Abstract
The prevalence of chronic trapezius myalgia is high in women with high exposure to awkward working positions, repetitive movements and movements with high precision demands. The mechanisms behind chronic trapezius myalgia are not fully understood. The purpose of this study was to explore the differences in protein content between healthy and myalgic trapezius muscle using proteomics. Muscle biopsies from 12 female cleaners with work-related trapezius myalgia and 12 pain free female cleaners were obtained from the descending part of the trapezius. Proteins were separated with two-dimensional differential gel electrophoresis (2D-DIGE) and selected proteins were identified with mass spectrometry. In order to discriminate the two groups, quantified proteins were fitted to a multivariate analysis: partial least square discriminate analysis. The model separated 28 unique proteins which were related to glycolysis, the tricaboxylic acid cycle, to the contractile apparatus, the cytoskeleton and to acute response proteins. The results suggest altered metabolism, a higher abundance of proteins related to inflammation in myalgic cleaners compared to healthy, and a possible alteration of the contractile apparatus. This explorative proteomic screening of proteins related to chronic pain in the trapezius muscle provides new important aspects of the pathophysiology behind chronic trapezius myalgia.
Introduction
Trapezius myalgia remains a major problem in work tasks with high exposure to awkward working positions, repetitive movements and movements with high precision demands [1]. Although several pathophysiological models have been suggested (reviewed by Visser and Van Dieen 2006) [2], the aetiology behind trapezius myalgia is insufficiently elucidated. Several studies have investigated trapezius muscle structure and biochemistry in both healthy subjects and in patients with chronic trapezius myalgia [3], [4]. In myalgic muscle, an increased fiber area of type 1 muscle fibers has been recognized [4], [5]. An inability to utilize oxygen in myalgic trapezius has been suggested based on changes in cyclooxygenase (COX) protein complex IV of the mitochondrial respiration chain. COX negative fibers have been found in muscle biopsies from women suffering from trapezius myalgia [6]. Other studies have shown COX negative fibers to be connected to work exposure, as COX negative fibers prevailed in both pain and pain free subjects with the same kind of work exposure [7]. However, there seems to be a substantial biological variation in the presence of COX-negative fibers among subjects with and without trapezius myalgia [7]. There are also reports of muscle alterations probably due to mitochondrial disturbances such as moth-eaten and ragged-red fibers [7]. In more recent studies, using microdialysis (MD) [8], increased concentrations of pyruvate and lactate in the myalgic trapezius muscle have been reported [9], [10], [11], suggesting alterations in energy metabolism. Furthermore, several studies using MD report increased interstitial levels of serotonin and glutamate in chronic trapezius myalgia [12]. The majority of biochemical studies conducted on myalgic trapezius muscle has addressed a few biochemical parameters in each study [1], [12]. A more complete picture of the underlying mechanisms behind trapezius myalgia has not yet been obtained; hence more comprehensive studies are needed.
Advances in research technology allow investigation of a substantial part of the protein content in a tissue. Two-dimensional difference gel electrophoresis (2D-DIGE) [13] in combination with mass spectrometry has been used for simultaneous screening of differentially expressed proteins in different chronic pain conditions. The proteome of the cerebrospinal fluid in healthy subjects, in patients with herniated disc and idiopathic back pain have been investigated [14], [15], [16]. Also, nerve samples from patients with complex regional pain syndrome (CRPS) have been analyzed in order to detect up/down regulated proteins [17]. Proteomics of serum have been used for the identification of possible biomarkers of chronic endometriosis [18], [19]. In addition, several proteomic studies have investigated human skeletal muscle exposed to high altitude [20], bed rest [21], [22], and exercise [23]. Also, differences in protein content comparing different human muscles has been studied [24], [25], showing protein patterns related to muscle function.
The aim was to explore differentially expressed proteins in muscle biopsies of the trapezius muscle in female cleaners with chronic trapezius myalgia compared to healthy female cleaners using a combination of a proteomic screening method and multivariate modeling.
This explorative comprehensive screening is expected to provide new clues regarding differences in protein content between myalgic and healthy trapezius muscle. The clues presented allows the creation of new hypotheses for the pathophysiology of myalgic muscle, which in the future will provide a better understanding regarding the maintenance of myalgia, and facilitate the creation of diagnostic tools and treatment. This screening provides an important hallmark as it presents a novel exploration of the myalgic muscle tissue proteome.
Subjects and Methods
Subjects
Twelve female cleaners with work-related trapezius myalgia (MYA) and twelve female cleaners without work-related trapezius myalgia (CON) participated in the study. The mean (± one standard deviation, ±1 SD) age, height and mass for MYA was 42±8 years, 165±6 cm, 71±12 kg and for CON 41±8 years, 165±6 cm and 68±16 kg. The main work duty of all cleaners was manual floor cleaning requiring a high work demand on the trapezius muscle [26]. Trapezius myalgia was considered work related when reported to have started while working as a cleaner and when it worsened during the working day. Pain duration for the participants in the myalgia group was on average 7 years. The female cleaners comprising the control group reported no neck pain at all or neck pain for a maximum of 2–3 days during the previous 12 months.
Subjects taking oral steroids or non-steroidal anti-inflammatory drugs were excluded from this study. No one suffered from trauma to the neck region, diabetes mellitus, rheumatic diseases, generalized pain, or fibromyalgia, hence fulfilling the criteria of the American College of Rheumatism 1990 [27]. After receiving written information about the study, all subjects signed a consent form that was in accordance with the Declaration of Helsinki. The study was granted ethical clearance by the ethics committees of the University of Lund and University of Linköping (dnr: Dm131-07).
Clinical Examination
Participants underwent a standardised clinical examination [28] to ensure that the myalgic patients and healthy subjects met the inclusion criteria. The clinical examination included questions on pain, tiredness and stiffness on the day of examination. Also, physical tests were performed, including range of motion and tightness of muscles, pain threshold and sensitivity, muscle strength and palpation of tender points. The trapezius myalgia diagnosis included: neck pain, tightness of the trapezius muscle (a feeling of stiffness in the descending part of the trapezius muscle during lateral flexion of the head) and palpable tender parts in the trapezius muscle. The range of motion of the columna had to be normal or just slightly decreased [28]. The clinical examination protocol allows the examiner to exclude subjects with pain in the trapezius region likely referred from painful tendons or nerve compressions in the neck and shoulder area.
Biopsy Collection
Open surgical samples biopsies were taken from the descending part of the trapezius muscle. In the MYA group samples were taken on the myalgic side and in the CON group samples were obtained from the dominant side. If the subjects in the MYA group had bilateral trapezius myalgia the most painful side was used for biopsy collection. The incision was placed 2 cm lateral of the midpoint between the 7th process of the cervical spine and the lateral part of the acromion process. The skin and the subcutaneous area were infiltrated with 3–5 ml 5% xylocaine. Care was taken not to infiltrate the fascia or the muscle with xylocaine. A piece of muscle tissue, approximately 0.5 cm×0.5 cm×0.5 cm, was carefully removed and placed in a humid chamber for 15 min during transportation. The samples were then oriented and mounted for transverse sectioning in Optimal Cutting Temperature (OTC) compound (Tissue Tek, Miles laboratories, Naperville, Ill., USA) and frozen in chilled liquid propane and stored at −80°C until use.
Two-Dimensional Difference Gel Electrophoresis (2-D DIGE) and Image Analysis
Unless otherwise stated, all chemicals were from GE healthcare, Uppsala, Sweden and of proteomic grade quality. The protocol has previously been evaluated [29]. The frozen muscle samples were suspended in lysis buffer (9.5 M Urea, 4% (v/w) CHAPS and 30 mM Tris Base) and homogenized with a Grinding Kit. Quantification of protein content was made with a 2D-Quant Kit. Labelling of protein samples, containing 50 µg of protein, was made with CyDye minimal dyes, Cy2, Cy3 and Cy5, in accordance to manufacturers’ protocol. The internal standard method was used [30] where a pooled internal standard containing sample from each of all the biopsy samples included in the analysis, labelled with Cy2 was incorporated. Twelve biopsies from twelve different patients suffering from trapezius myalgia and twelve biopsies from twelve different healthy subjects were alternately labelled with Cy5 and Cy3. Added to each gel three samples, Cy2, Cy3 and Cy5, from healthy, myalgic and pooled internal standard were pooled to be simultaneously separated. An equal volume of lysis buffer was added. An IPG-buffer pH 3–11 was added to reach a concentration of 2% (w/v). DeSteak™ rehydration solution was added to a final volume of 450 µl. The samples were applied onto 24 cm 3–11 Non-Linear (NL) Immobilised pH gradient (IPG) strips and rehydrated at room temperature in the dark for 16 hours. The first dimension was run using Amersham Ettan™ IPGphor unit applying 300 V for 900 Vhrs, 600 V for 1800 Vhrs, 1000 V for 3000 Vhrs, 5000 V for 55000 Vhrs. Prior to the second dimension the gel strips was equilibrated for 10 minutes in equilibration buffer (50 mM 1.5 M Tris HCl pH 8.8, 6 M Urea, 30% (v/v) Glycerol (87%), 2% (w/v) SDS, trace of Bromophenol Blue and 0.5% DTT). Gels were then alkylated by further equilibration for 10 minutes in the same buffer, containing 4.5% (w/v) Iodoacetamide instead of DTT. The second dimension was conducted by using the Ettan DALT six apparatus as stips were loaded onto a 12.5% acrylamide gel. Gels were run simultaneously at 5 W per gel, 600 V, 400 mA for 30 minutes, followed by additional 5 hours or until the blue front reached the bottom of the gel, with 17 W per gel 600 V 400 mA of a constant temperature of 15°C. Gels were then immediately scanned with a Typhoon™ 9410 scanner, using 488 nm laser and emission filter of 520 BP40 for Cy2 labelled proteins, 532 nm laser and emission filter 580 nm BP30 for Cy3 and 633 nm laser and 670 nm BP30 for Cy5. The scanned image was further processed using ImageQuant™ V5.2, before protein abundance was determined and statistical evaluation was made using DeCyder™ V6.5. After differential in gel analysis (DIA), gels were batch processed with exclusion filters set at max slope: 1.0, min spot volume 20.000. Protein spots that occurred in >50% of the gels were included in the analysis.
Protein Identification by LC-MS/MS
In gels used for protein identification 450 µg unlabelled proteins were loaded and analysed as above. Protein spots were excised using Ettan™ Spot Handling Workstation, with a ∅ 2.0 mm picker head. The picked protein spots were digested with trypsin (Promega/SDS Biosciences, Falkenberg, Sweden). Briefly, the gel pieces were washed with a mixture of acetonitrile/ammonium bicarbonate, dehydrated with acetonitrile and incubated with 20 µl of 20 µg/ml trypsin overnight at 37°C. The supernatant was transferred to a new tube and the peptides further extracted from the gel by incubation in 50% acetonitrile/5% trifluoroacetic acid for about 3 hours at room temperature with occasional mixing. The supernatant obtained by the two steps pooled, dried by SpeedVac and dissolved in 6 µl of 0.1% formic acid. Peptides were analysed using an on-line nano-flow HPLC system (EASY-nLC; Proxeon, Bruker Daltonics) in conjugation with the mass spectrometer HCTultra PTM Discovery System (Bruker Daltonics). A 100 mm×75 µm C18 column was used for separation at a flow rate 300 nL/min. The gradient buffers used were 0.1% formic acid in water (buffer A) and 0.1% formic acid in acetonitrile (buffer B) and a linear gradient from 0–100% buffer B in 40 min was used for separation. The automated online tandem MS analysis was performed using collision induced dissociation of peptide ions.
Statistical Analysis
Statistical evaluation of the protein abundance in the 2D-DIGE analysis was made using the Biological Variation Analysis (BVA) module in DeCyder™ V6.5 and multivariate modeling was performed using SIMCA-P version 12 (Umetrics AB, Umeå, Sweden). In all statistical analyses, the log of the standardized abundance derived from the ratio of each pooled protein group (each gel) normalized by the internal standard was used. In the Differential In-gel Analysis (DIA) 1330 protein spots were included in the BVA. Spots with slope values <1.0 were considered non-protein spots and not included in the analysis. In data-sets from 2D-DIGE analysis the number of variables (protein spots) greatly exceeds the number of observation and the variables are often highly correlated.
Multivariate projection methods have the advantage in being able to deal with such data. In this study both principal component analysis (PCA) and projections to latent structures with discriminant analysis (PLS-DA) was used. PCA is an unsupervised projection method to extract and display systematic variation between×variables. PLS-DA is a supervised linear regression method to find a relationship between×variables and in this case a binary Y vector using class membership data. This method, in contrast to classical statistical methods, does not assume that a high subject-to-variables ratio is present (5–10). Such requirements are not required for PCA or PLS, in fact PLS-DA can handle ratios lower than 1.0. In the multivariate data analysis (PCA and PLS-DA) the spot volume ratios were mean centered and scaled for unified variance. PCA was used to detect multivariate outliers among the observations - e.g., due to technical problems with gels - and as a general overview of the data. In the PLS-DA modeling the number of PLS-DA components was determined by full cross-validation (SIMCA-P version 12, Umetrics AB, Umeå, Sweden). This method keeps part of the data out from the model development to assess the predictive power of the model and was used to test the significance of the components. Hence, this validation technique increases the stability of the results. Such validation is not implemented in other common statistical packages e.g., SPSS. The PLS-DA model was validated by both response permutation tests and CV-ANOVA on the residuals [31]. Only protein spots present in at least 50% of the gels and with ratio of between subject variance vs. within subject variance falling within the 15th percentile of an F-distribution (equal to using a cut-off at p-value = 0.3 in a one-way ANOVA) were selected for PLS-DA modeling and identification.
Database Searches
Spectra were processed by Bruker Daltonics DataAnalysis 3.4 (Bruker Daltonics, Bremen, Germany) and resulting MS/MS data were searched in NCBInr and Swiss-Prot database on MASCOT server (www.matrixscience.com). Database search parameters were set as follows: the enzyme trypsin was used; up to one missed cleavage was allowed; fixed modification included were carbamidomethylation of cysteine and oxidation of methionine; mass tolerance for MS precursor ion was 0.8 Da and for MS/MS fragment ion was 0.6 Da; and charge states were varied. Criteria for identification of a protein were at least 3 peptides of the protein should be identified with a MASCOT score over 25 and an expectation value <1.
Results
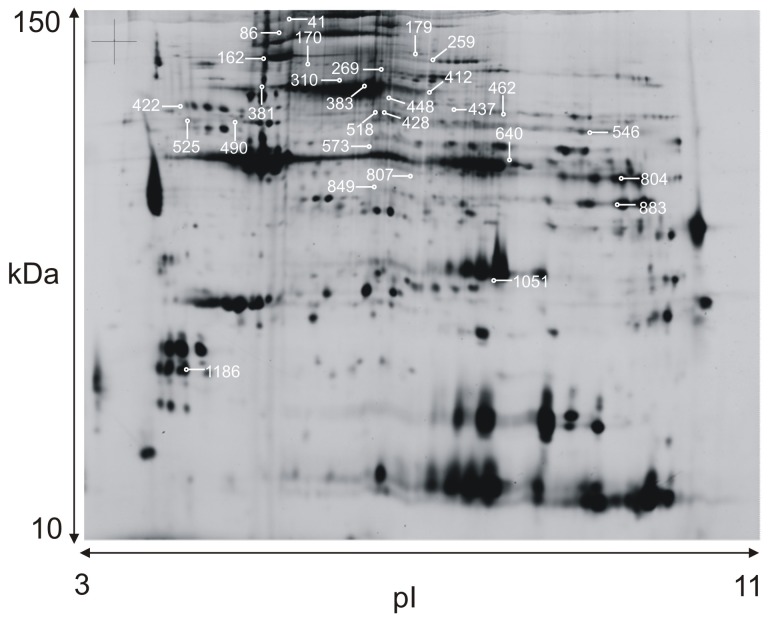
A typical 2-DE protein pattern of human trapezius muscle is shown in figure 1 . Altogether 1330 protein spots were detected. Eight-hundred forty-seven protein spots that were presented in at least 50% of the gels were included in the analysis of protein spot intensity in biopsy samples taken from controls and myalgic subjects. Of these, 170 protein spots met the criteria for identification, generating 162 identified proteins (table S1).
Figure 1. A typical 2-DE gel pattern of human trapezius muscle homogenate.
(pH 3–11 and 12.5% SDS). Identified proteins marked with spot ID numbers, available in table 1.
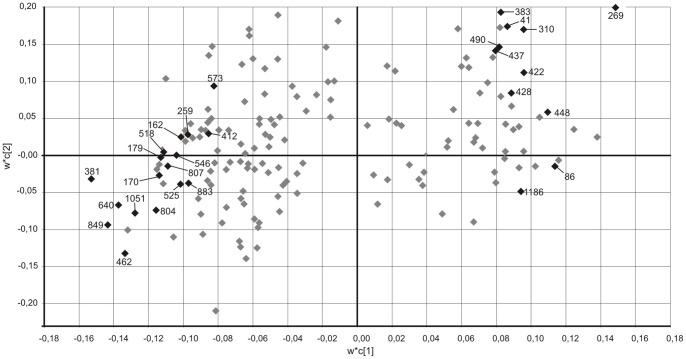
Of selected protein spots for identification 37 showed VIP-values of 1 or higher and 7 with a VIP-value of 1.5 or higher and yielding 28 unique proteins separating healthy and myalgic muscle ( figure 2 ). Some proteins were identified in multiple isoforms. The 28 identified proteins were considered to belong to five groups based on their function: metabolic (n = 10), contractile (n = 8), acute response (n = 3), structural (n = 5), and other (n = 2) proteins ( table 1 ).
Figure 2. PLS-DA weight plot.
Weight plot (w*c[1]/w*c[2]) from the PLS-DA model of healthy and myalgic muscle based on 170 protein spots. Situated on the left in the figure are proteins with a higher abundance in the myalgic muscle and on the right, proteins with a higher abundance in healthy muscle. Analyzed spots (◊) with variable of importance values (VIP)<1.
Table 1. Protein Spot with Variable of importance (VIP) value >1.0 in the PLS-DA multivariate model.
| ID (spotnr) | Protein ID (SwissProt) | Protein | Mw/pI | MS Score | No. of peptides | Av. Ratio (MYA/CON) | VIP PLS-DA |
| Metabolic | |||||||
| 269 | P11217 | Glycogen phosporylase, muscle form | 97.5/6.6 | 134 | 4 | 1.47 | 1.9 |
| 462 | P14618 | Pyruvate kinase isoenzymes M1/M2 | 58.5/8.0 | 472 | 14 | −1.16 | 1.7 |
| 640 | P13929 | Beta Enolase | 46.8/7.7 | 1010 | 16 | −1.11 | 1.6 |
| 804 | P04075 | Fructose bisphosphate adolase A&C | 39.4/8.3 | 2795 | 14 | −1.18 | 1.4 |
| 170 | Q99798 | Aconitate hydratase, mitochondrial | 86.1/7.4 | 146 | 4 | −1.40 | 1.3 |
| 310 | Q02218 | 2-oxoglutarate dehydrogenase, mitochondrial | 117/6.4 | 282 | 7 | 1.23 | 1.3 |
| 518 | P13645 | Creatine kinase, M-type | 43/6.8 | 142 | 2 | −1.16 | 1.3 |
| 546 | P25705 | ATP synthase, subunit alpha | 55/8.3 | 129 | 4 | −1.46 | 1.2 |
| 883 | P04406 | Glyceraldehyde-3-phosphate dehydrogenase | 35.9/8.6 | 2062 | 14 | −1.16 | 1.2 |
| 437 | P36871 | Phosphoglucomutase-1 | 61.7/6.3 | 797 | 19 | 1.07 | 1.1 |
| Contractile | |||||||
| 381 | P60709 | Actin, cytoplasmic 1 | 41.6/5.3 | 64 | 2 | −1.24 | 1.8 |
| 41 | P62736 | Actin aortic smooth muscle | 42.4/5.23 | 99 | 3 | 1.36 | 1.3 |
| 383 | Q00872 | Myosin binding protein C, slow type | 129/5.8 | 273 | 10 | 1.18 | 1.3 |
| 448 | P12883 | Myosin-7, slow | 223/5.6 | 207 | 6 | 1.25 | 1.3 |
| 1186 | Q96A32 | Myosin regulatory light chain 2, skeletal muscle isoform | 19.01/4.9 | 93 | 13 | 1.83 | 1.2 |
| 162 | P13533 | Myosin-6, fast | 223.7/5.6 | 140 | 8 | −1.42 | 1.2 |
| 573 | Q9UKX2 | Myosin-2 | 224/5.6 | 638 | 23 | −1.12 | 1.1 |
| 412 | P12883 | Myosin-7, slow | 223/5.6 | 93 | 6 | −1.52 | 1.0 |
| Acute response | |||||||
| 86 | P08107 | Heat shock 70 kDa protein | 70.2/5.5 | 944 | 25 | 1.16 | 1.4 |
| 1051 | P07451 | Carbonic anhydrase 3 | 29.4/6.9 | 146 | 13 | −1.32 | 1.5 |
| 422 | P01009 | Alpha-1-antitrypsin | 46.7/5.4 | 99 | 2 | 1.31 | 1.2 |
| Structural | |||||||
| 849 | P04264 | Keratin, type II cytoskeletal 1 | 66/8.1 | 382 | 13 | −1.28 | 1.7 |
| 259 | O75112 | LIM domain-binding protein 3 | 77/8.5 | 155 | 2 | −1.40 | 1.2 |
| 525 | P07437 | Tubulin beta chain | 50/4.8 | 787 | 16 | −1.17 | 1.2 |
| 428 | P40123 | Adenylyl cyclase associated protein 2 | 53/5.9 | 135 | 4 | 1.20 | 1.1 |
| 490 | Q8TD99 | Desmin | 53.5/5.2 | 1059 | 39 | 1.28 | 1.1 |
| Other | |||||||
| 179 | P02768 | Serum albumin | 66.5/5.7 | 381 | 13 | −1.13 | 1.4 |
| 807 | P82650 | 28S ribosomal protein S22, mitochondrial | 41/7.7 | 130 | 4 | −1.27 | 1.3 |
Proteins identified with LC-MS/MS, MALDI-TOF-MS/MS. ID is the same as in figure 2 (Weight-plot PLS-DA).
The Metabolic proteins identified and of interest for separating healthy trapezius from myalgic trapezius were mainly related to glycolysis. In the myalgic muscle there were a higher abundance of glycogen phosphorylase; muscle form, 2-oxoglutarate dehydrogenase; mitochondrial, and phosphoglucomutase-1. In healthy muscle there were a higher abundance of pyruvate kinase isoenzymes M1/M2, beta enolase, fructose bisphosphate adolase A&C, aconitate hydratase; mitochondrial, creatine kinase; M-type, ATP synthase; subunit alpha and glyceraldehyde-3-phosphate dehydrogenase. Contractile proteins that were more abundant in myalgic muscle were actin aortic smooth muscle, myosin binding protein C slow type, myosin-7 slow and myosin regulatory light chain 2 fast; skeletal muscle isoform. In healthy muscle there was a higher abundance of the contractile proteins: myosin-6 fast, myosin-2, myosin-7 slow and actin; cytoplasmic 1.
The identified acute response proteins that separated the two groups were heat shock 70 kDa protein and alpha-1-antitrypsin, more abundant in myalgic trapezius; and carbonic anhydrase 3 more abundant in healthy trapezius. Structural proteins found to be more abundant in myalgic trapezius were adenylyl cyclase associated protein 2 and desmin. In healthy trapezius muscle keratin; type II cytoskeletal 1, LIM domain-binding protein 3 and tubulin beta chain were more abundant.
Two other proteins of relevance for separation between healthy and myalgic trapezius were serum albumin and 28S ribosomal protein S22, mitochondrial, both more abundant in healthy trapezius muscle.
Through PLS-DA model validation using response permutation test (Q2 intercept = -0.05, Q2(cum) = 0.65) and CV-ANOVA of residuals (F = 2.94, p = 0.0023) a two component model with a total of 29% explained variance (R2(cum) = 0.29)(Component 1∶16%, Component 2∶13%) was used to avoid over fitting the data.
Discussion
In this study, muscle biopsies from women with and without chronic trapezius myalgia and with the same external work exposure were compared using 2D-DIGE and PLS-DA. Proteins correlating to either myalgic or healthy subjects in the PLS-DA ( figure 2 ) were grouped as metabolic, contractile, regulatory, structural, acute response and other proteins, based on their biochemical function ( table 1 ).
Acute Response Proteins
The expression levels of heat shock 70 kDa (HSP 70) protein and alpha-1-antitrypsin, proteins involved in stress and inflammatory responses, were increased in cleaners with chronic trapezius myalgia compared to healthy cleaners. HSP70 kDa protein is an ATP binding protein that stabilizes pre-existing proteins against aggregation, prevents mis-location and facilitates protein folding, [23], [32]. Its expression level is highly inducible and the synthesis is increased in response to multiple stressors e.g., hyperthermia [33], energy depletion [34], hypoxia [35] and reactive oxygen species [36]. Alpha-1-antitrypsin is an acute phase protein with a broad anti-inflammatory spectrum [37]. It is released by macrophages in response to inflammation and has been suggested to control inflammatory components associated with fibromyalgia in musculoskeletal connective tissue [38].
A decreased level of carbonic anhydrase III, involved in oxidative processes, was found in cleaners with chronic trapezius myalgia. Carbonic anhydrase III is expressed predominantly in skeletal muscle and has been suggested to be an indicator of muscle damage [39]. It has been proposed that this protein functions as an essential anti-oxidant agent in skeletal muscle [40]. Decreased levels of carbonic anhydrase III in the myalgic muscle may indicate an imbalance in the cellular redox potential in this condition.
Taken together, these facts might indicate activated inflammatory mechanisms and alterations in anti-oxidant protection in chronic trapezius myalgia. These results have to be confirmed in future studies.
Alterations in Metabolic Proteins
Glycogen phosphorylase and phosphoglucomutase-1 were increased in cleaners with trapezius myalgia ( table 1 ). The catalytic action of glycogen phosphorylase is to break down muscle glycogen to Glucose-1-phosphate (G1P) and is the main regulatory step in glycogenesis ( figure 3 ). Glycogen phosphorylase is activated by adrenaline and insulin through phosphorylation allosterically by adenosine monophosphate (AMP) or by substrate control through available inorganic phosphate. In healthy subjects glycogen phosphorylase transformation is related to energy state of the muscle cell [41]. The other major enzyme occurring with a higher abundance in myalgic muscle is phosphoglucomutase-1, which catalyzes the conversion of G1P to Glucose-6-phosphate which enters the glycolysis. The changes in the glycolytic enzymes of both glycogen phosphorylase and phosphoglucomutase-1 in skeletal muscle suggest an increased need or utilization of glucose from glycogen storages in the myalgic trapezius muscle in habitual daily activities. This is in contrast to skeletal muscles in healthy subjects where glycogen utilization at rest is low or absent [42]. Also, the energy utilization when the maximal oxygen consumption is below 35% relies mainly on plasma free fatty acid and glucose and not glycogen storages [43].
Figure 3. Schematic figure of the metabolic pathways: glycogenesis and glycolysis.
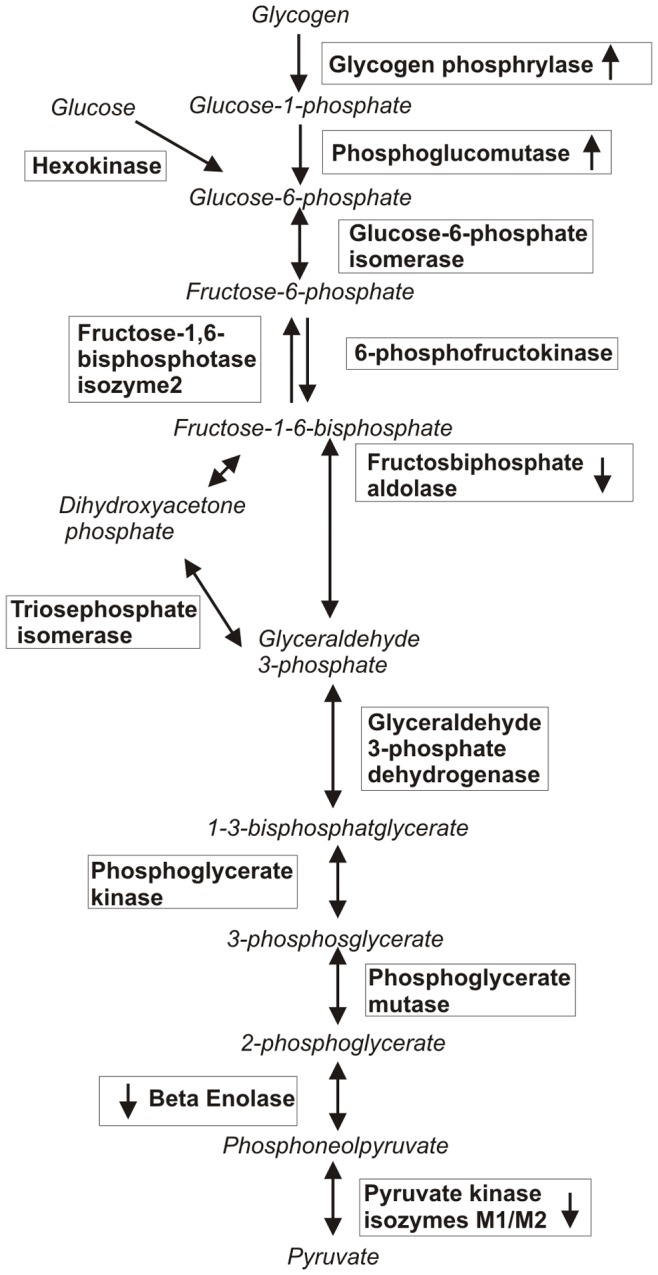
Regulating enzymes are written in frames, arrows within frames indicate increased or decreased abundance in myalgic muscle compared to healthy muscle.
The other glycolytic enzymes that differed in abundance were fructose bisphosphate adolase A&C, beta enolase and pyruvate kinase isoenzymes M1/M2, all in the later part of the glycolysis ( figure 3 ). The levels of abundance of these proteins were lower in MYA than in CON. Increased lactate concentrations have been shown to dissociate the key regulatory enzyme phosphofructokinase tetramers into dimers and thus reducing the enzymes activity and the glycolytic flux later part of the glycolysis [44]. Interstitial trapezius muscle concentrations of lactate have been reported to be significantly increased in chronic trapezius myalgia in six out of seven previous studies [45].
Pyruvate kinase isoenzyme M1/M2, showing a lower abundance in myalgic muscle compared to healthy, is a main glycolysis regulatory enzyme ( figure 3 ) [46]. In muscle fibers, M1 is the major isoform [47]. In MD studies, increased pyruvate and lactate levels in myalgic trapezius muscle have been found [9], [10]. MD studies determine the extracellular concentrations of these substances while proteomics reflect both intra- and extracellular processes. One often proposed mechanism behind the increased interstitial concentrations of lactate is an increased reliance on anaerobic energy production in the myalgic muscle. However, increased concentrations of lactate can also occur during adequate oxygen provision [48], as lactate is also considered a systemically active metabolite capable of moving between cells and tissues, where it may be oxidized as a fuel or reconverted to form pyruvate or glucose [49], [50], [51]. Furthermore the interstitial concentrations of lactate is also dependent upon lactate dehydrogenase (LDH) and monocarboxylate transporters [52]. An increased anaerobic metabolism is not supported by the data presented in this study. It has been suggested that accumulation of pyruvate and lactate could occur if there is a higher flux through the glycolysis than the aerobic oxidative system can handle. Since none of the identified proteins related to the mitochondrial respiratory chain differed in abundance between cleaners with myalgic trapezius and healthy trapezius, this screening shows no evidence of pain related differences in the oxidative metabolism of female cleaners. Previous results regarding complex IV of the respiratory chain cyclooxygenase (COX) are in coherence with our results [6], [7].
Alterations in Contractile and Structural Proteins
Comparative proteomic studies of different muscles, proteins related to oxidative metabolism have been shown to relate to the unique fiber type composition of the muscles [24], [25]. Previous results regarding fiber type composition of myalgic muscle in comparison to healthy are diverse [4], [6]. The main hypothesis supports an alteration towards more or larger type 1 fibers [5], [53]. Although there have been contradictory results both when comparing the fiber type content between myalgic and healthy muscle [54], and also when investigating differences in myosin heavy chain abundance [55]. The results of a higher abundance of type 1 fibers in myalgic muscle supports the theory that more type 1 fibers are recruited due to low load work [53]. As these results are not reproduced in other studies using larger research material [54], [56] this opens for other mechanisms explaining the muscle adaptation towards low-load work and muscle stiffness experienced by myalgic patients. Our results indicate a higher abundance of myosin light chain fast 2 regulatory in myalgic muscles. Myosin light chain fast 2 is predominant in fast contracting type 2 fibers [24], [25], [57], [58]. A higher occurrence of the protein in myalgic muscle might be due to an altered contractility of the myalgic muscle.
Creatine kinase M-type, was more abundant in the healthy muscle compared to the myalgic and is a metabolic protein more abundant in fast twitch fibers compared to slow twitch. Creatine kinase is used as a marker of energy turnover in the cell. Creatine kinase M produces phosphocreatine from mitochondrial ATP processes together with creatine. Phosphocreatine provides ATP for muscle contraction, or ion pumps, like the calcium pump for muscle relaxation and also serves as an energy buffer and an energy transporter [59].
Other contractile proteins that were more abundant in muscle biopsies from cleaners with trapezius myalgia were aortic smooth muscle actin, myosin binding protein C slow type and fragments of myosin 7 slow. Contractile proteins that were more abundant in healthy muscle were actin cytoplasmic 1 and fragments of myosin-6 fast, myosin-2 and myosin-7 slow. Proteins building the intermediate filaments and cytoskeleton are grouped as structural proteins. Healthy muscle has a higher abundance of keratin, LIM-binding protein 3 and tubulin beta chain. Myalgic muscle has a higher abundance of desmin and adenylyl cyclase associated protein 2. Desmin is a major intermediate filament of muscle fibers. The lack of desmin results in muscle dystrophy with disruption of muscle fiber integrity. Muscles from knock-out mice lacking desmin also become progressively stiffer and accumulate increased collagen in a degenerating process [60]. The increased abundance of desmin in the biopsies from myalgic may indicate remodeling of the cytoskeleton or a muscle regeneration process. In agreement with this an increased myogenic activity and increased myonuclear content have been reported when comparing myalgic and healthy muscle [61]. Moreover, a recent proteomic study of the interstitium of chronic trapezius myalgia indicated profound proteomic alterations in myalgia [62]. At the present it is unclear whether these alterations in contractile and structural proteins are primarily linked to nociceptive/inflammatory or metabolic processes or whether they may be secondary consequences of having a muscle in persistent pain e.g., deconditioning, altered activation patterns etc.
Methodological Considerations
Two-dimensional gel electrophoresis is a useful comprehensive method, but choice of gel composition and buffer solutions will determine the type of proteins detected [63]. High molecular weight proteins are difficult to isolate on 2-DE and low molecular weight proteins are difficult to identify because only a few proteolytic fragments are generated by the tryptic digestion and so the database search leads to an uncertain identification. It is also important to keep in mind that what can be visualized by the staining method used in this study is only a top fraction of the proteins expressed in human trapezius muscle, and it can be expected that there are still several proteins at low abundance below the detection level that have not been evaluated.
Conclusions
Proteomic analyses of biopsies from women with trapezius myalgia provide new insights into biological mechanisms that might reveal important aspects of the pathophysiology of trapezius myalgia. A variety of proteins that are involved in glycolysis, tricaboxylic acid cycle, contractile apparatus, cytoskeleton and acute response processes showed differential expression on the 2-DE gels. The results suggest altered metabolism, a higher abundance of proteins related to inflammation in myalgic cleaners compared to healthy, and a possible alteration of the contractile apparatus. Further studies will be required to provide a complete picture of the protein patterns of myalgic muscle. However, results presented here show extensive alterations in the proteome of myalgic muscles and provide new clues concerning the pathophysiological mechanisms behind chronic myalgia. These results are important in the creation of new hypotheses for the pathophysiology of myalgic muscle which in a future aspect could facilitate diagnosis and treatment of myalgic patients.
Supporting Information
Identified proteins by LC-MS/MS or MALDI-TOF-MS/MS.
(DOCX)
Funding Statement
This study was funded by the Swedish Research Council (K2011-69X-21874-01-6) and the Swedish Council for Working life and Social research (2009-1761, 2010-0913). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Larsson B, Sogaard K, Rosendal L (2007) Work related neck-shoulder pain: a review on magnitude, risk factors, biochemical characteristics, clinical picture and preventive interventions. Best Pract Res Clin Rheumatol 21: 447–463. [DOI] [PubMed] [Google Scholar]
- 2. Visser B, van Dieen JH (2006) Pathophysiology of upper extremity muscle disorders. J Electromyogr Kinesiol 16: 1–16. [DOI] [PubMed] [Google Scholar]
- 3. Lindman R, Eriksson A, Thornell LE (1991) Fiber type composition of the human female trapezius muscle: Enzyme-histochemical characteristics. Am J Anat 190: 385–392. [DOI] [PubMed] [Google Scholar]
- 4. Kadi F, Waling K, Ahlgren C, Sundelin G, Holmner S, et al. (1998) Pathological mechanisms implicated in localized female trapezius myalgia. Pain 78: 191–196. [DOI] [PubMed] [Google Scholar]
- 5. Andersen LL, Suetta C, Andersen JL, Kjaer M, Sjogaard G (2008) Increased proportion of megafibers in chronically painful muscles. Pain 139: 588–593. [DOI] [PubMed] [Google Scholar]
- 6. Kadi F, Hägg G, Håkansson R, Holmner S, Butler-Browne GS, et al. (1998) Structural changes in male trapezius muscle with work-related myalgia. Acta Neuropathologica 95: 362–360. [DOI] [PubMed] [Google Scholar]
- 7. Larsson B, Bjork J, Henriksson KG, Gerdle B, Lindman R (2000) The prevalences of cytochrome c oxidase negative and superpositive fibres and ragged-red fibres in the trapezius muscle of female cleaners with and without myalgia and of female healthy controls. Pain 84: 379–387. [DOI] [PubMed] [Google Scholar]
- 8. Ungerstedt U (1991) Microdialysis-principles and applications for studies in animals and man. J Intern Med 230: 365–373. [DOI] [PubMed] [Google Scholar]
- 9. Rosendal L, Larsson B, Kristiansen J, Peolsson M, Sogaard K, et al. (2004) Increase in muscle nociceptive substances and anaerobic metabolism in patients with trapezius myalgia: microdialysis in rest and during exercise. Pain 112: 324–334. [DOI] [PubMed] [Google Scholar]
- 10. Sjogaard G, Rosendal L, Kristiansen J, Blangsted AK, Skotte J, et al. (2009) Muscle oxygenation and glycolysis in females with trapezius myalgia during stress and repetitive work using microdialysis and NIRS. Eur J Appl Physiol 108: 657–669. [DOI] [PubMed] [Google Scholar]
- 11. Flodgren G, Crenshaw AG, Hellström F, Fahlström M (2010) Combining microdialysis and near-infrared spectroscopy for studying effects of low-load repetitive work on the intramuscular chemistry in trapezius myalgia. J Biomed Biotechnol 2010: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerdle B, Larsson B (2012) Potential muscle biomarkers of chronic myalgia in humans - a systematic review of microdialysis studies; Khan APTK, editor. INTECH open Access publisher. 103–132 p.
- 13. Unlu M, Morgan M, Minden J (1997 ) Difference gel electrophoresis: a single gel method for detecting changes in protein extracts. Electrophoresis 18: 2071–2077. [DOI] [PubMed] [Google Scholar]
- 14. Finehout EJ, Franck Z, Lee KH (2004) Towards two-dimensional electrophoresis mapping of the cerebrospinal fluid proteome from a single individual. Electrophoresis 25: 2564–2575. [DOI] [PubMed] [Google Scholar]
- 15. Yuan X, Russell T, Wood G, Desiderio DM (2002) Analysis of the human lumbar cerebrospinal fluid proteome. Electrophoresis 23: 1185–1196. [DOI] [PubMed] [Google Scholar]
- 16. Liu J, Jia H, Yang Y, Dai W, Su X, et al. (2009) Cerebrospinal fluid cytology and clinical analysis of 34 cases with leptomeningeal carcinomatosis. J Int Med Res 37: 1913–1920. [DOI] [PubMed] [Google Scholar]
- 17. Oki G, Wada T, Iba K, Aiki H, Sasaki K, et al. (2012) Metallothionein deficiency in the injured peripheral nerves of complex regional pain syndrome as revealed by proteomics. Pain 153: 532–539. [DOI] [PubMed] [Google Scholar]
- 18. Ferrero S, Gillott DJ, Remorgida V, Anserini P, Ragni N, et al. (2008) Peritoneal fluid proteome in women with different ASRM stages of endometriosis. Gynecol Endocrinol 24: 433–441. [DOI] [PubMed] [Google Scholar]
- 19. Seeber B, Sammel MD, Fan X, Gerton GL, Shaunik A, et al. (2009) Proteomic analysis of serum yields six candidate proteins that are differentially regulated in a subset of women with endometriosis. Fertil Steril 93: 2137–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Viganò A, Ripamonti M, De Palma S, Capitanio D, Vasso M, et al. (2008) Proteins modulation in human skeletal muscle in the early phase of adaptation to hypobaric hypoxia. Proteomics 8: 4668–4679. [DOI] [PubMed] [Google Scholar]
- 21.Brocca L, Cannavino J, Coletto L, Biolo G, Sandri M, et al.. (2012) The time course of the adaptations of human muscle proteome to bed rest and the underlying mechanisms. J Physiol: 6. [DOI] [PMC free article] [PubMed]
- 22. Moriggi M, Vasso M, Fania C, Capitanio D, Bonifacio G, et al. (2010) Long term bed rest with and without vibration exercise countermeasures: effects on human muscle protein dysregulation. Proteomics 10: 3756–3774. [DOI] [PubMed] [Google Scholar]
- 23. Holloway KV, O’Gorman M, Woods P, Morton JP, Evans L, et al. (2009) Proteomic investigation of changes in human vastus lateralis muscle in response to interval-exercise training. Proteomics 9: 5155–5174. [DOI] [PubMed] [Google Scholar]
- 24. Capitanio D, Vigano A, Ricci E, Cerretelli P, Wait R, et al. (2005) Comparison of protein expression in human deltoideus and vastus lateralis muscles using two-dimensional gel electrophoresis. Proteomics 5: 2577–2586. [DOI] [PubMed] [Google Scholar]
- 25. Hadrevi J, Hellstrom F, Kieselbach T, Malm C, Pedrosa-Domellof F (2011) Protein differences between human trapezius and vastus lateralis muscles determined with a proteomic approach. BMC Musculoskelet Disord 12: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Unge J, Ohlsson K, Nordander C, Hansson GA, Skerfving S, et al. (2007) Differences in physical workload, psychosocial factors and musculoskeletal disorders between two groups of female hospital cleaners with two diverse organizational models. Int Arch Occup Environ Health 81: 209–220. [DOI] [PubMed] [Google Scholar]
- 27. Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, et al. (1990) The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 33: 160–172. [DOI] [PubMed] [Google Scholar]
- 28. Ohlsson K, Attewell RG, Johnsson B, Ahlm A, Skerfving S (1994) An assessment of neck and upper extremity disorders by questionnaire and clinical examination. Ergonomics 37: 891–897. [DOI] [PubMed] [Google Scholar]
- 29. Malm C, Hadrevi J, Bergström S-A, Pedrosa-Domellöf F, Antti H, et al. (2008) Evaluation of 2-D DIGE for skeletal muscle: Protocol and repeatability. Scand J Clin Lab Invest 68: 793–800. [DOI] [PubMed] [Google Scholar]
- 30. Alban A, David SO, Bjorkesten L, Andersson C, Sloge E, et al. (2003) A novel experimental design for comparative two-dimensional gel analysis: two-dimensional difference gel electrophoresis incorporating a pooled internal standard. Proteomics 3: 36–44. [DOI] [PubMed] [Google Scholar]
- 31. Eriksson L, Trygg J, Wold S (2008) CV-ANOVA for significance testing of PLS and OPLS® models. J Chemometrics 22: 594–600. [Google Scholar]
- 32. Morton JP, Kayani AC, McArdle A, Drust B (2009) The exercise-induced stress response of skeletal muscle, with specific emphasis on humans. Sports Med 39: 643–662. [DOI] [PubMed] [Google Scholar]
- 33. Skidmore R, Gutierrez JA, Guerriero V Jr, Kregel KC (1995) HSP70 induction during exercise and heat stress in rats: role of internal temperature. Am J Physiol 268: R92–97. [DOI] [PubMed] [Google Scholar]
- 34. Schlesinger MJ (1990) Heat shock proteins. J Biol Chem 265: 12111–12114. [PubMed] [Google Scholar]
- 35. Iwaki K, Chi SH, Dillmann WH, Mestril R (1993) Induction of HSP70 in cultured rat neonatal cardiomyocytes by hypoxia and metabolic stress. Circulation 87: 2023–2032. [DOI] [PubMed] [Google Scholar]
- 36. Wallen ES, Buettner GR, Moseley PL (1997) Oxidants differentially regulate the heat shock response. Int J Hyperthermia 13: 517–524. [DOI] [PubMed] [Google Scholar]
- 37. Hunt JM, Tuder R (2012) Alpha 1 anti-trypsin: one protein, many functions. Curr Mol Med 12: 827–835. [DOI] [PubMed] [Google Scholar]
- 38. Blanco I, Canto H, de Serres FJ, Fernandez-Bustillo E, Rodriguez MC (2004) Alpha1-antitrypsin replacement therapy controls fibromyalgia symptoms in 2 patients with PI ZZ alpha1-antitrypsin deficiency. J Rheumatol 31: 2082–2085. [PubMed] [Google Scholar]
- 39. Brancaccio P, Lippi G, Maffulli N (2010) Biochemical markers of muscular damage. Clin Chem Lab Med 48: 757–767. [DOI] [PubMed] [Google Scholar]
- 40. Zimmerman UJ, Wang P, Zhang X, Bogdanovich S, Forster R (2004) Anti-oxidative response of carbonic anhydrase III in skeletal muscle. IUBMB Life 56: 343–347. [DOI] [PubMed] [Google Scholar]
- 41. Howlett RA, Parolin ML, Dyck DJ, Hultman E, Jones NL, et al. (1998) Regulation of skeletal muscle glycogen phosphorylase and PDH at varying exercise power outputs. Am J Physiol 275: R418–425. [DOI] [PubMed] [Google Scholar]
- 42. van Loon LJ, Greenhaff PL, Constantin-Teodosiu D, Saris WH, Wagenmakers AJ (2001) The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol 536: 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, et al. (1993) Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol 265: E380–391. [DOI] [PubMed] [Google Scholar]
- 44. Costa Leite T, Da Silva D, Guimaraes Coelho R, Zancan P, Sola-Penna M (2007) Lactate favours the dissociation of skeletal muscle 6-phosphofructo-1-kinase tetramers down-regulating the enzyme and muscle glycolysis. Biochem J 408: 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larsson B, Bjork J, Borsbo B, Gerdle B (2012) A systematic review of risk factors associated with transitioning from regional musculoskeletal pain to chronic widespread pain. Eur J Pain: 1532–2149. [DOI] [PubMed]
- 46. Erlandsen H, Abola EE, Stevens RC (2000) Combining structural genomics and enzymology: completing the picture in metabolic pathways and enzyme active sites. Curr Opin Struct Biol 10: 719–730. [DOI] [PubMed] [Google Scholar]
- 47. Maughan DW, Henkin JA, Vigoreaux JO (2005) Concentrations of glycolytic enzymes and other cytosolic proteins in the diffusible fraction of a vertebrate muscle proteome. Mol Cell Proteomics 4: 1541–1549. [DOI] [PubMed] [Google Scholar]
- 48. Philp A, Macdonald AL, Watt PW (2005) Lactate–a signal coordinating cell and systemic function. J Exp Biol 208: 4561–4575. [DOI] [PubMed] [Google Scholar]
- 49. Brooks GA (2009) Cell-cell and intracellular lactate shuttles. J Physiol 587: 5591–5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gladden LB (2004) Lactate metabolism: a new paradigm for the third millennium. J Physiol 558: 5–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Robergs RA, Ghiasvand F, Parker D (2004) Biochemistry of exercise-induced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol 287: R502–516. [DOI] [PubMed] [Google Scholar]
- 52. Draoui N, Feron O (2012) Lactate shuttles at a glance: from physiological paradigms to anti-cancer treatments. Dis Model Mech 4: 727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hägg GM (2000) Human muscle fibre abnormalities related to occupational load. Eur J Appl Physiol 83: 159–165. [DOI] [PubMed] [Google Scholar]
- 54. Larsson B, Bjork J, Elert J, Lindman R, Gerdle B (2001) Fibre type proportion and fibre size in trapezius muscle biopsies from cleaners with and without myalgia and its correlation with ragged red fibres, cytochrome-c-oxidase-negative fibres, biomechanical output, perception of fatigue, and surface electromyography during repetitive forward flexions. Eur J Appl Physiol 84: 492–502. [DOI] [PubMed] [Google Scholar]
- 55. Larsson B, Andersen JL, Kadi F, Bjork J, Gerdle B (2002) Myosin heavy chain isoforms influence surface EMG parameters: a study of the trapezius muscle in cleaners with and without myalgia and in healthy teachers. Eur J Appl Physiol 87: 481–488. [DOI] [PubMed] [Google Scholar]
- 56. Larsson B, Bjork J, Henriksson KG, Gerdle B, Lindman R (2000) The prevalences of cytochrome c oxidase negative and superpositive fibres and ragged-red fibres in the trapezius muscle of female cleaners with and without myalgia and of female healthy controls. Pain 84: 379–387. [DOI] [PubMed] [Google Scholar]
- 57. Pette D, Staron R (2000) Myosin isoforms, muscle fiber types, and transitions. Microsc Res Tech 50: 500–509. [DOI] [PubMed] [Google Scholar]
- 58. Sweeney H, Bowman B, Stull J (1993) Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function. Am J Physiol 264: 1085–1095. [DOI] [PubMed] [Google Scholar]
- 59. Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM (1992) Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J 28: 21–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Meyer GA, Lieber RL (2012) Skeletal muscle fibrosis develops in response to desmin deletion. Am J Physiol Cell Physiol 302: 1609–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mackey AL, Andersen LL, Frandsen U, Suetta C, Sjogaard G (2010) Distribution of myogenic progenitor cells and myonuclei is altered in women with vs. those without chronically painful trapezius muscle. J Appl Physiol 109: 1920–1929. [DOI] [PubMed] [Google Scholar]
- 62. Olausson P, Gerdle B, Ghafouri N, Larsson B, Ghafouri B (2012) Identification of proteins from interstitium of trapezius muscle in women with chronic myalgia using microdialysis in combination with proteomics. PLoS One 7: e52560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ahmed F (2009) Sample preparation and fractionation for proteome analysis and cancer biomarker discovery by mass spectrometry. J Sep Sci 32: 771–798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Identified proteins by LC-MS/MS or MALDI-TOF-MS/MS.
(DOCX)