Abstract
To characterize MK-801’s effect on social behavior in mice, we examined adult male ICR mice for interaction with companion mice (juvenile male). Test mice were injected with either saline or MK-801 (0.1 mg/kg), and were tested 30 min later for their social behavior during a 5-min session. A second encounter took place 30 min later, with either a familiar companion mouse (the same as in the initial encounter) or a novel mouse. In saline controls, second encounter with a familiar companion mouse showed reduced social investigative behaviors (anogenital sniffing and staying together), indicating habituation toward a familiar mouse. Second encounter with a novel companion mouse did not show habituation in social investigative behaviors. Pretreatment with MK-801 reduced anogenital sniffing during the first encounter. At the second encounter, these mice displayed non-discriminative habituation of social investigative behaviors, with reduced anogenital sniffing and staying together, regardless of whether the companion mouse was a familiar or a novel one. These results indicate that MK-801 affected exploratory activities of mice, resulting in both reduced social investigative behaviors during first encounter with a companion mouse, and diminished discriminative capacities for a familiar vs. a novel companion mouse during subsequent encounter.
Keywords: Social interaction, Social recognition, MK-801, Anogenital sniffing, Mice
1. Introduction
The ability for recognizing conspecific identity is an important survival skill for animals. Studies in rodents have demonstrated their remarkable sensitivity to discriminate between familiar vs. unfamiliar individuals (File and Hyde, 1978; Thor and Holloway, 1982).
When two mice encounter for the first time, they engage in a series of social investigative behaviors to acquaint with each other; in subsequent encounters, mice spend less time investigating the same companion. This decrease in social interaction upon repeat encounters can be viewed as an index for social recognition. Social recognition may be a form of short-term memory with a limited duration (30 min – 2 hrs) in individually housed mice and rats (Thor and Holloway, 1982; Sekiguchi et al., 1991; Bluthe et al., 1993). Group-housed mice can display robust long-term (7 days) social memories for juvenile conspecifics (Kogan et al., 2000).
In social interaction, olfactory cues are a critical aspect for rodents (Matochik, 1988; Bluthe et al., 1993). It has been demonstrated that soiled bedding or urine samples from a juvenile rodent can be used as olfactory cues for recognition (Sawyer et al., 1984; Popik et al., 1991). These and other studies indicate that sensory inputs from olfactory cues form the foundation of social recognition in rodents, and the ability to recognize conspecifics is critical for social relationships (Yamamuro, 2006).
Deficits in social capacities are considered a negative symptom in schizophrenia (Sams-Dodd, 1995). In laboratory studies, rats treated with NMDA antagonists such as PCP and MK-801 display behaviors related to symptoms in schizophrenia, including decreased social behavior (Sams-Dodd, 1995). Employing a range of MK-801 doses (0.2, 0.3, and 0.4 mg/kg), Rung et al. showed that test rats exhibit decreased proximity toward companion rats, and considered this a model for negative symptoms of schizophrenia (Rung et al., 2005). Hlinak et al. showed that, when MK-801 was injected immediately after rats had initial encounter, social recognition in subsequent encounter was impaired (Hlinak and Krejci, 1994; Hlinak and Krejci, 2003). Chronic administration of MK-801 (0.13mg kg/day, over 14 days) also reduced social interaction in rats (Matsuoka et al., 2005). However, how MK-801 may impact social interactions in mice has not been reported.
In this study, we adapted a published method (Thor and Holloway, 1982), and examined MK-801 effect on exploration and social investigative behaviors in mice. We chose a relatively low dose (0.1 mg/kg), because MK-801 is known to influence a variety of psychomotor behaviors in rodents, and our previous work in mice indicated a complex dose-related effect for MK-801 (Wu et al., 2005). Specifically, at doses of 0.3 mg/kg or higher, stereotypy and ataxia occurred, and doses of 0.15 mg/kg or higher induced substantial hyperlocomotion (Wu et al., 2005). Based on these observations and our pilot studies (data not shown), a low dose of MK-801 at 0.1 mg/kg would likely minimize drug influence on psychomotor activities, allowing its effect on social behaviors to be examined. Our choice of MK-801 dose also reflected evidence from other studies. Using a range of MK-801 doses from 0.2 mg/kg to 0.4 mg/kg, Rung and colleagues showed that rats displayed decreased proximity toward companion animals (Rung et al., 2005). Chronic administration of MK-801 at 0.13 mg/kg also reduced social interaction in rats (Matsuoka et al., 2005). In addition, Hlinak and Krejci showed that MK-801 at 0.1 mg/kg impacted social recognition in rats when administered immediately after the initial social encounter, while a higher dose of MK-801 at 0.15 mg/kg reduced investigation time regardless of whether the rats were re-exposed to the same or novel companion animals, and signs of ataxia were observed at 0.2 mg/kg dose of MK-801 (Hlinak and Krejci, 1994; Hlinak and Krejci, 2003). These reports thus also suggest that MK-801 at 0.1 mg/kg was sufficient to influence social behavior, while higher does may further impact psychomotor activities.
2. Materials and Methods
2.1. Animals
Male ICR mice were obtained from Shanghai Laboratory Animal Center, Chinese Academy of Sciences, Shanghai, China. Male adult ICR mice, weighing 35–40 g at the time of experiment were used as test mice. Male juvenile ICR mice, weighing 28–31g at the time of experiment were used as companion mice to minimize aggression (Terranova et al., 1998). Animals were housed in standard housing conditions (in plastic cages with sawdust bedding and free access to food and water, 12 h light–dark cycle) and 2–3 mice per cage for a week before experiments were conducted. Principles of laboratory animal care were followed in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996), the PRC National Standards for Laboratory Animal Quality, and Guidelines for the Use of Experimental Animals. All experimental protocols were approved by the Ethics Committee for Biological Science, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences.
2.2. Drugs
MK-801 (Tocris Cookson, Inc., Ellisville, MO) was stored as a 4-mg/ml stock solution at − 70°C. Predilution of stocks with saline to the final injection concentrations was done at the beginning for each experiment, and these solutions were stored at 4 °C. Dilutions were designed to give injection volumes of 100 µl per 20 g of animal weight.
2.3. Experiment procedure
Experiments were performed in plastic cages (l, w, h: 48cm×24cm×20cm), covered with a piece of glass on the top, and with bedding inside. Each cage was placed inside a wooden enclosure (l, w, h: 60cm×40cm×44cm), equipped with a video camera on the top. Test and companion mice never met before. Social interaction was conducted over two encounter sessions, lasting for 5 min in each encounter. Two encounter sessions were separated by 30 min, with mice returned to their respective home cages. For the second encounter to observe social interaction, companion mice were either from the same pairing as the first social interaction (familiar companion mice) or different (novel companion mice). Prior to the first social interaction, test mice received a 15-min acclimation period in plastic cages, were administered an i.p. injection of either MK-801 (0.1 mg/kg) or saline, and were returned to their home cages. Thirty-min later, a test mouse and a companion mouse were placed in the plastic cage that the test mouse has acclimated to, with two mice about 10 cm apart in initial placement positions.
Pairing of mice consisted of four groups: saline/familiar, saline/novel, MK-801/familiar, and MK-801/novel. All experiments were conducted between 9:30 am and 3:00 pm.
2.4. Social interaction behaviors
Video tapes were scored by three observers, who were blind to the experimental pairing or drug treatment. Scoring consisted of seven behaviors, all regarding the test mouse only, with 5 min per observation session.
Rearing: cumulative time when a mouse lifted both of its front paws.
Square crossing: The surface area of the plastic cage was visually divided into eight equal squares. Each square crossing was scored when the four limbs of the test mouse moved from one square to another.
Self-grooming: cumulative time for the test mouse’s self-grooming activities (coat, paw grooming).
Anogenital sniffing: cumulative time for the test mouse to investigate the tail/anogenital area of the companion mouse.
Approaching: total count for the test mouse to approach the companion mouse, starting from a body separation distance of at least 1 cm apart (about half of the body width).
Staying together: cumulative time for the test mouse to be with the companion mouse. Two mice were within 1-cm distance, and can be either stationary or engaged in other interactive activities (sniffing head, body, paw, or tail/anogenital area, or climbing on top of each other).
Rearing, square crossing, and self-grooming were considered individual-directed behaviors. Anogenital sniffing, approaching, and staying together were considered social interaction behaviors.
2.5. Statistics
Statistical analysis used Student's un-paired t-test to compare the results during the initial encounter. Wilcoxon test was used to compare the results between the initial and the second encounters.
3. Results
3.1 First-encounter social investigative behaviors in mice and the impact of MK-801
In order to study MK-801’s effect on social behaviors, test mice (adult male ICR) were injected with either saline or MK-801 (0.1 mg/kg). First encounter began 30min later when a test mouse and a companion mouse (juvenile male ICR) were placed in the test arena at the same time.
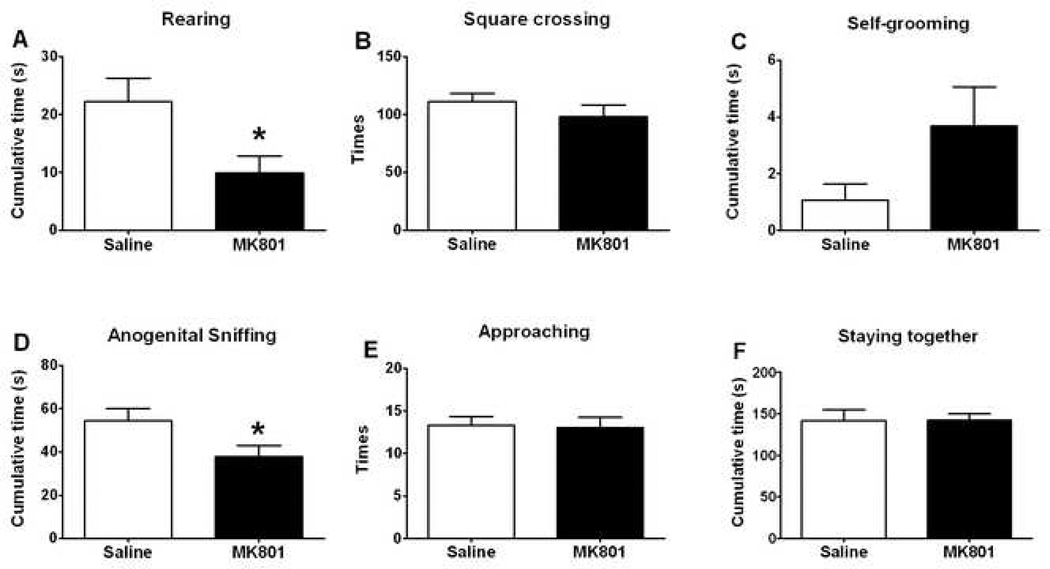
Fig. 1 showed various behaviors of the test mice, both for their individual-oriented behaviors (top row: rearing, square crossing, and self-grooming) and social behaviors with the companion mouse (bottom row: anogenital sniffing, approaching companion mice, and staying together with companion mice). Compared with the saline group, MK-801 mice showed significant reduction in rearing (t(23)=2.515, p<0.05) and anogenital sniffing (t(23)=2.167, p<0.05). Other behaviors were not significantly different, although self-grooming showed a tendency of increase (p=0.10).
Figure 1.
First-encounter social investigative behaviors in mice and the impact of MK-801. Test mice (adult male ICR) were placed in the testing arena to acclimate for 15 min, followed by i.p. injection of either saline or MK-801 (0.1 mg/kg), and placed in home cage for 30 min. First encounter began when a test mouse and a companion mouse (juvenile male ICR) were placed into the test arena at the same time. Animals were allowed to move and interact freely, and were videotaped for 5 min. The video was analyzed offline for various behaviors of the test mouse only. Data are shown as mean ± SEM (n = 12 – 13). Open boxes: saline-injected control mice. Closed boxes: MK-801-injected mice. *, significant difference (p < 0.05) between MK-801 mice and saline controls (Student's un-paired t-test). Top row: individual-oriented behaviors; bottom row: social behaviors.
3.2 Second-encounter social investigative behaviors and the impact of MK-801
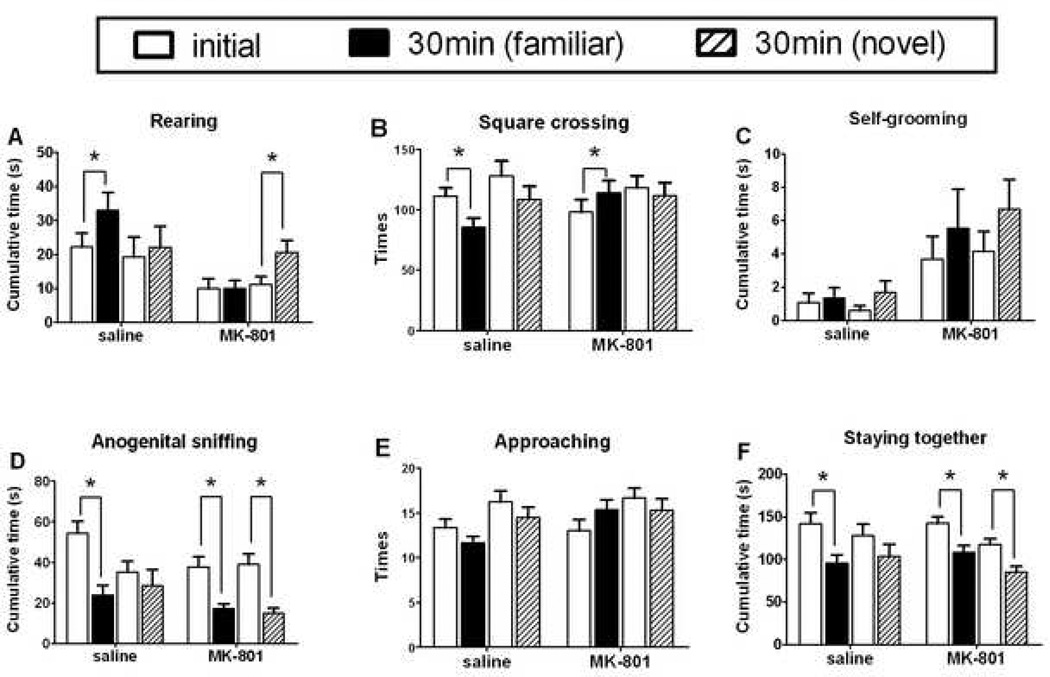
After 30 min in the home cage following the first-encounter, second-encounter testing began. A test mouse was placed in the same testing arena as in the first encounter, together with a companion mouse. The companion mouse was either the same as in the first encounter (“familiar”), or different (“novel”). At this low dose of MK-801, we did not observe any impairment of motor performance or ataxia. Results of individual-oriented and social behaviors are shown in Figure 2, and the overall tendency of behavior changes is summarized in Table 1. Compared with behaviors during the initial encounter, significant reduction in second encounter was seen for anogenital sniffing for saline over familiar (p<0.001, n=12), MK-801 over familiar (p<0.001, n=15), and MK-801 over novel (p<0.001, n=14); also reduced was the time staying together for saline over familiar (p<0.01, n=12), MK-801 over familiar (p<0.01, n=15), and MK-801 over novel (p<0.001, n=14). Whereas, increased rearing was observed for saline over familiar (p<0.01, n=12), and MK-801 over novel (p<0.05, n=14).
Figure 2.
Second-encounter social investigative behaviors and the impact of MK-801. After the first-encounter social interaction described in Figure 1, mice were returned to their home cage for 30 min. Second-encounter testing began when a test mouse was placed in the same testing arena as before, and a companion mouse was also placed in the arena at the same time. The companion mouse was either the same as in the first encounter (“familiar”), or different (“novel”). Animals were allowed to move and interact freely, and were videotaped for 5 min. The video was analyzed offline for various animal behaviors of the test mouse only. Data are shown as mean ± SEM (n = 12 – 13). Open bars: first-encounter behaviors. Closed bars: second-encounter behaviors with a familiar companion mouse. Hatched bars: second-encounter behaviors with a novel companion mouse. *, significant difference (p < 0.05) between the first- and the second-encounter behaviors (Wilcoxon test). Top row: individual-oriented behaviors; bottom row: social behaviors.
Table 1.
Overall tendency of behavior changes between the first- and the second- encounter with the companion mouse
| Saline/familiar | Saline/novel | MK-801/familiar | MK-801/novel | |
|---|---|---|---|---|
| Rearing | ↑ | — | — | ↑ |
| Square crossing | ↓ | — | ↑ | — |
| Self-grooming | — | — | — | — |
| Anogenital sniffing | ↓ | — | ↓ | ↓ |
| Approaching | — | — | — | — |
| Staying together | ↓ | — | ↓ | ↓ |
Note: up- and down- arrows indicate significant increase or decrease in behaviors. Dash indicates no significant difference.
4. Discussion
To characterize MK-801’s effect on social behavior in mice, we examined adult male ICR mice for their interaction with companion mice (juvenile male ICR). Both the initial encounter (Fig. 1) and subsequent encounter (Fig. 2) were observed, and various behaviors were scored. The behaviors observed include individual-oriented behaviors for the test mouse (rearing, locomotion, and self-grooming), as well as social behaviors directed toward the companion mouse (sniffing the anogenital area of the companion mouse, approaching the companion mouse, and staying together with the companion mouse). For the second encounter with the companion mouse, familiar pairing (with the same companion mouse as the initial encounter) and paring with a novel mouse were compared. This way, the memory from the first encounter with the familiar mouse would be a factor influencing the subsequent social behaviors.
For the initial encounter, pretreatment with MK-801 reduced sniffing anogenital area among the social behaviors (Fig. 1D). Rodents possess rather sensitive olfactory senses, both as a way for exploring the environment, and for encounter with other members of the same species. Sniffing the anogenital and tail area of a companion mouse is a common way for gathering olfactory signature of the companion, because urinary odors and preputial gland are the predominant component of the olfactory signature of a rodent (Engelmann et al., 1995). Low dose of MK-801 reduced anogenital sniffing (Fig. 1D), without significantly altering other social behaviors (Fig. 1E,F). This suggests that olfactory sense serves as a subtle yet important indicator, and MK-801-induced reduction in anogenital sniffing represents a sign of early stages of MK-801-related social withdrawal, possibly contributing to MK-801 influence on social memory. These results are in agreement with the olfactory bulb distribution of NMDA receptors (Monaghan and Cotman, 1985; Trombley and Westbrook, 1990), to which MK-801 is a non-competitive antagonist.
Pretreatment with MK-801 before the initial encounter also reduced rearing among the individual-oriented behaviors (Fig. 1, top row). As a behavioral activity, it is unclear in the literature how to interpret rearing behavior. It is known that locomotion can interfere with rearing (Wu et al., 2005). In this regard, rearing is part of the repertoire of movement behavior. Edsbagge et al. suggested to consider rearing as a form of exploratory behavior (Edsbagge et al., 2004). Our observation also indicated that as a form of exploration, rearing behavior parallels that of hole-poking exploratory behavior (data not shown). Reduction of rearing by low dose of MK-801 (Fig. 1A) supports the notion that rearing represents a sensitive indicator of mouse’s exploration of the surrounding environment.
During the second encounter with a companion mouse, different patterns of MK-801 impact were seen for repeating the encounter with a familiar mouse vs. meeting a novel mouse (Fig. 2 and Table 1), especially for the two behaviors of anogenital sniffing and staying together.
Social investigative behaviors (anogenital sniffing and staying together) of the test mouse during the second encounter session with a familiar companion mouse were decreased for saline group compared with the first encounter session (Fig. 2D & 2F, saline panel, left 2 bar comparisons, significant difference). On the other hand, there was no difference for the saline group during the second encounter with a novel companion mouse (Fig. 2D & 2F, saline panel, right 2 bar comparisons, no difference). Similar findings have been reported before (Thor and Holloway, 1982; Dantzer et al., 1987; Sekiguchi et al., 1991; Engelmann et al., 1995; Kogan et al., 2000), with two possible interpretations — social memory and habituation. Social memory (social recognition) is defined as a decrease of social investigation during a second encounter session with the same animals, without a decrease to different animals (Thor and Holloway, 1982). Habituation is thought as a widely adapted form of learning where a decrease in response takes place to repeated stimuli that lack reinforcing quality (Thorpe, 1963; Liebrecht and Askew, 1980). Social memory formed during the initial encounter serves companion recognition during subsequent encounters. Thus, during the second encounter with the same companion mouse, the familiarity from social memory resulted in habituation, leading to reduced social investigative behaviors; a novel companion represents a new form of social stimuli, therefore no such habituation took place for social investigative behaviors.
It is noteworthy that, with the pretreatment of MK-801, the social investigative behaviors (anogenital sniffing and staying together) during a second encounter were all decreased compared to the first encounter, regardless of whether the companion mouse was a familiar or a novel one (Fig. 2D, MK801 panel, pair-wise comparisons for both familiar and novel pairing in second encounter, significant differences). This indicates that after MK-801 treatment, testing mice regarded both familiar and novel companion mice as if they were the same, and habituation took place for both types of companion mice. Taking into account that MK-801 reduced anogenital sniffing during the first encounter session (Fig. 1D), one possibility is that MK-801 may have impaired a mouse’s olfactory discriminative capability, thus hampering the formation of social memory to discriminate between a familiar vs. novel companion mouse; that is, during a second encounter, the memory of first encounter gave rise to habituation for both familiar and novel companion mice, without the benefit of social memory to distinguish whether a companion mouse was a familiar or a novel one.
When MK-801 was administered immediately after the first encounter session, it was observed that MK-801 at 0.1mg/kg in rats resulted in ‘amnesia’, i.e., the time spent by animals in social investigation during re-exposure to the same juvenile companion was comparable with the time measured during the initial exposure and with the time of animals re-exposed to a novel juvenile (Hlinak and Krejci, 1994; Hlinak and Krejci, 2003). That is, both familiar and novel companion rats were treated as if they were novel during re-exposure. These results indicate that no habituation took place, because MK-801 administered immediately after initial encounter impaired the consolidation of social memory.
Taken together, our results indicate that exploratory behaviors in mice, both individual-oriented (as in rearing) and social investigation (anogenital sniffing), are sensitive to low dose of MK-801 effect. In particular, pretreatment with MK-801 resulted in both a reduction in social investigative behaviors during first encounter with a companion mouse, and a diminishment of discriminative capacities for a familiar vs. a novel companion mouse during subsequent encounter.
Acknowledgement
This work was supported in part by grants from the National Natural Science Foundation of China (30670755), and the National Institutes of Health of the United States (DA013471 and DA020555).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bluthe RM, Gheusi G, Dantzer R. Gonadal steroids influence the involvement of arginine vasopressin in social recognition in mice. Psychoneuroendocrinology. 1993;18:323–335. doi: 10.1016/0306-4530(93)90028-j. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Koob GF, Le Moal M. Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology (Berl) 1987;91:363–368. doi: 10.1007/BF00518192. [DOI] [PubMed] [Google Scholar]
- Edsbagge J, Zhu S, Xiao MY, Wigstrom H, Mohammed AH, Semb H. Expression of dominant negative cadherin in the adult mouse brain modifies rearing behavior. Mol Cell Neurosci. 2004;25:524–535. doi: 10.1016/j.mcn.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Landgraf R. Social discrimination procedure: an alternative method to investigate juvenile recognition abilities in rats. Physiol Behav. 1995;58:315–321. doi: 10.1016/0031-9384(95)00053-l. [DOI] [PubMed] [Google Scholar]
- File SE, Hyde JR. Can social interaction be used to measure anxiety? Br J Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlinak Z, Krejci I. Effects of excitatory amino acid antagonists on social recognition of male rats. Behav Pharmacol. 1994;5:239–244. doi: 10.1097/00008877-199406000-00002. [DOI] [PubMed] [Google Scholar]
- Hlinak Z, Krejci I. Kynurenic acid prevented social recognition deficits induced by MK-801 in rats. Physiol Res. 2003;52:805–808. [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Kogan JH, Frankland PW, Silva AJ. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 2000;10:47–56. doi: 10.1002/(SICI)1098-1063(2000)10:1<47::AID-HIPO5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Liebrecht BC, Askew HR. Habituation from a comparative perspective. In: Denny MR, editor. Comparative Psychology. New York: Wiley; 1980. [Google Scholar]
- Matochik JA. Role of the main olfactory system in recognition between individual spiny mice. Physiol Behav. 1988;42:217–222. doi: 10.1016/0031-9384(88)90073-x. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Sumiyoshi T, Tanaka K, Tsunoda M, Uehara T, Itoh H, Kurachi M. NC-1900, an arginine-vasopressin analogue, ameliorates social behavior deficits and hyperlocomotion in MK-801-treated rats: therapeutic implications for schizophrenia. Brain Res. 2005;1053:131–136. doi: 10.1016/j.brainres.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Monaghan DT, Cotman CW. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985;5:2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popik P, Vetulani J, Bisaga A, Van Ree JM. Recognition cue in the rat's social memory paradigm. J Basic Clin Physiol Pharmacol. 1991;2:315–327. doi: 10.1515/jbcpp.1991.2.4.315. [DOI] [PubMed] [Google Scholar]
- Rung JP, Carlsson A, Ryden MK, Carlsson ML. (+)-MK-801 induced social withdrawal in rats; a model for negative symptoms of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:827–832. doi: 10.1016/j.pnpbp.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F. Distinct effects of d-amphetamine and phencyclidine on the social behaviour of rats. Behav Pharmacol. 1995;6:55–65. [PubMed] [Google Scholar]
- Sawyer TF, Hengehold AK, Perez WA. Chemosensory and hormonal mediation of social memory in male rats. Behav Neurosci. 1984;98:908–913. doi: 10.1037//0735-7044.98.5.908. [DOI] [PubMed] [Google Scholar]
- Sekiguchi R, Wolterink G, Van Ree JM. Short duration of retroactive facilitation of social recognition in rats. Physiol Behav. 1991;50:1253–1256. doi: 10.1016/0031-9384(91)90591-b. [DOI] [PubMed] [Google Scholar]
- Terranova ML, Laviola G, de Acetis L, Alleva E. A description of the ontogeny of mouse agonistic behavior. J Comp Psychol. 1998;112:3–12. doi: 10.1037/0735-7036.112.1.3. [DOI] [PubMed] [Google Scholar]
- Thor DH, Holloway WR. Social memory of the male laboratory rat. J Comp Physiol Psychol. 1982;96:1000–1006. [Google Scholar]
- Thorpe WH. Learning and Instinct in Animals. Cambridge, Massachusetts: Harvard University Press; 1963. [Google Scholar]
- Trombley PQ, Westbrook GL. Excitatory synaptic transmission in cultures of rat olfactory bulb. J Neurophysiol. 1990;64:598–606. doi: 10.1152/jn.1990.64.2.598. [DOI] [PubMed] [Google Scholar]
- Wu J, Zou H, Strong JA, Yu J, Zhou X, Xie Q, Zhao G, Jin M, Yu L. Bimodal effects of MK-801 on locomotion and stereotypy in C57BL/6 mice. Psychopharmacol. 2005;177:256–263. doi: 10.1007/s00213-004-1944-1. [DOI] [PubMed] [Google Scholar]
- Yamamuro Y. Social behavior in laboratory rats: Applications for psycho-neuroethology studies. Animal Science Journal. 2006;77:386–394. [Google Scholar]