 1,500-fold; Fig. 11C) due to agroinfiltration, that the accumulation of anthocyanins is considerably decreased (Fig. 11A) at the expense of lignin production (Fig. 11B). Overall, the results demonstrated that manipulation of the lignin biosynthesis pathway enzymes can yield strawberry fruit with increased levels of soluble phenolic compounds and thus provide potential health benefits.
CONCLUSION
Comparison of the transcript patterns of different strawberry genotypes combined with metabolite profiling analysis revealed novel candidate genes that might affect the accumulation of flavonoid and anthocyanin in strawberry fruit. Interestingly, neither structural genes nor transcription factors of the phenolics pathway appeared among the top 20 candidates. The majority of the novel genes have not been implicated in the secondary metabolism. A putative peroxidase gene was analyzed in detail to test the validity of the approach. FaPRX27 encodes a functional enzyme that is required for the polymerization of phenylpropanoids in ripening strawberry fruit and thus competing with flavonoid/anthocyanin pathway enzymes for common precursors. In this respect, FaPRX27 could be a trigger in balancing the flux toward soluble and insoluble (lignin) phenolic compounds. Future studies will show whether other candidates affect phenolics accumulation in a similar or different fashion.
MATERIALS AND METHODS
Chemicals
Except where otherwise stated, all chemicals, solvents, and reference compounds were obtained from Sigma-Aldrich, Fluka, Riedel de Haёn, Merck, or Roth.
Sample Extraction
Ripe strawberry (Fragaria × ananassa) fruits were harvested in 2009 from 16 selected cultivars (Fig. 2) cultivated in the south of Spain and in 2010 from two segregating populations, an F2 and a “pseudo test-cross F1,” from Spain and France, respectively. Fruits were individually frozen, lyophilized for 48 h, and homogenized with a mill (Retsch MM 200) to a fine powder. An aliquot of 50 mg of lyophilized fruit powder was used for each of the three biological replicates. Biochanin A (250 µL of a solution in methanol, 0.2 mg mL−1) was added as an internal standard, yielding 50 µg of internal standard in each sample. After the addition of 250 µL of methanol, vortexing, and sonication for 10 min, the sample was centrifuged at 16,000g for 10 min. The supernatant was removed, and the residue was reextracted with 500 µL of methanol. The supernatants were combined, concentrated to dryness in a vacuum concentrator, and redissolved in 35 µL of water. After 1 min of vortexing, 10 min of sonication, and 10 min of centrifugation at 16,000g, the clear supernatant was used for LC-MS analysis. Each extract was injected twice (technical replicate).
LC-Electrospray Ionization-Multiple Stage Mass Spectrometry Analysis
Samples were analyzed on an Agilent 1100 HPLC/UV system (Agilent Technologies) equipped with a reverse-phase column [Luna 3u C18(2) 100A, 150 × 2 mm; Phenomenex] and connected to a Bruker esquire3000plus ion-trap mass spectrometer (Bruker Daltonics). As mobile phase, 0.1% formic acid in water (A) and 0.1% formic acid in methanol (B) were used. Injection volume was 5 µL, and flow rate was 0.2 mL min−1. A gradient was applied that started at 0% B and went to 50% B in 30 min. Within the next 5 min, B was increased to 100%, where it was kept for 15 min. Afterward, B was decreased to 0% within 5 min. These initial conditions were kept for 10 min for system equilibration. UV signals were detected at 280 nm. MS spectra were recorded in alternating polarity mode. Nitrogen was used as nebulizer gas at 30 p.s.i. and as dry gas at 330°C and 9 L min−1. The electrospray ionization voltage of the capillary was set to −4,000 V, the end plate to −500 V, the skimmer to 40 V, and the capillary exit to 121 V. The full scan ranged from 100 to 800 mass-to-charge ratio with a resolution of 13,000 mass-to-charge ratio per second. Ions were accumulated until an ion charge control target of 20,000 (positive mode) or 10,000 (negative mode) was achieved or the maximum time of 200 ms was reached. For tandem MS, helium was used as the collision gas at 4 × 10−6 mbar and a collision voltage of 1 V. Data were analyzed with Data Analysis 5.1 software (Bruker Daltonics). Metabolites were identified by comparing their retention times and mass spectra (MS and tandem MS) with those of measured reference compounds. Phenylpropanoyl Glc esters were enzymatically synthesized with FaGT2 (Lunkenbein et al., 2006a). Pelargonidin 3-O-glucoside, quercetin 3-O-glucuronide, quercetin 3-O-glucoside, kaempferol 3-O-glucuronide, kaempferol 3-O-glucoside, catechin, and epicatechin were obtained from Roth. Proanthocyanidins and pelargonidin 3-O-glucoside-6-O-malonate were isolated from strawberry and identified according to Fossen et al. (2004). Statistical evaluation was performed using independent Student’s t test of SOFA Statistics (Paton-Simpson & Associates). The major known phenolic metabolites were quantified in the positive (anthocyanins) or negative (phenylpropanoids and flavonoids) MS mode by the internal standard method and were expressed as per mil equivalent of dry weight assuming a response factor of 1. The metabolite concentrations did not always lie within the linear range of the detector, and the calculation of their relative levels did not allow immediate comparison with absolute levels for phenolics provided by other studies, but the method offered the advantage of obtaining relative values in a short period of time that is sufficient to rank the varieties according to their metabolite levels (Fig. 3). The consumption of substrates by FaPRX27 was also quantified by LC-MS under the same conditions. Samples were analyzed prior to the addition of FaPRX27 and 5 min after the addition of FaPRX27. Ion counts of the substrates were used for the calculation.
Quantitative PCR Analysis
Two grams of frozen plant material was ground to a fine powder in liquid nitrogen using a mortar and pestle for total RNA extraction. Total RNA was prepared as described (Liao et al., 2004). First-strand cDNA was synthesized from 1 μg of DNase I (Fermentas)-treated total RNA by Moloney murine leukemia virus reverse transcriptase H− (Promega) and random hexamer primer according to the manufacturer’s instructions. Real-time PCR was performed on a 96-well reaction plate (Applied Biosystems) with the StepOnePlus real-time PCR system (Applied Biosystems) using Fast SYBR Green Master Mix (Applied Biosystems) to monitor double-stranded cDNA synthesis. Two microliters of a 50-fold dilution and 2 μL of a 4,000-fold dilution cDNA were used as templates for targeting FaPRX27 and interspacer genes, respectively. The following primers were used: FaPRX27 gene-specific primers (forward, 5′-ATTTCCATGATTGCTTTGTCA-3′; reverse, 5′-CAACGGCTAAGATGTCAGAAC-3′) and interspacer primers (forward, 5′-ACCGTTGATTCGCACAATTGGTCATCG-3′; reverse, 5′-TACTGCGGGTCGGCAATCGGACG-3′). PCR (three technical replicates) was performed using the following thermal cycling conditions: 95°C for 10 min (holding stage), then 95°C for 15 s and 60°C for 1 min (40 cycles), followed by 95°C for 15 s, 60°C for 1 min, and 95°C for 15 s (melt curve stage). Relative gene expression was quantified using the 2-ΔΔCT method (Livak and Schmittgen, 2001), and values were calculated as fold change of each sample relative to the selected reference sample (white fruit).
Construction of the FaPRX27 Expression Plasmid
The full-length sequence of FaPRX27 was isolated from cDNA of red fruit of strawberry (cv Elsanta) using primers for_19455 (5′-ATGGCTGCTACTTCAA-3′) and rev_19455 (5′-CTAATTGATCTTGCTGC-3′). The amplification product was A tailed and cloned into the pGEM-T Easy (Promega) vector, and the ligation product was transformed into Escherichia coli NEB10 beta (New England Biolabs). The identity of the cloned gene was confirmed by sequencing the complete insert (MWG Biotech) and by restriction enzyme digestion with PstI/SacI and NotI. SmaI and XhoI restriction sites were introduced by sense and antisense primers, respectively, and the PCR product was cloned in frame with a coding region for a C-terminal GST tag into pGEX expression vector (GE Healthcare). Correct insertion was confirmed by sequencing, and FaPRX27 expression vector was finally transformed into E. coli Rosetta(DE3)pLysS (Merck).
Heterologous Protein Expression
E. coli Rosetta(DE3)pLysS cells carrying the FaPRX27 expression vector were grown overnight at 37°C in Luria-Bertani medium containing 50 µg mL−1 carbenicillin and 34 µg mL−1 chloramphenicol. The next day, the cultures were diluted to an optical density at 600 nm of 0.06 with Luria-Bertani medium containing the appropriate antibiotics in a final volume of 400 mL. This culture was grown at 37°C to an optical density at 600 nm of 0.3 to 0.4, and 200 µL of 1 m isopropylthio-β-galactoside was added to induce protein expression. After overnight (19–24 h) incubation at 16°C to 18°C, cells were harvested by centrifugation and stored at −80°C. The pellet was resuspended in 10 mL of extraction buffer (100 mm potassium phosphate buffer, pH 6.6, 0.5% Triton X-100, and 2 mm EDTA) containing 10 µL of the proteinase inhibitor phenylmethylsulfonyl fluoride (10 mm in 2-propanol). Cells were lysed by sonication (3 × 30 min, 18% intensity; Bandelin Sonoplus) followed by vigorous vortexing. Crude protein extract was centrifuged (6,000g at 4°C), and the supernatant finally was used in PRX enzyme assays.
Enzyme Assays
Enzyme activity was assayed according to a published procedure (Vitali et al., 1998) in solutions consisting of 2.8 mL of buffer (0.1 m potassium phosphate buffer, pH 7.0), 50 µL of substrate (18 mm), 50 µL of H2O2 (30%, 1:100 diluted, optical density at 240 nm = 0.4), and 100 µL of crude FaPRX27 extract (1 mg mL−1). Reactions were monitored photometrically at the wavelength of maximum absorption of the respective substrates. In addition, after 5 min, 50 µL of the assays was withdrawn, and 25 µL of methanol was added, vortexed, and centrifuged (14,000g for 5 min). The supernatant was analyzed by LC-multiple stage mass spectrometry. As a control, Rosetta(DE3)pLysS cells were transformed with an empty pGEX vector, and the resulting protein extract was assayed under the same conditions. As an additional control, assays were conducted without the addition of protein extracts. FaPRX27 activity was quantified photometrically by measuring the increase in A470 due to the formation of oxidation products of guaiacol. The 300-μL reaction mixture contained 230 μL of 0.1 m potassium phosphate buffer (pH 7.0), 25 μL of 18 mm guaiacol, 25 μL of 9.8 mm H2O2 (30% solution), and 20 μL of crude protein extract. The reaction was started by the addition of the protein extract at room temperature. Enzymatic activity was calculated with an extinction coefficient of 26.6 mm−1 cm−1 at 470 nm (Vitali et al., 1998). Values are means ± se of three replicates.
Quantification of Lignin
Extraction of lignin was adapted from published procedures (Meyer et al., 1998; Franke et al., 2002). Fifty milligrams of lyophilized fruit powder was suspended in 1.5 mL of 0.1 m phosphate buffer (pH 7.2), sonicated for 1 min, and kept agitated at 500 rpm and 40°C for 30 min. The suspension was centrifuged for 30 min at 16,000g, and the supernatant was discarded. The sample was extracted a second time with 1.5 mL of 0.1 m phosphate buffer (pH 7.2). The residue was washed twice with 1.5 mL of 80% ethanol, sonicated for 1 min, and incubated at 80°C for 10 min at 500 rpm. The sample was centrifuged at 16,000g for 10 min, and the supernatant was removed. The residue was then washed with 1.5 mL of acetone, sonicated for 1 min, and centrifuged for 30 min at 16,000g, and the supernatant was discarded. The residue was incubated with 950 µL of 1 m sodium hydroxide solution for 16 h at room temperature. The solution was neutralized with 950 µL of 1 m hydrochloric acid and centrifuged at 16,000g for 10 min. The residue was washed twice with 1.5 mL of water followed by sonication for 1 min and centrifugation for 15 min at 16,000g. Quantification of lignin was done according to a published report (Campbell and Ellis, 1992). The pellet was suspended with 750 µL of water, 250 µL of concentrated hydrochloric acid (32%), and 100 µL of thioglycolic acid and incubated for 3 h at 80°C. After centrifugation (5 min at 16,000g), 1 mL of water was added to the residue, sonicated for 1 min, and centrifuged. The residue was dissolved in 1 mL of 1 m sodium hydroxide solution for 12 h of agitation at 500 rpm at room temperature. After centrifugation (5 min at 16,000g), the supernatant was placed into a new tube, mixed with 200 µL of concentrated hydrochloric acid (32%), and kept for 4 h at 4°C for precipitation of lignin. After centrifugation (10 min at 16,000g and 4°C), the residue was finally dissolved in 1 mL of 1 m sodium hydroxide solution and its absorbance measured photometrically at 280 nm, being diluted if necessary. A calibration curve was prepared with 1, 2.5, 5, 7.5, and 10 mg of hydrolytic lignin following the same procedure (Sigma-Aldrich). Six biological replicates were analyzed.
RNAi-Mediated Gene Silencing
For transient transfection of strawberry fruit, Agrobacterium tumefaciens strain AGL0 suspensions containing pBI-CHSi (Hoffmann et al., 2006) were injected into one-half of several fruits, whereas the other one-half remained untreated. The octoploid strawberry cv Elsanta was used for transfections. Fruits remained attached to the plants after agroinfiltration. A detailed description of the agroinfiltration procedure (timing and sampling) has been published (Hoffmann et al., 2006).
Determination of Fruit Firmness
Fruit firmness was determined by a TA-XT2i texture analyzer (Singh and Reddy, 2006). The measuring force was made with a probe of 0.5 mm in diameter to penetrate the surface of the fruit. Each fruit was penetrated at a speed rate of 1 to 10 mm s−1. Based on the bioyield point, the maximum force developed during the measurement was recorded and expressed in Newton. Each sample was measured twice on the two opposite sides.
Bioinformatics of EST Sequences
A total of 29,741 raw strawberry EST sequences were processed with the programs included in the EGassembler Web page (http://egassembler.hgc.jp/; Masoudi-Nejad et al., 2006). This included a masking process in which poly(T) tails, vector and adapter sequences, simple repeats, rolling circles, interspersed repeats, small RNA, low-complexity sequences, and those sequences having less than 100 bp in length and containing more than 3% of N were removed. After that, we excluded sequences that were present in the Arabidopsis RepBase repeats library (mostly retroelement and DNA transposon sequences) using the slow method (0%–5% more sensitive, two to three times slower than default) and eliminated vector and organelle sequences using the Core NCBI vector and the Arabidopsis plastid database, respectively. Once the sequences were masked, the ESTs were assembled and clustered using the CAP3 program included in the EGassembler service using a maximum gap length in any overlap of 20 bases, with a cutoff of more than 75% identity and an overlap similarity score cutoff of 700. This service provided the sequences of the contigs fully aligned as a text file, and this was visually inspected to get a rough idea about the quality of the contig sequences obtained. A similarity search and functional annotation were performed with the assembled sequences containing contigs and singletons using the JAVA package Blast2Go run under Windows (Götz et al., 2008). This was done in several consecutive steps in an attempt to unravel the putative function of the maximum number of sequences. In a first step, a regular BLASTX search was used using the corresponding default NCBI-deposited protein databases with a cutoff expect value of 1.0E-05 for the significance similarity using the nr (for nonredundant) database located at the NCBI. All sequences that gained a BLASTX hit were used to get additional Gene Ontology, InterPro, Enzyme, and KEGG functional annotations. However, for further consideration, analyses, and generation of tables and graphics, we excluded those sequences with hits with e-values higher than 1.0E-15. Gene Ontology annotation was done using the Gene Ontology Blast2Go database deposited in the Spanish server dated May 2010. Finally, with the set of sequences lacking BLASTX hits, we conducted searches using both BLASTN and TBLASTX separately with the only intention of assigning them a putative candidate not present in protein databases. Since BLASTN and TBLASTX account for DNA sequences, they could not be further annotated for Gene Ontology like the rest of the sequences.
Microarray Generation and Analysis
A custom-made oligonucleotide-based (60-mer length) platform representing a total of 18,152 unigenes of strawberry was designed (Roche NimbleGen) from the nonredundant sequences. For each of the sequences, four oligonucleotides were printed per block, and four blocks were printed for each data set. Total RNA samples were treated with DNase I and purified by Qiagen columns according to the manufacturer’s instructions. Labeling (Cy3) of samples, hybridization, and data normalization were performed according to the procedures described in the expression analysis section published by Roche NimbleGen (http://www.nimblegen.com/). Briefly, 10 μg of total receptacle RNA was processed using the Roche cDNA Synthesis System. The cDNA was purified using the High Pure PCR Product Purification Kit according to the manufacturer’s protocol. Afterward, the samples were processed using full-size reverse transcription reactions. Three replicate reverse transcription reactions were performed for each total RNA input amount. Each cDNA sample was random primer labeled with Cy3 nonamers according to Roche NimbleGen’s standard protocol using NimbleGen One-Color DNA Labeling Kits. One microgram of cDNA was used in the labeling reaction. Using random assignment, each Cy3-labeled cDNA sample was applied to the custom-made strawberry 12x135K arrays format (each slide contains 12 independent arrays, each with 140,572 probes covering 35,143 ESTs, with four probes per target gene). The array was then hybridized for 16 h at 42°C, washed, dried, and scanned at 2-μm resolution using a NimbleGen MS 200 Microarray Scanner (Roche NimbleGen). The NimbleScan version 2.6 software (Roche NimbleGen) was used to extract fluorescence intensity signals from the scanned images and perform robust multiarray (RMA) analysis to generate gene expression values. RMA was performed across replicate arrays within each test condition, sample, and input amount (e.g. separate RMA analyses were performed for the data sets from the three replicate hybridizations using cDNA derived from 10 μg of total RNA). Data analysis of the microarray expression studies was performed with the DNASTAR software for gene expression analysis (http://www.dnastar.com/). The moderated Student’s t test and false discovery rate (Benjamini and Hochberg, 1995) for multiple testing corrections were used with a confidence of P < 0.01 to identify statistically significant differences.
Genotype and QTL Analysis
A genomic sequence of Fragaria vesca was analyzed to find the sequence around the gene FaPRX27. A SSR was detected 50 kb upstream of the gene using Tandem Repeat Finder software (http://tandem.bu.edu/trf/trf.html). The design of primers flanking the SSR was made using Primer 3 software (http://frodo.wi.mit.edu). The Spanish population of cultivated strawberry was genotyped with the SSRCL317CGf marker. DNA from 93 individuals of an F2 Dover × Camarosa population was extracted and amplified by PCR using SSRCL317CGf primers (forward, 5′-AGTGTGCAGTTTCCACAACG-3′; reverse, 5′-TGCGGAATTGATGTTCTGTC-3′). SSRCL317CGf was mapped in an F2 Dover × Camarosa population using JOINMAP software (van Ooijen, 2006). QTL detection was performed by composite interval mapping (Zeng, 1994) using QTL Cartographer software (Basten et al., 1997) separately for each parent using the female and male maps for the French pseudo test-cross segregating population and using the linkage maps for the Spanish F2 segregating population. A forward-backward stepwise regression was performed to choose the five cofactors with the highest F values before performing QTL detection by composite interval mapping. A window size of 10 centimorgan around the test interval, where the cofactors were not considered, was chosen (model 6 of QTL Cartographer). The statistical significance threshold for declaring a putative QTL was determined by permutations of the data set. After 1,000 permutations, mean log of the odds thresholds of 3.1 and 3.0 were chosen for the French and Spanish data, respectively. This corresponded to a genome-wise significance level of α = 0.05. The proportion of phenotypic variation explained by each significant marker was estimated as the coefficient of determination (r2) at the peak QTL position estimated by QTL Cartographer.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number JX290513.1.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Nucleotide sequence alignment of gene 19544, FaPRX27, and homologous sequences.
Supplemental Figure S2. Nucleotide (top) and protein (bottom) sequences of functionally characterized FaPRX27.
Supplemental Figure S3. Nucleotide sequences of scaffold 0513171 (partial sequence), FaPRX27, and gene 19544 as predicted by the F. vesca genome sequence (Shulaev et al., 2011).
Supplemental Figure S4. SDS-PAGE analysis of four different samples of soluble crude protein extract obtained from FaPRX27-expressing E. coli.
Supplemental Figure S5. FaPRX27 enzyme assays with cinnamic acid.
Supplemental Figure S6. FaPRX27 enzyme assays with 4-coumaric acid.
Supplemental Figure S7. FaPRX27 enzyme assays with caffeic acid.
Supplemental Figure S8. FaPRX27 enzyme assays with ferulic acid.
Supplemental Figure S9. FaPRX27 enzyme assays with guaiacol.
Supplemental Figure S10. FaPRX27 enzyme assays with vanillin.
Supplemental Figure S11. FaPRX27 enzyme assays with coniferyl alcohol.
Supplemental Figure S12. FaPRX27 enzyme assays with cinnamaldehyde.
Supplemental Figure S13. FaPRX27 enzyme assays with 4-hydroxybenzaldehyde.
Supplemental Figure S14. FaPRX27 enzyme assays with 3,4-dihydroxybenzaldehyde.
Supplemental Figure S15. Description of candidate genes.
Supplemental Table S1. Strawberry varieties used for comparative microarray analyses.
Acknowledgments
We thank Aurélie Petit and Philippe Chartier from Ciref for providing the strawberry fruits and are grateful to Miguel Angel Hidalgo from Planasa for providing Spanish strawberry fruits.
Glossary
- QTL
quantitative trait locus
- LC
liquid chromatography
- MS
mass spectrometry
- H2O2
hydrogen peroxide
- NCBI
National Center for Biotechnology Information
- cDNA
complementary DNA
- GST
glutathione S-transferase
- SSR
simple sequence repeat
- RNAi
RNA interference
- RMA
robust multiarray
References
- Aaby K, Ekeberg D, Skrede G. (2007) Characterization of phenolic compounds in strawberry (Fragaria × ananassa) fruits by different HPLC detectors and contribution of individual compounds to total antioxidant capacity. J Agric Food Chem 55: 4395–4406 [DOI] [PubMed] [Google Scholar]
- Aharoni A, De Vos CH, Wein M, Sun Z, Greco R, Kroon A, Mol JN, O’Connell AP. (2001) The strawberry FaMYB1 transcription factor suppresses anthocyanin and flavonol accumulation in transgenic tobacco. Plant J 28: 319–332 [DOI] [PubMed] [Google Scholar]
- Aharoni A, Giri AP, Verstappen FWA, Bertea CM, Sevenier R, Sun Z, Jongsma MA, Schwab W, Bouwmeester HJ. (2004) Gain and loss of fruit flavor compounds produced by wild and cultivated strawberry species. Plant Cell 16: 3110–3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni A, Keizer LC, Bouwmeester HJ, Sun Z, Alvarez-Huerta M, Verhoeven HA, Blaas J, van Houwelingen AM, De Vos RC, van der Voet H, et al. (2000) Identification of the SAAT gene involved in strawberry flavor biogenesis by use of DNA microarrays. Plant Cell 12: 647–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni A, Keizer LC, Van Den Broeck HC, Blanco-Portales R, Muñoz-Blanco J, Bois G, Smit P, De Vos RC, O’Connell AP. (2002) Novel insight into vascular, stress, and auxin-dependent and -independent gene expression programs in strawberry, a non-climacteric fruit. Plant Physiol 129: 1019–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni A, O’Connell AP. (2002) Gene expression analysis of strawberry achene and receptacle maturation using DNA microarrays. J Exp Bot 53: 2073–2087 [DOI] [PubMed] [Google Scholar]
- Allan AC, Hellens RP, Laing WA. (2008) MYB transcription factors that colour our fruit. Trends Plant Sci 13: 99–102 [DOI] [PubMed] [Google Scholar]
- Almeida JRM, D’Amico E, Preuss A, Carbone F, de Vos CH, Deiml B, Mourgues F, Perrotta G, Fischer TC, Bovy AG, et al. (2007) Characterization of major enzymes and genes involved in flavonoid and proanthocyanidin biosynthesis during fruit development in strawberry (Fragaria × ananassa). Arch Biochem Biophys 465: 61–71 [DOI] [PubMed] [Google Scholar]
- Basten C, Weir BS, Zeng ZB (1997) QTL Cartographer: A Reference Manual and Tutorial for QTL Mapping. Department of Statistics, North Carolina State University, Raleigh. http: //statgen.ncsu.edu/qtlcart/(January 2013)
- Benjamini Y, Hochberg Y. (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57: 289–300 [Google Scholar]
- Besseau S, Hoffmann L, Geoffroy P, Lapierre C, Pollet B, Legrand M. (2007) Flavonoid accumulation in Arabidopsis repressed in lignin synthesis affects auxin transport and plant growth. Plant Cell 19: 148–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombarely A, Merchante C, Csukasi F, Cruz-Rus E, Caballero JL, Medina-Escobar N, Blanco-Portales R, Botella MA, Muñoz-Blanco J, Sánchez-Sevilla JF, et al. (2010) Generation and analysis of ESTs from strawberry (Fragaria × ananassa) fruits and evaluation of their utility in genetic and molecular studies. BMC Genomics 11: 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudet AM. (2007) Evolution and current status of research in phenolic compounds. Phytochemistry 68: 2722–2735 [DOI] [PubMed] [Google Scholar]
- Bunzel M, Seiler A, Steinhart H. (2005) Characterization of dietary fiber lignins from fruits and vegetables using the DFRC method. J Agric Food Chem 53: 9553–9559 [DOI] [PubMed] [Google Scholar]
- Cai C, Xu C, Li X, Ferguson I, Chen KS. (2006) Accumulation of lignin in relation to change in activities of lignification enzymes in loquat fruit flesh after harvest. Postharvest Biol Technol 40: 163–169 [Google Scholar]
- Campbell MM, Ellis BE. (1992) Fungal elicitor-mediated responses in pine cell cultures. I. Induction of phenylpropanoid metabolism. Planta 186: 409–417 [DOI] [PubMed] [Google Scholar]
- Capocasa F, Diamanti J, Tulipani S, Battino M, Mezzetti B. (2008) Breeding strawberry (Fragaria × ananassa Duch) to increase fruit nutritional quality. Biofactors 34: 67–72 [DOI] [PubMed] [Google Scholar]
- Carbone F, Mourgues F, Biasioli F, Gasperi F, Märk T, Rosati C, Perrotta G. (2006) Development of molecular and biochemical tools to investigate fruit quality traits in strawberry elite genotypes. Mol Breed 18: 127–142 [Google Scholar]
- Chabannes M, Barakate A, Lapierre C, Marita JM, Ralph J, Pean M, Danoun S, Halpin C, Grima-Pettenati J, Boudet AM. (2001) Strong decrease in lignin content without significant alteration of plant development is induced by simultaneous down-regulation of cinnamoyl CoA reductase (CCR) and cinnamyl alcohol dehydrogenase (CAD) in tobacco plants. Plant J 28: 257–270 [DOI] [PubMed] [Google Scholar]
- Cosio C, Dunand C. (2009) Specific functions of individual class III peroxidase genes. J Exp Bot 60: 391–408 [DOI] [PubMed] [Google Scholar]
- Dinkel H, Michael S, Weatheritt RJ, Davey NE, Van Roey K, Altenberg B, Toedt G, Uyar B, Seiler M, Budd A, et al. (2012) ELM: the database of eukaryotic linear motifs. Nucleic Acids Res 40: D242–D251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fait A, Hanhineva K, Beleggia R, Dai N, Rogachev I, Nikiforova VJ, Fernie AR, Aharoni A. (2008) Reconfiguration of the achene and receptacle metabolic networks during strawberry fruit development. Plant Physiol 148: 730–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornalé S, Shi X, Chai C, Encina A, Irar S, Capellades M, Fuguet E, Torres JL, Rovira P, Puigdomènech P, et al. (2010) ZmMYB31 directly represses maize lignin genes and redirects the phenylpropanoid metabolic flux. Plant J 64: 633–644 [DOI] [PubMed] [Google Scholar]
- Fossen T, Rayyan S, Andersen ØM. (2004) Dimeric anthocyanins from strawberry (Fragaria ananassa) consisting of pelargonidin 3-glucoside covalently linked to four flavan-3-ols. Phytochemistry 65: 1421–1428 [DOI] [PubMed] [Google Scholar]
- Franke R, Hemm MR, Denault JW, Ruegger MO, Humphreys JM, Chapple C. (2002) Changes in secondary metabolism and deposition of an unusual lignin in the ref8 mutant of Arabidopsis. Plant J 30: 47–59 [DOI] [PubMed] [Google Scholar]
- Gil-Ariza DJ, Amaya I, López-Aranda JM, Sánchez-Sevilla JF, Botella MA, Valpuesta V. (2009) Impact of plant breeding on the genetic diversity of cultivated strawberry as revealed by expressed sequence tag-derived simple sequence repeat markers. J Am Soc Hortic Sci 134: 337–347 [Google Scholar]
- Gonzalez A, Zhao M, Leavitt JM, Lloyd AM. (2008) Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J 53: 814–827 [DOI] [PubMed] [Google Scholar]
- Götz S, García-Gómez JM, Terol J, Williams TD, Nagaraj SH, Nueda MJ, Robles M, Talón M, Dopazo J, Conesa A. (2008) High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res 36: 3420–3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesser M, Hoffmann T, Bellido ML, Rosati C, Fink B, Kurtzer R, Aharoni A, Muñoz-Blanco J, Schwab W. (2008a) Redirection of flavonoid biosynthesis through the down-regulation of an anthocyanidin glucosyltransferase in ripening strawberry fruit. Plant Physiol 146: 1528–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesser M, Vitzthum F, Fink B, Bellido ML, Raasch C, Munoz-Blanco J, Schwab W. (2008b) Multi-substrate flavonol O-glucosyltransferases from strawberry (Fragaria × ananassa) achene and receptacle. J Exp Bot 59: 2611–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock JF (1999) Strawberries. CABI Publishing, Wallingford, UK [Google Scholar]
- Hanhineva K, Rogachev I, Kokko H, Mintz-Oron S, Venger I, Kärenlampi S, Aharoni A. (2008) Non-targeted analysis of spatial metabolite composition in strawberry (Fragaria × ananassa) flowers. Phytochemistry 69: 2463–2481 [DOI] [PubMed] [Google Scholar]
- Hernanz D, Recamales ÁF, Meléndez-Martínez AJ, González-Miret ML, Heredia FJ. (2007) Assessment of the differences in the phenolic composition of five strawberry cultivars (Fragaria × ananassa Duch.) grown in two different soilless systems. J Agric Food Chem 55: 1846–1852 [DOI] [PubMed] [Google Scholar]
- Hoffmann T, Kalinowski G, Schwab W. (2006) RNAi-induced silencing of gene expression in strawberry fruit (Fragaria × ananassa) by agroinfiltration: a rapid assay for gene function analysis. Plant J 48: 818–826 [DOI] [PubMed] [Google Scholar]
- Hoffmann T, Kurtzer R, Skowranek K, Kiessling P, Fridman E, Pichersky E, Schwab W. (2011) Metabolic engineering in strawberry fruit uncovers a dormant biosynthetic pathway. Metab Eng 13: 527–531 [DOI] [PubMed] [Google Scholar]
- Horvath A, Sánchez-Sevilla JF, Punelli F, Richard L, Sesmero-Carrasco R, Leone A, Höefer M, Chartier P, Balsemin E, Barreneche T, et al. (2011) Structured diversity in octoploid strawberry cultivars: importance of the old European germplasm. Ann Appl Biol 149: 358–371 [Google Scholar]
- Hu WJ, Harding SA, Lung J, Popko JL, Ralph J, Stokke DD, Tsai CJ, Chiang VL. (1999) Repression of lignin biosynthesis promotes cellulose accumulation and growth in transgenic trees. Nat Biotechnol 17: 808–812 [DOI] [PubMed] [Google Scholar]
- Janz D, Behnke K, Schnitzler JP, Kanawati B, Schmitt-Kopplin P, Polle A. (2010) Pathway analysis of the transcriptome and metabolome of salt sensitive and tolerant poplar species reveals evolutionary adaption of stress tolerance mechanisms. BMC Plant Biol 10: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogel KH, Voll LM, Schäfer P, Jansen C, Wu Y, Langen G, Imani J, Hofmann J, Schmiedl A, Sonnewald S, et al. (2010) Transcriptome and metabolome profiling of field-grown transgenic barley lack induced differences but show cultivar-specific variances. Proc Natl Acad Sci USA 107: 6198–6203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhou Y, Cheng X, Sun J, Marita JM, Ralph J, Chiang VL. (2003) Combinatorial modification of multiple lignin traits in trees through multigene cotransformation. Proc Natl Acad Sci USA 100: 4939–4944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z, Chen M, Guo L, Gong Y, Tang F, Sun X, Tang K. (2004) Rapid isolation of high-quality total RNA from taxus and ginkgo. Prep Biochem Biotechnol 34: 209–214 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Lunkenbein S, Bellido ML, Aharoni A, Salentijn EMJ, Kaldenhoff R, Coiner HA, Muñoz-Blanco J, Schwab W. (2006a) Cinnamate metabolism in ripening fruit. Characterization of a UDP-glucose:cinnamate glucosyltransferase from strawberry. Plant Physiol 140: 1047–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunkenbein S, Coiner H, de Vos CH, Schaart JG, Boone MJ, Krens FA, Schwab W, Salentijn EM. (2006b) Molecular characterization of a stable antisense chalcone synthase phenotype in strawberry (Fragaria × ananassa). J Agric Food Chem 54: 2145–2153 [DOI] [PubMed] [Google Scholar]
- Lüthje S, Meisrimler CN, Hopff D, Möller B. (2011) Phylogeny, topology, structure and functions of membrane-bound class III peroxidases in vascular plants. Phytochemistry 72: 1124–1135 [DOI] [PubMed] [Google Scholar]
- Mansouri IE, Mercado JA, Santiago-Dómenech N, Pliego-Alfaro F, Valpuesta V, Quessada MA. (1999) Biochemical and phenotypical characterization of transgenic tomato plants overexpressing a basic peroxidase. Physiol Plant 106: 335–362 [Google Scholar]
- Marjamaa K, Kukkola EM, Fagerstedt KV. (2009) The role of xylem class III peroxidases in lignification. J Exp Bot 60: 367–376 [DOI] [PubMed] [Google Scholar]
- Masoudi-Nejad A, Tonomura K, Kawashima S, Moriya Y, Suzuki M, Itoh M, Kanehisa M, Endo T, Goto S. (2006) EGassembler: online bioinformatics service for large-scale processing, clustering and assembling ESTs and genomic DNA fragments. Nucleic Acids Res 34: W459–W462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathé C, Barre A, Jourda C, Dunand C. (2010) Evolution and expression of class III peroxidases. Arch Biochem Biophys 500: 58–65 [DOI] [PubMed] [Google Scholar]
- Meyer K, Shirley AM, Cusumano JC, Bell-Lelong DA, Chapple C. (1998) Lignin monomer composition is determined by the expression of a cytochrome P450-dependent monooxygenase in Arabidopsis. Proc Natl Acad Sci USA 95: 6619–6623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panjehkeh N, Backhouse D, Taji A. (2010) Role of proanthocyanidins in resistance of the legume Swainsona formosa to Phytophthora cinnamomi. J Phytopathol 158: 365–371 [Google Scholar]
- Passardi F, Longet D, Penel C, Dunand C. (2004) The class III peroxidase multigenic family in rice and its evolution in land plants. Phytochemistry 65: 1879–1893 [DOI] [PubMed] [Google Scholar]
- Pincemail J, Kevers C, Tabart J, Defraigne JO, Dommes J. (2012) Cultivars, culture conditions, and harvest time influence phenolic and ascorbic acid contents and antioxidant capacity of strawberry (Fragaria × ananassa). J Food Sci 77: C205–C210 10.1111/j.1750-3841.2011.02539.x [DOI] [PubMed] [Google Scholar]
- Quiroga M, Guerrero C, Botella MA, Barceló A, Amaya I, Medina MI, Alonso FJ, de Forchetti SM, Tigier H, Valpuesta V. (2000) A tomato peroxidase involved in the synthesis of lignin and suberin. Plant Physiol 122: 1119–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekika D, Khanizadeh S, Deschênes M, Levasseur A, Charles MT, Tsao R, Yang R. (2005) Antioxidant capacity and phenolic content of selected strawberry genotypes. HortScience 40: 1777–1781 [Google Scholar]
- Rohrmann J, Tohge T, Alba R, Osorio S, Caldana C, McQuinn R, Arvidsson S, van der Merwe MJ, Riaño-Pachón DM, Mueller-Roeber B, et al. (2011) Combined transcription factor profiling, microarray analysis and metabolite profiling reveals the transcriptional control of metabolic shifts occurring during tomato fruit development. Plant J 68: 999–1013 [DOI] [PubMed] [Google Scholar]
- Rousseau-Gueutin M, Lerceteau-Köhler E, Barrot L, Sargent DJ, Monfort A, Simpson D, Arús P, Guérin G, Denoyes-Rothan B. (2008) Comparative genetic mapping between octoploid and diploid Fragaria species reveals a high level of colinearity between their genomes and the essentially disomic behavior of the cultivated octoploid strawberry. Genetics 179: 2045–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salentijn EMJ, Aharoni A, Schaart JG, Boone MJ, Krens FA. (2003) Differential gene expression analysis of strawberry cultivars that differ in fruit-firmness. Physiol Plant 118: 571–578 [Google Scholar]
- Schwab W, Hoffmann T, Kalinowski G, Preuß A. (2011) Functional genomics in strawberry fruit through RNAi-mediated silencing. Genes Genomes Genomics 5: 91–101 [Google Scholar]
- Shulaev V, Sargent DJ, Crowhurst RN, Mockler TC, Folkerts O, Delcher AL, Jaiswal P, Mockaitis K, Liston A, Mane SP, et al. (2011) The genome of woodland strawberry (Fragaria vesca). Nat Genet 43:109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KK, Reddy BS. (2006) Post-harvest physico-mechanical properties of orange peel and fruit. J Food Eng 73: 112–120 [Google Scholar]
- Solovchenko A, Schmitz-Eiberger M. (2003) Significance of skin flavonoids for UV-B-protection in apple fruits. J Exp Bot 54: 1977–1984 [DOI] [PubMed] [Google Scholar]
- Stushnoff C, Ducreux LJ, Hancock RD, Hedley PE, Holm DG, McDougall GJ, McNicol JW, Morris J, Morris WL, Sungurtas JA, et al. (2010) Flavonoid profiling and transcriptome analysis reveals new gene-metabolite correlations in tubers of Solanum tuberosum L. J Exp Bot 61: 1225–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thévenin J, Pollet B, Letarnec B, Saulnier L, Gissot L, Maia-Grondard A, Lapierre C, Jouanin L. (2011) The simultaneous repression of CCR and CAD, two enzymes of the lignin biosynthetic pathway, results in sterility and dwarfism in Arabidopsis thaliana. Mol Plant 4: 70–82 [DOI] [PubMed] [Google Scholar]
- Tulipani S, Mezzetti B, Capocasa F, Bompadre S, Beekwilder J, de Vos CHR, Capanoglu E, Bovy A, Battino M. (2008) Antioxidants, phenolic compounds, and nutritional quality of different strawberry genotypes. J Agric Food Chem 56: 696–704 [DOI] [PubMed] [Google Scholar]
- Valério L, De Meyer M, Penel C, Dunand C. (2004) Expression analysis of the Arabidopsis peroxidase multigenic family. Phytochemistry 65: 1331–1342 [DOI] [PubMed] [Google Scholar]
- Vanholme R, Demedts B, Morreel K, Ralph J, Boerjan W. (2010) Lignin biosynthesis and structure. Plant Physiol 153: 895–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ooijen JW (2006) Joinmap: Software for the Calculation of Genetic Linkage Maps in Experimental Populations. Kyazma, Wageningen, The Netherlands [Google Scholar]
- Vassão DG, Davin LD, Lewis NG. (2008) Metabolic engineering of plant allyl/propenyl phenol and lignin pathways: future potential for biofuels/bioenergy, polymer intermediates, and specialty chemicals? Adv Plant Biochem Mol Biol 1: 385–428 [Google Scholar]
- Vattem DA, Shetty K. (2005) Biological functionality of ellagic acid: a review. J Food Biochem 29: 234–266 [Google Scholar]
- Veitch NC. (2004) Structural determinants of plant peroxidase function. Phytochem Rev 3: 3–18 [Google Scholar]
- Ververidis F, Trantas E, Douglas C, Vollmer G, Kretzschmar G, Panopoulos N. (2007) Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I. Chemical diversity, impacts on plant biology and human health. Biotechnol J 2: 1214–1234 [DOI] [PubMed] [Google Scholar]
- Vitali A, Botta B, Delle Monache G, Zappitelli S, Ricciardi P, Melino S, Petruzzelli R, Giardina B. (1998) Purification and partial characterization of a peroxidase from plant cell cultures of Cassia didymobotrya and biotransformation studies. Biochem J 331: 513–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt T. (2010) Phenylpropanoid biosynthesis. Mol Plant 3: 2–20 [DOI] [PubMed] [Google Scholar]
- Ward G, Hadar Y, Bilkis I, Konstantinovsky L, Dosoretz CG. (2001) Initial steps of ferulic acid polymerization by lignin peroxidase. J Biol Chem 276: 18734–18741 [DOI] [PubMed] [Google Scholar]
- Willson MF, Whelan CJ. (1990) The evolution of fruit color in fleshy-fruited plants. Am Nat 136: 790–809 [Google Scholar]
- Yonekura-Sakakibara K, Tohge T, Matsuda F, Nakabayashi R, Takayama H, Niida R, Watanabe-Takahashi A, Inoue E, Saito K. (2008) Comprehensive flavonol profiling and transcriptome coexpression analysis leading to decoding gene-metabolite correlations in Arabidopsis. Plant Cell 20: 2160–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng ZB. (1994) Precision mapping of quantitative trait loci. Genetics 136: 1457–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zifkin M, Jin A, Ozga JA, Zaharia LI, Schernthaner JP, Gesell A, Abrams SR, Kennedy JA, Constabel CP. (2012) Gene expression and metabolite profiling of developing highbush blueberry fruit indicates transcriptional regulation of flavonoid metabolism and activation of abscisic acid metabolism. Plant Physiol 158: 200–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla-Fontanesi Y, Cabeza A, Domínguez P, Medina JJ, Valpuesta V, Denoyes-Rothan B, Sánchez-Sevilla JF, Amaya I. (2011) Quantitative trait loci and underlying candidate genes controlling agronomical and fruit quality traits in octoploid strawberry (Fragaria × ananassa). Theor Appl Genet 123: 755–778 [DOI] [PubMed] [Google Scholar]