Abstract
Ghrelin acts on the growth hormone secretagogue receptor (GHSR) in the brain to elicit changes in physiological functions. It is associated with the neural control of appetite and metabolism, however central ghrelin also affects fertility. Central ghrelin injection in rats suppresses luteinizing hormone (LH) concentrations and pulse frequency. Although ghrelin suppresses LH and regulates kisspeptin mRNA in the anteroventral periventricular/periventricular nucleus (AVPV/PeN), there is no neuroanatomical evidence linking GHSR neural circuits to kisspeptin neurons. In this study, we first determined coexpression of GHSR and GnRH neurons using a GHSR-eGFP reporter mouse line. Using dual-label immunohistochemistry, we saw no coexpression. GHSR-eGFP expressing cells were present in the AVPV/PeN and over 90% of these expressed estrogen receptor-α (ERα). Despite this, we observed no evidence of GHSR-eGFP/kisspeptin coexpressing neurons in the AVPV/PeN. To further examine the phenotype of GHSR-eGFP cells in the AVPV/PeN, we determined coexpression with tyrosine hydroxylase (TH) and showed virtually no coexpression in the AVPV/PeN (<2%). We also observed no coexpression of GHSR-eGFP and RFamide-related peptide-3 (RFRP3) neurons in the dorsomedial hypothalamic nucleus. Importantly, we observed that approximately half of the GHSR-eGFP cells in the AVPV coexpressed Ghsr mRNA (as determined by in situ hybridization) so these data should be interpreted accordingly. Although ghrelin influences the hypothalamic reproductive axis, our data using a GHSR-eGFP reporter suggests ghrelin regulates neurons expressing ERα but does not directly act on GnRH, kisspeptin, TH, or RFRP3 neurons, as little or no GHSR-eGFP coexpression was observed.
Keywords: ghrelin, GHSR, GFP, Kisspeptin, AgRP, reproduction
Introduction
Ghrelin is a stomach hormone commonly associated with the neural control of appetite and metabolism [1, 8]. However, recent studies highlight that ghrelin targets the brain to regulate a diverse range of physiological functions (for review see [2]) including chronic stress [12, 45], acute stress [71], reward [20, 36, 53, 64], learning and memory [17, 18] and neuroprotection [3, 17, 48]. Central ghrelin also affects the reproductive system. For example, icv ghrelin injection to ovariectomized rats or ovariectomized rats treated with 17β-estradiol suppressed luteinizing hormone (LH) concentration and pulse frequency [27, 49]. Similar inhibitory effects on LH secretion were observed throughout the estrus cycle [23]. Here, the authors demonstrated that ghrelin significantly inhibited gonadotropin-releasing hormone (GnRH) from hypothalamic explants and ghrelin suppressed GnRH-induced LH release in vitro [23]. The central inhibitory effects of ghrelin on LH secretion also occur in sheep [35]. These studies collectively demonstrate that central ghrelin negatively influences the reproductive axis. Moreover, this is clinically relevant to human health, as repeated intravenous ghrelin injections reduced LH concentrations and pulsatility in males and females [39, 40].
The mechanisms through which central ghrelin inhibits the reproductive system remain unresolved, although kisspeptin neurons in the hypothalamus may be a primary target. Kisspeptin is a hypothalamic peptide that activates GnRH neurons and triggers puberty [29, 30, 61]. Kisspeptin neurons are found predominantly in the anteroventral periventricular/periventricular nucleus (AVPV/PeN) and the arcuate nucleus (ARC) [29] and appear critical for the feedback control of GnRH secretion [65, 66]. Moreover, kisspeptin stimulates GnRH release directly from the ovine median eminence and is essential for the tonic release of LH and the full LH preovulatory surge [16, 58, 69]. Previous studies highlight that ghrelin could inhibit LH secretion by directly suppressing the effects of kisspeptin on the reproductive axis. For example, ghrelin significantly reduced the duration of the LH secretory response to kisspeptin-10 [46]. Because conditions of negative energy balance increase plasma ghrelin concentrations and suppress the reproductive axis, it is tempting to speculate that ghrelin feeds back to the hypothalamus and relays metabolic information pertinent to ongoing reproductive function. In support of this notion, Forbes et al [25] demonstrated that fasting, which elevates plasma ghrelin [9], exogenous ghrelin, or the combination of both, suppressed kisspeptin gene (Kiss1 mRNA) in the medial preoptic area (mPOA, likely to contain the AVPV/PeN), without affecting Kiss1 mRNA in the ARC, indicating that ghrelin may target AVPV/PeN kisspeptin neurons to suppress LH secretion.
Ghrelin acts on the growth hormone secretagogue receptor (GHSR) in the brain to elicit changes in physiological functions. Indeed, the effects of ghrelin on hypothalamic neuropeptide Y levels, appetite and adiposity are absent in Ghsr knockout mice [4, 79]. Although ghrelin suppresses LH secretion and regulates Kiss1 mRNA, there is no neuroanatomical evidence linking GHSR neural circuits to reproductive neural circuits. In the current study, we first examined direct coexpression of the GHSR and GnRH neurons using a GHSR-eGFP reporter mouse line, which is currently the best model available to visualize GHSR expressing neurons. Estrogen receptive neurons in the AVPV/PeN are key components of the neuroendocrine machinery that govern GnRH neurons [74] and hence reproduction. For these reasons we further proposed that ghrelin responsive neurons would also be estrogen responsive. We next determined if kisspeptin neurons in the AVPV/PeN express GHSR-eGFP and/or if GHSR-eGFP expressing neurons are dopaminergic (coexpress tyrosine hydroxylase, TH) as these neurons represent an estrogen responsive population relatively distinct from kisspeptin neurons (56% of TH cells in the AVPV/PeN and only 36% in the PeN coexpress Kiss1 mRNA)[15, 37, 60]. Finally, we examined if any GHSR-eGFP neurons in the dorsomedial hypothalamic nucleus (DMH) express RF-amide related peptide-3 (RFRP3), a hypothalamic neuropeptide though to inhibit gonadotropin secretion [14, 54]. Our results clearly illustrate that GnRH neurons or AVPV/PeN kisspeptin, TH, and RFRP3 neurons do not express GHSR-eGFP, although the majority of GHSR-eGFP neurons are estrogen responsive based on coexpression with estrogen receptor alpha (ERα).
Materials and Methods
Animals
Experiments were conducted in accordance with the National Health and Medical Research Council Australia Code of Practice for the Care of Experimental Animals and were approved by the Monash University School of Biomedical Sciences Animal Ethics Committee. GHSR-eGFP mice were obtained from the Mouse Mutant Regional Resource Center at University of California at Davis. This mouse was generated by the GENSAT project at Rockefeller University and contains a modified BAC in which a GFP reporter is inserted immediately upstream of the coding sequence for the Ghsr gene (http://www.mmrrc.org/catalog/sds.php?mmrrc_id=30942). The GHSR-eGFP mouse model is on a C57BL/J6 background and GHSR cells were previously confirmed in the pituitary gland [56]. Additionally, GHSR-eGFP expression in the reporter mouse was confirmed using GHSR-eGFP and Ghsr mRNA dual label immunohistochemistry/in situ hybridization (see below). Mice were maintained at 22 °C on a 12 h light/dark cycle (0700 – 1900 h) with pelleted mouse chow and water available ad libitum. All experiments were conducted using adult GHSR-eGFP mice at 8–10 wks age.
GHSR-eGFP and Ghsr mRNA dual label immunohistochemistry/in situ hybridization
Animal experiments were approved by University of Texas Southwestern Medical Center Institutional Animal Care and Use Committee. Mice were housed under 12 h dark- 12 h light under standard environmentally controlled conditions. Male GHSR-eGFP mice (12–16 weeks of age) were subjected to 60% calorie restriction for 2 weeks as described previously [77]. In brief, the mice were individually housed and daily food intake of standard chow (Teklad Global Diet #2016 Madison, WI) was monitored for a week. During calorie restriction, the mice were provided with 40% of their average daily food intake during the week of acclimatization. The mice were then deeply anesthetized with intraperitoneal injection of chloral hydrate (500 mg/kg for mice) and transcardially perfused with diethylpyrocarbonate (DEPC)-treated 0.9% phosphate-buffered saline (PBS) followed by 10% neutral buffered formalin. Brains were removed immediately and stored in the same fixative for 4–6 hours at 4°C, immersed in 20% sucrose in DEPC-treated PBS, pH 7.0 at 4°C overnight, and sectioned coronally into five equal series at a thickness of 25 μm on a sliding microtome. The sections were stored at −20°C in an antifreeze solution [63] until further processing.
Free-floating sections of mouse brains were subjected sequentially to immunohistochemistry/in situ hybridization using procedures reported previously [21, 78]. The Ghsr cRNA probes were generated as previously described [12, 13, 52]. Sectioned series of three different mouse brains were first rinsed in DEPC-treated PBS, pH 7.0, and were pretreated with 0.1% sodium borohydride (Sigma, St. Louis, MO) in DEPC-treated PBS for 15 minutes at room temperature. After thorough washing in DEPC-treated PBS the sections were rinsed in 0.1 M triethanolamine (TEA, pH 8.0), incubated in 0.25% acetic anhydride in 0.1 M TEA for 10 minutes, then washed again in 2× saline-sodium citrate buffer (SSC). The sections were then incubated at 50°C for 16 hours with 33P-labelled mouse Ghsr riboprobe diluted to 106 cpm/mL in hybridization solution [78]. Subsequently, sections were rinsed in 4× SSC and incubated in 0.002% RNase A (Roche Molecular Biochemicals, Indianapolis, IN) solution for 30 minutes at 37°C. Sections were then rinsed in 2×SSC and submitted to sequential stringency washes with 2X SSC and 0.2X SSC for one hour each at 55°C.
Immunohistochemistry was performed after first washing the in situ processed-processed sections in PBS. Sections were pretreated with 0.3% hydrogen peroxide in PBS, pH 7.4, for 30 minutes at room temperature and then were incubated in 3% normal donkey serum (Jackson ImmunoResearch Laboratories, West Grove, PA) with 0.3% Triton X-100 in PBS (PBT) for 2 hours at room temperature. The sections were then incubated overnight at room temperature in chicken anti-GFP primary antibody (Aves laboratories, Tigard, OR; 1:5,000 in PBT). After washing with PBS, sections were incubated in biotin-conjugated donkey anti-chicken IgG (Jackson ImmunoResearch Laboratories; 1:1,000) for 2 h at room temperature, followed by incubation for 1 h in a solution of avidin-biotin complex (Vectastain Elite ABC Kit, Vector Laboratories, Burlingame, CA; 1:500) diluted in PBS. The sections were next washed in PBS and incubated in diaminobenzidine (DAB) using an enhanced DAB substrate kit (Thermo Scientific, Pittsburg, PA). Sections were mounted onto SuperFrost slides and then dehydrated in increasing concentrations of ethanol. Slides were air dried, and placed in an X-ray cassette with BMR-2 film (Kodak, Rochester, NY) for 3 days. Slides were then dipped in NTB2 photographic emulsion (Kodak) and stored in the dark at 4°C for 2 weeks. The slides were then developed with D-19 developer (Kodak) to precipitate the silver granules, dehydrated in graded ethanols, cleared with xylenes and cover-slipped with permount mounting medium (Thermo Scientific, Pittsburg, PA).
The brain sections were viewed with a Zeiss Axioskop microscope using both brightfield and darkfield optics. Photomicrographs were produced with a Zeiss digital camera attached to the microscope and a desktop computer. Criteria used to determine co-localization of eGFP and Ghsr mRNA included both 1) brightfield visualization of silver granules overlying the DAB-stained cell at ≥ 3x the background density of silver granule deposition and 2) conformation of the overlying silver granules to the shape of the DAB-stained cell. An image editing software program, Adobe Photo-Shop CS5.1 (San Jose, CA), was used to adjust sharpness, contrast and brightness of the photomicrographs.
Dual label immunohistochemistry
All animals were deeply anaesthetized with isoflurane and perfused through the left ventricle of the heart with 0.05 M phosphate buffered saline (PBS), followed by 0.05 M PBS buffered 4% paraformaldehyde. The brain was post-fixed in 0.05 M PBS buffered 4% paraformaldehyde overnight at 4 °C, then placed in 0.05 M PBS buffered 30% sucrose. Brains were frozen on a bed of crushed dry ice, stored at −80 °C, and cut at 40 μm on a cryostat. Serial sections from the front of the forebrain through the hypothalamus to the brainstem were collected (grouped into 4 sets, adjacent sections 160 μm apart) and stored in cryoprotectant at −20 °C.
Experiment 1: GnRH and GHSR-eGFP coexpression in the mouse forebrain
Double-label immunohistochemistry was performed for GnRH and eGFP to determine the level of coexpression in the diagonal band of Brocca (DBB) and medial preoptic area (mPOA). A set of coronal sections from brains of ovary-intact (diestrus) females and colchicine treated intact male mice were used (n = 4 per group). Colchicine was used to maximize the expression of kisspeptin in the male AVPV/PeN [50](see below) and was diluted in aCSF to a final concentration of 5μg/1μl and 1.5μl was injected into the left lateral ventricle (coordinates from bregma; −0.3 mm anterior/posterior, 1 mm lateral and 2.5mm deep) and mice were perfused 24 hours later. One set of sections through the DBB-mPOA was examined in each animal and the immunohistochemistry procedure performed sequentially. First, GnRH immuno-detection was performed as previously described [69]. Briefly, sections were incubated for 48 h at 4 °C in a mouse monoclonal antibody against GnRH (1:1000; HU11B, Urbanski, Oregon Regional Primate Research, Beaverton, OR) followed by a goat anti-mouse red fluorescent secondary antibody (dilution 1:500; Alexa 594, Molecular Probes, Inc., Eugene). Following Tris-buffered saline washes, a second round of immunohistochemistry was performed. A polyclonal chicken anti-GFP antiserum (dilution 1:1000; cat. number ab13970, Abcam, Sapphire Biosciences, NSW Australia) was applied and followed by a goat anti-chicken green fluorescent secondary antibody (1:400; cat. number A-11039, Invitrogen, Victoria, Australia). Negative controls included primary or secondary antibody omission and no staining was observed.
Experiment 2: Estrogen receptor α and GHSR-eGFP coexpression in the mouse forebrain
To examine if GHSR is present on estrogen receptor positive cells in the AVPV/PeN we examined ERα and GHSR-eGFP coexpression in the AVPV/PeN of intact (diestrus), ovariectomized (OVX) and OVX plus estradiol (E2) replaced (OVX+E2) female and colchicine treated intact male mice. OVX and steroid replacement procedures were performed as previously described [68] with subcutaneous E2 implants designed to increase E2 within the physiological limit. A set of 4 coronal sections through the AVPV/PeN (n = 4 per group) was incubated with a rabbit anti-ERα antiserum (1:20,000; C1355, Millipore, Australia). ERα-positive cells were visualized with appropriate fluorescent secondary antibodies (1:400, donkey anti-rabbit red fluorescent secondary antibody, Alexa 594, Molecular Probes, Inc.). Immunodetection of GHSR-eGFP was performed as above.
Experiment 3: Kisspeptin and GHSR-eGFP coexpression in the mouse forebrain
Double-label immunohistochemistry was performed for kisspeptin and eGFP to determine the level of coexpression in the AVPV/PeN. A set of coronal sections from brains of ovary-intact (diestrus), OVX+E2 mice and colchicine treated intact male mice were used (n = 4 per group). Intact and OVX+E2 mice were used to examine coexpression in the AVPV/PeN because these treatments allow for optimal visualization of kisspeptin protein. One set of 4 sections through the AVPV/PeN was examined in each animal and the immunohistochemistry procedure performed as above. First, kisspeptin immuno-detection was performed as previously described [70]. Sections were incubated for 48 h at 4 °C in the polyclonal rabbit anti-kisspeptin-10 antiserum (dilution 1:2000; #566; a gift from A. Caraty, Universite’ Tours, Nouzilly, France). Kisspeptin-positive cells were visualized with appropriate fluorescent secondary antibodies (1:400, goat anti-rabbit red fluorescent secondary antibody, Alexa 594, Molecular Probes, Inc.). Immunodetection of GHSR-eGFP was performed as above.
Experiment 4: TH and GHSR-eGFP coexpression in the mouse forebrain
Because dopaminergic neurons represent an estrogen responsive population relatively distinct from kisspeptin neurons [15, 37, 60], we next sought to determine if GHSR-eGFP cells coexpressed TH. A set of 4 coronal sections from brains of ovary-intact, OVX and OVX +E2 female mice and colchicine treated intact male mice were used (as above, n = 4 per group). For TH immunohistochemistry, sections were incubated for 48 h at 4 °C with a mouse monoclonal antibody against TH (1:1000; cat. number NCL-TH, Novocastra, Leica Microsystems Pty Ltd, Wetzlar, Germany) followed by a donkey goat anti-mouse red fluorescent secondary antibody (dilution 1:400, Alexa 594, Molecular Probes, Inc.). Immunodetection of GHSR-eGFP was performed as above.
Experiment 5: RFRP3 and GHSR-eGFP coexpression in the mouse forebrain
We sought to determine if GHSR was coexpressed in RFRP3 neurons, which are proposed regulators of GnRH neurons. A set of 4 coronal sections through the DMH was examined from brains of ovary-intact, OVX and OVX +E2 female mice and colchicine treated intact male mice (n = 4 per group. For immunohistochemistry, sections were incubated for 48 h at 4 °C with a rabbit polyclonal anti-RFRP3 antiserum (dilution 1:1000; PAC 123a; GE Bentley, University of California Berkley, Berkley, CA) followed by a goat anti-rabbit red fluorescent secondary antibody (dilution 1:400; Alexa 594, Molecular Probes, Inc., Eugene). Immunodetection of GHSR-eGFP was performed as above.
Data Analysis
Analysis of dual-labeled sections was performed with epifluorescence microscopy using a Zeiss Apotome microscope (Carl Zeiss, Inc., North Ryde, Sydney, Australia) with appropriate excitation for green and red fluorescence. A single observer then counted the total number of immunopositive cell bodies, and the number of cells containing both GHSR-eGFP and GnRH, ERα, kisspeptin, TH or RFRP3. For each the mean number of cells per section was reported. Where appropriate, the total number of coexpressing cells in a single animal was divided by the total number of cells to generate a percentage. This was then averaged for each animal and a group mean (±SEM) was calculated. The percentage of coexpression in intact, OVX and OVX+E2 animals was examined by one-way ANOVA using the GraphPad Prism 5.00 for Mac OS X (GraphPad Software, San Diego CA). P<0.05 was considered statistically significant.
Results
Validation of GHSR-eGFP model in the AVPV/PeN
In order to validate the expected expression of eGFP within GHSR-containing neurons in the AVPV/PeN region, we performed dual-label histochemistry experiments. Both signals were visualized simultaneously in dual-label studies using 3 different brains from GHSR-eGFP mice. In order to assess the degree of co-expression, we focused on the portion of the AVPV/PeN located approximately 0.02 mm rostral to bregma, as in the Paxinos and Franklin atlas [51]. Sections at this level contained 80 – 105 DAB-marked eGFP-immunoreactive cells in the AVPV/PeN. Of those eGFP-expressing cells, 44 ± 7% were co-labeled by the antisense Ghsr riboprobe, as indicated by overlying silver granules at 3× the background density of silver granule deposition (Figure 1). We also examined eGFP expression, Ghsr mRNA expression and their co-localization in the ARC. Despite strong Ghsr mRNA signal, only scattered eGFP-expressing cells were observed in the ARC (10.7 ± 1.3 eGFP immunopositive cells in the ARC/section), and 91.67 ± 4.8% of the eGFP cells demonstrated co-localized Ghsr mRNA expression (data not shown). Similarly, visualization of kisspeptin cell bodies in the mouse ARC is difficult due to the extremely high and dense kisspeptin fibers present [50]. For these reasons, data in the ARC were not examined in this study. Very few eGFP-immunoreactive and GHSR-containing cells were seen in the DBB. Similarly, very few eGFP-immunoreactive were present in the DMH and 20% showed co-localization with Ghsr mRNA.
Figure 1.
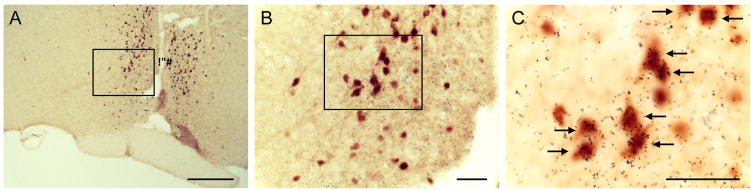
Co-expression of growth hormone secretagogue receptor (GHSR) mRNA and eGFP protein in the anteroventral periventricular/periventricular nucleus. Photomicrographs of a representative GHSR-eGFP mouse brain, sectioned coronally, at approximately 0.02 mm rostral to Bregma. Cells with eGFP-immunoreactivity have been stained with DAB and appear orange-brown in color (A–C). Those eGFP-immunoreactive cells that co-express GHSR mRNA have overlying black silver granules, and are indicated by arrows. Subsequent images on the right (B and C) are magnified images of the boxed areas in the images on the left (A). 3V, Third ventricle. Scale: 250 μm (A), 50 μm (B and C).
GnRH and GHSR-eGFP coexpression in the mouse forebrain
We determined if GnRH neurons located the DBB and mPOA coexpress GHSR in intact females and males. No coexpression was observed (Supplementary Figure 1). A mean of 6±2 GnRH neurons/section and 7±3 GHSR-eGFP cells/section were examined in females and 10±1 GnRH neurons and 3±2 GHSR-eGFP cells examined in males (n=4 for each).
Estrogen receptor α and GHSR-eGFP coexpression in the mouse forebrain
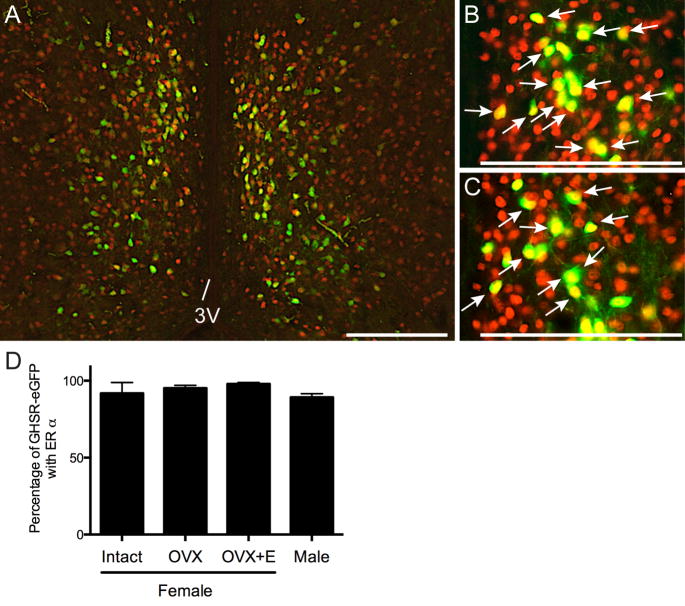
Neuronal cell bodies expressing ERα and GHSR-eGFP were readily detected in the AVPV/PeN of female and male mice (Figure 2A–C). In the female AVPV/PeN, over 90% of GHSR-eGFP cells coexpressed ERα and this did not differ among E2 treatments (Intact 91.8±7.0%, OVX 95.2±1.8%, OVX+E2 97.9±0.9%; Figure 2D). Similarly, in males 89.3±2.0% of GHSR-eGFP cells in the AVPV/PeN coexpressed ERα (Figure 2D). No significant difference was noted in the mean number of GHSR-eGFP neurons/section in the AVPV/PeN in females following steroid manipulations or males (Female: Intact 120±6 cells, OVX 153±5, OVX+E2 152±17; Male: 115±8; n=4 per group).
Figure 2.
Growth hormone secretagogue receptor (GHSR)-eGFP coexpression with ERα in the anteroventral periventricular/periventricular nucleus (AVPV/PeN). Representative photomicrographs of the AVPV/PeN from an intact female (A, B) and male (C) showing GHSR-eGFP cells (green) and ERα expressing cells (red) and coexpression (indicated by arrows in merged B, C). 3V, third ventricle. Scale bars = 200 μm. D, A high percentage of GHSR-eGFP cells coexpressed ERα in the AVPV/PeN and this was similar in intact, OVX and OVX+E2 females and males.
Kisspeptin and GHSR-eGFP coexpression in the mouse forebrain
Kisspeptin neuronal cell bodies were readily detected in the AVPV/PeN of intact and OVX+E2 mice (Figure 3A, B). We were unable to detect any kisspeptin-immunoreactive cells in the AVPV/PeN of OVX mice (data not shown). GHSR-eGFP was also detected in the AVPV/PeN (Figure 3A, B). We saw no examples of co-localization between AVPV/PeN kisspeptin neurons and GHSR-eGFP cells. We examined a mean of 42±12 kisspeptin neurons/section in intact mice and 56±3 kisspeptin neurons/section in OVX+E mice. For GHSR-eGFP cells, 39±14 and 77±10 cells/section were examined in the AVPV/PeN of intact and OVX+E mice respectively (n=4 per group).
Figure 3.
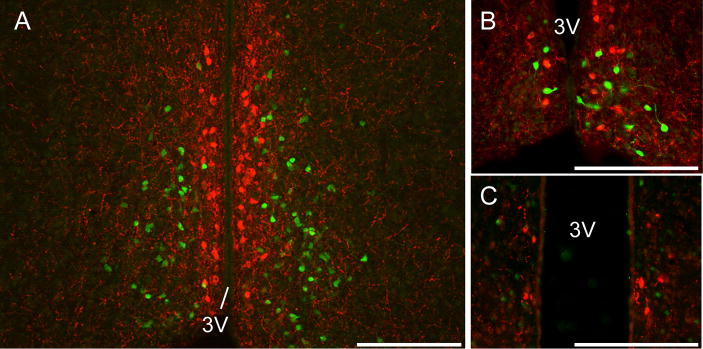
Kisspeptin and growth hormone secretagogue receptor (GHSR)-eGFP expression in the anteroventral periventricular/periventricular nucleus (AVPV/PeN). Representative photomicrographs of the AVPV/PeN from intact females (A, B) and males (C). Note the absence of any coexpression of kisspeptin (red) and GHSR-eGFP (green). 3V, third ventricle. Scale bars = 200 μm.
To confirm the lack of coexpression of kisspeptin in GHSR-eGFP cells, we repeated the above experiments in colchicine treated male mice. Few kisspeptin immunoreactive cell bodies were observed in the AVPV/PeN (not to the extent of that seen in females; Figure 3C). Again, we saw no examples of co-localization between AVPV/PeN kisspeptin neurons and GHSR-eGFP cells. Sporadic kisspeptin immunoreactive cells were also seen in the medial amygdala (Supplementary Figure 2), as shown with mRNA expression [38], and these did not co-localize with GHSR-eGFP although GHSR-eGFP expression was observed in this region. On average, we examined 6±3 kisspeptin neurons/section and 79±15 GHSR-eGFP cells/section in the AVPV/PeN from colchicine-treated mice (n=4).
TH and GHSR-eGFP coexpression in the mouse forebrain
Because we saw no coexpression between kisspeptin and GHSR-eGFP, yet a high degree of coexpression between GHSR-eGFP and ERα, we sought to determine if GHSR-eGFP was expressed on hypothalamic neurons expressing TH, which express ERα. TH immunoreactive cell bodies were observed in the AVPV/PeN of intact (Figure 4A–B), OVX and OVX+E2 mice. Very few TH neurons coexpressed GHSR-eGFP in the AVPV/PeN (Intact 2.3±1.6%, OVX 2.1±0.9%, OVX+E2 2.1±0.7%). No significant difference was noted in the number of TH neurons in the AVPV/PeN following steroid manipulations (Intact 73±24 cells/section, OVX 98±9, OVX+E2 56±5; n=4 per group). The mean number of detectable GHSR-eGFP cells was also similar in the AVPV/PeN after steroid manipulations (Intact 151±26 cells/section, OVX 102±32, OVX+E2 54±21; n=4 per group).
Figure 4.
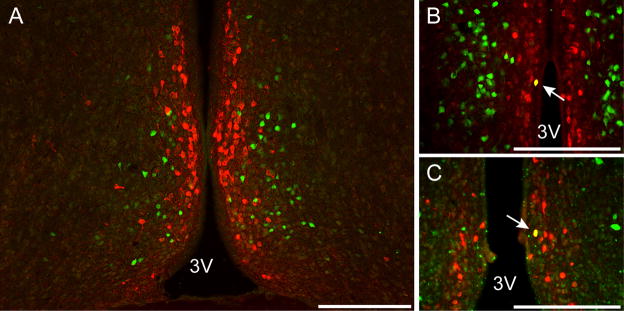
Tyrosine hydroxylase (TH) and growth hormone secretagogue receptor (GHSR)-eGFP expression in the anteroventral periventricular/periventricular nucleus (AVPV/PeN). Representative photomicrographs of the AVPV/PeN from intact females (A, B) and males (C). Note the examples of coexpression of TH (red) and GHSR-eGFP (green)(white arrow). 3V, third ventricle. Scale bars = 200 μm.
TH immunoreactive cell bodies were also observed in the AVPV/PeN of male mice (Figure 4C). Again, very few TH neurons coexpressed GHSR-eGFP in the AVPV/PeN (1.6±1.1%). A mean of 34±12 TH neurons and 59±33 GHSR cells/section were observed in the male AVPV/PeN (n=4).
RFRP3 and GHSR-eGFP coexpression in the mouse forebrain
GHSR expression was examined in RFRP3 neurons in the DMH. We saw no evidence for co-localization between RFRP3 neurons and GHSR-eGFP cells (Figure 5A, B). In females, a mean of 7±1 RFRP3 cells/section and 2±1 GHSR-eGFP cells/section were examined (n=4). In males, a mean of 9±2 RFRP3 cells/section and 3±1 GHSR-eGFP cells/section were examined (n=4).
Figure 5.
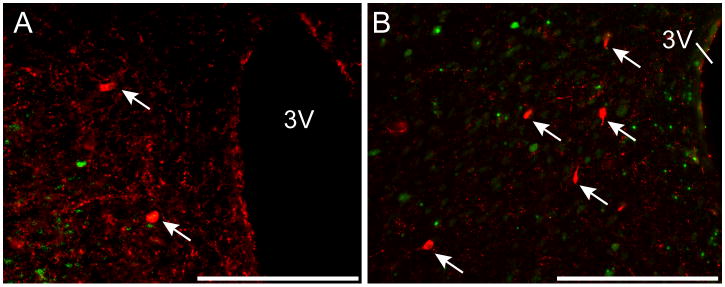
RFamide-related peptide 3 (RFRP3) and growth hormone secretagogue receptor (GHSR)-eGFP coexpression in the dorsomedial hypothalamic nucleus (DMH). Representative photomicrographs of the DMH from intact females (A) and males (C). Note the absence of any coexpression of RFRP3 (red, indicated by arrows) with GHSR-eGFP (green). 3V, third ventricle. Scale bars = 100μm.
Discussion
Although central ghrelin suppresses gonadotropin secretion from the anterior pituitary gland, the neural mechanisms responsible remain unknown. This is due, in part, to the lack of reliable and specific GHSR antibodies to identify GHSR-expressing cells in the hypothalamus. To circumvent this issue, we have used a novel GHSR-eGFP mouse model to examine whether or not key reproductive neural elements directly express GHSR-eGFP. We saw that no GnRH neurons express GHSR-eGFP, however, greater than 90% of GHSR-eGFP expressing neurons in the AVPV/PeN express ERα. Intriguingly, we describe for the first time that kisspeptin neurons in the AVPV/PeN do not express GHSR-eGFP. Similarly, virtually all TH neurons in the AVPV/PeN also do not express GHSR-eGFP. If the GFP signal observed in the GHSR-GFP mouse model faithfully reports on the expression of GHSR, then these data suggest that central ghrelin does not inhibit the reproductive axis by directly suppressing kisspeptin neuronal activity in the AVPV/PeN. Previous studies show that ghrelin reduces kiss1 mRNA in the mPOA (likely to represent the AVPV/PeN) [25] and based on our studies we postulate that ghrelin acts indirectly via unknown circuitry to inhibit these kisspeptin neurons. The ability of the GHSR neurons to respond to estrogen may indirectly influence kisspeptin and GnRH expression and neuronal activity.
The ability to control reproduction and metabolism simultaneously ensures offspring are born into an environment with sufficient energy supplies to maintain survival of both the mother and offspring [22]. Ghrelin is a peripheral metabolic hormone that conveys negative energy balance to the brain [8] and kisspeptin promotes puberty and reproductive function [29, 30, 61, 65, 66]. Therefore, plasma ghrelin, which suppresses reproductive function [23, 27, 39, 40, 49], serves an important feedback signal to the brain to suppress reproduction. If ghrelin does not directly inhibit kisspeptin neurons, what are the possible indirect mechanisms? A recent study suggests that agouti-related peptide (AgRP) neurons are involved in an important link between reproduction and metabolism [75]. AgRP neurons are critical to initiate food intake [5, 6] and genetic ablation of AgRP neurons in adulthood results in starvation [44]. In order to examine the mechanisms underpinning hyperphagia in genetically obese and infertile ob/ob mice, Wu et al discovered that ablating AgRP neurons in these mice caused a prolonged period of starvation and remarkably restored fertility in both male and female ob/ob mice [75]. The majority of AgRP neurons in the ARC contain GHSRs and leptin receptors [52, 73] and ghrelin promotes food intake by acting on GHSR in AgRP neurons [1, 4, 11, 43]. Thus, ghrelin activation of AgRP neurons may modulate the reproductive axis by suppressing kisspeptin neuronal activity. Of note, plasma ghrelin levels are elevated during negative energy balance [9, 45, 55, 77] and intraperitoneal ghrelin reduces Kiss1 mRNA in the AVPV/PeN [25]. Moreover, ob/ob mice have low Kiss1 mRNA expression in the ARC [67] and high AgRP, NPY or GABA release from AgRP neurons [59, 62, 72] and kisspeptin directly inhibits NPY neurons [26]. Collectively, these studies suggest a reciprocal circuit linking metabolism and reproduction, a circuit that has been anatomically demonstrated in sheep [7]. Under conditions of negative energy balance, elevated NPY and AgRP would act to suppress kisspeptins and switch off reproduction in order to conserve energy stores, whereas kisspeptin neurons release this reproductive brake by decreasing AgRP neuronal firing.
In addition to kisspeptin neurons, we show that TH, RFRP3 and GnRH neurons do not coexpress GHSR-eGFP. Thus, the neuronal mechanism that enables ghrelin signaling to inhibit reproduction remains unresolved. Alternatively, we cannot discount the existence of unknown ghrelin receptors, other than GHSR. GABAergic neurons are the likely candidate neurons expressing GHSR-eGFP in the mPOA and AVPV/PeN, where the ability of ghrelin (or estrogen) to activate these neurons would in turn inhibit kisspeptin neuronal activity or potentially GnRH neuronal activity. In support of this, studies have recently reported GABAergic inhibition of kisspeptin neurons [19, 41] and GABA regulation of GnRH neurons is well established [33]. Moreover, the vast majority of GABAergic neurons in the AVPV/PeN respond to estradiol [42]. GABAergic neurons in the AVPV/PeN express ERα [24, 34], and estradiol appears to exert a predominant stimulatory effect upon these neurons [32, 47].
Our current data exclude the possibility that AVPV kisspeptin neurons express GHSR. We realize that these findings are reliant on the validity of the GHSR-eGFP model. In our study, we observed that approximately half of the GHSR-eGFP cells in the AVPV coexpressed Ghsr mRNA (as determined by in situ hybridization). However, far fewer eGFP cells were localized to the ARC than expected (data not shown). Given this we can make no claim to the degree of GHSR-eGFP coexpression in kisspeptin neurons of the ARC. Moreover, we remain cautious with the GHSR-eGFP mouse model and the interpretation of our data. Importantly, although the moderate level of coexpression between eGFP and mRNA could signify at least some degree of ectopic eGFP expression, it more likely reflects known limitations of the in situ hybridization technique, especially when used in dual-label studies such as in combination with immunohistochemistry. Reassuringly, the anatomical distribution of GHSR-eGFP in the AVPR/PeN mirrors that of Ghsr mRNA, so we remain confident that the mouse is an adequate model to examine ghrelin targets in this region of the brain. We have also recently shown that gonadotropes in the anterior pituitary gland express GHSR-eGFP in both males and females [56]. Furthermore, the majority of GHSR-eGFP pituitary cells also express ERα [56]. Despite the modest level of coexpression between GHSR-eGFP and gonadotropes (~20%), these findings confirm that ghrelin can regulate the reproductive axis at both the hypothalamic and pituitary level.
We show the majority of GHSR-eGFP neurons also express ERα in the AVPV/PeN. Thus, it is possible that the central effects of estrogen on reproduction, including those on kisspeptin neurons, may be mediated indirectly by ghrelin responsive neurons. Moreover, in addition to their clear effects on reproduction, estrogens regulate energy homeostasis, exerting anti-obesity effects. Menopause is characterized by the loss of estrogen production and is also associated with obesity [10]. Ovariectomy in rodents causes obesity, increasing food intake and decreasing energy expenditure [57] and E2 replacement prevents this effect [28]. The effects of estrogen on energy balance are primarily mediated by neuronal ERα signaling [31], particularly in hypothalamic steroidogenic factor-1 (SF1) and POMC neurons [76]. Our data potentially extend the scope of these findings because the majority of GHSR-eGFP neurons also express ERα. Thus, estrogen may regulate ghrelin signaling in these neurons to alter energy intake and metabolism.
In conclusion, our studies highlight that neither kisspeptin nor TH neurons in the AVPV/PeN express GHSR-eGFP. Furthermore, neither GnRH neurons in the DBB nor RFRP3 neurons in the DMH express GHSR-eGFP. We observed that the majority of GHSR-eGFP neurons the AVPV/PeN express ERα. Taken together, our data suggest that ghrelin is able to interact with the reproductive system via a number of afferent GHSR neuronal systems, which are estrogen sensitive neuronal populations.
Supplementary Material
We have used a novel GHSR-eGFP mouse to colocalize GHSR-eGFP cells
No coexpression of GHSR-eGFP with kisspeptin neurons in the AVPV
No coexpression of GHSR-eGFP with kisspeptin neurons
More than 90% of GHSR-eGFP neurons in the AVPV express estrogen receptor alpha.
Ghrelin regulates the reproductive axis via an indirect mechanism.
Acknowledgments
JTS is supported by a NHMRC project grant (606538) and an ARC Future Fellowship (FT0990986) and discovery project (DP120100521).
ZBA is supported by a Monash Fellowship, Monash University, Australia, an ARC Future Fellowship (FT100100966) and NHMRC grants (NHMRC 1011274, NHMRC 1030037).
BKM and JMZ would like to acknowledge the excellent technical guidance and assistance of Carol Elias, PhD, and Charlotte E. Lee at UT Southwestern Medical Center. We would also like to acknowledge support from the NHMRC (1030337) and the NIH (R01DK071320).
Footnotes
Disclosure Statement
The authors have no conflicts of interest to disclose.
Kisspeptin neurons do not express GHSR
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andrews ZB. Central mechanisms involved in the orexigenic actions of ghrelin. Peptides. 2011;32:2248–55. doi: 10.1016/j.peptides.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Andrews ZB. The extra-hypothalamic actions of ghrelin on neuronal function. Trends Neurosci. 2011;34:31–40. doi: 10.1016/j.tins.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Andrews ZB, Erion D, Beiler R, Liu ZW, Abizaid A, Zigman J, et al. Ghrelin promotes and protects nigrostriatal dopamine function via a UCP2-dependent mitochondrial mechanism. J Neurosci. 2009;29:14057–65. doi: 10.1523/JNEUROSCI.3890-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, et al. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–51. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–5. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–7. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Backholer K, Smith JT, Rao A, Pereira A, Iqbal J, Ogawa S, et al. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology. 2010;151:2233–43. doi: 10.1210/en.2009-1190. [DOI] [PubMed] [Google Scholar]
- 8.Briggs DI, Andrews ZB. Metabolic status regulates ghrelin function on energy homeostasis. Neuroendocrinology. 2011;93:48–57. doi: 10.1159/000322589. [DOI] [PubMed] [Google Scholar]
- 9.Briggs DI, Lemus MB, Kua E, Andrews ZB. Diet-induced obesity attenuates fasting-induced hyperphagia. J Neuroendocrinol. 2011;23:620–6. doi: 10.1111/j.1365-2826.2011.02148.x. [DOI] [PubMed] [Google Scholar]
- 10.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–11. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 11.Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145:2607–12. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- 12.Chuang JC, Perello M, Sakata I, Osborne-Lawrence S, Savitt JM, Lutter M, et al. Ghrelin mediates stress-induced food-reward behavior in mice. J Clin Invest. 2011;121:2684–92. doi: 10.1172/JCI57660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuang JC, Sakata I, Kohno D, Perello M, Osborne-Lawrence S, Repa JJ, et al. Ghrelin directly stimulates glucagon secretion from pancreatic alpha-cells. Mol Endocrinol. 2011;25:1600–11. doi: 10.1210/me.2011-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke IJ, Qi Y, Puspita Sari I, Smith JT. Evidence that RF-amide related peptides are inhibitors of reproduction in mammals. Front Neuroendocrinol. 2009;30:371–8. doi: 10.1016/j.yfrne.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Clarkson J, Herbison AE. Dual phenotype kisspeptin-dopamine neurones of the rostral periventricular area of the third ventricle project to gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2011;23:293–301. doi: 10.1111/j.1365-2826.2011.02107.x. [DOI] [PubMed] [Google Scholar]
- 16.Clarkson J, d’Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28:8691–7. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis JF, Choi DL, Clegg DJ, Benoit SC. Signaling through the ghrelin receptor modulates hippocampal function and meal anticipation in mice. Physiol Behav. 2011;103:39–43. doi: 10.1016/j.physbeh.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–8. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 19.Ducret E, Gaidamaka G, Herbison AE. Electrical and morphological characteristics of anteroventral periventricular nucleus kisspeptin and other neurons in the female mouse. Endocrinology. 2010;151:2223–32. doi: 10.1210/en.2009-1480. [DOI] [PubMed] [Google Scholar]
- 20.Egecioglu E, Jerlhag E, Salome N, Skibicka KP, Haage D, Bohlooly YM, et al. Ghrelin increases intake of rewarding food in rodents. Addict Biol. 2010;15:304–11. doi: 10.1111/j.1369-1600.2010.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, et al. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998;21:1375–85. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- 22.Evans JJ, Anderson GM. Balancing ovulation and anovulation: integration of the reproductive and energy balance axes by neuropeptides. Hum Reprod Update. 2012;18:313–32. doi: 10.1093/humupd/dms004. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Fernandez R, Tena-Sempere M, Navarro VM, Barreiro ML, Castellano JM, Aguilar E, et al. Effects of ghrelin upon gonadotropin-releasing hormone and gonadotropin secretion in adult female rats: in vivo and in vitro studies. Neuroendocrinology. 2005;82:245–55. doi: 10.1159/000092753. [DOI] [PubMed] [Google Scholar]
- 24.Flugge G, Oertel WH, Wuttke W. Evidence for estrogen-receptive GABAergic neurons in the preoptic/anterior hypothalamic area of the rat brain. Neuroendocrinology. 1986;43:1–5. doi: 10.1159/000124500. [DOI] [PubMed] [Google Scholar]
- 25.Forbes S, Li XF, Kinsey-Jones J, O’Byrne K. Effects of ghrelin on Kisspeptin mRNA expression in the hypothalamic medial preoptic area and pulsatile luteinising hormone secretion in the female rat. Neurosci Lett. 2009;460:143–7. doi: 10.1016/j.neulet.2009.05.060. [DOI] [PubMed] [Google Scholar]
- 26.Fu LY, van den Pol AN. Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J Neurosci. 2010;30:10205–19. doi: 10.1523/JNEUROSCI.2098-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furuta M, Funabashi T, Kimura F. Intracerebroventricular administration of ghrelin rapidly suppresses pulsatile luteinizing hormone secretion in ovariectomized rats. Biochem Biophys Res Commun. 2001;288:780–5. doi: 10.1006/bbrc.2001.5854. [DOI] [PubMed] [Google Scholar]
- 28.Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, et al. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med. 2007;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- 29.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–7. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 30.Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–56. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97:12729–34. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev. 1998;19:302–30. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- 33.Herbison AE, Moenter SM. Depolarising and hyperpolarising actions of GABA(A) receptor activation on gonadotrophin-releasing hormone neurones: towards an emerging consensus. J Neuroendocrinol. 2011;23:557–69. doi: 10.1111/j.1365-2826.2011.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herbison AE, Robinson JE, Skinner DC. Distribution of estrogen receptor-immunoreactive cells in the preoptic area of the ewe: co-localization with glutamic acid decarboxylase but not luteinizing hormone-releasing hormone. Neuroendocrinology. 1993;57:751–9. doi: 10.1159/000126433. [DOI] [PubMed] [Google Scholar]
- 35.Iqbal J, Kurose Y, Canny B, Clarke IJ. Effects of central infusion of ghrelin on food intake and plasma levels of growth hormone, luteinizing hormone, prolactin, and cortisol secretion in sheep. Endocrinology. 2006;147:510–9. doi: 10.1210/en.2005-1048. [DOI] [PubMed] [Google Scholar]
- 36.Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D, et al. Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci U S A. 2009;106:11318–23. doi: 10.1073/pnas.0812809106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, et al. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148:1774–83. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- 38.Kim J, Semaan SJ, Clifton DK, Steiner RA, Dhamija S, Kauffman AS. Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology. 2011;152:2020–30. doi: 10.1210/en.2010-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kluge M, Schussler P, Uhr M, Yassouridis A, Steiger A. Ghrelin suppresses secretion of luteinizing hormone in humans. J Clin Endocrinol Metab. 2007;92:3202–5. doi: 10.1210/jc.2007-0593. [DOI] [PubMed] [Google Scholar]
- 40.Kluge M, Schussler P, Schmidt D, Uhr M, Steiger A. Ghrelin suppresses secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in women. J Clin Endocrinol Metab. 2012;97:E448–51. doi: 10.1210/jc.2011-2607. [DOI] [PubMed] [Google Scholar]
- 41.Kurian JR, Keen KL, Guerriero KA, Terasawa E. Tonic control of kisspeptin release in prepubertal monkeys: implications to the mechanism of puberty onset. Endocrinology. 2012;153:3331–6. doi: 10.1210/en.2012-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu X, Herbison AE. Estrous cycle- and sex-dependent changes in pre- and postsynaptic GABAB control of GnRH neuron excitability. Endocrinology. 2011;152:4856–64. doi: 10.1210/en.2011-1369. [DOI] [PubMed] [Google Scholar]
- 43.Luquet S, Phillips CT, Palmiter RD. NPY/AgRP neurons are not essential for feeding responses to glucoprivation. Peptides. 2007;28:214–25. doi: 10.1016/j.peptides.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 44.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–5. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 45.Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11:752–3. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martini AC, Fernandez-Fernandez R, Tovar S, Navarro VM, Vigo E, Vazquez MJ, et al. Comparative analysis of the effects of ghrelin and unacylated ghrelin on luteinizing hormone secretion in male rats. Endocrinology. 2006;147:2374–82. doi: 10.1210/en.2005-1422. [DOI] [PubMed] [Google Scholar]
- 47.Mitsushima D, Shwe TT, Funabashi T, Shinohara K, Kimura F. GABA release in the medial preoptic area of cyclic female rats. Neuroscience. 2002;113:109–14. doi: 10.1016/s0306-4522(02)00160-4. [DOI] [PubMed] [Google Scholar]
- 48.Moon M, Choi JG, Nam DW, Hong HS, Choi YJ, Oh MS, et al. Ghrelin ameliorates cognitive dysfunction and neurodegeneration in intrahippocampal amyloid-beta1–42 oligomer-injected mice. J Alzheimers Dis. 2011;23:147–59. doi: 10.3233/JAD-2010-101263. [DOI] [PubMed] [Google Scholar]
- 49.Ogata R, Matsuzaki T, Iwasa T, Kiyokawa M, Tanaka N, Kuwahara A, et al. Hypothalamic ghrelin suppresses pulsatile secretion of luteinizing hormone via beta-endorphin in ovariectomized rats. Neuroendocrinology. 2009;90:364–70. doi: 10.1159/000257421. [DOI] [PubMed] [Google Scholar]
- 50.Overgaard A, Tena-Sempere M, Franceschini I, Desroziers E, Simonneaux V, Mikkelsen JD. Comparative analysis of kisspeptin-immunoreactivity reveals genuine differences in the hypothalamic Kiss1 systems between rats and mice. Peptides. 2013;45C:85–90. doi: 10.1016/j.peptides.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 51.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Elsevier Academic Press; 2004. [Google Scholar]
- 52.Perello M, Scott MM, Sakata I, Lee CE, Chuang JC, Osborne-Lawrence S, et al. Functional implications of limited leptin receptor and ghrelin receptor coexpression in the brain. J Comp Neurol. 2012;520:281–94. doi: 10.1002/cne.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perello M, Sakata I, Birnbaum S, Chuang JC, Osborne-Lawrence S, Rovinsky SA, et al. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol Psychiatry. 2010;67:880–6. doi: 10.1016/j.biopsych.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poling MC, Kim J, Dhamija S, Kauffman AS. Development, Sex Steroid Regulation, and Phenotypic Characterization of RFamide-Related Peptide (Rfrp) Gene Expression and RFamide Receptors in the Mouse Hypothalamus. Endocrinology. 2012 doi: 10.1210/en.2011-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Redman LM, Veldhuis JD, Rood J, Smith SR, Williamson D, Ravussin E. The effect of caloric restriction interventions on growth hormone secretion in nonobese men and women. Aging Cell. 2010;9:32–9. doi: 10.1111/j.1474-9726.2009.00530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reichenbach A, Steyn FJ, Sleeman MW, Andrews ZB. Ghrelin receptor expression and colocalization with anterior pituitary hormones using a GHSR-GFP mouse line. Endocrinology. 2012;153:5452–66. doi: 10.1210/en.2012-1622. [DOI] [PubMed] [Google Scholar]
- 57.Rogers NH, Perfield JW, 2nd, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150:2161–8. doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, et al. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29:3920–9. doi: 10.1523/JNEUROSCI.5740-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwartz MW, Baskin DG, Bukowski TR, Kuijper JL, Foster D, Lasser G, et al. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 1996;45:531–5. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- 60.Semaan SJ, Murray EK, Poling MC, Dhamija S, Forger NG, Kauffman AS. BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice. Endocrinology. 2010;151:5807–17. doi: 10.1210/en.2010-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–27. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 62.Shutter JR, Graham M, Kinsey AC, Scully S, Luthy R, Stark KL. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 1997;11:593–602. doi: 10.1101/gad.11.5.593. [DOI] [PubMed] [Google Scholar]
- 63.Simmons DM, Arrizza JL, Swanson LW. A complete protocol for in situ hybridization of messenger RNAs in brain and other tissues with radio-labelled single-stranded RNA probes. The Journal of Histotechnology. 1989;12:169–81. [Google Scholar]
- 64.Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience. 2011;180:129–37. doi: 10.1016/j.neuroscience.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 65.Smith JT. Kisspeptin signalling in the brain: steroid regulation in the rodent and ewe. Brain Res Rev. 2008;57:288–98. doi: 10.1016/j.brainresrev.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 66.Smith JT. Sex steroid control of hypothalamic Kiss1 expression in sheep and rodents: comparative aspects. Peptides. 2009;30:94–102. doi: 10.1016/j.peptides.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 67.Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18:298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- 68.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–92. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 69.Smith JT, Li Q, Yap KS, Shahab M, Roseweir AK, Millar RP, et al. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology. 2011;152:1001–12. doi: 10.1210/en.2010-1225. [DOI] [PubMed] [Google Scholar]
- 70.Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, et al. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology. 2008;149:5770–82. doi: 10.1210/en.2008-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spencer SJ, Xu L, Clarke MA, Lemus M, Reichenbach A, Geenen B, et al. Ghrelin regulates the hypothalamic-pituitary-adrenal axis and restricts anxiety after acute stress. Biol Psychiatry. 2012;72:457–65. doi: 10.1016/j.biopsych.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 72.Takahashi KA, Cone RD. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology. 2005;146:1043–7. doi: 10.1210/en.2004-1397. [DOI] [PubMed] [Google Scholar]
- 73.Willesen MG, Kristensen P, Romer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70:306–16. doi: 10.1159/000054491. [DOI] [PubMed] [Google Scholar]
- 74.Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–80. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu Q, Whiddon BB, Palmiter RD. Ablation of neurons expressing agouti-related protein, but not melanin concentrating hormone, in leptin-deficient mice restores metabolic functions and fertility. Proc Natl Acad Sci U S A. 2012;109:3155–60. doi: 10.1073/pnas.1120501109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–65. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao TJ, Liang G, Li RL, Xie X, Sleeman MW, Murphy AJ, et al. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci U S A. 2010;107:7467–72. doi: 10.1073/pnas.1002271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–48. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115:3564–72. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.