Abstract
A screening method using LC-DAD-ESI/MS was applied to the analysis of flavonoids in celery, Chinese celery, and celery seeds (Apium graveolens L. and varieties). Fifteen flavonoid glycosides were detected in the three celery materials. They were identified as luteolin 7-O-apiosylglucoside, luteolin 7-O-glucoside, apigenin 7-O-apiosylglucoside, chrysoeriol 7-O-apiosylglucoside, chrysoeriol 7-O-glucoside, and more than 10 malonyl derivatives of these glycosides. The identification of the malonyl derivatives was confirmed by their conversion into glycosides upon heating and by comparison of some of the malonates with malonates that had previously been identified in red bell pepper and parsley. The concentrations of the glycosides and the malonyl glycosides in the three materials were estimated by comparison to aglycone standards. This is the first report of the presence of these glycosylated flavonoid malonates in celery.
Keywords: Chinese celery, celery and celery seeds, Apium graveolens L. and varieties, glycosylated flavonoids, flavonoid malonates, LC-DAD-ESI/MS
Introduction
The flavonoid content of food plants has been reported to offer positive biological benefits such as reduced risk of cancer and cardiovascular disease (1–3). A common, highly consumed vegetable with a high flavonoid content is celery, Apium graveolens L. (Umbelliferae or Apiaceae). A. graveolens var. dulce (Mill.) Pers., with the name of celery, is a popular and common vegetable throughout the world. The aerial part of A. graveolens L., including the small stems and leaves, is known as Chinese celery and is also a common vegetable in China and other Asian countries. Celery seed is a commercially available spice of which the powder, oil, and extract are widely used as flavoring ingredients in major food products (4).
Studies of celery have identified the flavonoids 7-O-apiosylglucosides and 7-O-glucosides of luteolin, apigenin, and chryseoriol, as well as the phenolic acids chlorogenic acid, cinnamic acids, coumarins, and their glycosides (4–7). Quantitative determinations of some of the flavonoids have been reported on the basis of analysis of the aglycones produced by hydrolysis of the glycosylated flavonoids (8–15). Malonated apiosylglucosides have been reported in parsley and red bell pepper, but not in celery (16–18).
As a part of a project to systematically identify glycosylated flavonoids and other phenolic compounds in food plants, we examined celery, Chinese celery, and celery seed using a screening method (19) that is based on liquid chromatography with diode array and electrospray ionization mass spectrometry detection (LC-DAD-ESI/MS). Besides the previously reported apiosylglucosides and glucosides of luteolin, apigenin, and chrysoeriol, more than 10 glycosylated flavone malonates were also found in the celery samples for the first time. The identification and quantification of these glycosylated flavonoids and glycosylated flavonoid malonates in celery food materials were accomplished by chemical and physical means.
Materials and Methods
Plant Materials
Fresh celery (three samples), fresh Chinese celery (three samples), dried celery seeds (one sample), fresh parsley, and fresh red bell pepper were purchased from local food stores in Maryland. The fresh materials (for the celery, stalk, and leaves) were cut into small pieces and dried at room temperature, and all of the plant materials were finely powdered and passed through a 20 mesh sieve prior to extraction. The moisture contents for the celery and Chinese celery samples were determined to be 93.95 and 90.63%, respectively.
Flavonoid Standards and Chemicals
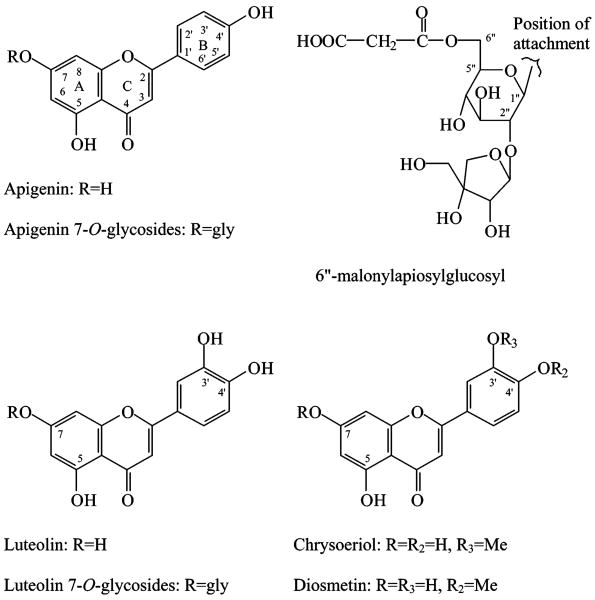
Apigenin (Figure 1) (purity 95% by TLC) was purchased from Sigma Chemical Co. (St. Louis, MO). Luteolin 7-O-glucoside, chrysoeriol, and diosmetin (allof HPLC grade) were purchased from Extrasynthese (Genay, Cedex, France). Luteolin and apiin were purchased from Indofine Chemical Co. (Somerville, NJ). The apiin standard was analyzed in this laboratory and determined to contain approximately 15% chrysoeriol 7-O-apioglucoside and 4% diosmetin 7-O-apioglucoside.
Figure 1.
Structures of the flavonoids.
Formic acid, hydrochloric acid (37%), HPLC solvents (acetonitrile, methanol, acetone), ethanol, and dimethyl sulfoxide were purchased from VWR Scientific (Seattle, WA). HPLC grade water was prepared from distilled water using a Milli-Q system (Millipore Laboratory, Bedford, MA).
LC-DAD and ESI-MS Conditions
The LC-DAD-ESI/MS instrument and operating parameters have been previously described (19). Briefly, the LC-DAD-ESI/MS consisted of an Agilent 1100 HPLC coupled to a diode array detector and mass spectrometer (MSD, SL mode) (Agilent, Palo Alto, CA). A 250 × 4.6 mm i.d., 5 μm Symmetry C18 column (C18, 5 μm,) with a 20 × 3.9 mm i.d., 5 μm sentry guard column (Symmetry, 3.9 × 20 mm) (Waters Corp., Milford, MA), was used at flow rate of 1.0 mL/min. The column oven temperature was set at 25 °C. The mobile phase consisted of a combination of A (0.1% formic acid in water) and B (0.1% formic acid in acetonitrile). The gradient was varied linearly from 10 to 26% B (v/v) in 40 min, to 65% B at 70 min, and finally to 100% B at 71 min and held at 100% B to 75 min. The DAD was set at 350, 310, and 270 nm to record the peak intensity, and UV spectra were recorded from 190 to 650 nm for plant component identification. Mass spectra were simultaneously acquired using electrospray ionization in the positive and negative ionization (PI and NI) modes at low and high fragmentation voltages (70 and 250 V) over the range of m/z 100-2000. A drying gas flow of 13 L/min, a drying gas temperature of 350 °C, a nebulizer pressure of 50 psi, and capillary voltages of 4000 V for PI and 3500 V for NI were used. The LC system was directly coupled to the MSD without stream splitting.
Plant Extracts
Dried ground material (50 mg for celery stalks and Chinese celery, 25 mg for celery seeds and parsley, and 500 mg for red bell pepper) was extracted with methanol/water (5.00 mL, 60:40, v/v) using an FS30 Ultrasonic sonicator (Fisher Scientific, Pittsburgh, PA) at 40 kHz and 100 W for 60 min at room temperature (from 22 to <35 °C at the end). The extract was filtered through a 0.45 μm Nylon Acrodisk 13 filter (Gelman, Ann Arbor, MI). A 50 μL sample of the extract was injected onto the analytical column for analysis. To avoid errors from unexpected degradation of the phenolic compounds, the LC determinations were completed within 24 h of the extraction. On the basis of extraction of 100 mg or less of the dried plant material with 5.0 mL of solvent, the sample preparation method was confirmed to be suitable for the quantitative determination of the glycosylated flavonoids in the tested celery samples(19).
Heat-Induced Hydrolysis of Samples
The filtered extract (1 mL, pH around 5.5–6.0) was heated in a covered glass tube at 85 °C for 16 h. The extract was allowed to cool by standing at room temperature for 30 min and then filtered and injected onto the analytical column.
Acid-Hydrolyzed Samples
The filtered extract (0.5 mL) was mixed with concentrated HCl (37%, 0.1 mL) and heated in a covered tube at 85 °C for 2 h. Then, 0.4 mL of methanol was added to the mixture, which was then sonicated for 10 min. The solution was refiltered prior to HPLC injection.
Quantitative Analysis
Three samples of celery and Chinese celery and one sample of celery seeds were analyzed. Each of the samples was prepared in triplicate, and each preparation was analyzed in duplicate. Luteolin 7-O-glucoside (HPLC grade) and apiin (81% purity) were used as standards for the quantification of celery flavonoids. Luteolin 7-O-glucoside and apiin standard stock solutions [2.2 and 2.0 mg in 10.0 mL of methanol/water (60:40, v/v), respectively] were diluted to provide calibration curves. The two calibration curves were linear with respect to absorbance at 350 nm.
Results and Discussion
Identification of Celery Flavonoids
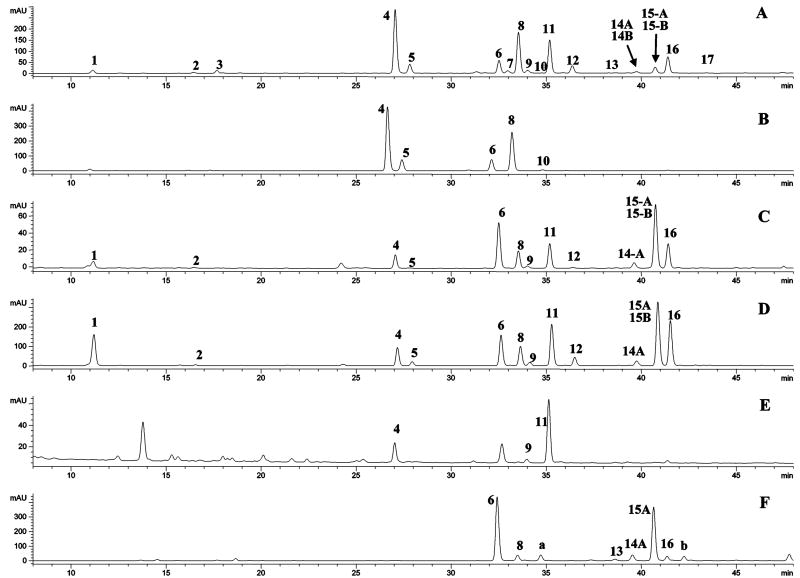
HPLC chromatograms of the extracts from celery, Chinese celery, and celery seed, and the malonate-free celery seed sample (from heat-induced hydrolysis) are shown in Figure 2. The retention times (tR), wavelength of maximum absorbance (λmax), protonated/deprotonated molecules ([M + H]+/[M − H]−), and major fragment ions (PI/NI aglycone) are listed in Table 1. The 7-O-apiosylglucosides and glucosides of apigenin, luteolin, and chrysoeriol have been reported in celery previously (4, 5). On the basis of the UV and mass spectrometric data for peak 4 (i.e., λmax at 256, 266, and 348 nm, PI and NI protonated and deprotonated molecules at m/z 581 and 579, and aglycone ions at m/z 287 and 285) (Table 1), it was identified as luteolin 7-O-apiosylglucoside. The mass difference between the molecular ions and aglycone ions was 294 amu, as expected for the loss of the apiosylglucosyl derivative. Similarly, peaks 6 and 8 were identified as apigenin 7-O-apiosylglucoside (apiin) and chrysoeriol 7-O-apiosylglucoside, respectively. Peak 5 (m/z 449/447, 287/285) was identified as luteolin 7-O-glucoside, and peak 10 (m/z 463/461, 301/299) was identified as chrysoeriol 7-O-glucoside. The identification of peaks 5, 6, and 9 was based on the match of their retention times and UV–vis and mass spectra with those of pure standards. This identification was further confirmed by the fact that apigenin, luteolin, and chrysoeriol were the only aglycones detected in the hydrolyzed Chinese celery (Figure 4E), celery, and celery seed extracts (data not shown). Thus, the glycosides of the trihydroxy-monomethoxyflavone in the tested celery samples are formed from chrysoeriol. Peaks in the same acid-hydrolyzed extract confirmed that both diosmetin 7-O-apiosylglucoside (peak a, Figure 2F) and chrysoeriol 7-O-apiosylglucoside (peak 8) existed in parsley samples.
Figure 2.
Chromatograms with UV detection at 350 nm for extracts of (A) celery seed, (B) heat-induced hydrolyzed sample, (C) celery, (D) Chinese celery, (E) red bell pepper, and (F) parsley.
Table 1. Peak Assignment for Aqueous Methanol Extracts of Celery Samples.
| peak | tR (min) | [M + H]+/[M − H]− (m/z) | PI/NI aglycone (m/z) | λmax (nm) | identification |
|---|---|---|---|---|---|
| 1 | 11.14 | −/353 | −/191, 179 | 240, 298 sh, 326 | Chlorogenic acid |
| 2 | 16.43 | −/337 | −/191, 163 | 228, 312 | coumaroylquinic acid |
| 3 | 17.66 | 409/407 | 247/245 | 250, 360 | un-identified |
| 4 | 27.05 | 581/579 | 287/285 | 256, 266, 348 | luteolin 7-O-apiosylglucoside |
| 5 | 27.82 | 449/447 | 287/285 | 256, 266, 348 | luteolin 7-O-glucosidea |
| 6 | 32.51 | 565/563 | 271/269 | 266, 338 | apiina |
| 7 | 32.90 | 667/665 | 287/285 | 256, 266, 348 | luteolin 7-O-malonyl apiosylglucoside A |
| 8 | 33.53 | 595/593 | 287/285 | 254, 266, 346 | chrysoeriol 7-O-apiosylglucoside |
| 9 | 34.03 | 667/665 | 287/285 | 256, 266, 348 | luteolin 7-O-malonyl-apiosylglucoside B |
| 10 | 35.10 | 463/461 | 301/299 | ndb | chrysoeriol 7-O-glucoside |
| 11 | 35.19 | 667/665 | 287/285 | 256, 266, 348 | luteolin 7-O-6′-malonyl-apiosylglucoside |
| 12 | 36.38 | 535/533 | 287/285 | 256, 266, 348 | luteolin 7-O-6′-malonyl glucoside |
| 13 | 38.71 | 651/649 | 271/269 | nd | malonylapiin A |
| 14A | 39.76 | 651/649 | 271/269 | 266, 338 | malonylapiin B |
| 14B | 39.88 | 681/679 | 301/299 | nd | chrysoeriol 7-O-malonyl apiosylglucoside A |
| 15A | 40.74 | 651/649 | 271/269 | 266, 338 | 6″-malonylapiin |
| 15B | 40.79 | 681/679 | 301/299 | nd | chrysoeriol 7-O-malonyl apiosylglucoside B |
| 16 | 41.41 | 681/679 | 301/299 | 254, 266, 348 | chrysoeriol 7-O-6″-malonyl apiosylglucoside |
| 17 | 43.44 | 549/547 | 301/299 | nd | chrysoeriol 7-O-6″-malonyl glucoside |
Ientified with standard.
UV data were not determined.
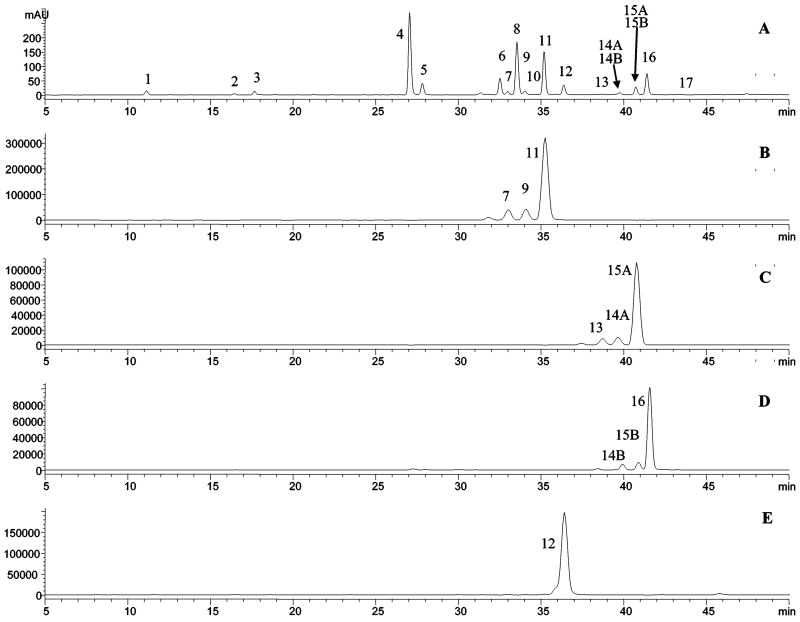
Figure 4.
Chromatograms with UV detection at 350 nm for (A) Chinese celery extract and for heat-induced hydrolysis at 85 °C for (B) 2 h, (C) 8 h, and (D) 16 h and for (E) acid-hydrolyzed Chinese celery extract.
Peak 12 had molecular and aglycone ions at m/z 535/533 and 287/285, respectively, that is, a difference of 248 amu. The molecular ion mass is 86 amu larger than that of the glucosyl group (162 amu) and corresponds to a malonyl group (−OCCH2-COOH) (20–25). Therefore, peak 12 was identified as luteolin 7-O-glucoside malonate. Similarly, the minor peak 17 (m/z 549/547 and 301/299) of celery seed can be identified as chrysoeriol 7-O-glucoside malonate.
Peaks 7, 9, and 11 had molecular ions that were 380 amu larger than their aglycones. The mass difference suggested that they were the malonates of luteolin 7-O-apiosylglucosides. Similarly, peaks 13, 14A, and 15A and peaks 14B, 15B, and 16 were identified as the malonates of apigenin 7-O-apiosylglucoside and chrysoeriol 7-O-apiosylglucoside, respectively.
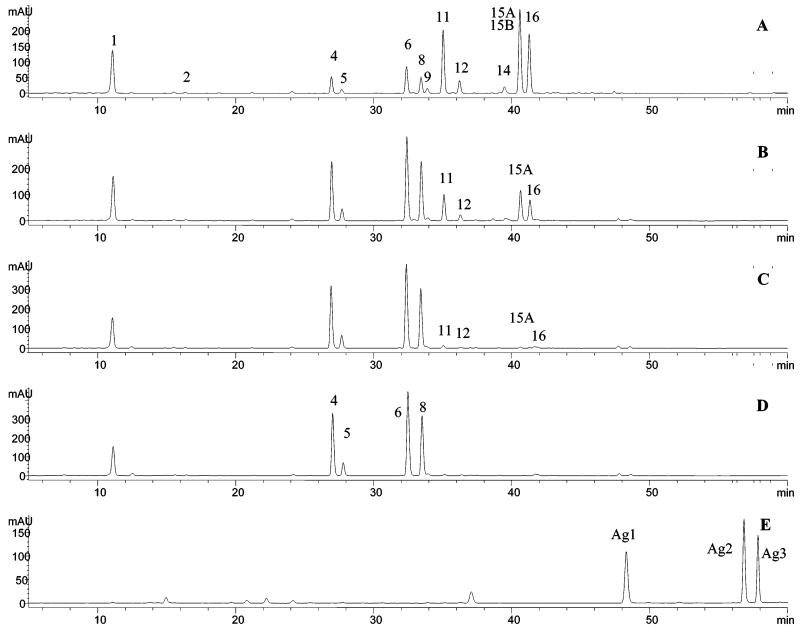
The specificity and enhanced signal-to-noise ratios for selective ion monitoring (SIM) detection, compared to TIC detection, allow trace components to be detected in the presence of more concentrated molecules with similar retention times. SIM detection was used to detect each group of isomeric malonates in celery seeds by monitoring the positive ions at m/z 651, 667, 681, and 535 (Figure 3). The existence of these malonates was further confirmed by the fact that all of the malonates were converted into their related glycosides by a heat-induced hydrolysis (Figure 2B).
Figure 3.
Chromatograms of celery seed extract: (A) UV detection at 350 nm; SIM detection at (B) m/z 667, (C) m/z 651, (D) m/z 681, and (E) m/z 535.
To determine the heating time required for complete removal of the malonates, the extracts were initially heated for 2, 4, 8, 12, 16, and 30 h and analyzed by LC-DAD-ESI/MS with the DAD at 350 nm and SIM detection to detect the possible aglycones produced. The results indicated that only 50–60% of each malonate was converted to its related glycosides at 2 h, and a complete conversion required 12–16 h. At this time, no detectable decomposition of the glycosides into the aglycones was observed (Figure 4).
It has been reported that acylation of the glycoside is most frequently reported at the 6″-position of the sugar (16, 22, 23, 25, 26). Therefore, peaks 11, 15A, 16, and 12 could be provisionally identified as luteolin 7-O-6″-malonyl apiosylglucoside, 6″-malonylapiin, chrysoeriol 7-O-6″-malonyl apiosylglucoside, and luteolin 7-O-6″-malonylglucoside, respectively. The identification of peaks 11 and 15A was further confirmed by a direct comparison of their retention time and UV–vis and mass spectra with those of the luteolin 7-O-6″-malonylapiosylglucoside in red bell pepper (17, 26) and 6″-malonylapiin in parsley (16) (Figure 2E,F). Because their structures have been completely determined by NMR analysis (16–18), it confirmed that the apiosyl group for celery was connected at the 2″-position of the glucosyl as shown in Figure 1. This comparison also indicated the existence (previously unreported) of a minor isomer of luteolin 7-O-6″-malonylapiosylglucoside (peak 9) in red bell pepper and two minor malonylapiins (peaks 13 and 14A) in parsley (Figure 2E). Diosmetin 7-O-6″-malonylapiosylglucoside (peak b in Figure 2F) was also identified in parsley extract.
The isomeric malonates of each 7-O-apiosylglucoside (Figures 2 and 3) have the same molecular and mass spectra as its major isomeric malonate (6″-malonate). The malonyl linkage for the minor isomers might be at the 3- or 4-position of the glucose, or some position of the apinose. The position cannot be determined using MS or MSn. Instead, further NMR analysis would be required for position identification.
Besides the flavones, chlorogenic acid and a trace amount of coumaroylquinic acid were also detected in the three celery samples (peaks 1 and 2 in Table 1 and Figure 2).
Quantification of Flavonoids in Celery Materials
The concentrations of the flavonoids identified in the three celery materials are listed Table 2. Because the malonyl group and the secondary sugar do not influence the molecular absorption, the diglycosylated flavones and the malonated forms will have the same UV λmax, and the molecular extinction coefficients are affected only by the difference in mass (18). Thus, apiin is suitable as a standard for quantifying the malonates of apiin, and luteolin 7-O-glucoside is suitable for the 7-O-apiosylglucosides and malonates of luteolin and chrysoeriol. In both cases the concentration has to be corrected by the molecular mass ratios of analyte and standard compounds.
Table 2. Glycosylated Flavonoid Contents of Dried Celery, Chinese Celery, and Celery Seeds (Milligrams per Gram of Dry Weight)a.
| compound | celery | Chinese celery | celery seed |
|---|---|---|---|
| luteolin 7-O-apiosylglucosideb | 0.14 | 0.70 | 6.32 |
| luteolin 7-O-6″-malonylapiosylglucosideb | 0.74 | 3.98 | 4.27 |
| luteolin 7-O-malonylapiosylglucoside(s)b | 0.06 | 0.28 | 0.40 |
| luteolin 7-O-glucoside | –c | 0.13 | 0.80 |
| luteolin 7-O-6″-malonylglucosideb | 0.02 | 0.74 | 0.92 |
| total luteolin glycosides | 0.96 ± 0.05 | 5.83 ± 0.03 | 12.71 ± 0.07 |
| apigenin 7-O-apiosylglucoside | 0.53 | 0.93 | 1.11 |
| apigenin 7 -O-6″-malonylapiosylglucosided | 1.40 | 3.51 | 0.47 |
| apigenin 7-O-malonylapiosylglucoside(s)d | 0.12 | 0.34 | 0.09 |
| total apigenin glycosides | 2.28 ± 0.03 | 5.36 ± 0.05 | 1.75 ± 0.04 |
| chrysoeriol 7-O-apiosylglucosideb | 0.27 | 0.88 | 4.67 |
| chrysoeriol 7-O-6″-malonylapiosylglucosideb | 0.71 | 3.95 | 2.38 |
| chrysoeriol 7-O-malonylapiosylglucoside(s)b | – | – | 0.31 |
| chrysoeriol 7-O-glucosideb | – | – | – |
| chrysoeriol 7-O-6′-malonylglucosideb | – | – | – |
| total chrysoeriol glycoside | 0.98 ± 0.05 | 4.83 ± 0.04 | 7.36 ± 0.06 |
| total glycosylated flavonoids | 4.22 ± 0.08 | 16.02 ± 0.07 | 21.82 ± 0.10 |
Unless otherwise stated, all values are based on UV absorption. Values for individual compounds are expressed as the mean value (n = 6 for celery seed and n = 18 for Chinese celery and celery) in mg/g on a dry weight basis. All standard deviations for the individual values were <0.06 mg/g. For total values, mean ± standard deviation is provided
Calculated as the molar equivalent of luteolin 7-O-glucoside.
Not quantified; below the limitation of quantification.
Calculated as the molar equivalent of apiin.
Two of the peaks in Figure 2A are composed of unresolved, overlapping peaks (peaks 14A and 14B and peaks 15A and 15B) and could not be quantified by UV absorption directly. For these peaks, SIM detection was used to determine the percentage contribution of each compound to the peak. The total concentration of the peak, based on UV absorption, was then multiplied by the appropriate fraction to provide a concentration for each compound.
Table 2 shows that, for the limited number of samples analyzed in this study, the total flavone content for the three celery materials ranged from 4.22 to 16.02 to 21.82 mg/g on a dry-weight basis, increasing from celery to Chinese celery to celery seed. The value of 4.22 mg/g (0.17 mg/g, wet weight) for the total flavonoid content of celery agrees with published data (9, 14). The total concentrations of the luteolin, apigenin, and chrysoeriol glycosides were 6.1, 2.4, and 4.9 times greater, respectively, for Chinese celery compared to celery. Celery seed had 36% more flavones than Chinese celery and contained more luteolin and chrysoeriol glycosides and less apigenin glycosides. Compared to the celery, Chinese celery contained many more leaves with smaller stalks, suggesting that the flavonoid content is highest in the leaves. This, however, was not tested directly.
The data in Table 2 show that the most abundant (60–80%) forms of each glycoside in Chinese celery and celery were the malonated flavonoids. In celery seeds, the malonated fraction was lower, but still constituted more than half of the total flavonoids.
This is the first report of 10 naturally occurring glycosylated flavone malonates in celery, Chinese celery, and celery seeds. Flavonoid glycoside malonates are known to widely exist in food plants, but the determination has been difficult because they are unstable and easy to convert to their corresponding glycosides. The malonate concentration will depend on sample storage and handling and, especially, the sample preparation methods (25, 27, 28). The sample preparation and detection method used in this study seem to be convenient for the identification of the flavonoid glycoside malonates in food plants.
Acknowledgments
This research was supported by the Office of Dietary Supplements at the National Institutes of Health under an Interagency Agreement.
Literature Cited
- 1.Robards K, Prenzler PD, Tucker G, Swatsitang P, Glover W. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999;66:401–436. [Google Scholar]
- 2.Robards K. Strategies for the determination of bioactive phenols in plants, fruits and vegetables. J Chromatogr A. 2003;1000:657–691. doi: 10.1016/s0021-9673(03)00058-x. [DOI] [PubMed] [Google Scholar]
- 3.Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2003;13:572–584. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 4.Galensa R, Hermann K. Flavone glycosides of leaves and tubers of celery (Apium graveolens L.). 9. Flavonols and flavones of vegetables. Z Lebensm-Unters -Forsch. 1979;169:170–172. Chem Abstr 91, 171672. [Google Scholar]
- 5.Garg SK, Gupt SR, Sharma ND. Coumarins from Apium graveolens seed. Phytochemistry. 1979;18:1580–1581. [Google Scholar]
- 6.Garg SK, Gupt SR, Sharma ND. Glucoside of Apium graveolens. Planta Med. 1980;38:363–365. [Google Scholar]
- 7.Leung AY, Foster S. Celery seed. In: Leung AY, Foster S, editors. Encyclopedia of Common Natural Ingredients Used in Food, Drugs and Cosmetics. 2nd. Wiley; New York: 1996. pp. 141–143. [Google Scholar]
- 8.Hertog MGL, Hollman PCH, Vernema DP. Optimization of a quantitative HPLC determination of potentially anticarcinogenic flavonoids in vegetables and fruits. J Agric Food Chem. 1992;40:1591–1599. [Google Scholar]
- 9.Crozier A, Lean MEJ, McDonald MS, Black C. Quantitative analysis of the flavonoid content of commercial tomatoes, onions, lettuce, and celery. J Agric Food Chem. 1997;45:590–595. [Google Scholar]
- 10.Miean KH, Mohamed S. Flanonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agric Food Chem. 2001;49:3106–3112. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- 11.Chu YF, Sun J, Wu X, Liu RH. Antioxidant and antiproliferative activities of common vegetables. J Agric Food Chem. 2002;50:6910–6916. doi: 10.1021/jf020665f. [DOI] [PubMed] [Google Scholar]
- 12.Ninfali P, Bacchiocca M. Polyphenols and antioxidant capacity of vegetables under fresh and frozen conditions. J Agric Food Chem. 2003;51:2222–2226. doi: 10.1021/jf020936m. [DOI] [PubMed] [Google Scholar]
- 13.Justesen U, Knuthsen P, Leth T. Quantitative analysis of flavonols, flavones, and flavanones in fruits, vegetables and beverages by high-performance liquid chromatography with photo-diode array and mass spectrometric detection. J Chromatogr A. 1998;799:101–110. doi: 10.1016/s0021-9673(97)01061-3. [DOI] [PubMed] [Google Scholar]
- 14.Sakakibara H, Honda Y, Nakagawa S. Simultaneous determination of all polyphenols in vegetable, fruits and teas. J Agric Food Chem. 2003;51:571–581. doi: 10.1021/jf020926l. [DOI] [PubMed] [Google Scholar]
- 15.Hollman PCH, Arts ICW. Flavonols, flavones and flavanols–nature, occurrence and dietary burden. J Agric Food Chem. 2002;50:1081–1093. [Google Scholar]
- 16.Eckey-Kaltenbach H, Heller W, Sonnenbichler J, Zetl I, Schaefer W, Ernst D, Sandermann H., Jr Oxidative stress and plant secondary metabolism: 6″-malonylapiin in parsley. Phytochemistry. 1993;34:687–691. [Google Scholar]
- 17.Matin A, Ferrenes F, Tomas-Barberan FA, Gil MI. Characterization and quantification of antioxidant constituents of sweet pepper (Capsicum annuum L.) J Agric Food Chem. 2004;52:3861–3869. doi: 10.1021/jf0497915. [DOI] [PubMed] [Google Scholar]
- 18.Papagiannopoulos P, Wollseifen HR, Mellenthin A, Haber B, Galensa R. Identification and quantification of polyphenolis in caros fruit (Ceratonia siliqua L.) and derived products by HPLC-UV-ESI/MSn. J Agric Food Chem. 2004;52:3784–3791. doi: 10.1021/jf030660y. [DOI] [PubMed] [Google Scholar]
- 19.Lin LZ, Harnly J. A screening method for the systematic identification of glycosylated flavonoids and other phenolic compounds using a standard analytical approach for all plant materials. J Agric Food Chem. 2007 doi: 10.1021/jf062431s. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Justesen U. Negative atmospheric pressure chemical ionization low-energy collision activation mass spectrometry for the characterization of flavonoids in extracts of fresh herbs. J Chromatogr A. 2000;902:369–379. doi: 10.1016/s0021-9673(00)00861-x. [DOI] [PubMed] [Google Scholar]
- 21.Cuyckems F, Cleays M. Mass spectrometry in the structural analysis of flavonoids. J Mass Spectrom. 2004;39:1–15. doi: 10.1002/jms.585. [DOI] [PubMed] [Google Scholar]
- 22.Svelikova V, Bennett RN, Mellon FA, Needs PW, Piacente S, Kroon PA, Bao Y. Isolation, identification and stability of acylated derivatives of apigenin 7-O-glucoside from chamomile (Chamomilla recutita [L.] Rauschert) Phytochemistry. 2004;65:2323–2332. doi: 10.1016/j.phytochem.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Lin LZ, He XQ, Lindenmaier M, Yang J, Cleary M, Qiu SX, Cordell GA. Liquid chromatography–elctrospray ionization mass spectroscopy study of the flavonoids of the roots of Atragalus mongholicus and A. membraceus. J Chromatogr A. 2000;876:87–95. doi: 10.1016/s0021-9673(00)00149-7. [DOI] [PubMed] [Google Scholar]
- 24.Lin LZ, He XQ, Lindenmaier M, Yang J, Cleary M, Qiu SX, Cordell GA. Study of the flavonoid glycoside malonates of red clover (Trifolium pretense) J Agric Food Chem. 2000;48:3541–365. doi: 10.1021/jf991002+. [DOI] [PubMed] [Google Scholar]
- 25.de Rijke E, Kamter FD, Ariese F, Brinkman UA, Gooijer C. Liquid chromatography coupled to nuclear magnetic resonance spectroscopy for the identification of isoflavone glucoside malonates in T. pretense L. leaves. J Sep Sci. 2004;27:1061–1070. doi: 10.1002/jssc.200401844. [DOI] [PubMed] [Google Scholar]
- 26.Materska M, Piacente S, Stochmal A, Pizza C, Oleszek W, Perucka I. Isolation and structure eluciadation of flavonoid and phenolic acid glycosides from pericarp of hot pepper fruit Capsicum annuum L. Phytochemistry. 2003;63:893–898. doi: 10.1016/s0031-9422(03)00282-6. [DOI] [PubMed] [Google Scholar]
- 27.Klejdus B, Vitamvásová-Štěrbová D, Kubáň V. Idnetification of isoflavone conjugates in red clover (Trifolium pretense) by liquid chromatography–mass spectrometry after two-dimensional solid-phase extraction. Anal Chim Acta. 2001;450:81–97. [Google Scholar]
- 28.Toebes AHW, Boer VD, Verkleij JAC, Lingeman H, Ernst WHO. Extraction of isolfavone malonyglucosides from Trifolium pretense L. J Agric Food Chem. 2003;53:4660–4666. doi: 10.1021/jf047995f. [DOI] [PubMed] [Google Scholar]