Abstract
Objective
This review aimed to comprehensively assess the literature examining a possible link between the rs1801133 polymorphism (677C→T) in the gene encoding the methylenetetrahydrofolate reductase (MTHFR) gene and risk of type 2 diabetes mellitus (DM).
Research Design and Methods
Several research databases were systematically searched for studies examining the genotype at the rs1801133 polymorphism in healthy control individuals and individuals with type 2 DM. Genotype frequency data were examined across all studies and across subsets of studies according to ethnicity and presence of serious DM-related complications. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated.
Results
A total of 4855 individuals with type 2 DM and 5242 healthy controls from 15 countries comprising Asian, Caucasian and African ethnicities were found to satisfy the inclusion criteria and included in the review. Genotype at the rs1801133 polymorphism was not consistently associated with either increased or reduced risk of type 2 DM; the OR across all studies was 0.91 (95%CI 0.82 to 1.00) for the C- vs. T-allele, 0.88 (0.75 to 1.03) for CC vs. CT+TT, 0.82 (0.71 to 0.95) for CC vs. TT, and 1.15 (1.03 to 1.29) for TT vs. CC+CT. Similar results were found when the meta-analysis was repeated separately for each ethnic subgroup, and for subgroups with or without serious DM-related complications.
Conclusions
There does not appear to be compelling evidence of an association between the genotype at the rs1801133 polymorphism of the MTHFR gene and risk of type 2 DM.
Introduction
Diabetes mellitus (DM) is a global health epidemic, affecting approximately 171 million people in 2000 and projected to affect more than 360 million in 2030 [1]. Approximately 90% of people with DM have type 2 disease (T2DM) [2]. In contrast to T1DM, which is genetically inherited, T2DM has a complex aetiology that appears to involve numerous environmental risk factors and potentially some genetic risk factors.
Predicting T2DM risk is important because the disease can severely affect quality of life. T2DM is associated with a broad array of cardiovascular diseases, including retinopathy, nephropathy, neuropathy, acute myocardial infarction, stroke and atherosclerosis. It is important to diagnose and manage T2DM as early as possible to ensure therapeutic efficacy and avoid more serious long-term complications.
The rising prevalence of T2DM and the importance of early detection and management has led many investigators to search for environmental and genetic risk factors for T2DM and T2DM-related complications. Elevated plasma levels of homocysteine, a condition known as hyperhomocysteinaemia (HHcy), have been linked with such T2DM features as endothelial dysfunction and arterial stiffness [3], insulin resistance [4], [5], prothrombotic inflammation and hypercoagulability [6], macroangiopathy [4], [7] and nephropathy [8], [9]. HHcy has also been associated with atherosclerosis [10], coronary heart disease [11] and death [12] among individuals with T2DM.
The enzyme methylenetetrahydrofolate reductase (MTHFR) methylates homocysteine to generate methionine [13], and its dysfunction can lead to HHcy. Therefore numerous studies have investigated whether reduced MTHFR activity is a risk factor for T2DM. The single nucleotide polymorphism (SNP) rs1801133 (677C→T) leads to an Ala222Val substitution in the N-terminal catalytic domain of the enzyme. This mutation reduces enzyme activity, such that the activity in individuals with CT and TT genotypes is approximately 65% and 35%, respectively, that of individuals with the wild-type CC genotype [14], [15], [16]. As a result, individuals with the TT genotype have significantly higher Hcy levels than do individuals with CT and CC genotypes [17]. More recent genome-wide association studies (GWAS) have confirmed the association between rs1801133 genotype and homocysteine levels in healthy populations [18], [19].
Numerous studies around the world have examined whether an association exists between the 677C→T SNP and risk of T2DM. These studies have arrived at different conclusions, with some suggesting a significant association and others no association. This discrepancy is doubtless due in part to the wide variation in genotype frequencies at the rs1801133 locus. The TT genotype, for example, is present in 9–13% of Brazilians [20], 15–17% of north Indians [21], 18–20% of Chinese [7], and 20–30% of Turkish [22]. This makes it particularly important to systematically assess the association between this polymorphism and risk of T2DM across a range of ethnicities.
Despite the divergent results among single-country studies and strong evidence that rs1801133 genotype depends on ethnicity, no systematic review has been undertaken to determine conclusively whether this MTHFR SNP is associated with risk of T2DM, and whether the association is universal or specific to particular ethnic groups. To address this question as comprehensively as possible, we carried out a systematic review of case-control studies in the medical literature.
Methods
Literature Search Strategy
The most recent on-line versions of the following research databases were searched in April 2013 without language restrictions: Chinese National Knowledge Infrastructure (CNKI), Cochrane Library (http://onlinelibrary.wiley.com/cochranelibrary/search), Directory of Open-Access Journals (www.doaj.org), Embase, Public Library of Science (www.plosmedicine.org), PubMed, SciELO (www.scielo.org), Scopus, and Web of Knowledge. The following search terms were used to identify studies: “methylenetetrahydrofolate reductase” or MTHFR, gene or polymorphism or variation or genotype or genetic or mutation, diabetes or mellitus or “diabetes mellitus”. We also searched the Catalog of Published Genome-Wide Association Studies (www.genome.gov/gwastudies) of the US National Human Genome Research Institute.
Inclusion Criteria
We included in the systematic review full-length research studies that satisfied the following criteria: (a) they assessed the association between T2DM and the 677C→T polymorphism of the MTHFR gene; (b) they used a case-control design in which cases were T2DM patients and controls were healthy individuals; and (c) they provided sufficient published data for estimating an odds ratio (OR) with a 95% confidence interval (95%CI). Conference abstracts or other forms of summary publication were not included.
If studies included case groups of DM patients with serious DM-related complications or control groups other than healthy individuals, data for those additional groups were not extracted. Serious DM-related complications, which included cardiovascular disease, coronary heart disease, nephropathy and diabetic retinopathy, were defined as complications aside from the more frequent clinical manifestations of T2DM such as hyperlipidaemia, hypertension and obesity. In the case of multiple studies apparently based on the same case or control population, we included only the study with the largest number of participants.
Data Extraction
Two authors (J-HZ, ACR) independently extracted the following data from included studies: first author’s family name, year of publication, numbers of cases and controls, presence of serious complications among cases, duration of T2DM at the time of the study, Hardy-Weinberg equilibrium (HWE) of controls, and rs1801133 genotype frequencies in cases and controls. Extracted data were compared and discrepancies resolved by discussion.
Statistical Methods and Bias Testing
The unadjusted OR with 95%CI was used to assess the strength of the association between the 677C→T polymorphism of the MTHFR gene and T2DM risk based on the genotype frequencies in cases and controls. The meta-analysis examined the association of different genotypes at 677C→T MTHFR with T2DM risk by comparing the C allele with the T allele, comparing homozygous genotypes, and applying recessive and dominant genetic models.
All statistical tests for this meta-analysis were performed using RevMan 5.14 (Cochrane Collaboration) and Stata 11.0 (StataCorp, College Station, USA). Pooled ORs were calculated using fixed- or random-effect models, and the significance of those ORs was assessed using the Z-test. The threshold for significance in the Z-test was defined as P<0.05. We used a chi squared-based Q-test to assess heterogeneity among studies. In this test, P>0.10 was taken to suggest that effect sizes were larger than those expected by chance [23], [24], indicating the absence of statistical heterogeneity. In this case, a pooled OR was calculated for each study using the fixed-effect model. Otherwise, the random-effect model was used. HWE in the control group was assessed using the asymptotic test, with P<0.05 considered significant.
Publication bias was assessed by visual inspection of Begg’s funnel plots. Small-study bias was assessed by Harbord’s modified test [25].
Subgroup and Sensitivity Analysis
To detect associations that might be masked in the overall sample, we performed subgroup analyses based on subsets of the included studies defined according to ethnicity (African, Asian, and Caucasian) and according to whether the studies included T2DM cases with serious DM-related complications, such as cardiovascular disease, coronary heart disease, nephropathy and diabetic retinopathy. For subgroup analysis based on the presence or absence of serious DM-related complications, we defined two subgroups of studies: one subgroup in which the authors explicitly stated that such complications were absent, and another subgroup in which the authors either reported the presence of such complications or did not report on the presence or absence of complications at all. We also performed subgroup analysis separately on studies in which the MTHFR alleles in the control group were in HWE and on studies in which they were not in HWE. To assess the reliability of the outcomes in the meta-analysis, a sensitivity analysis was performed by excluding one study at a time.
Results
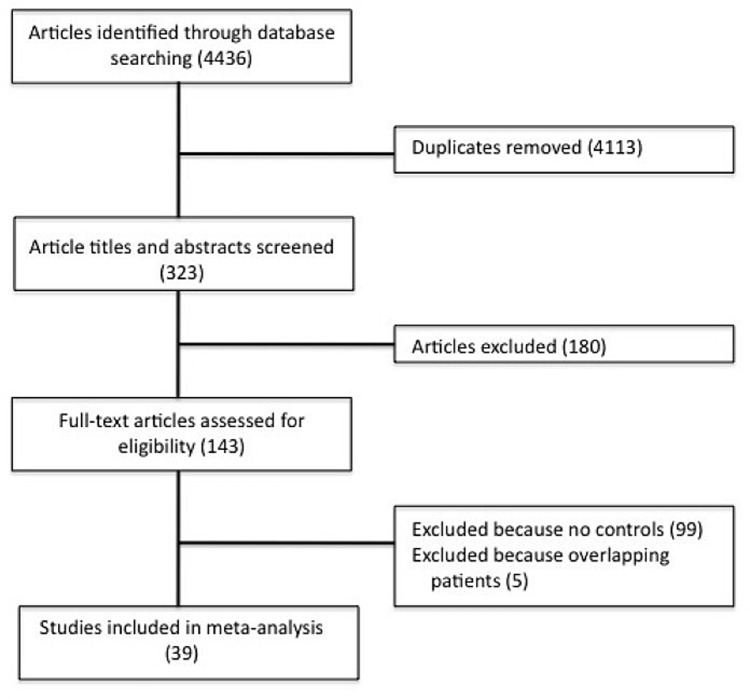
Several research databases were searched without language restrictions to identify case-control studies assessing the possible association between the rs1801133 polymorphism in the MTHFR gene and risk of T2DM. A total of 4436 studies were identified, none of which was a GWAS. This list was reduced to 143 after removing duplicates and screening based on the title and abstract review. These articles were read in full, and 99 studies were removed because they did not include a healthy control group without T2DM, while another 5 studies were removed because they analysed overlapping patient populations. In the end, 39 studies were included in the meta-analysis (Fig. 1) [7], [8], [21], [22], [26]–[60]. The main characteristics of the included studies are shown in Table 1.
Figure 1. Flow chart of study selection.
Table 1. Description of cases and controls and their genotypes at MTHFR polymorphism rs1801133.
| Study (ref.) | Ethnicity | Cases/Controls | Serious DM-related complicationsa | Duration of DM (yr)b | Genotype by PCR-RFLP | PHWE c | |||||||
| Cases | Controls | ||||||||||||
| CC | CT | TT | CC | CT | TT | ||||||||
| Asian | |||||||||||||
| Bazzaz 2010 [46] | Iranian | 281/207 | NR | NR | 148 | 102 | 31 | 113 | 80 | 14 | 1.000 | ||
| Chang 2011 [27] | Taiwanese | 56/62 | NR | NR | 1 | 25 | 30 | 3 | 23 | 36 | 1.000 | ||
| Chauhan 2012 [43] | Indian | 1018/1006 | NR | NR | 31 | 310 | 677 | 45 | 335 | 626 | 1.000 | ||
| Chen 2010 [29] | Chinese | 40/55 | No metabolic syndrome, inflammatory disease, kidney disease, or coronary heart disease | NR | 23 | 13 | 4 | 34 | 17 | 4 | 0.439 | ||
| Dai 2012 [26] | Chinese | 60/60 | No diabetic nephropathy | 11.7±0.9 | 29 | 28 | 3 | 31 | 27 | 2 | 0.310 | ||
| Eroglu 2007 [47] | Turkish | 56/128 | No diabetic nephropathy | NR | 25 | 25 | 6 | 63 | 58 | 7 | 0.272 | ||
| Hasegawa 2003 [45] | Japanese | 62/200 | No renal failure or macroangiopathy. Retinopathy present in 77.4% of cases. | 18.5 (10–40) | 23 | 32 | 7 | 78 | 96 | 26 | 0.762 | ||
| Ho 2005 [36] | Taiwanese | 20/42 | Normal renal function; no deep vein thrombosis or coronary artery disease | NR | 13 | 5 | 2 | 28 | 11 | 3 | 0.326 | ||
| Luo 2009 [31] | Chinese | 71/85 | No cardiovascular disease or coronary heart disease | NR | 39 | 26 | 6 | 43 | 31 | 11 | 0.204 | ||
| Mao 2004 [37] | Chinese | 41/47 | No cerebrovascular disease | 5.68±4.22 | 19 | 17 | 5 | 26 | 18 | 3 | 1.000 | ||
| Mei 2012 [28] | Chinese | 116/124 | NR | NR | 19 | 70 | 27 | 14 | 73 | 37 | 0.025 | ||
| Movva 2011 [44] | Indian | 100/100 | No history or family history of renal complications | 12.16±4.07 | 68 | 32 | 0 | 91 | 9 | 0 | 1.000 | ||
| Raza 2012 [21] | Indian | 87/88 | NR | NR | 35 | 37 | 15 | 49 | 26 | 13 | 0.009 | ||
| Shi 2006 [33] | Chinese | 104/110 | No microvascular complications | NR | 70 | 29 | 5 | 68 | 34 | 8 | 0.273 | ||
| Sun 2006 [7] | Chinese | 104/114 | No overt nephropathy, cerebrovascular disease, coronary heart disease or peripheral vascular disease | 7.04±4.71 | 60 | 27 | 17 | 63 | 31 | 20 | <0.001 | ||
| Tutuncu 2005 [48] | Turkish | 87/91 | Coronary artery disease present in 18% of cases; cerebrovascular disease, 3%; diabetic retinopathy, 18%; neuropathy, 9%. | 8.7±7.1 | 39 | 37 | 11 | 47 | 39 | 5 | 0.593 | ||
| Xiao 2006 [34] | Chinese | 41/73 | No nephropathy, cerebrovascular disease, coronary heart disease or peripheral vascular disease | NR | 8 | 31 | 2 | 47 | 25 | 1 | 0.439 | ||
| Xu 2003 [34] | Chinese | 54/52 | No diabetic nephropathy | 6.77±4.08 | 24 | 21 | 9 | 20 | 25 | 7 | 1.000 | ||
| Wang 2001 [42] | Chinese | 117/85 | No overt nephropathy | 5.42±3.81 | 57 | 48 | 12 | 37 | 38 | 10 | 1.000 | ||
| Wen 2008 [32] | Chinese | 59/57 | No nephropathy | 4.8±5.3 | 21 | 32 | 6 | 27 | 25 | 5 | 1.000 | ||
| Yang 2001 [41] | Chinese | 102/62 | No diabetic nephropathy, diabetic retinopathy, and other microvascular complications | >10 | 32 | 56 | 14 | 26 | 28 | 8 | 1.000 | ||
| Yilmaz 2004 [22] | Turkish | 249/214 | Left ventricular hypertrophy present in 11.2% of cases | 9.5±7.7 | 121 | 98 | 30 | 101 | 93 | 20 | 1.000 | ||
| Yue 2006 [35] | Chinese | 140/30 | No diabetic nephropathy, diabetic retinopathy, or other microvascular complications | NR | 43 | 76 | 21 | 17 | 11 | 2 | 1.000 | ||
| Zhang 2002 [40] | Chinese | 100/100 | No macroangiopathy complications | NR | 32 | 55 | 13 | 40 | 49 | 11 | 0.662 | ||
| Zhang 2010 [30] | Chinese | 206/194 | NR | NR | 73 | 98 | 35 | 53 | 103 | 38 | 0.388 | ||
| Zhou 2004 [38] | Chinese | 67/69 | No macroangiopathy complications | NR | 5 | 42 | 20 | 8 | 31 | 30 | 1.000 | ||
| Caucasian | |||||||||||||
| Beneš 2001 [53] | Czech | 49/209 | No coronary artery disease | NR | 24 | 20 | 5 | 86 | 106 | 17 | 0.062 | ||
| Blüthner 1999 [55] | German and Polish | 146/150 | No diabetic nephropathy | 17.0±7.0 | 63 | 65 | 18 | 67 | 68 | 15 | 0.853 | ||
| Cenerelli 2002 [52] | Italy | 30/43 | No diabetic retinopathy, neuropathy, nephropathy, cardiovascular disease or coronary heart disease | 3.1±6.5 | 8 | 14 | 8 | 13 | 21 | 9 | 1.000 | ||
| Helfenstein 2005 [57] | Brazilian | 50/56 | No atherosclerosis, myocardial infarction, or diabetic retinopathy | NR | 26 | 20 | 4 | 26 | 24 | 6 | 1.000 | ||
| Książek 2004 [50] | Polish | 155/170 | No diabetic nephropathy. Retinopathy present in 8.4% of cases. | 9.8±5.1 | 82 | 58 | 15 | 71 | 83 | 16 | 0.304 | ||
| Mazza 2005 [54] | Italian | 105/120 | No coronary artery disease, cerebrovascular disease, kidney dysfunction, or diabetic proliferative retinopathy | 11.4±8.0 | 35 | 47 | 23 | 35 | 66 | 19 | 0.264 | ||
| Russo 2008 [49] | Italian | 90/91 | No cardiovascular disease | NR | 28 | 42 | 20 | 23 | 50 | 18 | 0.403 | ||
| Soares 2008 [56] | Brazilian | 7/16 | No coronary disease, stroke, or chronic renal failure | >1 | 5 | 2 | 0 | 9 | 5 | 2 | 0.530 | ||
| Wirta 2002 [51] | Finnish | 81/114 | Background retinopathy present in 3.6% of cases | 3 mos. (range, 1–11 mos.) | 44 | 29 | 8 | 60 | 47 | 7 | 0.811 | ||
| African | |||||||||||||
| Benrahma 2012 [58] | Moroccan | 282/262 | Neuropathic complications present in 30.5% of cases; cardiovascular complications, 17.4%; nephropathic complications, 2.5%. | NR | 160 | 97 | 25 | 114 | 122 | 26 | 0.487 | ||
| Mackawy 2011 [59] | Egyptian | 40/40 | No history of ischemic stroke | NR | 24 | 10 | 6 | 32 | 6 | 2 | 0.090 | ||
| Mehri 2010 [60] | Tunisian | 115/116 | History of cardiovascular disease present in 8.7% of cases; nephropathy, 26.1%; retinopathy, 26.1%; neuropathy, 19.1%. | 9.3±5.7 | 50 | 49 | 16 | 66 | 38 | 12 | 0.095 | ||
| Mtiraoui 2007 [8] | Tunisian | 267/400 | No nephropathy. Neuropathy present in 23.6% of cases; retinopathy, 19.1%. | 12.3±4.1 | 152 | 79 | 36 | 270 | 94 | 36 | <0.001 | ||
Abbreviations: NR, not reported; T2DM, type 2 diabetes mellitus; PCR-RFLP, polymerase chain reaction assay based on restriction fragment length polymorphism.
Defined as serious complications aside from the more frequent clinical manifestations of T2DM such as hyperlipidaemia, hypertension and obesity.
Reported as either “median (range)” or as “mean ± standard deviation”, unless indicated otherwise.
P value for Hardy-Weinberg equilibrium for rs1801133 genotype among controls.
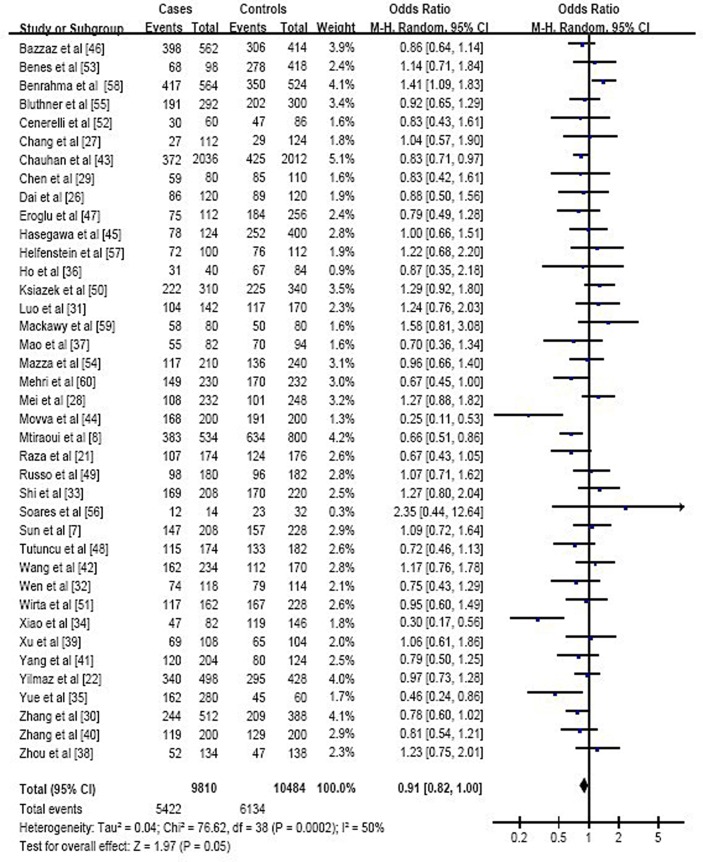
These studies involved 4855 individuals with T2DM and 5242 healthy controls from 15 countries in Asia (26 studies), Europe (7), North Africa (4), and Brazil (2). Of the 39 studies, 14 were published in Chinese and 25 in English. Meta-analysis of all included studies indicated that the genotype at MTHFR polymorphism rs1801133 was not consistently associated with either increased or reduced risk of T2DM across the genetic models tested: the OR across all studies was 0.91 (95%CI 0.82 to 1.00) for the C- vs. T-allele (Fig. 2), 0.88 (0.75 to 1.03) for CC vs. CT+TT, 0.82 (0.71 to 0.95) for CC vs. TT, and 1.15 (1.03 to 1.29) for TT vs. CC+CT (Table 2).
Figure 2. Forest plot assessing the potential association between the genotype at MTHFR polymorphism rs1801133 and risk of T2DM in all included studies (C-allele vs. T-allele).
Table 2. Overall and stratified meta-analyses of the association between methylenetetrahydrofolate reductase polymorphism 677C→T and risk of type 2 diabetes mellitus.
| Genotype comparison | OR [95% CI] | Z (P value) | Heterogeneity of study design | Analysis model | ||
| χ2 | df (P value) | I2 | ||||
| All studies (4855 cases, 5242 controls) | ||||||
| C-allele vs. T-allele | 0.91 [0.82, 1.00] | 1.97 (0.05) | 76.62 | 38 (<0.001) | 50% | Random |
| CC vs. CT+TT | 0.88 [0.75, 1.03] | 1.56 (0.12) | 95.21 | 38 (<0.001) | 60% | Random |
| CC vs. TT | 0.82 [0.71, 0.95] | 2.62 (0.009) | 35.40 | 37 (0.54) | 0% | Fixed |
| TT vs. CC+CT | 1.15 [1.03, 1.29] | 2.57 (0.01) | 26.07 | 37 (0.91) | 0% | Fixed |
| Subgroups by ethnicity | ||||||
| Asian (3438 cases, 3455 controls) | ||||||
| C-allele vs. T-allele | 0.86 [0.76, 0.96] | 2.62 (0.009) | 45.38 | 25 (0.008) | 45% | Random |
| CC vs. CT+TT | 0.81 [0.66, 0.98] | 2.14 (0.03) | 59.41 | 25 (<0.001) | 58% | Random |
| CC vs. TT | 0.82 [0.68, 0.99] | 2.11 (0.04) | 24.34 | 24 (0.44) | 1% | Fixed |
| TT vs. CC+CT | 1.12 [0.99, 1.27] | 1.76 (0.08) | 19.40 | 24 (0.73) | 0% | Fixed |
| Caucasian (713 cases, 969 controls) | ||||||
| C-allele vs. T-allele | 1.06 [0.91, 1.22] | 0.73 (0.47) | 4.19 | 8 (0.84) | 0% | Fixed |
| CC vs. CT+TT | 1.22 [0.99, 1.49] | 1.91 (0.06) | 3.63 | 8 (0.89) | 0% | Fixed |
| CC vs. TT | 0.92 [0.67, 1.27] | 0.51 (0.61) | 2.25 | 8 (0.97) | 0% | Fixed |
| TT vs. CC+CT | 1.23 [0.91, 1.65] | 1.35 (0.18) | 2.06 | 8 (0.98) | 0% | Fixed |
| African (704 cases, 818 controls) | ||||||
| C-allele vs. T-allele | 0.75 [0.46, 1.24] | 1.12 (0.26) | 23.04 | 3 (<0.001) | 87% | Random |
| CC vs. CT+TT | 0.75 [0.39, 1.43] | 0.88 (0.38) | 23.67 | 3 (<0.001) | 87% | Random |
| CC vs. TT | 0.70 [0.37, 1.31] | 1.13 (0.26) | 8.05 | 3 (0.05) | 63% | Random |
| TT vs. CC+CT | 1.32 [0.95, 1.83] | 1.65 (0.10) | 3.59 | 3 (0.31) | 16% | Fixed |
| Subgroups by DM-related complications | ||||||
| T2DM with serious complications (3062 cases, 3248 controls) | ||||||
| C-allele vs. T-allele | 0.91 [0.79, 1.04] | 1.37 (0.17) | 32.40 | 13 (0.002) | 60% | Random |
| CC vs. CT+TT | 0.96 [0.77, 1.20] | 0.38 (0.71) | 38.46 | 13 (<0.001) | 66% | Random |
| CC vs. TT | 0.83 [0.69, 1.00] | 1.99 (0.05) | 20.32 | 13 (0.09) | 36% | Fixed |
| TT vs. CC+CT | 1.17 [1.02, 1.33] | 2.32 (0.02) | 12.21 | 13 (0.51) | 0% | Fixed |
| T2DM without serious complications (1793 cases, 1994 controls) | ||||||
| C-allele vs. T-allele | 0.90 [0.78, 1.04] | 1.43 (0.15) | 44.11 | 24 (0.007) | 46% | Random |
| CC vs. CT+TT | 0.83 [0.66, 1.03] | 1.68 (0.09) | 55.21 | 24 (<0.001) | 57% | Random |
| CC vs. TT | 0.82 [0.65, 1.03] | 1.71 (0.09) | 15.09 | 23 (0.89) | 0% | Fixed |
| TT vs. CC+CT | 1.13 [0.92, 1.39] | 1.13 (0.26) | 13.78 | 23 (0.93) | 0% | Fixed |
| Subgroups by HWE | ||||||
| Alleles in control group in HWE (4281 cases, 4516 controls) | ||||||
| C-allele vs. T-allele | 0.91 [0.82, 1.01] | 1.75 (0.08) | 64.89 | 34 (0.001) | 48% | Random |
| CC vs. CT+TT | 0.89 [0.75, 1.05] | 1.36 (0.17) | 83.85 | 34 (<0.001) | 59% | Random |
| CC vs. TT | 0.83 [0.70, 0.97] | 2.33 (0.02) | 28.57 | 33 (0.69) | 0% | Fixed |
| TT vs. CC+CT | 1.16 [1.04, 1.31] | 2.53 (0.01) | 21.44 | 33 (0.94) | 0% | Fixed |
| Alleles in control group not in HWE (574 cases, 726 controls) | ||||||
| C-allele vs. T-allele | 0.88 [0.63, 1.24] | 0.74 (0.46) | 10.80 | 3 (0.01) | 72% | Random |
| CC vs. CT+TT | 0.83 [0.54, 1.26] | 0.88 (0.38) | 7.81 | 3 (0.05) | 62% | Random |
| CC vs. TT | 0.88 [0.51, 1.51] | 0.46 (0.64) | 6.80 | 3 (0.08) | 56% | Random |
| TT vs. CC+CT | 1.09 [0.81, 1.48] | 0.57 (0.57) | 4.52 | 3 (0.21) | 34% | Fixed |
To test the robustness of these findings, we recalculated ORs and 95% CIs across all studies after systematically removing each of them, one at a time. The results after deleting each study were similar to those obtained across all studies.
Subgroup Analysis by Ethnicity
We performed subgroup analysis based on ethnicity in order to uncover any evidence of an association between the MTHFR SNP and risk of T2DM that might go undetected in the overall sample. We loosely classified the study populations as African, Asian, or Caucasian based on the majority ethnicity of the participants. Meta-analysis of each of the three subgroups failed to provide clear, consistent evidence that the genotype at MTHFR polymorphism rs1801133 was associated with either increased or reduced risk of T2DM (Table 2).
Subgroup Analysis by Presence or Absence of Serious Complications of T2DM
Our original intention was to include only studies in which cases were T2DM without serious complications, which we defined as complications such as nephropathy, retinopathy, and coronary heart disease–in other words, complications aside from the hyperlipidaemia, HHcy, and obesity typically observed in patients with T2DM. Many studies, however, included cases with T2DM and serious complications, even when the study purported to assess only the association between the MTHFR SNP and T2DM per se. Therefore we divided the 39 studies into two subgroups: 14 studies that explicitly reported the presence of serious complications among cases or failed to report on such complications at all [8], [21], [22], [27], [28],[30],[43],[45],[46],[48],[50],[51],[58],[60], and 25 studies that explicitly reported the absence of serious complications [7], [26], [29], [31]–[42], [44], [47], [49], [52]–[57], [59]. Meta-analysis of these two subgroups, like the meta-analysis across all included studies, failed to provide clear, consistent evidence that the genotype at MTHFR polymorphism rs1801133 was associated with either increased or reduced risk of T2DM (Table 2).
Since the rs1801133 alleles in the control groups of several studies were not in HWE, we analysed those studies separately from those in which the alleles in the control groups were in HWE. The results were similar to those obtained across all included studies (Table 2).
Bias Testing
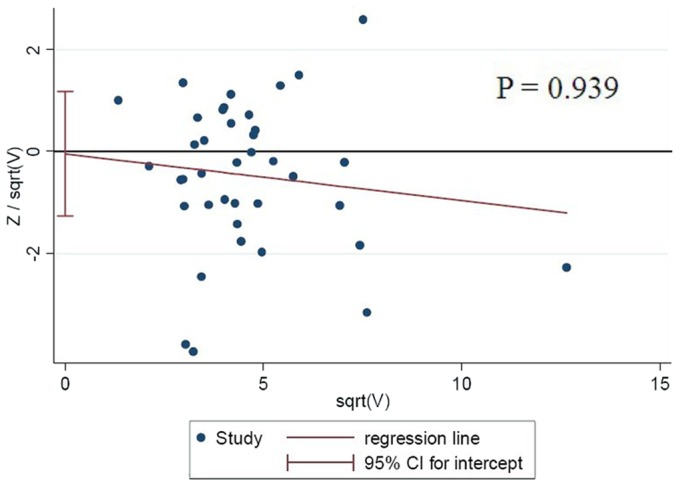
Begg’s funnel plots were prepared for the 39 studies to assess publication bias for studies about 677C→T MTHFR and T2DM risk. The shape of the funnel plots appeared to be symmetrical for allele contrast, homozygous comparison, and recessive and dominant genetic models, suggesting the absence of publication bias. Small-study bias tests showed no significant bias (P = 0.939, Figure 3).
Figure 3. Analysis to detect small-scale study bias across all included studies, based on the allele contrast genetic model.
Discussion
This systematic review sought to assess the evidence for an association between the rs1801133 polymorphism in the MTHFR gene and risk of T2DM. We failed to find any clear, consistent evidence of such an association across all 39 studies conducted in 15 countries. In addition, no compelling evidence of an association was found specifically for African, Asian, or Caucasian populations, or specifically for populations with T2DM in the presence or absence of serious DM-related complications.
One of the challenges in conducting this systematic review was taking into account the clinical profile of the T2DM cases in the included studies. Such characterisation is important, because diabetes is a syndrome that can have far-reaching effects on various organ systems. For example, individuals with T2DM have approximately 2-fold higher risk of cardiovascular events than do individuals without diabetes [61]. This may make it difficult to determine whether the rs1801133 polymorphism is associated with T2DM, a T2DM-related complication or both. In order to isolate as much as possible an association between the MTHFR polymorphism and onset of T2DM per se, we analysed results separately for studies in which cases were explicitly described as having or lacking serious DM-related complications. The results of this subgroup meta-analysis were similar to those of the meta-analysis across all studies, further supporting the lack of an association between the MTHFR 677C→T SNP and risk of T2DM.
Our finding of a lack of association between this SNP and risk of T2DM contrasts with studies suggesting that this polymorphism is associated with certain serious DM-related complications. A meta-analysis of 29 studies found the TT genotype to be associated with moderately elevated risk for diabetic nephropathy and retinopathy [62]. Individual studies have reported the T allele to be associated with diabetic nephropathy [50], [63] and diabetic macroangiopathy [7], [45], but not with T2DM per se. These findings, in light of our meta-analysis results, highlight the need for rigourous, large-scale prospective studies that separate genetic risk of disease onset from genetic risk of complications. This work is crucial for clarifying whether MTHFR can affect long-term T2DM progression and patient prognosis.
Genotype frequencies at the rs1801133 locus of MTHFR vary widely by ethnicity [7], [20]–[22], raising the possibility that any association between this SNP and risk of T2DM may likewise depend on ethnicity. Therefore we repeated our meta-analysis separately for the ethnic groups that emerged from our literature searches: African, Asian, and Caucasian. We were restricted to these large, loosely defined ethnic categories because of the lack of detailed ethnicity data within the included studies. Meta-analysis results for each of the three ethnic groups failed to provide compelling evidence of an association between the MTHFR SNP and risk of T2DM. These findings are important because diabetes prevalence is projected to increase at substantially different rates in different ethnic groups. For example, diabetes prevalence is projected to increase between 2000 and 2030 by 26% in Italy, 71% in USA, 104% in China, 148% in Brazil, and 205% in Iran [1]. If this differential increase has a genetic basis, it seems unlikely to involve polymorphism in the MTHFR gene.
Another SNP in the MTHFR gene, 1298A→C (Glu429Ala), was reported to reduce enzyme activity based on studies of endogenous enzyme in lymphocyte extracts [15], [16], raising the possibility that it might be a risk factor for HHcy just like the 677C→T SNP. However, the 1298A→C SNP lowers MTHFR activity substantially less than does 677C→T [15], [16], [64], and biochemical studies with purified recombinant enzyme suggest that the originally reported lower activity for Glu429Ala enzyme may have been an artifact [65]. Furthermore, compelling evidence that the 1298A→C SNP is associated with HHcy is lacking [66]. The much milder effects observed with 1298A→C than with 677C→T are consistent with the fact that 1298A→C causes a mutation in the C-terminal regulatory domain of the enzyme, whereas 677C→T causes a mutation in the catalytic domain. Thus the 1298A→C SNP has not been the focus of studies of genetic risk factors of T2DM, and it was not considered in this systematic review.
The lack of an association between the 677C→T SNP and risk of T2DM may be consistent with studies calling into question whether HHcy plays a role in the disease. For example, although some authors have associated HHcy with macroangiopathy [4], [67], other authors have reported no such association [68]. Some studies have reported a positive association between total homocysteine levels and insulin levels in blood [69], while others have found a negative association [70] or no association at all [4]. To make the situation more complex, the negative effects of HHcy, at least with respect to increased risk of stroke, appear to be exacerbated by low folate [71]. Most of the studies included in the present systematic review did not analyze Hcy levels in cases and controls, making it difficult to gain a comprehensive picture. Of the studies that did examine whether Hcy levels were associated with the rs1801133 polymorphism, 11 found the TT genotype to be associated with higher Hcy levels than the CT or CC genotypes in patients with T2DM [7], [8], [31], [32], [35], [45], [46], [51], [54], [59], [60], while 6 found no such association [27], [48], [49], [52], [56], [57]. A meta-analysis published in 1998 concluded there was an association between the TT genotype and elevated plasma homocysteine levels in individuals with T2DM [17], while a more recent prospective study found no such association [72]. Thus, we second the conclusions of previous authors that the link between Hcy levels and T2DM remains unclear [48], [53], [56], [60]. The recommendation of the College of American Pathologists to measure Hcy only in patients with documented atherosclerotic disease [73] seems reasonable, given that HHcy is commonly accepted to be an independent risk factor for atherosclerosis and thromboembolism [74].
The findings in this systematic review are limited by the designs of the included studies. Only three studies [21], [43], [57] reported statistical power (76–98%), raising the possibility that other included studies were underpowered, which might lead to inaccuracy in our meta-analysis results. Nearly all included studies examined polymorphism only in the MTHFR gene, even though evidence suggests that this gene may interact with others in conferring risk or protection from diabetes. For example, the ID polymorphism in the gene encoding angiotensin-converting enzyme may act synergistically with the MTHFR 677C→T polymorphism to enhance diabetes risk [60]. Another design limitation is that most included studies looked only at a genetic association between MTHFR SNP genotype and T2DM risk. This is different from examining the downstream effects of different MTHFR SNP genotypes. For example, since more than half the included studies did not examine plasma Hcy levels in individuals with different genotypes, it is difficult to gain further insight into whether these levels are directly linked to risk of T2DM and thereby resolve the contradictory information in the literature. Several studies in our meta-analysis included patients with T2DM who had been managing their disease with medication, while other studies excluded such patients; studies also diverged significantly with respect to how long their participants had been living with the disease. Further studies should aim to control for as many possible confounders as possible.
Our findings fail to provide compelling evidence for an association between the MTHFR polymorphism rs1801133 and risk of T2DM, regardless of the ethnicity of the patient or the presence of serious DM-related complications. The possibility remains that MTHFR does affect risk of T2DM in concert with other genetic risk factors, which might include the 1298A→C SNP or additional polymorphisms that recent GWAS have linked to homocysteine levels [18].
Reporting of this meta-analysis has been guided by the PRISMA statement (Checklist S1).
Supporting Information
PRISMA 2009 Checklist.
(DOC)
Funding Statement
The authors have no support or funding to report.
References
- 1.World Health Organization. “Country and regional data on diabetes: Prevalence of diabetes worldwide”. Available: www.who.int/diabetes/facts/world_figures/en/index.html, accessed 2013 February 17.
- 2.World Health Organization. “Genetics and diabetes”. Available: www.who.int/genomics/about/Diabetis-fin.pdf, accessed 2013 February 17.
- 3. Doupis J, Eleftheriadou I, Kokkinos A, Perrea D, Pavlatos S, et al. (2010) Acute hyperhomocysteinemia impairs endothelium function in subjects with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 118: 453–458. [DOI] [PubMed] [Google Scholar]
- 4. Buysschaert M, Dramais AS, Wallemacq PE, Hermans MP (2000) Hyperhomocysteinemia in type 2 diabetes: relationship to macroangiopathy, nephropathy, and insulin resistance. Diabetes Care 23: 1816–1822. [DOI] [PubMed] [Google Scholar]
- 5. Masaki T, Anan F, Anai M, Higuchi K, Tsubone T, et al. (2007) Hyperhomocysteinemia is associated with visceral adiposity in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 77: 168–173. [DOI] [PubMed] [Google Scholar]
- 6. Aso Y, Yoshida N, Okumura K, Wakabayashi S, Matsutomo R, et al. (2004) Coagulation and inflammation in overt diabetic nephropathy: association with hyperhomocysteinemia. Clin Chim Acta 348: 139–145. [DOI] [PubMed] [Google Scholar]
- 7. Sun J, Xu Y, Zhu Y, Lu H (2006) Methylenetetrahydrofolate reductase gene polymorphism, homocysteine and risk of macroangiopathy in Type 2 diabetes mellitus. J Endocrinol Invest 29: 814–820. [DOI] [PubMed] [Google Scholar]
- 8. Mtiraoui N, Ezzidi I, Chaieb M, Marmouche H, Aouni Z, et al. (2007) MTHFR C677T and A1298C gene polymorphisms and hyperhomocysteinemia as risk factors of diabetic nephropathy in type 2 diabetes patients. Diabetes Res Clin Pract 75: 99–106. [DOI] [PubMed] [Google Scholar]
- 9. Ukinc K, Ersoz HO, Karahan C, Erem C, Eminagaoglu S, et al. (2009) Methyltetrahydrofolate reductase C677T gene mutation and hyperhomocysteinemia as a novel risk factor for diabetic nephropathy. Endocrine 36: 255–261. [DOI] [PubMed] [Google Scholar]
- 10. Akalin A, Alatas O, Colak O (2008) Relation of plasma homocysteine levels to atherosclerotic vascular disease and inflammation markers in type 2 diabetic patients. Eur J Endocrinol 158: 47–52. [DOI] [PubMed] [Google Scholar]
- 11. Sun J, Xu Y, Xue J, Zhu Y, Lu H (2005) Methylenetetrahydrofolate reductase polymorphism associated with susceptibility to coronary heart disease in Chinese type 2 diabetic patients. Mol Cell Endocrinol 229: 95–101. [DOI] [PubMed] [Google Scholar]
- 12. Hoogeveen EK, Kostense PJ, Jakobs C, Dekker JM, Nijpels G, et al. (2000) Hyperhomocysteinemia increases risk of death, especially in type 2 diabetes: 5-year follow-up of the Hoorn Study. Circulation 101: 1506–1511. [DOI] [PubMed] [Google Scholar]
- 13. Goyette P, Sumner JS, Milos R, Duncan AM, Rosenblatt DS, et al. (1994) Human methylenetetrahydrofolate reductase: isolation of cDNA, mapping and mutation identification. Nat Genet 7: 195–200. [DOI] [PubMed] [Google Scholar]
- 14. Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, et al. (1995) A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 10: 111–113. [DOI] [PubMed] [Google Scholar]
- 15. Weisberg I, Tran P, Christensen B, Sibani S, Rozen R (1998) A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab 64: 169–172. [DOI] [PubMed] [Google Scholar]
- 16. Weisberg IS, Jacques PF, Selhub J, Bostom AG, Chen Z, et al. (2001) The 1298A–>C polymorphism in methylenetetrahydrofolate reductase (MTHFR): in vitro expression and association with homocysteine. Atherosclerosis 156: 409–415. [DOI] [PubMed] [Google Scholar]
- 17. Brattstrom L, Wilcken DE, Ohrvik J, Brudin L (1998) Common methylenetetrahydrofolate reductase gene mutation leads to hyperhomocysteinemia but not to vascular disease: the result of a meta-analysis. Circulation 98: 2520–2526. [DOI] [PubMed] [Google Scholar]
- 18. Paré G, Chasman DI, Parker AN, Zee RRY, Mälarstig A, et al. (2009) Novel associations of CPS1, MUT, NOX4, and DPEP1 with plasma homocysteine in a healthy population: a genome-wide evaluation of 13 974 participants in the Women’s Genome Health Study. Circ Cardiovasc Genet 2: 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lange LA, Croteau-Chonka DC, Marvelle AF, Qin L, Gaulton KJ, et al. (2010) Genome-wide association study of homocysteine levels in Filipinos provides evidence for CPS1 in women and a stronger MTHFR effect. Hum Mol Genet 19: 2050–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Errera FI, Silva ME, Yeh E, Maranduba CM, Folco B, et al. (2006) Effect of polymorphisms of the MTHFR and APOE genes on susceptibility to diabetes and severity of diabetic retinopathy in Brazilian patients. Braz J Med Biol Res 39: 883–888. [DOI] [PubMed] [Google Scholar]
- 21. Raza ST, Abbas S, Ahmed F, Fatima J, Zaidi ZH, et al. (2012) Association of MTHFR and PPARgamma2 gene polymorphisms in relation to type 2 diabetes mellitus cases among north Indian population. Gene 511: 375–379. [DOI] [PubMed] [Google Scholar]
- 22. Yilmaz H, Agachan B, Ergen A, Karaalib ZE, Isbir T (2004) Methylene tetrahydrofolate reductase C677T mutation and left ventricular hypertrophy in Turkish patients with type II diabetes mellitus. J Biochem Mol Biol 37: 234–238. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 25. Harbord RM, Egger M, Sterne JA (2006) A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 25: 3443–3457. [DOI] [PubMed] [Google Scholar]
- 26. Dai SH, Y Z (2012) [An Association Study of MTHFR and eNOS Genes Polymorphism with Diabetic Nephropathy]. Chin J Trauma Disability Med 20: 4–6. [Google Scholar]
- 27. Chang YH, Fu WM, Wu YH, Yeh CJ, Huang CN, et al. (2011) Prevalence of methylenetetrahydrofolate reductase C677T and A1298C polymorphisms in Taiwanese patients with Type 2 diabetic mellitus. Clin Biochem 44: 1370–1374. [DOI] [PubMed] [Google Scholar]
- 28. Mei QB, Chen P, LH Z (2012) [Correlation study between gene polymorphism of methylene tetrahydrofolate reductase and type 2 diabetes]. China Medical Herald 9: 162–163. [Google Scholar]
- 29. Chen AR, Zhang HG, Wang ZP, Fu SJ, Yang PQ, et al. (2010) C-reactive protein, vitamin B12 and C677T polymorphism of N-5,10-methylenetetrahydrofolate reductase gene are related to insulin resistance and risk factors for metabolic syndrome in Chinese population. Clin Invest Med 33: E290–297. [DOI] [PubMed] [Google Scholar]
- 30. Zhang YD, Shi L, Yu M, Wong DH, Liu C, et al. (2010) [Correlation between Single Nucleotide Polymorphisms in Methylenetetrahydrofolate Reductase Gene and Type 2 Diabetes Mellitus in Chinese Han Nationality]. J Mod Lab Med 25: 14–16. [Google Scholar]
- 31. Luo D, Yan S, Cheng X, Song Y (2009) [Levels of homocysteine and polymorphisms of homocysteine metabolism-related enzymes in patients with type 2 diabetes mellitus and coronary heart disease]. Wei Sheng Yan Jiu 38: 39–42. [PubMed] [Google Scholar]
- 32. Wen J, Lu ZQ, LI XM, Wu D, Zhang YW, et al. (2008) [Correlation of MTHFR gene polymorphism and plasma homocysteine with microalbuminuria in type 2 diabetes]. Shanghai Med J 31: 47–51. [Google Scholar]
- 33. Shi CJ, He WH, Cheng G, Wang WQ, Liu J, et al. (2006) [Detection of the 677 C→T variant of MTHFR gene in Chinese diabetic patients with fluorescent MGB probe real time PCR]. Chin J Diabetes 14: 258–260. [Google Scholar]
- 34. Xiao Y, Wu ZH, Dan KR, Guan ZS, XL R (2006) [An Investigation on the Relationship Between the Polymorphism of MTHFR Gene and Type II Diabetic Cardiovascular Disease]. J Guiyang Med College 31: 317–319. [Google Scholar]
- 35. Yue H, Liu J, Kang WJ, Hu L, Qin J, et al. (2006) [Relationship between plasma level of homocysteine and urine microalbumin in incipient type 2 diabetic nephropathy]. Chin J Gen Pract 5: 725–729. [Google Scholar]
- 36. Ho CH, Kuo BI, Kong CW, Chau WK, Hsu HC, et al. (2005) Influence of methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism, B vitamins and other factors on plasma homocysteine and risk of thromboembolic disease in Chinese. J Chin Med Assoc 68: 560–565. [DOI] [PubMed] [Google Scholar]
- 37. Mao L, Gao YF, Qin WH, H S (2004) [The Association of Methlenetetrahydrofolate Reductase Gene Polymorphism with Cerebral Infarction in Type II Diabetes Mellitus]. Acta Academiae Medicinae Nantong 24: 146–150. [Google Scholar]
- 38. Zhou J, Li XX, JC Z (2004) [Relationship between the C677T polymorphism in the methylenetetrahydrofolate reductase gene and cerebral infarction complicated type 2 diabetes]. J Apoplexy Nervous Diseases 21: 136–138. [Google Scholar]
- 39. Xu JS, Zhang J, Shan DS, HC M (2003) [Relationship between methylenetetrahydrofolate reductase gene polymorphism and diabetic nephropathy in type 2 diabetes mellitus in the Hans of Hebei Province]. Clinical Focus 18: 787–789. [Google Scholar]
- 40. Zhang GD, Xiang SS, Wong Q, J L (2002) [Association between 677C/T polymorphism of methylenetetrahydrofolate reductase gene and type 2 diabetes with macrovascular complications in Shanghai]. Chin J Endocrinol Metab 18: 362–365. [Google Scholar]
- 41. Yang GQ, Lu JM, CY P (2001) [Study on the relationship between N5, 10-methylenetetrahydrofolate reductase gene polymorphism and the susceptibility to microangiopathy in type 2 diabetes mellitus]. Chin J Endocrinol Metab 17: 224–227. [Google Scholar]
- 42. Wang L, Wang J, Xue Y, Chen Y, Zou H (2001) [Relationship between methylenetetrahydrofolate reductase gene polymorphism and diabetic nephropathy]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 18: 276–278. [PubMed] [Google Scholar]
- 43. Chauhan G, Kaur I, Tabassum R, Dwivedi OP, Ghosh S, et al. (2012) Common variants of homocysteine metabolism pathway genes and risk of type 2 diabetes and related traits in Indians. Exp Diabetes Res 2012: 960318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Movva S, Alluri RV, Venkatasubramanian S, Vedicherla B, Vattam KK, et al. (2011) Association of methylene tetrahydrofolate reductase C677T genotype with type 2 diabetes mellitus patients with and without renal complications. Genet Test Mol Biomarkers 15: 257–261. [DOI] [PubMed] [Google Scholar]
- 45. Hasegawa G, Obayashi H, Kamiuchi K, Nakai M, Kanatsuna T, et al. (2003) The association between end-stage diabetic nephropathy and methylenetetrahydrofolate reductase genotype with macroangiopathy in type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 111: 132–138. [DOI] [PubMed] [Google Scholar]
- 46. Bazzaz JT, Shojapoor M, Nazem H, Amiri P, Fakhrzadeh H, et al. (2010) Methylenetetrahydrofolate reductase gene polymorphism in diabetes and obesity. Mol Biol Rep 37: 105–109. [DOI] [PubMed] [Google Scholar]
- 47. Eroglu Z, Erdogan M, Tetik A, Karadeniz M, Cetinalp S, et al. (2007) The relationship of the methylenetetrahydrofolate reductase C677T gene polymorphism in Turkish type 2 diabetic patients with and without nephropathy. Diabetes Metab Res Rev 23: 621–624. [DOI] [PubMed] [Google Scholar]
- 48. Tutuncu NB, Erbas T, Alikasifoglu M, Tuncbilek E (2005) Thermolabile methylenetetrahydrofolate reductase enzyme genotype is frequent in type 2 diabetic patients with normal fasting homocysteine levels. J Intern Med 257: 446–453. [DOI] [PubMed] [Google Scholar]
- 49. Russo GT, Di Benedetto A, Alessi E, Giandalia A, Gaudio A, et al. (2008) Menopause modulates homocysteine levels in diabetic and non-diabetic women. J Endocrinol Invest 31: 546–551. [DOI] [PubMed] [Google Scholar]
- 50. Książek P, Bednarek-Skublewska A, Buraczyńska M (2004) The C677T methylenetetrahydrofolate reductase gene mutation and nephropathy in type 2 diabetes mellitus. Med Sci Monit 10: BR47–51. [PubMed] [Google Scholar]
- 51. Wirta V, Saransaari P, Wirta O, Rantalaiho V, Oja SS, et al. (2002) Methylenetetrahydrofolate reductase gene polymorphism, hyperhomocysteinemia and occlusive retinal vascular disease in type 2 diabetic and non-diabetic subjects. Clin Nephrol 58: 171–178. [DOI] [PubMed] [Google Scholar]
- 52. Cenerelli S, Bonazzi P, Galeazzi R, Testa I, Bonfigli AR, et al. (2002) Helicobacter pylori masks differences in homocysteine plasma levels between controls and type 2 diabetic patients. Eur J Clin Invest 32: 158–162. [DOI] [PubMed] [Google Scholar]
- 53. Beneš P, Kaňková K, Mužík J, Groch L, Benedík J, et al. (2001) Methylenetetrahydrofolate reductase polymorphism, type II diabetes mellitus, coronary artery disease, and essential hypertension in the Czech population. Mol Genet Metab 73: 188–195. [DOI] [PubMed] [Google Scholar]
- 54. Mazza A, Bossone E, Mazza F, Distante A (2005) Reduced serum homocysteine levels in type 2 diabetes. Nutr Metab Cardiovasc Dis 15: 118–124. [DOI] [PubMed] [Google Scholar]
- 55. Blüthner M, Brüntgens A, Schmidt S, Strojek K, Grzeszczak W, et al. (1999) Association of methylenetetrahydrofolate reductase gene polymorphism and diabetic nephropathy in type 2 diabetes? Nephrol Dial Transplant 14: 56–57. [DOI] [PubMed] [Google Scholar]
- 56. Soares AL, Fernandes AP, Cardoso JE, Sousa MO, Lasmar MC, et al. (2008) Plasma total homocysteine levels and methylenetetrahydrofolate reductase gene polymorphism in patients with type 2 diabetes mellitus. Pathophysiol Haemost Thromb 36: 275–281. [DOI] [PubMed] [Google Scholar]
- 57. Helfenstein T, Fonseca FA, Relvas WG, Santos AO, Dabela ML, et al. (2005) Prevalence of myocardial infarction is related to hyperhomocysteinemia but not influenced by C677T methylenetetrahydrofolate reductase and A2756G methionine synthase polymorphisms in diabetic and non-diabetic subjects. Clin Chim Acta 355: 165–172. [DOI] [PubMed] [Google Scholar]
- 58. Benrahma H, Abidi O, Melouk L, Ajjemami M, Rouba H, et al. (2012) Association of the C677T polymorphism in the human methylenetetrahydrofolate reductase (MTHFR) gene with the genetic predisposition for type 2 diabetes mellitus in a Moroccan population. Genet Test Mol Biomarkers 16: 383–387. [DOI] [PubMed] [Google Scholar]
- 59. Mackawy AMH, Badawy M (2011) Methylene tetrahydrofolate reductase gene polymorphism and the risk of ischemic stroke in type 2 diabetic Egyptian patients. Global J Health Sci 3: 162–174. [Google Scholar]
- 60. Mehri S, Koubaa N, Nakbi A, Hammami S, Chaaba R, et al. (2010) Relationship between genetic polymorphisms of angiotensin-converting enzyme and methylenetetrahydrofolate reductase as risk factors for type 2 diabetes in Tunisian patients. Clin Biochem 43: 259–266. [DOI] [PubMed] [Google Scholar]
- 61. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, et al. (2010) Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375: 2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Niu W, Qi Y (2012) An updated meta-analysis of methylenetetrahydrofolate reductase gene 677C/T polymorphism with diabetic nephropathy and diabetic retinopathy. Diabetes Res Clin Pract 95: 110–118. [DOI] [PubMed] [Google Scholar]
- 63. Cui WP, Du B, Jia Y, Zhou WH, Liu SM, et al. (2012) Is C677T polymorphism in methylenetetrahydrofolate reductase gene a risk factor for diabetic nephropathy or diabetes mellitus in a Chinese population? Arch Med Res 43: 42–50. [DOI] [PubMed] [Google Scholar]
- 64. van der Put NM, Gabreels F, Stevens EM, Smeitink JA, Trijbels FJ, et al. (1998) A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? Am J Hum Genet 62: 1044–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yamada K, Chen Z, Rozen R, Matthews RG (2001) Effects of common polymorphisms on the properties of recombinant human methylenetetrahydrofolate reductase. Proc Natl Acad Sci USA 98: 14853–14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Friedman G, Goldschmidt N, Friedlander Y, Ben-Yehuda A, Selhub J, et al. (1999) A common mutation A1298C in human methylenetetrahydrofolate reductase gene: association with plasma total homocysteine and folate concentrations. J Nutr 129: 1656–1661. [DOI] [PubMed] [Google Scholar]
- 67. Smulders YM, Rakic M, Slaats EH, Treskes M, Sijbrands EJ, et al. (1999) Fasting and post-methionine homocysteine levels in NIDDM: Determinants and correlations with retinopathy, albuminuria, and cardiovascular disease. Diabetes Care 22: 125–132. [DOI] [PubMed] [Google Scholar]
- 68. Chico A, Pérez A, Córdoba A, Arcelús R, Carreras G, et al. (1998) Plasma homocysteine is related to albumin excretion rate in patients with diabetes mellitus: a new link between diabetic nephropathy and cardiovascular disease? Diabetologia 41: 684–693. [DOI] [PubMed] [Google Scholar]
- 69. Shimomura T, Anan F, Umeno Y, Eshima N, Saikawa T, et al. (2008) Hyperhomocysteinaemia is a significant risk factor for white matter lesions in Japanese type 2 diabetic patients. Eur J Neurol 15: 289–294. [DOI] [PubMed] [Google Scholar]
- 70. Bar-On H, Kidron M, Friedlander Y, Ben-Yehuda A, Selhub J, et al. (2000) Plasma total homocysteine levels in subjects with hyperinsulinemia. J Intern Med 247: 287–294. [DOI] [PubMed] [Google Scholar]
- 71. Holmes MV, Newcombe P, Hubacek JA, Sofat R, Ricketts SL, et al. (2011) Effect modification by population dietary folate on the association between MTHFR genotype, homocysteine, and stroke risk: a meta-analysis of genetic studies and randomised trials. Lancet 378: 584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mello AL, Cunha SF, Foss-Freitas MC, Vannucchi H (2012) Evaluation of plasma homocysteine level according to the C677T and A1298C polymorphism of the enzyme MTHRF in type 2 diabetic adults. Arq Bras Endocrinol Metabol 56: 429–434. [DOI] [PubMed] [Google Scholar]
- 73.College of American Pathologists. “Consensus Conference XXXVI: Diagnostic issues in thrombophilia (November 9–11, 2001)”. Available: www.caporg/apps/docs/committees/coagulation/pocketbookpdf, Accessed 2013 February 17. [DOI] [PubMed]
- 74. Hankey GJ, Eikelboom JW (1999) Homocysteine and vascular disease. Lancet 354: 407–413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA 2009 Checklist.
(DOC)