Abstract
Pseudomonas aeruginosa is an opportunistic pathogen that can cause a wide range of infections and inflammations in a variety of hosts, such as chronic biofilm associated lung infections in Cystic Fibrosis patients. Phosphate, an essential nutrient, has been recognized as an important signal that affects virulence in P. aeruginosa. In the current study we examined the connection between phosphate regulation and surface motility in P. aeruginosa. We focused on two important genes, pstS, which is involved in phosphate uptake, and phoB, a central regulator that responds to phosphate starvation. We found that a mutant lacking pstS is constantly starved for phosphate and has a hyper swarming phenotype. Phosphate starvation also induced swarming in the wild type. The phoB mutant, on the other hand, did not express phosphate starvation even when phosphate was limited and showed no swarming. A double mutant lacking both genes (pstS and phoB) showed a similar phenotype to the phoB mutant (i.e. no swarming). This highlights the role of phoB in controlling swarming motility under phosphate-depleted conditions. Finally, we were able to demonstrate that PhoB controls swarming by up-regulating the Rhl quorum sensing system in P. aeruginosa, which resulted in hyper production of rhamonlipids: biosurfactants that are known to induce swarming motility.
Introduction
Pseudomonas aeruginosa is a versatile bacterium that can adapt to a variety of niches and grows well in soil, water, plants and animals [1]. P. aeruginosa is also an opportunistic pathogen that can cause a variety of infections such as chronic infection of the lungs in Cystic Fibrosis (CF) patients [2], urinary tract infections resulting from catheterization [3], burn wounds [4] and Keratitis [5]. An important aspect of the P. aeruginosa “life style” is its ability to switch from a planktonic to a sessile mode of growth, depending on nutrient availability and its surrounding bacterial community. When nutrient availability is scarce, P. aeruginosa uses swarming motility, a coordinated movement on a surface, using flagella, biosurfactants [6] and Type-IV pili [7], in order to find an optimal niche [8]. In this mode of growth, planktonic cells differentiate to elongated and hyper-flagellated cells that spread across the surface. In order to facilitate the movement, P. aeruginosa secretes rhamnolipids [6], glycolipids that act as a biosurfactant and are produced by the rhlAB operon [9]. Rhamnolipid production is regulated by Quorum Sensing (QS) [10]–[12], a social behavior in which bacteria sense and respond to its surrounding population by producing and receiving signal molecules. There are three known QS systems in P. aeruginosa – Las and RhI, each consisting of an auto-inducer molecule from the acyl-homoserine lactone (AHL) family (synthesized by the LasI and RhlI enzymes) and a response regulator (LasR and RhlR) [13], and PQS (Pseudomonas Quinolone Signal), a secondary metabolite that functions as a QS molecule [14]. In addition to rhamnolipid production, QS in P. aeruginosa affects many bacterial processes, such as virulence [15] and biofilm formation [16]. While searching for irregular swarming phenotypes using a transposon mutant library, we came upon a hyper-swarming mutant. The mutated gene was mapped to pstS, a key component in the phosphate specific transport system (Pst) [17]. Phosphate is an essential nutrient, used in the assembly of ATP, LPS, nucleic acids and other cell components. P. aeruginosa has two phosphate uptake systems – Pit, a low-affinity, constitutively operating channel [18], and Pst – a high-affinity ABC transporter. The Pst system is encoded by the pst operon, containing the genes pstA, pstB, pstC, pstS and phoU. pstA, pstB and pstC encode the transporter proteins, while pstS encodes a periplasmic phosphate-binding protein that transfers the phosphate to the bacterial cytoplasm through the transporter. The system is regulated by PhoB/R – a two-component system activated by phosphate depletion [19]. When phosphate levels in the bacteria become low, PhoR phosphorylates PhoB, which in turn acts as a transcription factor and activates genes that have the consensus sequence ‘Pho Box’ in their promoters, such as the pst operon. In recent years, studies concerning the Pst system have uncovered a connection between phosphate depletion and virulence – over-expression of PstS has been found in ampicilin-resistant Streptococcus pneumoniae [20], a possible correlation between phosphate depletion and P. aeruginosa in a gut mouse model has also been suggested [21], and it has been shown that deletion of PhoB affects swarming [22]. Furthermore, the Pst system has been connected to virulence in many bacteria [23]. For example, in clinical strains of P. aeruginosa, PstS forms extracellular appendages that increase the strain’s virulence in a mouse model [24]. In this study, we establish the intricate connection between swarming, QS and phosphate availability in order to better understand the molecular mechanisms that regulate surface motility in P. aeruginosa. We show that in the absence of phosphate, PhoB up-regulates rhlR expression, which results in rhamnolipid production that promotes hyper swarming.
Materials and Methods
Bacterial Strains, Plasmids, and Media
The bacterial strains and plasmids used in this study are shown in Table 1. For Alkaline Phosphatase and Real-time assays, strains were grown on M9 minimal medium (20 mM NH4Cl; 12 mM Na2HPO4; 22 mM KH2PO4; 8.6 mM NaCl; 1 mM MgSO4; 1 mM CaCl2; 11 mM Dextrose) supplemented with 50 µM FeCl3. For swarming assays, we used M9 minimal medium or M9 depleted of phosphate (20 mM NH4Cl; 8.6 mM NaCl; 1 mM MgSO4; 1 mM CaCl2; 11 mM Dextrose), both supplemented with 0.5% Casamino acids, 50 µM FeCl3 and solidified with 0.5% Bacto Agar (Difco). For deletions, Luria-Bertani broth (LB, Difco), No Salt LB (NSLB, 0.5% Tryptone, 0.5% yeast extract), Vogel Bonner Minimal Medium (VBMM) and Psuedomonas Isolation Agar (PIA, Difco) were used. All strains were grown at 37°C with shaking, unless specified otherwise. Antibiotic concentrations used in this study were 300 µg/ml or 150 µg/ml carbenicillin for P. aeruginosa, 100 µg/ml ampicillin for E. coli, 100 µg/ml gentamicin for P. aeruginosa and 20 µg/ml for E. coli.
Table 1. Strains used in this study.
| Strain or plasmid | Description | Source or reference |
| P. aeruginosa strains | ||
| PAO1 | Wild type | [36] |
| ΔpstS | PAO1 with an unmarked deletion of pstS | This study |
| ΔphoB | PAO1 with an unmarked deletion of phoB | This study |
| ΔpstSΔphoB | PAO1 with an unmarked deletion of pstS and phoB | This study |
| ΔrhlA | PAO1 with an unmarked deletion of rhlA | This study |
| ΔrhlR | PAO1 with an unmarked deletion of rhlR | This study |
| ΔpstSΔrhlA | PAO1 with an unmarked deletion of pstS and rhlA | This study |
| ΔpstSΔrhlR | PAO1 with an unmarked deletion of pstS and rhlR | This study |
| E. coli strains | ||
| DH5α | F′/endA1 hsdR17 supE44 thi-1 recA1 gyrA relA1 Δ(lacZYA-argF) U169deoR (Φ80 dlacZ-M15 recA1) | [37] |
| S17.1 (λpir) | recA derivative of E. coli 294 (F- thi pro hsdR) carrying a modifiedderivative of IncPα plasmid pRP4 (Aps Tcs Kms) integrated in thechromosome, Tpr; lysogenized with bacteriophage λpir | Y. Irie and M. R. Parsek |
| Plasmids | ||
| pUCP18Ap | A broad-host range cloning vector. CbR/AmpR | [38] |
| DB3.1 pEX18GmGW | pEX18Gm containing the Gateway (GW) destination cloning site. GmR | Nan Fulcher and Matthew Wolfgang |
| pphoB | pUCP18Ap containing the phoB gene for complementation | This study |
| ppstS | pUCP18Ap containing the pstS gene for complementation | This study |
| prhlA | pUCP18Ap containing the rhlA gene for complementation | This study |
| prhlR | pUCP18Ap containing the rhlR gene for complementation | This study |
| pECP61.5 | Contains a rhlA-lacZ fusion, used for C4-HSL detection | [11] |
AmpR -Ampicilin resistance for E. coli CbR – Carbenicillin resistance for P. aeruginosa. GmR – Gentamicin resistance.
Construction of Strains and Plasmids
Deletion mutants of pstS, phoB, pstS+phoB, rhlA and rhlR were constructed as previously described [25]. Overlap extension PCR using the primers specified in Table S1 was used in order to generate a fragment containing the upstream and downstream regions of each gene. Each fragment was cloned into the allelic exchange vector DB3.1 pEX18GmGW [26] using BP-Clonase (Invitrogen). Each deletion was introduced to PAO1 or ΔpstS using bi-parental mating [27]. Deletions were generated using a standard method for two-step allelic exchange as described by Schweizer and Hoang [28] and were confirmed by PCR.
Swarming Motility Assay
Strains were grown overnight in M9 medium supplemented with Casamino acids, FeCl3, and carbenicillin if necessary. Afterwards, the bacteria were diluted 1∶10 into fresh medium and grown for an additional three hours in order to reach the logarithmic growth phase. 2.5 µl from each culture was plated in the middle of a swarming plate, which contained M9 medium or M9 depleted of phosphate, supplemented with 0.5% Casamino acids, 50 µM FeCl3 and carbenicillin if necessary, and solidified with 0.5% Bacto Agar. Plates were incubated at 37°C for 24 hours.
Alkaline Phosphatase Assay
Alkaline Phosphatase (AP) is a protein whose expression is enhanced under phosphate starvation [29]. We utilized AP activity in order to assay a strain’s phosphate starvation levels. AP activity was measured by sampling strains grown in a liquid culture or on swarming plates. To measure AP from a liquid culture, strains were grown overnight in M9 medium supplemented with FeCl3, and carbenicillin if necessary. Afterwards, bacteria were diluted to an O.D595nm of 0.04 into 50 ml of fresh M9 medium, containing 50 µM FeCl3 and grown for an additional 10 hours. Then, 6 ml from each strain were taken and centrifuged for 10 minutes at 2,200 g (Centrifuge 5418; eppendorf (. The pellet was re-suspended with 20 µl of Chloroform. After 15 minutes of incubation at room temperature, 20 µl of 0.01 M Tris-HCl (pH = 8) were added to each sample, and the samples were centrifuged for 20 minutes at 6,000 g. Following centrifugation, 30 µl from each sample’s supernatant were added to a 96-well plate, containing the reaction buffer (5 µl of 0.5 mM MgCl2 and 10 µl of 1 M Tris (pH = 9.5)). 5 µl of 500 mM p-Nitrophenyl Phosphate (PNPP, NEB) were added to each well and the reaction was read at 405 nm in an ELISA plate reader )Synergy™ 2 Multi-Detection Microplate Reader; Biotech). Results were normalized to each samples’ total protein concentration using Bradford assay (Thermo).
When measuring AP activity from swarming plates, bacteria grown for 24 hours on swarming plates were scraped off and suspended in 2 ml of M9, then submitted to the same procedure as the samples taken from liquid culture.
RNA Extraction, RT-PCR and Real-Time PCR Analysis
Strains were grown overnight in M9 medium supplemented with FeCl3. Then, bacteria were diluted to an O.D595nm of 0.04 into 50 ml of fresh M9 medium, containing FeCl3 and grown for an additional 48 hours. Afterwards, 2 ml from each culture was incubated with 4 ml of RNAprotect Bacteria Reagent (Qiagen). RNA extraction was performed using RNeasy Mini Kit (Qiagen). cDNA was synthesized from 500 ng of RNA using GoScript™ Reverse Transcription System (Promega). Real-time PCR was performed using CFX-96 Touch Real-Time PCR detection system (Bio-Rad). Results were normalized to the expression of PA1769 [30].
C4–HSL Quantification
The level of C4-HSL was measured using a bio-reporter strain as previously reported [31]. Briefly, strains were grown overnight in M9 medium supplemented with FeCl3. Then, bacteria were diluted to an O.D595nm of 0.04 into 50 ml of fresh M9 medium, supplemented with FeCl3 and grown for an additional 48 hours. Afterwards, 5 ml from each culture was extracted twice with an equal volume of ethyl acetate containing 0.1% glacial acetic acid, as previously described [32]. Ethyl acetate was evaporated using nitrogen gas. Overnight culture of DH5α harboring pECP61.5 was diluted into an O.D595nm of 0.1 into modified A medium [33] with ampicillin and 1 mM IPTG. 500 µl of the diluted bacteria was added to each sample and grown at 30°C for 5.5 hours. Cells were than lysed with chloroform and the β-galactosidase activity was measured using Tropix-Galacton kit according to the manufacturer guidelines (Applied Biosystems).
Statistical Analysis
Statistical analysis was carried out using unpaired t-test and Turkey’s Post Hoc test. P<0.05 was considered as significant.
Results and Discussion
Phosphate Starvation Promotes phoB-mediated Hyper Swarming
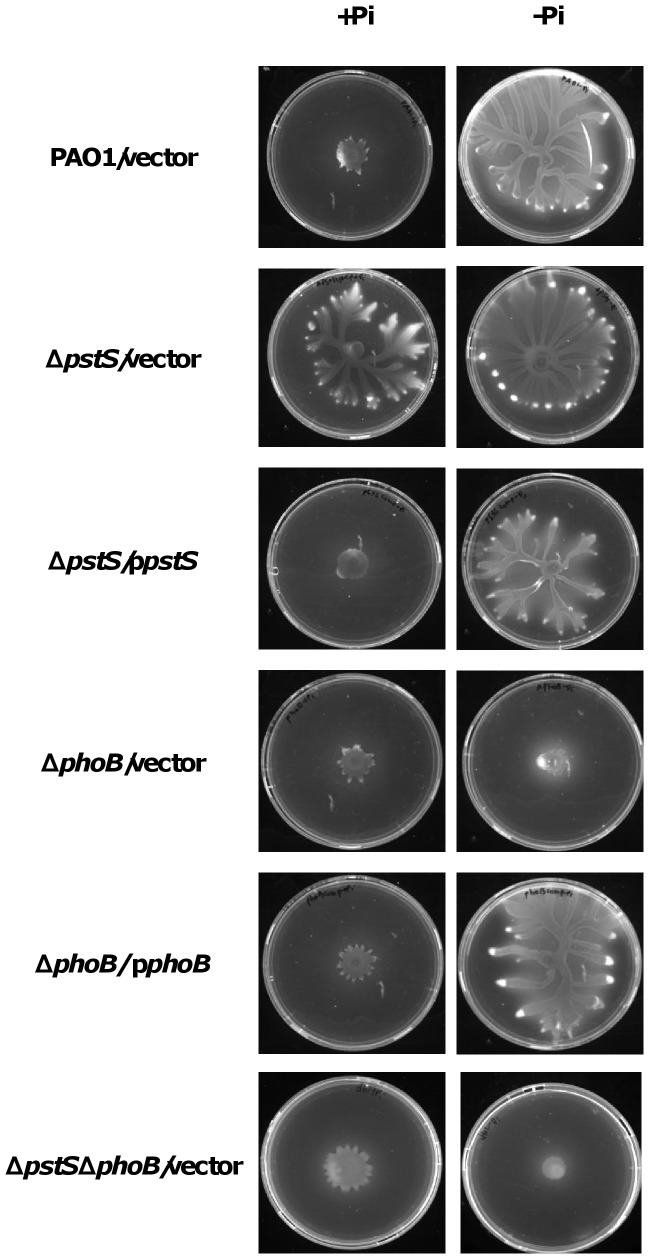
While scanning a transposon mutant library for irregular swarming phenotypes, we found that transposon insertion in the pstS gene promoted hyper-swarming. In order to ensure that the phenotype is indeed caused by the disruption of the gene, we generated a clean deletion of pstS in PAO1. The mutant showed a hyper-swarming phenotype both in standard M9 minimal medium representing phosphate-repleted conditions (20 mM Pi) and in M9 medium with phosphate depleted conditions (0.2 mM Pi). The wild type, on the other hand, showed swarming ability only under phosphate-depleted conditions (Fig. 1). Complementation of the ΔpstS strain with a plasmid encoding pstS restored the wild type phenotype.
Figure 1. Influence of pstS and phoB deletion on swarming motility.
PAO1, ΔpstS, ΔphoB and ΔpstSΔphoB carrying an empty vector (pUCP18Ap) or a complementation plasmid (ppstS and pphoB) were grown for 24 hours at 37°C on swarming plates containing M9 (20 mM) or phosphate-depleted M9 (0.2 mM) as described in Materials and Methods.
Next, we generated a clean deletion of phoB in PAO1, in order to see how deletion of a protein with a regulatory function in the phosphate uptake system affects the bacteria’s swarming pattern. We saw that deletion of phoB caused the bacteria to lose all swarming ability, even under phosphate depleted conditions. A similar result was demonstrated previously by Bains et al. [22], who showed that a phoB mutant loses its ability to swarm when grown on a BM2 medium with low (0.2 mM) phosphate concentration. Complementation of our ΔphoB strain with a plasmid encoding phoB restored the wild type phenotype (Fig. 1). We then wanted to see which one of the two genes – pstS or phoB – is responsible for controlling the swarming phenotype under phosphate depleted conditions. To do so, we generated a double mutant, lacking both pstS and phoB in PAO1. We assumed that if PstS controlled swarming in the presence or lack of phosphate, the double mutant would show the hyper-swarming phenotype presented by the pstS mutant, and if PhoB controlled swarming under the tested conditions, the double mutant would act like the phoB mutant and show a non-swarming phenotype. Plating the double mutant on swarming plates containing M9 or M9 with low levels of phosphate, generated a non-swarming phenotype under both conditions (Fig. 1), which led us to conclude that PhoB is responsible for controlling P. aeruginosa’s swarming patterns in the presence or lack of phosphate. When we complemented the double mutant with a plasmid containing the pstS gene, we received the phenotype presented by phoB, and when we complemented the double mutant with a plasmid containing the phoB gene (pphoB), we received the phenotype presented by pstS (Fig. S1A). From these experiments we can conclude that while both pstS and phoB have impact on swarming motility on media containing low levels of phosphate, PhoB is the protein that regulates swarming under the specified conditions.
The pstS Mutant is Constantly Starved for Phosphate, while the phoB Mutant cannot sense the Bacteria’s Phosphate Levels
The results from the swarming experiments led us to hypothesize that deletion of pstS causes P. aeruginosa to be constantly starved for phosphate, whereas deletion of phoB causes the bacteria to lose its ability to sense its intra-cellular phosphate levels. Because PhoB is activated under low phosphate levels, we assumed that deletion of phoB causes the bacteria to act as if they were in a constant state of phosphate saturation. To test this hypothesis, we plated PAO1, ΔpstS, ΔphoB and ΔpstSΔphoB on either phosphate depleted or repleted swarming plates in order to measure the Alkaline Phosphatase (AP) activity in each strain, as described in Materials and Methods. AP levels increase when intra-cellular phosphate levels are low, thus, AP levels point to the degree of phosphate starvation in bacteria. PAO1 was used as a reference – as expected, when grown on standard M9 plates, AP levels were low, and when grown on phosphate-depleted M9, AP levels increased dramatically (p<0.05) (Fig. 2). AP levels in the pstS mutant were higher than in PAO1 in both media (p<0.05), and AP levels in ΔphoB and ΔpstSΔphoB were similar to those of PAO1 grown on standard M9 plates, but did not increase under phosphate depleted conditions (Fig 2). Complementation of the deletion strains, with plasmids encoding pstS or phoB, reverted the phenotypes (Fig. S1B). The results suggest that absence of both pstS and phoB disrupts P. aeruginosa’s ability to respond to its intra-cellular phosphate levels, but in different ways. Deletion of pstS, the gene that encodes the phosphate-carrying protein, causes the bacteria to lose its ability to acquire phosphate through the Pst system, which puts the bacteria in a state of constant phosphate starvation. On the other hand, deletion of phoB causes P. aeruginosa to lose the regulatory part of the phosphate uptake system. Since PhoB/R are activated only when phosphate levels are low, bacteria with a phoB deletion will lose their ability to sense when there is a decrease in intra-cellular phosphate levels and therefore will always have a false sense of being in a phosphate saturated environment, hence the lack of swarming and the low AP levels in ΔphoB and ΔpstSΔphoB grown in media containing low levels of phosphate.
Figure 2. The effect of pstS and phoB deletion on phosphate starvation in P.aeruginosa.
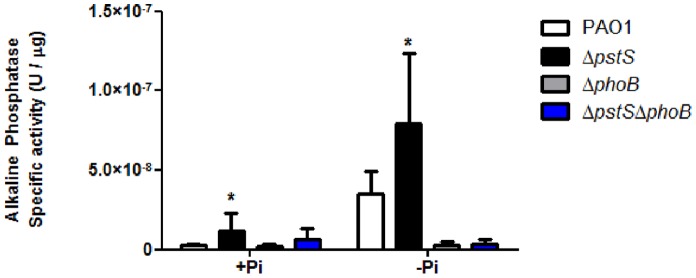
PAO1, ΔpstS, ΔphoB and ΔpstSΔphoB were grown for 24 hours at 37°C on swarming plates containing M9 or phosphate-depleted M9. After 24 hours, bacteria were scraped off the plates and Alkaline Phosphatase activity was measured using p-Nitrophenyl Phosphate as described in Materials and Methods. Results were normalized to each samples’ total protein concentration using Bradford assay. Results shown represent mean+standard deviation of six different experiments. Each experiment was performed in triplicate. Asterisks represent the significant rise in AP activity compared to the WT (p<0.05, student’s t-test).
rhlR and rhlA are Essential for Swarming Under Phosphate-deplete Conditions
After determining how deletion of pstS and phoB affects P. aeruginosa’s ability to sense phosphate, we were interested to study the molecular pathway that leads from the bacteria’s reaction to phosphate depletion to swarming motility. It was previously shown that the rhlR promoter contains a Pho Box, that rhlR transcription can be activated under phosphate-limiting conditions [34] and that RhlR controls rhamnolipid production [10]. Therefore, we wanted to see if up-regulation of rhlR, and in turn, up-regulation of rhlA and increase in rhamnolipid production, is the cause for hyper-swarming under phosphate-depleted conditions. In order to do so, we measured the transcription levels of rhlR and rhlA in PAO1, ΔpstS, ΔphoB and ΔpstSΔphoB grown in M9 medium, using Real-Time PCR. Results were normalized to those of PAO1, the reference strain (Fig. 3). Only in ΔpstS did we see a significant rise in transcription levels of rhlA and rhlR (2.5 and 3.9 fold elevation in gene transcription as opposed to the WT, respectively, p<0.05). Because ΔpstS remains constantly starved for phosphate, PhoB is activated in this strain, which strengthens our hypothesis that PhoB is indeed responsible for setting in motion the cascade that ultimately results in swarming in the absence of phosphate. This result is further supported by microarray studies done by Bains et al., showing that rhlA and rhlR transcription increases under minimal phosphate conditions in P. aeruginosa [22]. To further corroborate this data, we measured the C4-HSL levels in each of the strains (Fig. 4). Coinciding with the RT-PCR results, C4-HSL levels in ΔpstS were significantly higher than those of the other strains (p<0.05), which shows that the response to phosphate starvation involves all factors of the Rhl system.
Figure 3. pstS knockout results in elevated rhlA and rhlR expression.
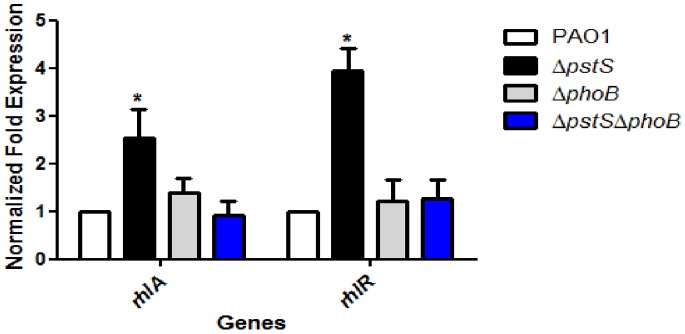
PAO1, ΔpstS, ΔphoB and ΔpstSΔphoB were grown for 48 hours at 37°C with shaking in M9 medium. Levels of rhlA and rhlR were measured by Real-Time PCR as described in Materials and Methods. Values were normalized to the expression of PA1769. The results shown are relative to PAO1 and represent mean+standard deviation of nine different experiments. Each experiment was performed in triplicate. Asterisks represent the significant elevation in rhlA and rhlR transcription compared to the WT (p<0.05, student’s t-test).
Figure 4. Phosphate starvation promotes C4-HSL production.
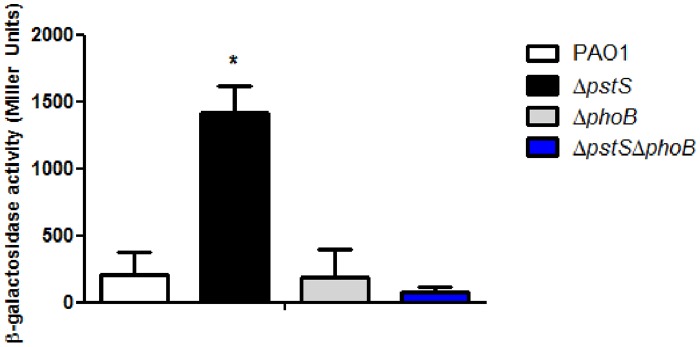
PAO1, ΔpstS, ΔphoB and ΔpstSΔphoB were grown for 48 hours at 37°C with shaking in M9 medium. C4-HSL in each strain was extracted and measured as described in Materials and Methods. β-galactosidade activity (Miller Units) represent C4-HSL levels. Results represent mean+standard deviation of three different experiments. Each experiment was performed in triplicate. Asterisks represent the significant elevation in C4-HSL production in ΔpstS as opposed to all other strains (p<0.05, Turkey’s post hoc test).
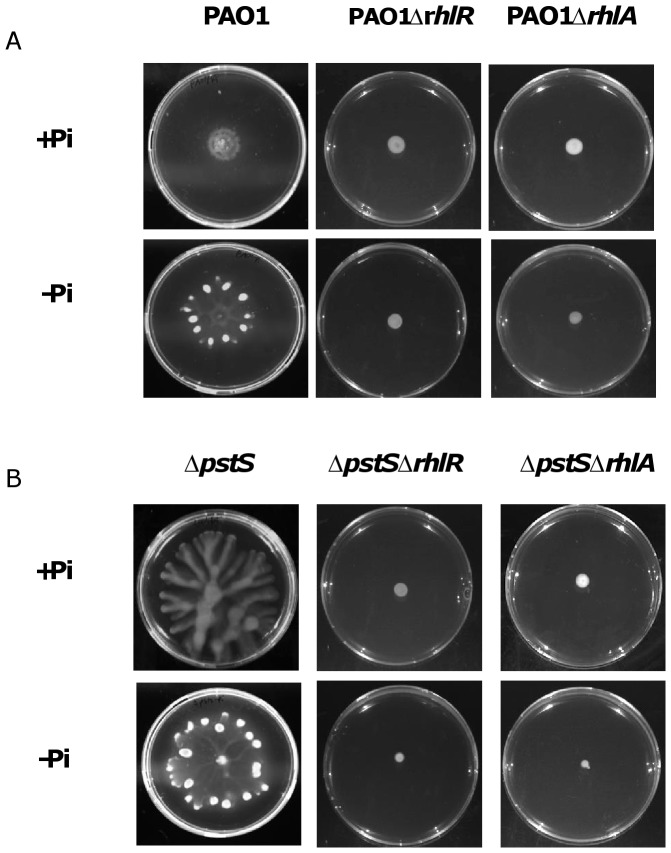
Next, in order to further prove that rhlR and rhlA are responsible for hyper-swarming when phosphate level is low, we generated clean deletions of rhlR and rhlA in PAO1 and in ΔpstS, and plated them on swarming plates containing M9 with either low or high levels of phosphate. The results (Fig. 5) clearly show that deletion of each of these genes abolished the swarming completely in both strains and in both media, which proves that these genes are crucial for phoB-mediated swarming when phosphate is low. Complementation of rhlA and rhlR restored the phenotypes of both PAO1 and ΔpstS (data not shown).
Figure 5. rhlA and rhlR deletion cause loss of swarming motility.
A. PAO1, PAO1ΔrhlA and PAO1ΔrhlR were grown for 24 hours at 37°C on swarming plates containing M9 or phosphate-depleted M9. B. ΔpstS, ΔpstSΔrhlA and ΔpstSΔrhlR were grown for 24 hours at 37°C on swarming plates containing M9 or phosphate-depleted M9.
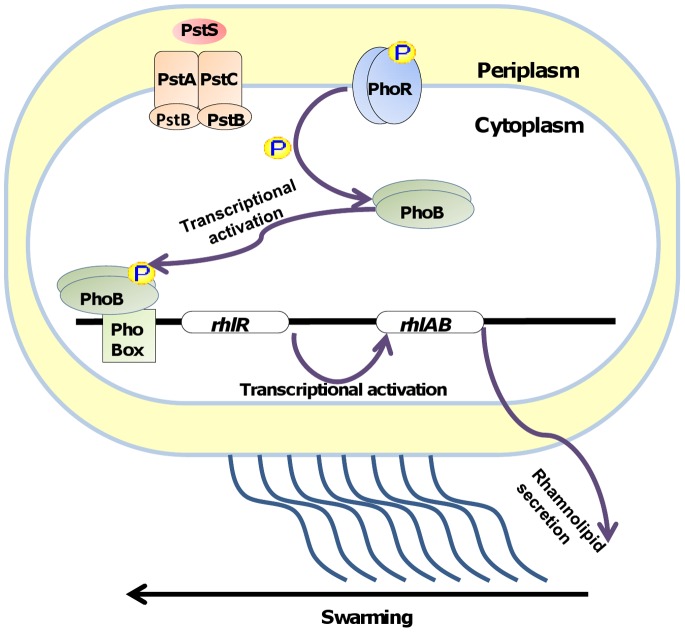
All of these results combined piece together the molecular mechanism that is initiated under phosphate-deplete conditions (Fig. 6) - PhoB is activated upon phosphorylation by PhoR, acts as a transcription factor and binds to promoters containing a Pho Box motif. One of these promoters is the promoter for rhlR. RhlR undergoes activation, and activates transcription of the rhlAB operon, causing rhamnolipid production, which results in swarming. Interestingly, we have previously shown in P. aeruginosa that iron depletion can also cause increased motility by QS-mediated rhamnolipid production [35]. The current study further links between environmental cues and QS, and sheds light on the complicated regulatory network in P. aeruginosa that makes this pathogen so versatile and highly adjustable to a variety of niches.
Figure 6. Model of the regulatory pathway leading to swarming under phosphate depletion.
Supporting Information
Complementation of pstS and phoB restores the wild type swarming phenotype.
(TIF)
Primers used in this study.
(DOCX)
Funding Statement
The work was supported by the Israel Science Foundation Grants No. 366/07 and 1124/12 to E.B. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. tover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, et al. (2000) Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406: 959–964. [DOI] [PubMed] [Google Scholar]
- 2. Kirov SM, Webb JS, O’May C Y, Reid DW, Woo JK, et al. (2007) Biofilm differentiation and dispersal in mucoid Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Microbiology 153: 3264–3274. [DOI] [PubMed] [Google Scholar]
- 3. Mittal R, Aggarwal S, Sharma S, Chhibber S, Harjai K (2009) Urinary tract infections caused by Pseudomonas aeruginosa: a minireview. J Infect Public Health 2: 101–111. [DOI] [PubMed] [Google Scholar]
- 4. Church D, Elsayed S, Reid O, Winston B, Lindsay R (2006) Burn wound infections. Clin Microbiol Rev 19: 403–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hazlett LD (2004) Corneal response to Pseudomonas aeruginosa infection. Prog Retin Eye Res 23: 1–30. [DOI] [PubMed] [Google Scholar]
- 6. Caiazza NC, Shanks RM, O’Toole GA (2005) Rhamnolipids modulate swarming motility patterns of Pseudomonas aeruginosa . J Bacteriol 187: 7351–7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kohler T, Curty LK, Barja F, van Delden C, Pechere JC (2000) Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J Bacteriol 182: 5990–5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Verstraeten N, Braeken K, Debkumari B, Fauvart M, Fransaer J, et al. (2008) Living on a surface: swarming and biofilm formation. Trends Microbiol 16: 496–506. [DOI] [PubMed] [Google Scholar]
- 9. Deziel E, Lepine F, Milot S, Villemur R (2003) rhlA is required for the production of a novel biosurfactant promoting swarming motility in Pseudomonas aeruginosa: 3-(3-hydroxyalkanoyloxy)alkanoic acids (HAAs), the precursors of rhamnolipids. Microbiology-Sgm 149: 2005–2013. [DOI] [PubMed] [Google Scholar]
- 10. Daniels R, Vanderleyden J, Michiels J (2004) Quorum sensing and swarming migration in bacteria. FEMS Microbiol Rev 28: 261–289. [DOI] [PubMed] [Google Scholar]
- 11. Pearson JP, Pesci EC, Iglewski BH (1997) Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol 179: 5756–5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soberon-Chavez G, Lepine F, Deziel E (2005) Production of rhamnolipids by Pseudomonas aeruginosa . Appl Microbiol Biotechnol 68: 718–725. [DOI] [PubMed] [Google Scholar]
- 13. Venturi V (2006) Regulation of quorum sensing in Pseudomonas . FEMS Microbiol Rev 30: 274–291. [DOI] [PubMed] [Google Scholar]
- 14. Pesci EC, Milbank JB, Pearson JP, McKnight S, Kende AS, et al. (1999) Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. . Proc Natl Acad Sci U S A 96: 11229–11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Antunes LC, Ferreira RB, Buckner MM, Finlay BB (2010) Quorum sensing in bacterial virulence. Microbiology 156: 2271–2282. [DOI] [PubMed] [Google Scholar]
- 16. de Kievit TR (2009) Quorum sensing in Pseudomonas aeruginosa biofilms. Environmental Microbiology 11: 279–288. [DOI] [PubMed] [Google Scholar]
- 17. Madhusudhan KT, McLaughlin R, Komori N, Matsumoto H (2003) Identification of a major protein upon phosphate starvation of Pseudomonas aeruginosa PAO1. Journal of Basic Microbiology 43: 36–46. [DOI] [PubMed] [Google Scholar]
- 18. Rosenberg H, Gerdes RG, Chegwidden K (1977) Two systems for the uptake of phosphate in Escherichia coli . J Bacteriol 131: 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hsieh YJ, Wanner BL (2010) Global regulation by the seven-component Pi signaling system. Current Opinion in Microbiology 13: 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Soualhine H, Brochu V, Menard F, Papadopoulou B, Weiss K, et al. (2005) A proteomic analysis of penicillin resistance in Streptococcus pneumoniae reveals a novel role for PstS, a subunit of the phosphate ABC transporter. Molecular Microbiology 58: 1430–1440. [DOI] [PubMed] [Google Scholar]
- 21. Long J, Zaborina O, Holbrook C, Zaborin A, Alverdy J (2008) Depletion of intestinal phosphate after operative injury activates the virulence of P. aeruginosa causing lethal gut-derived sepsis. Surgery 144: 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bains M, Fernandez L, Hancock RE (2012) Phosphate starvation promotes swarming motility and cytotoxicity of Pseudomonas aeruginosa . Appl Environ Microbiol 78: 6762–6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lamarche MG, Wanner BL, Crepin S, Harel J (2008) The phosphate regulon and bacterial virulence: a regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol Rev 32: 461–473. [DOI] [PubMed] [Google Scholar]
- 24. Zaborina O, Holbrook C, Chen Y, Long J, Zaborin A, et al. (2008) Structure-function aspects of PstS in multi-drug-resistant Pseudomonas aeruginosa . PLoS Pathog 4: e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rybtke MT, Borlee BR, Murakami K, Irie Y, Hentzer M, et al. (2012) Fluorescence-based reporter for gauging cyclic di-GMP levels in Pseudomonas aeruginosa . Appl Environ Microbiol 78: 5060–5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Choi KH, Schweizer HP (2005) An improved method for rapid generation of unmarked Pseudomonas aeruginosa deletion mutants. BMC Microbiol 5: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lewenza S, Falsafi RK, Winsor G, Gooderham WJ, McPhee JB, et al. (2005) Construction of a mini-Tn5-luxCDABE mutant library in Pseudomonas aeruginosa PAO1: a tool for identifying differentially regulated genes. Genome Res 15: 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schweizer HP, Hoang TT (1995) An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa . Gene 158: 15–22. [DOI] [PubMed] [Google Scholar]
- 29. Hou CI, Gronlund AF, Campbell JJ (1996) Influence of phosphate starvation on cultures of Pseudomonas aeruginosa . J Bacteriol 92: 851–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Son MS, Matthews WJ, Jr. Kang Y, Nguyen DT, Hoang TT (2007) In vivo evidence of Pseudomonas aeruginosa nutrient acquisition and pathogenesis in the lungs of cystic fibrosis patients. Infect Immun 75: 5313–5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parsek MR, Singh PK (2003) Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol 57: 677–701. [DOI] [PubMed] [Google Scholar]
- 32. Pearson JP, Passador L, Iglewski BH, Greenberg EP (1995) A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa . Proc Natl Acad Sci U S A 92: 1490–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pearson JP, Gray KM, Passador L, Tucker KD, Eberhard A, et al. (1994) Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci U S A 91: 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jensen V, Lons D, Zaoui C, Bredenbruch F, Meissner A, et al. (2006) RhlR expression in Pseudomonas aeruginosa is modulated by the Pseudomonas quinolone signal via PhoB-dependent and -independent pathways. J Bacteriol 188: 8601–8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Glick R, Gilmour C, Tremblay J, Satanower S, Avidan O, et al. (2010) Increase in rhamnolipid synthesis under iron-limiting conditions influences surface motility and biofilm formation in Pseudomonas aeruginosa . J Bacteriol 192: 2973–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holloway BW, Krishnapillai V, Morgan AF (1979) Chromosomal genetics of Pseudomonas . Microbiol Rev 43: 73–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Woodcock DM, Crowther PJ, Doherty J, Jefferson S, DeCruz E, et al. (1989) Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res 17: 3469–3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schweizer HP (1991) Escherichia-Pseudomonas shuttle vectors derived from pUC18/19. Gene 97: 109–121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Complementation of pstS and phoB restores the wild type swarming phenotype.
(TIF)
Primers used in this study.
(DOCX)