Abstract
Radial trajectories facilitate high-resolution balanced steady state free precession (bSSFP) because the efficient gradients provide more time to extend the trajectory in k-space. A number of radial bSSFP methods that support fat-water separation have been developed; however, most of these methods require an environment with limited B0 inhomogeneity. In this work, high-resolution bSSFP with fat-water separation is achieved in more challenging B0 environments by combining a 3D radial trajectory with the IDEAL chemical species separation method. A method to maintain very high resolution within the timing constraints of bSSFP and IDEAL is described using a dual-pass pulse sequence. The sampling of a unique set of radial lines at each echo time is investigated as a means to circumvent the longer scan time that IDEAL incurs as a multi-echo acquisition. The manifestation of undersampling artifacts in this trajectory and their effect on chemical species separation are investigated in comparison to the case in which each echo samples the same set of radial lines. This new bSSFP method achieves 0.63 mm isotropic resolution in a 5-minute scan and is demonstrated in difficult in vivo imaging environments, including the breast and a knee with ACL reconstruction hardware at 1.5 T.
Keywords: balanced steady state free precession, radial imaging, water-fat imaging, IDEAL
Introduction
Balanced Steady State Free Precision (bSSFP) is a powerful technique that produces rapid T2-like contrast with high signal-to-noise (SNR) performance, however, implementation of bSSFP presents several challenges. Foremost amongst these is the presence of signal nulls in the response spectrum that manifest as banding artifacts in regions of off-resonance. A second challenge is that very strong fat signal can obscure surrounding structures and thus must be suppressed, separated, or otherwise removed in order to obtain useful images. A number of novel methods (1–7) designed specifically to reduce banding artifacts and address the high fat signal in bSSFP have shown promise in initial clinical implementations. For example, Fluctuating Equilibrium MRI (FEMR) has provided improved SNR efficiency in articular cartilage and improved contrast-to-noise ratio (CNR) efficiency between fluid and cartilage in the knee (8). More recently, wideband SSFP has demonstrated consistent signal level and reduced off-resonance artifacts in coronary MR angiography (9).
Though effective in reducing banding artifacts and suppressing fat signal, many bSSFP methods have specific timing requirements that can hamper efforts at high-resolution imaging. As the width of the spectral passbands between bSSFP spectrum nulls is inversely proportional to the repetition time (TR), a shorter TR reduces the potential for banding artifacts. Thus, in many cases, allocating more time for high-resolution spatial encoding must be traded off with efforts to minimize the TR. This has led to efforts to achieve wide data acquisition windows along with effective fat suppression in bSSFP. Initial experiments of a dual acquisition bSSFP method achieved 1 mm isotropic resolution in peripheral angiography (7).
Radial trajectories present one option to address the competing requirements of increasing spatial encoding time and short TRs for high-resolution bSSFP. Radial trajectories can increase data acquisition efficiency in comparison to Cartesian trajectories, as data can be acquired throughout all of the imaging gradients including the prewinder and rewinder. In whole heart coronary MRI at 3T, a three-dimensional radial bSSFP acquisition achieved an isotropic resolution of 1.3 mm and improved image quality and sharpness in comparison to Cartesian bSSFP (10). The Steady-state Projection Imaging with Dynamic Echo-train Readout (SPIDER) technique incorporated a multi-echo radial readout with bSSFP to improve data acquisition efficiency for cardiac cine MRI (11). Also, Vastly Undersampled Isotropic Projection SSFP (VIPR-SSFP) has been investigated in the knee and breast (12–14) where 0.47 mm – 0.63 mm isotropic resolution was achieved in a 5-minute scan time.
These radial methods show promise for improving the diagnostic utility of bSSFP by providing high resolution and reducing banding artifacts, particularly in regions of modest B0 field inhomogeneity. However, additional techniques are often required to mitigate artifacts in regions of more significant off-resonance. A frequency scouting method was used to reduce off-resonance artifacts in whole-heart radial bSSFP acquisitions (10), while high order shims were utilized in applications of FEMR (8). Applications of VIPR-SSFP in both the knee and breast also reported the presence of artifacts in regions of significant field inhomogeneity. In the knee, off-resonance artifacts occurred near metal implants, while artifacts in the breast were present in the superior and inferior tissues close to the chest wall which are characteristically difficult to shim (12, 15). To further improve the acquisition of high-resolution radial bSSFP, obtaining more robust water/fat separation in regions of large B0 inhomogeneity is desirable.
In this work we combine IDEAL (Iterative Decomposition of Water and Fat With Echo Asymmetry and Least-Squares Estimation) (16–18) chemical species separation with 3D radial bSSFP as a means to achieve the high isotropic resolution of radial trajectories with water-fat separation that is more robust to B0 inhomogeneity. IDEAL relaxes requirements on B0 homogeneity by using multiple echo times to calculate and correct for off-resonance. IDEAL has been shown to be robust with numerous contrast mechanisms including RF spoiled T1-weighted imaging (19), fully refocused steady-state free precession (20–22), and spin echo imaging (23). As with many chemical species separation methods, IDEAL requires the acquisition of data at a series of echo times while the specific choice of echo times can improve the efficacy of water-fat separation. For the combination of IDEAL and radial bSSFP, we employ two strategies to balance timing requirements with the acquisition of high-resolution data while maintaining a clinically feasible scan time. First, we develop a 3D radial trajectory that collects four echoes over two passes so that high-resolution radial bSSFP can be achieved while maintaining echo times amenable to the IDEAL chemical species separation (18). Second, we investigate sampling a unique versus the same set of undersampled radial lines at each echo time as a means to accommodate the multi-echo acquisition in a clinically feasible scan time.
Unique sets of undersampled radial lines, acquired at a series of echo times have previously been utilized to accelerate multi-echo acquisitions. In the radial IDEAL-GRASE technique, T2 estimation is performed through view-sharing by utilizing only data from a single echo time at the center of k-space and filling the outer regions of k-space with radial data from surrounding echo times (24). Flask, et al. (25) investigated sampling a unique set of radial lines at each echo time as a means to accelerate two-point Dixon fat-water separation with steady-state imaging. They showed that resolution is maintained while acquisition time is reduced by a factor of two in comparison to the case in which the same set of radial lines is sampled for each echo.
The sampling of unique sets of undersampled radial lines at each echo time is well aligned with certain aspects of IDEAL but may also complicate other aspects of the algorithm. In IDEAL, the initial B0 estimate is based on a low-resolution field map, and in radially undersampled data the lower-resolution data is fully sampled. Therefore, utilizing a unique set of radially undersampled lines for each echo may not significantly degrade the field map estimation. However, while initial estimation of the field map may be robust to undersampling of the high spatial frequencies, the iterative least squares process that separates water and fat cannot properly decompose the spatially varying artifacts in each.
We call this new method VIPR-IDEAL to reflect the VIPR (Vastly Undersampled Isotropic Projection) (3, 26) trajectory on which it is based. The VIPR-IDEAL acquisition is developed with consideration to echo times, banding artifacts, resolution and scan time. The manifestation of radial undersampling artifacts of VIPR-IDEAL along with the consequence of these artifacts on the IDEAL chemical species separation are investigated and illustrated in digital and physical phantoms. Finally, an in vivo demonstration of VIPR-IDEAL is presented in the breast and knee at 1.5 T.
Methods
Dual-pass, Dual Half-echo 3D Radial Trajectory
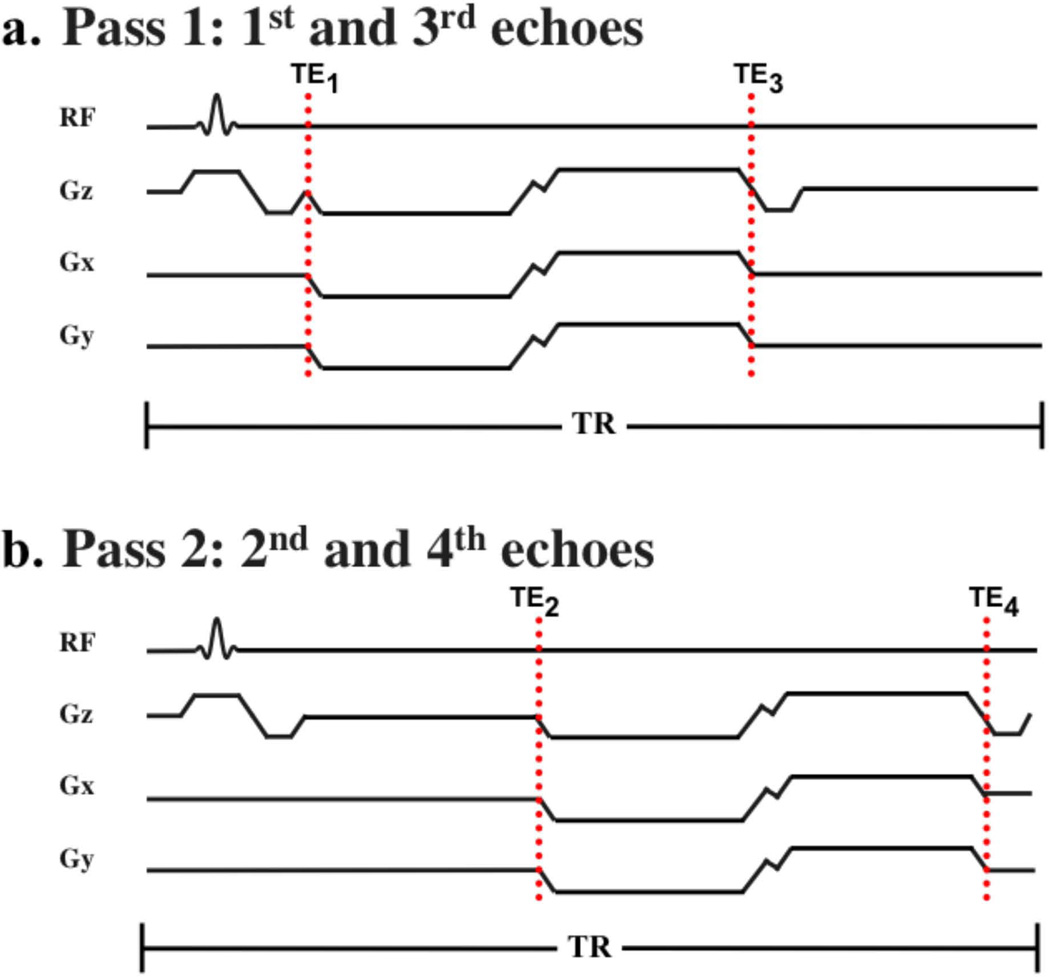
The VIPR-IDEAL trajectory is based on a previously described 3D radial dual half-echo trajectory (3). Two echoes are acquired per TR with a readout that encodes from the center of k-space out and then back in on a second radial line. A small tangential gradient blip is applied between echoes so a different radial line is sampled on the way back in to the center of k-space. At minimum, IDEAL requires 3 echo times, however multi-echo radial trajectories are amenable to even numbers of echo times. Therefore, in VIPR-IDEAL, 4 echoes are acquired in two passes with two echoes acquired per pass. The acquisition of 4 echoes is further advantageous as the efficacy of water-fat separation improves with the incorporation of more than three echo times (27). To balance the need for a short TR with the desire to acquire high-resolution data, the first and third echo times are acquired in a first pass and the second and fourth echo times are acquired in a second pass (Figure 1). Acquiring nonconsecutive pairs of echoes in each TR doubles the time available for spatial encoding in comparison to consecutive acquisition of echoes. The repetition time is kept constant for both passes by adding a delay after the readout in the first TR and before the readout in the second. The constant TR maintains the steady state and assures that the magnitude profile of the response spectrum is the same for both passes. The final trajectory is comprised of four sets of undersampled radial lines with the radial lines of the first and third echoes collected in the first pass and the radial lines of the second and fourth echoes collected in the second pass.
Figure 1.
In VIPR-IDEAL a dual-pass acquisition (a. pass 1, b. pass 2) acquires two unique radial lines per pass and also doubles the time available for spatial encoding, in comparison to the consecutive acquisition of echo times, by acquiring inconsecutive echoes in each pass (pass 1: 1st and 3rd echoes, pass 2: 2nd and 4th echoes). This trajectory balances the requirements of high-resolution, a short TR, and echo spacings that are amenable to IDEAL chemical species separation, all within a clinically feasible scan time.
Radial lines were acquired continuously from −kz to kz so change in gradient amplitude between successive TRs is minimal. While the dual echo out-and-back trajectory has a large first moment, the application of this trajectory with bSSFP is inherently nulled due to the π phase cycling of the excitation pulses (28, 29). Small variation of gradient amplitude between successive TRs is necessary for effective cancellation of first moment phase accrual. The robustness of the 3D dual-echo radial trajectory to flow artifacts in bSSFP, despite the large first moment has also been demonstrated previously in both the femoral and carotid arteries (3).
Selection of Echo Spacing and Repetition Time
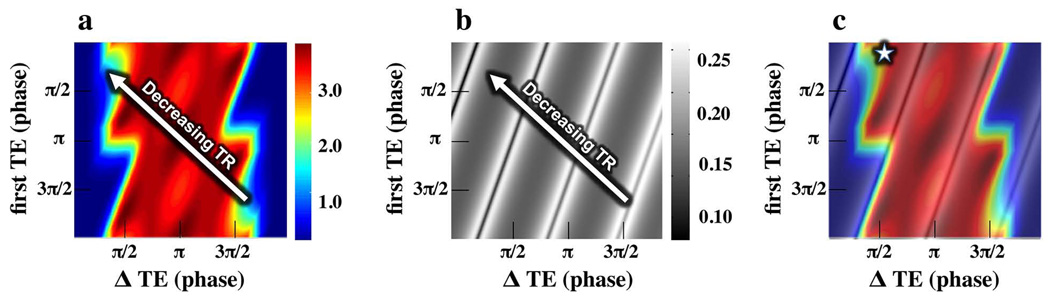
For the incorporation of IDEAL with bSSFP, the TR and echo times must be chosen with consideration to both the number of signal averages (NSA) performance and occurrence of banding artifacts. NSA is a measure of the noise efficiency of fat-water separation (30) and is used to determine the choice of echo spacing with IDEAL; NSA plots indicate the NSA associated with a range of initial echo times and echo spacing (Figure 2a). Because the location of the nulls in bSSFP varies with the TR, the choice of echo spacing will also affect the proximity of the nulls to the fat resonant peak in the spectrum. As the fat resonance peak approaches the nulls, the level of fat signal will decrease significantly and make robust water-fat separation with IDEAL much more difficult.
Figure 2.
In VIPR-IDEAL, when choosing echo spacing to maximize NSA for the 4 echo chemical species decomposition (a), consideration must also be given to the location of bSSFP banding artifacts. The banding artifacts are inversely proportional to the TR and thus are affected by echo spacing. A plot of the signal magnitude of fat at 1.5 T at the respective TR for each choice of echo spacing (b) is generated and overlaid on the NSA plot (c) to facilitate choosing echo spacing that achieves high NSA while avoiding nulls in the fat signal. For this initial implementation of VIPR-IDEAL (c - star), the phase of the first echo is chosen to be π/10 with phase between subsequent echoes of π/2. Resultant TR is 4.6 ms situating the fat peak at 1.5 T between two nulls in the response spectrum.
To incorporate consideration of the bSSFP nulls with NSA performance, a plot analogous to the NSA plot for 4 echoes was created showing the signal magnitude at the resonance frequency of fat at 1.5 T (Figure 2b). The anticipated TR can be calculated as a function of the first echo time and subsequent echo spacing from
| [1] |
where TE0 is the initial echo time, ΔTE is the echo spacing, and Tc is a constant based on the acquisition parameters, accounting for time between acquisition of the last echo and the middle of the next alpha pulse. A value of 600 µs was used for Tc to reflect the typical duration of this interval in the VIPR-IDEAL acquisition. The magnitude of the fat signal at −220 Hz at 1.5 T (T1 ≈ 260 ms, T2 ≈ 80 ms) in a bSSFP acquisition was then calculated for this range of TRs. With this approximation, the effect of the initial echo time and echo spacing on the TR, and thus on the location of the signal nulls, can be aligned with the respective NSA performance (Figure 2c). Based on this analysis, a TR of 4.6 ms and echo times of 0.3 ms, 1.5 ms, 2.9 ms, and 4.1 ms were chosen for VIPR-IDEAL at 1.5 T. These timing parameters simultaneously provide relatively, high NSA performance and optimal placement of nulls halfway between water and fat in the frequency response.
For this determination of echo spacing with consideration to bSSFP nulls we assumed a single spectral peak to represent fat. IDEAL does accommodate a multi-peak fat spectrum (31) and this method for VIPR-IDEAL parameter determination could be further refined by calculating the response spectrum magnitude for a range of off-resonant frequencies. Echo spacing would then be chosen based on the maximum points of the sum of these magnitude response spectrums. A similar method could be utilized for cases involving more than two chemical species.
Digital Phantom Simulations
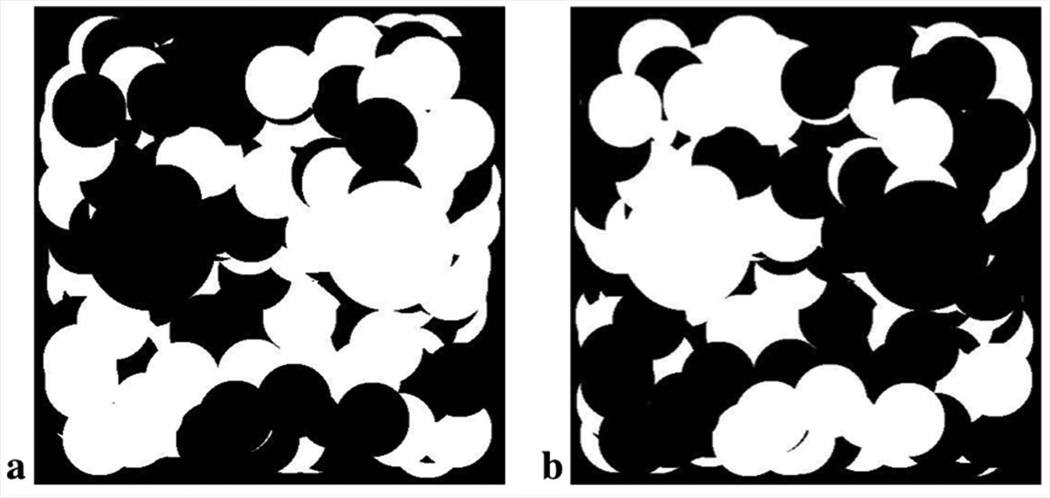
A reduction in undersampling artifacts for on resonance spins by sampling a different set of radial lines at each echo time has been previously shown for two-point radial Dixon imaging (25). As with Dixon imaging, IDEAL is a multi-echo technique. In particular, with radial imaging, which also includes sampling redundancy, acquiring fully sampled datasets at all time points may lead to prohibitively long scan times. Sampling unique versus the same set of radial lines at each echo time was investigated, as a means to lessen artifact if some degree of undersampling is necessary. As opposed to the straightforward combination of echoes utilized in 2-point Dixon imaging, in IDEAL, the individual echoes are first utilized at a low resolution for an initial field map estimate. Subsequently, an iterative least squares fitting determines the chemical species and B0 components at each voxel. Variable streak artifacts from sampling a unique set of radial lines at each echo time should not affect the initial low-resolution field map determination but may manifest during voxel by voxel least squares fitting. To investigate sampling the same versus unique radial lines at each echo time with IDEAL, a 2D digital phantom (Figure 3) was developed with variable distribution of fat and water regions and multiple high-resolution interfaces. The phantom was replicated at 4 times points with phase accrual in the fat regions reflecting an echo spacing of π/2 to reflect the echo spacing of VIPR-IDEAL.
Figure 3.
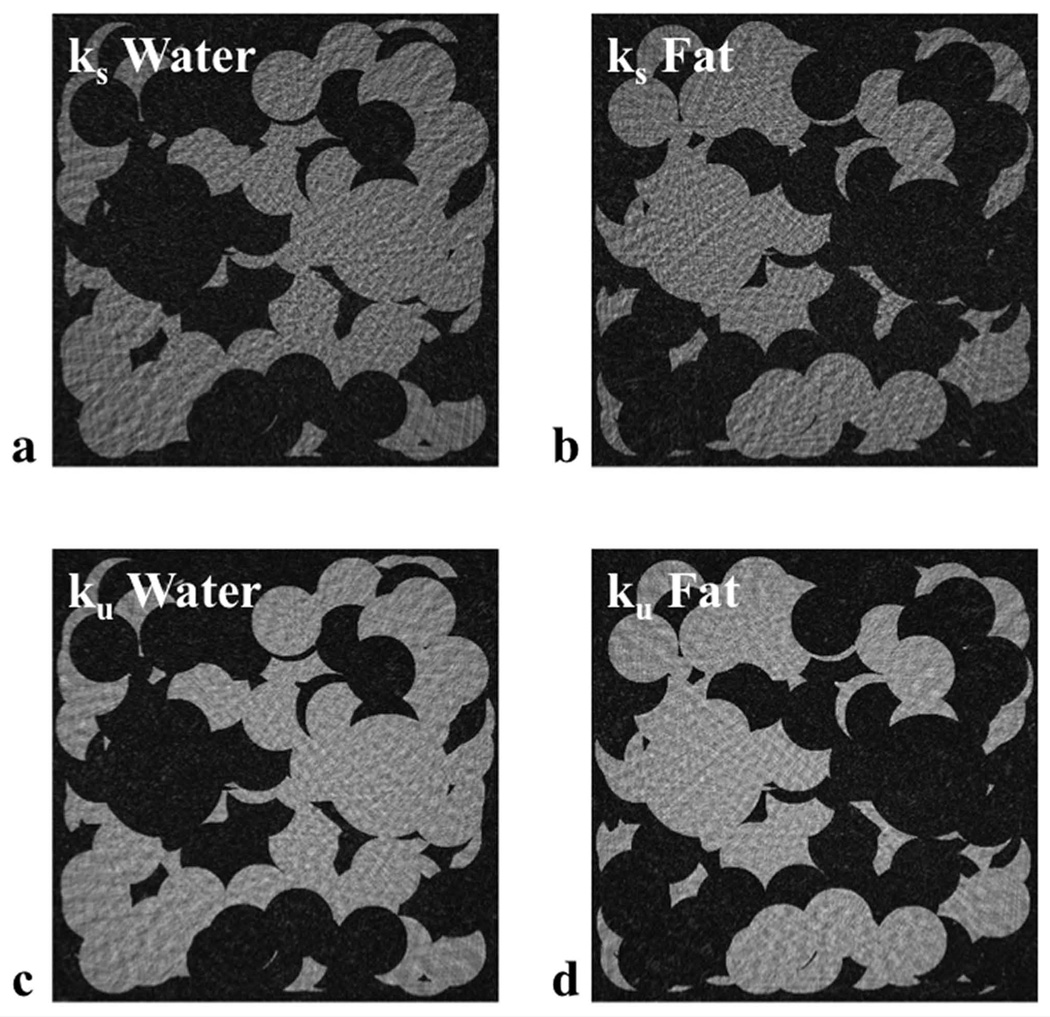
2D digital phantom comprised of variable distribution of water (a) and fat (b) regions designed to investigate the radial undersampling artifact and fat-water separation of VIPR-IDEAL. Multiple high-resolution interfaces are incorporated to represent in-vivo image data. The phantom was comprised of 4 time points with phase accrual in fat regions reflective of the echo times utilized in VIPR-IDEAL.
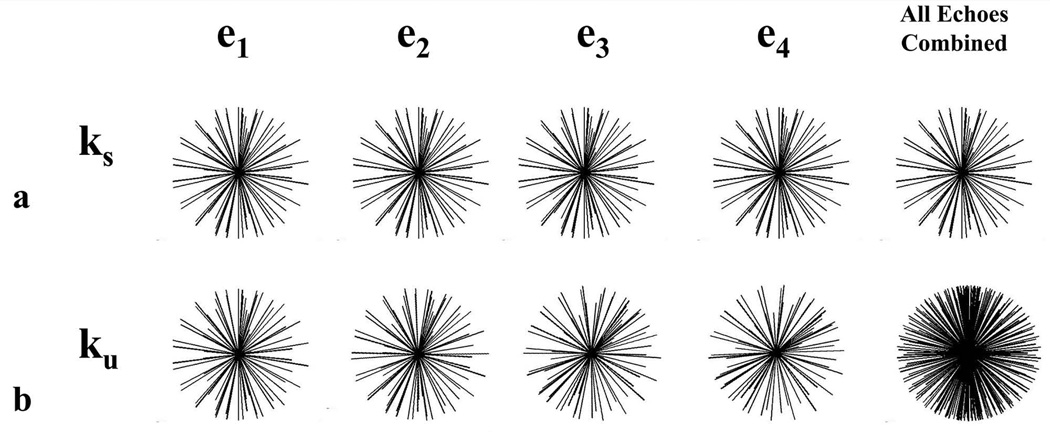
Two radial sampling strategies were applied to the phantom for a range of undersampling factors and then the images were processed with the commercially available IDEAL algorithm. In the first sampling strategy (ks) the same set of radial lines was acquired for each echo, while in the second strategy (ku) a unique set of radial lines was acquired for each echo (Figure 4). Three degrees of undersampling (1/4, 1/8, 1/16) were investigated where the undersampling factors indicate the degree to which a single echo is undersampled with respect to the fully sampled trajectory. To assess the presence of undersampling artifact, RMS error was calculated for both the fat and water images with respect to the fat and water images from a fully sampled case. Fat-water separation was also measured for each case based on the signal ratio of fat to water ROIs in the water images.
Figure 4.
Representation of the two radial sampling strategies investigated for VIPR-IDEAL. In ks (a) the same set of undersampled radial lines is acquired for each echo while in ku (b) a unique set of undersampled radial lines is acquired for each echo.
Phantom Experiments
VIPR-IDEAL acquisitions utilizing both trajectories (ks and ku) were performed in a fat-water phantom on a clinical 1.5 T Scanner (HDx MR450, GE Healthcare, Waukesha, WI) with a single channel quadrature transmit/receive head coil (GE Healthcare, Waukesha, WI). The fat-water phantom was comprised of half peanut oil and half water doped with gadolinium. Plastic drinking straws were placed in the phantom to provide some high-resolution structures. The imaging parameters were as follows: 20 cm field of view, 320 isotropic matrix, receive bandwidth of ±83.33 kHz, 15° flip angle, 4.5 ms TR, and echo times of 0.3 ms, 1.5 ms, 2.6 ms, and 3.8 ms. The total scan time was 3 minutes. In both experiments, each echo was undersampled by a factor of 16 with respect to the fully sampled trajectory. Fat-water separation was measured by the ratio of fat to water signal based on ROIs placed in the water image. Images were also qualitatively assessed for the presence of artifact.
In Vivo Experiments
VIPR-IDEAL acquisitions in the knee and breast were performed on a 1.5 T Scanner (HDx MR450 or HDx Echospeed, GE Healthcare, Waukesha, WI) in asymptomatic volunteers. Both sampling strategies (ks and ku) were acquired in a single breast volunteer while all other acquisitions utilized the ku trajectory in which a unique set of radial lines was acquired at each echo time. Knee volunteers included an individual with metal orthopedic hardware from prior ACL reconstruction surgery. Breast acquisitions were performed unilaterally in the sagittal plane utilizing an HD 8-channel breast coil (GE Healthcare, Waukesha, WI). Knee acquisitions were performed in the axial plane utilizing an eight-channel phased array extremity coil (GE Healthcare, Waukesha, WI). All volunteers provided informed consent following IRB protocols at our institution. Imaging parameters for both breast and knee acquisitions were as follows: 320 isotropic matrix, 20 cm field of view, 15 ° flip angle, receive bandwidth ± 83.33 kHz, echo times 0.3 ms, 1.5 ms, 2.9 ms, 4.0 ms, TR 4.5 ms and scan time of 5 minutes. VIPR-IDEAL breast and knee images were assessed for presence of undersampling artifact, depiction of fine structures, and water-fat separation, particularly in regions of known B0 inhomogeneity.
As noted previously, a number of studies have demonstrated the high resolution available by utilizing a radial trajectory with bSSFP. The development of VIPR-IDEAL builds on one of these methods, VIPR-SSFP which utilized the same 3D radial dual-echo trajectory as VIPR-IDEAL. To provide an initial assessment of VIPR-IDEAL in comparison to another radial bSSFP acquisition, both VIPR-IDEAL and VIPR-SSFP (3, 4) were acquired in a breast volunteer. The VIPR-SSFP acquisitions were performed with equivalent matrix, field-of-view, flip angle and scan time to the VIPR-IDEAL acquisitions. The two sets of images were qualitatively assessed for consistency of fat suppression, and for the depiction of fine structures.
Results
Digital Phantom Simulations
In the digital phantom, IDEAL effectively separated the fat and water regions for both trajectories (Figure 5). RMS error was lower with the ku trajectory for both fat and water images across all undersampling factors (Table 1). Fat suppression ratio was also improved across all acceleration factors with the ku trajectory (Table 2). These results demonstrate the improvement in image quality that may be gained without an increase in scan time by sampling a unique set of undersampled radial lines at each echo time, and that the sampling of a unique set of undersampled radial lines at each echo time does not compromise the ability of IDEAL to separate water and fat. For both trajectories, small features can be distinguished from surrounding signal reflecting the expected equivalent resolution, however the increased level of fat suppression with the ku trajectory improves the contrast between these small features and surrounding tissue, improving their conspicuity.
Figure 5.
Water (a,c) and fat (b,d) images from digital phantom for the ks (a,b) and ku (c,d) cases in which each echo is undersampled by a factor of 8 with respect to a fully sampled dataset. Streak artifact is less evident and level of fat suppression is improved when a unique set of radial lines is sampled at each echo time (ku) (c,d) in comparison to the case in which the same set of radial lines is sampled at each echo time (ks) (a,b).
Table 1.
RMS error is reduced in both the fat and water digital phantom images by sampling a unique set of radial lines at each echo time (ku) in comparison to sampling the same set of radial lines at each echo time (ks).
| Degree of Undersampling | |||
|---|---|---|---|
| 1/4 | 1/8 | 1/16 | |
| ks Water | 0.28 | 0.46 | 0.60 |
| ks Fat | 0.31 | 0.51 | 0.66 |
| ku Water | 0.17 | 0.33 | 0.51 |
| ks Fat | 0.18 | 0.35 | 0.56 |
Table 2.
Fat suppression ratio (in water image: ROI of fat signal/ROI of water signal) is improved for all degrees of undersampling by sampling a unique set of radial lines at each echo time (ku) in comparison to sampling the same set of radial lines at each echo time (ks).
| Degree of Undersampling | |||
|---|---|---|---|
| 1/4 | 1/8 | 1/16 | |
| ks | 0.16 | 0.25 | 0.40 |
| ku | 0.11 | 0.19 | 0.33 |
Phantom Experiments
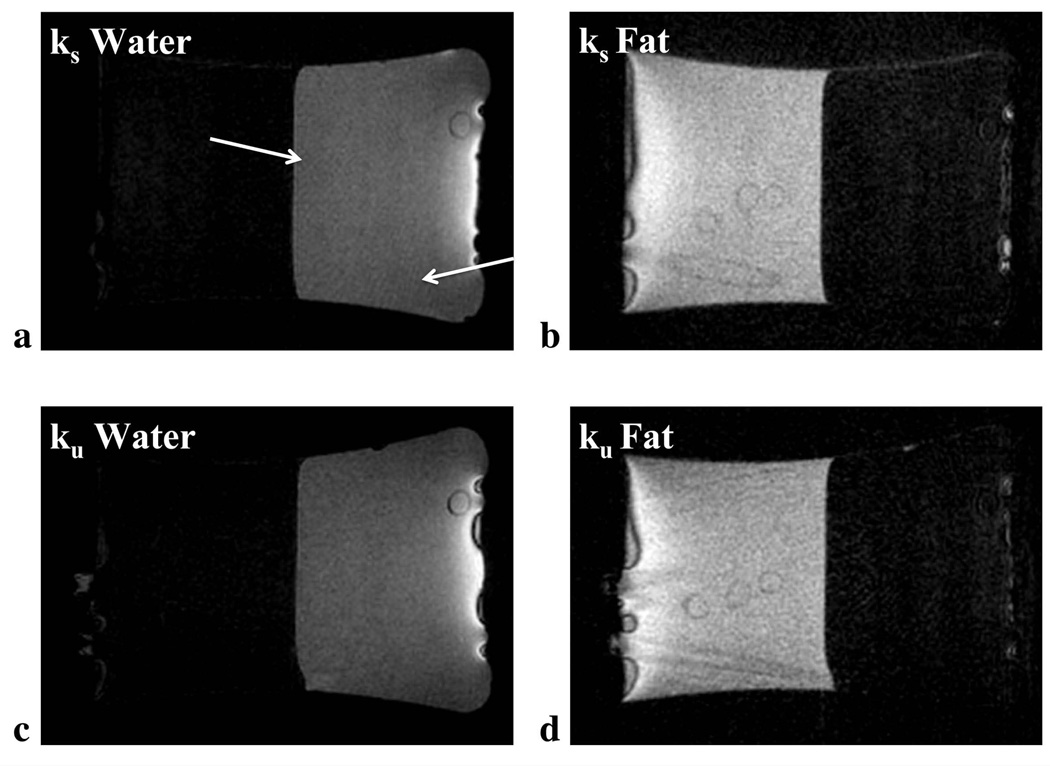
VIPR-IDEAL effectively separated fat and water regions in the phantom with both the ks and ku trajectories. (Figure 6). The fat suppression ratios were roughly equivalent with both trajectories (ks: 0.21, ku: 0.18) possibly due to the small amount of high-resolution structure in the phantom. Undersampling artifacts were reduced for the ku water images, which is evident in the reduced streak artifacts at the edge of the phantom as well as in the improved contrast of the cross section of a straw.
Figure 6.
Water and fat are effectively separated with VIPR-IDEAL in the fat-water phantom for both the ks (a,b) and ku (c,d) trajectories. Presence of undersampling artifact is reduced in the ku water images (c) in comparison to the ks water images (a - arrows). Level of fat suppression is roughly equivalent between the two cases possibly due to the small amount of structural detail from which undersampling artifacts would be expected.
In Vivo Experiments
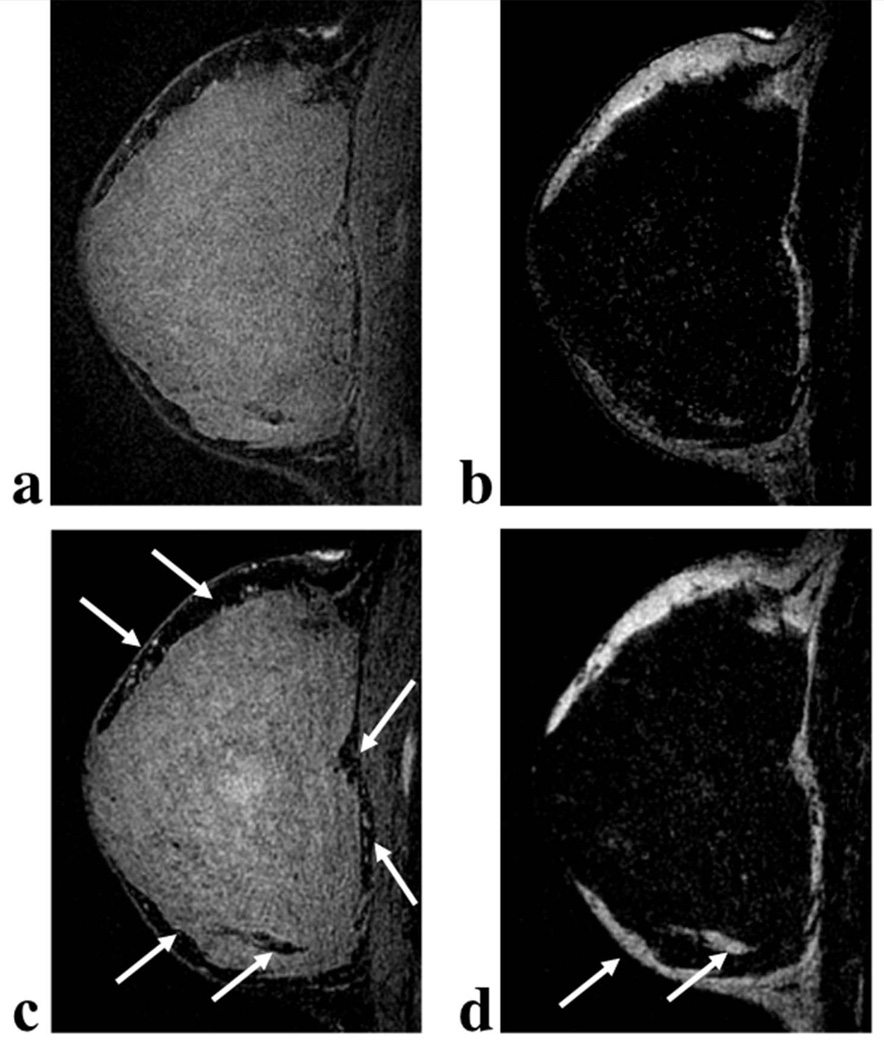
For the case in which both trajectories were acquired in the same breast volunteer (Figure 7) the level of fat suppression in the on resonance images is improved with the ku trajectory and fine structures including vessel cross-sections and tissue edges are more clearly depicted. In the respective fat images, the ku trajectory provides more consistent image quality across all fat regions but fat signal is noisier in comparison to the ks trajectory. The slight signal intensity variation at the center of the ku water image (Figure 7c) is due to a density compensation error. The artifact is present at the center of both the ks and ku image volumes, however, due to a shift of the volunteer between the two acquisitions the artifact did not appear in the same set of slices in the two image volumes.
Figure 7.
Water (a,c) and fat (b,d) VIPR-IDEAL sagittal breast images at 1.5 T with two different sampling strategies ks (a,b) and ku (c,d). Sampling a unique set of radial lines at each echo time (ku) improves the level of fat suppression and conspicuity of very small structures and tissue edges for the on resonance spins (c) in comparison to the case acquired in the same amount of scan time which sampled the same set of radial lines at each echo time (a). Consistency of level of fat signal across the fat image is also improved with the ku trajectory (d), but the local fat signal exhibits less noise in the ks fat image (b).
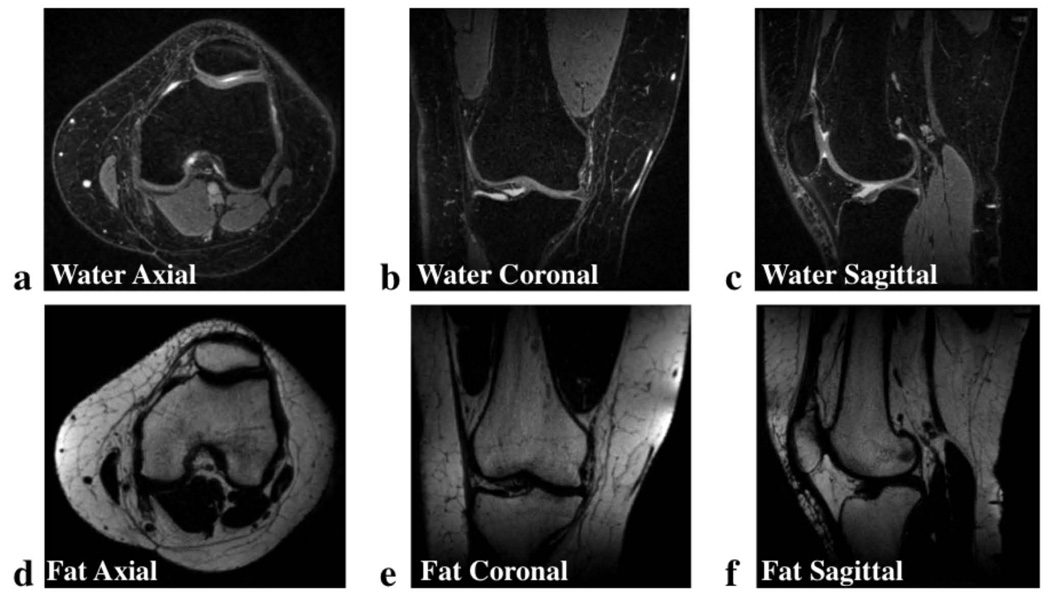
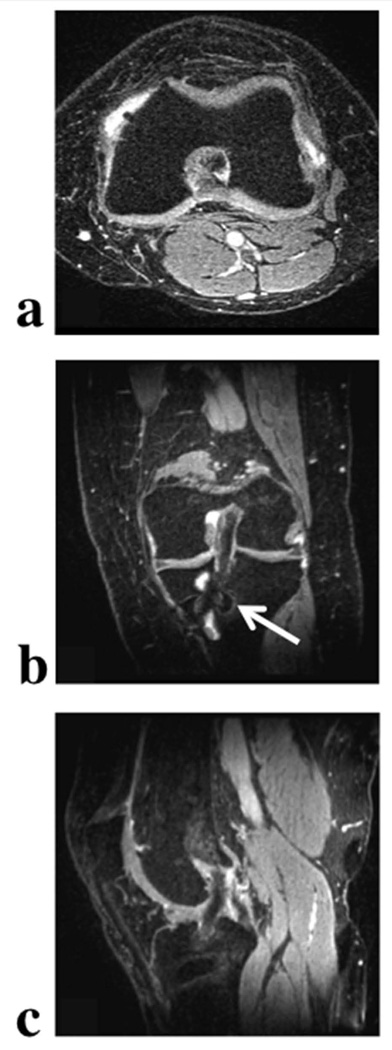
As in the breast, VIPR-IDEAL images of the knee demonstrate effective water-fat separation and clear depiction of fine structures in both the fat and water images. Further, reformats of the water and fat knee images in the axial, sagittal and coronal orientations, demonstrate that this very high image quality is achieved throughout the entire 3D image volume (Figure 8). Similar image quality is also achieved with VIPR-IDEAL in the more challenging case in which the knee contains metallic orthopedic hardware from ACL reconstruction surgery (Figure 9). In the axial, sagittal, and coronal reformats of the image volume, a small amount of fat-water swapping occurs immediately adjacent to the screws, but the artifact is limited to the region of severe B0 inhomogeneity very close to the metallic hardware. Undersampling artifacts from these regions that would propagate the frequency variations to other areas of the image volume are not prominent enough to degrade over all image quality.
Figure 8.
VIPR-IDEAL water (a-c) and fat (d-f) knee images acquired at 1.5 T reformatted in the axial (a,d), coronal (b,e), and sagittal (c,f) planes demonstrate the robust fat-water separation across the entire volume and high-resolution (0.63 mm isotropic) available in the 5 minute scan. Artifact from the undersampling of each echo time is not apparent and the isotropic resolution allows for the clear depiction of structures of interest in any orientation.
Figure 9.
VIPR-IDEAL water axial (a), coronal (b), and sagittal (c) reformats of a knee with ACL reconstruction hardware at 1.5 T. Image volume was acquired in 5 minutes with 0.63 mm isotropic resolution. Fat-water swaps (arrow) are present in regions of severe off-resonance surrounding titanium screw but effective fat-water separation and high isotropic resolution are still achieved throughout the rest of the image volume, demonstrating the robust performance of VIPR-IDEAL even in this challenging B0 environment.
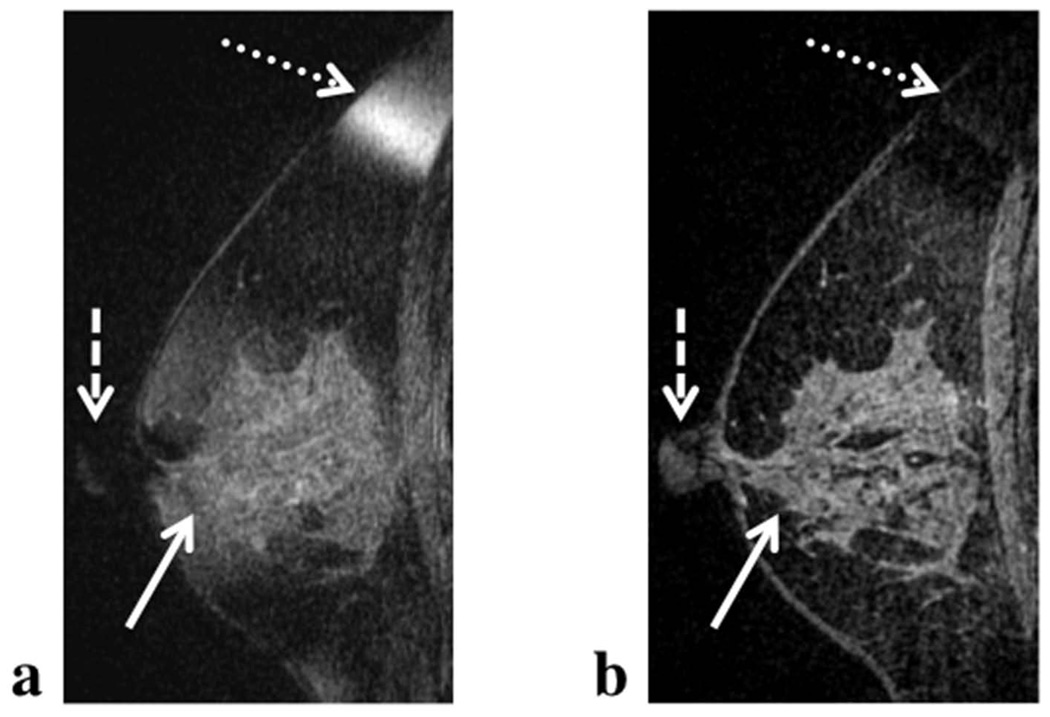
Comparison between VIPR-IDEAL and VIPR-SSFP in the breast (Figure 10), demonstrate equivalent depiction of fine structures and improvement in fat-water separation with VIPR-IDEAL. In the VIPR-SSFP images, regions of B0 inhomogeneity across the breast result in shading, signal drop-out, and water-fat swaps (Figure 10a.). The shading, in particular, which occurs through the central region of the breast, reduces image quality as the clear delineation of fibroglandular tissue from fat is obscured by the variable level of fat suppression. All three of these artifacts are resolved in the VIPR-IDEAL image from the same volunteer (Figure 10b). With VIPR-IDEAL, edges and fine structure of fibroglandular tissue within the breast as well as the nipple are clearly depicted while the signal swap in the superior region of the breast has been resolved.
Discussion
This work aims to provide high isotropic resolution bSSFP images with robust fat-water separation by combining a 3D radial trajectory with IDEAL chemical species separation. The dual-pass, dual half-echo trajectory of VIPR-IDEAL is designed to meet the competing requirements of specific echo timing, high-resolution and short TRs. Furthermore, the sampling of a unique set of undersampled radial lines at each echo time is utilized to reduce the prominence of undersampling artifacts while achieving a clinically feasible scan time. The initial in vivo acquisitions demonstrate the high isotropic resolution available and the excellent tissue contrast in both the breast and the knee that is not greatly affected by 3D radial undersampling artifact.
While no pathology is present in the normal breast volunteer, the clear depiction of fine extensions of tissue indicate that diagnostically significant morphologic details such as lesion margin may be effectively depicted with VIPR-IDEAL. Another consideration for the clinical implementation of VIPR-IDEAL, specifically in the breast, is that image volumes for this investigation were acquired unilaterally in the sagittal plane. However, a bilateral acquisition may be more clinically useful. Bilateral breast acquisitions would add further B0 inhomogeneity challenges, as shimming of both breasts is notoriously difficult and the current spherical trajectory of VIPR-IDEAL would result in more tissue from outside the breasts being excited (32). While all implementations of VIPR-IDEAL to date have utilized an isotropic field of view, the technique is compatible with an anisotropic field of view (33). An elliptical field of view is well-tailored for bilateral breast acquisitions and would greatly increase the feasibility of moving from two unilateral to a single bilateral acquisition.
In comparison to VIPR-SSFP, VIPR-IDEAL demonstrated improved consistency of fat suppression that notably improved image quality in a number of regions in the breast (Figure 10). While the efficacy of IDEAL in regions of large B0 is evident through the successful resolution of fat and water signal at the nipple and superiorly near the chest wall, the improved consistency of fat suppression across the center of the breast may be most diagnostically significant. In this region the conspicuity of fine structures of the fibroglandular tissue are greatly improved. This shading is due to magnitude variations in the frequency response spectrum. VIPR-IDEAL does maintain a single steady state across all four echoes and is therefore also susceptible to magnitude variations, but the variation will not be severe for the expected off resonance in the breast and knee (± 20–40 Hz). Magnitude variation in the VIPR-IDEAL images can be seen at the edge of the fat water phantom (Figure 6) and at the very superior edge of the breast in Figure 7 where off resonance approaches the edge of the bSSFP passband (± 80Hz).
The trajectory of VIPR-IDEAL differs from some previous implementations of radial bSSFP (25, 34) in that a bipolar gradient is utilized to acquire two distinct radial lines per TR. Bipolar gradients present explicit challenges for phase dependent chemical species separation methods as the acquisition order of k-space points is reversed between the two echoes. In 2008, Lu, et al. (35) identified three specific problems introduced by using a Cartesian multi-echo bipolar readout with IDEAL: misalignment of k-space data due to gradient delays and eddy currents, misregistration between echoes due to chemical shift, and misregistration between echoes due to field inhomogeneity. All three of these issues are addressed in VIPR-IDEAL for the current implementation at 1.5 T. Non-Cartesian trajectories often require a means to correct for gradient delay and eddy current induced k-space shifts due to the continually varying readout direction. Therefore a calibration and corrections method for gradient delay and eddy current errors utilized previously with a 3D dual echo radial trajectory (36, 37) was incorporated in VIPR-IDEAL. As with Cartesian bipolar trajectories, misregistration between echoes due to field inhomogenity with VIPR-IDEAL is accounted for by basing field map estimation on low-spatial frequency signal where B0-induced misregistration is substantially blurred out. Finally, noticeable chemical shift artifacts were not expected in this initial implementation of VIPR-IDEAL at 1.5 T as the relatively high receive bandwidth of +/-83.33 kHz and matrix size of 320 resulted in a chemical shift of well under a voxel in the readout direction. However, chemical shift artifacts, would be detrimental in an implementation of VIPR-IDEAL at 3T where only a slight reduction in the receive bandwidth or increase in matrix size would cause chemical shift on the order of a voxel. In 2008, Brodsky, et al. (38) reported the manifestation of chemical shift artifacts in IDEAL with non-Cartesian trajectories and presented a method for the correction of these errors by determining B0 inhomogeneity in image space prior to performing a point-by-point demodulation in k-space based on acquisition time. This type of method will be necessary for future implementations of VIPR-IDEAL at 3T to correct for the variable manifestation of chemical shift artifacts along the dual echo radial trajectory (14).
In our phantom and in vivo experiments, the level of artifact in the VIPR-IDEAL images did not lead to significant degradation of image quality, however, in some instances, the undersampling artifact that remains in the image may still introduce sufficient variation as to be detrimental to image quality. Sampling interleaved radial trajectories at each echo time is based on the assumption that field inhomogeneities are slowly-varying and thus k-space is fully sampled for the portion of the signal data utilized for field map determination within each echo. Although a number of other commonly used imaging techniques have been based on this assumption, a large region where field inhomogeneity varies rapidly may yield radial undersampling artifacts that are more detrimental to image quality. For example, in the presence of metal, severe B0 inhomogeneity can lead to fat-water swaps in the region of inhomogeneity, as is evident in the volunteer with metallic orthopedic hardware from prior ACL reconstruction surgery (Figure 9). In this case, the region of inhomogeneity was small enough and B0 variation was slow enough that the aliased signal did not affect overall image quality. Larger regions or more severe B0 inhomogeneity may lead to significant image degradation, for example a steel implant may be more problematic than the titanium screws in the volunteer scanned in our study. Regions of severe B0 inhomogeneity outside the field of view should also be considered as signal from extraneous tissue excitation may wrap back across tissues of interest. For example susceptibility (39) at the air-tissue interface of the lungs (~9ppm) at 3T could result in over 1 kHz frequency variation and must be considered for further implementation of VIPR-IDEAL in the breast. The noisier appearance of the VIPR-IDEAL breast images (Figure 7) in comparison to the VIPR-IDEAL knee images (Figure 8) may be due in part to off-resonance artifacts wrapping into the breast image from tissue outside the field of view, as well as the lower intrinsic SNR from using a breast coil compared to a dedicated phased-array extremity coil.
The undersampling artifact in VIPR-IDEAL did not significantly affect image quality, however, the residual artifact may prove to be more problematic with quantitative IDEAL imaging. Increasingly, IDEAL is being utilized for tissue parameter mapping such as hepatic fat quantization. A number of techniques are available to refine the VIPR-IDEAL reconstruction so that undersampling artifact may be quantified and removed. Since both water and fat volumes are available with VIPR-IDEAL, one possibility is to retrospectively model streak artifacts from each chemical species image volume. The fat volume could then be utilized to predict the presence of off-resonance undersampling artifacts to be subtracted from the original images, perhaps iteratively, until a specified consistency of signal level is achieved. The very high image quality already achieved with this initial implementation of VIPR-IDEAL indicates that subsequent modifications including incorporation of a multi-peak fat spectrum, chemical shift corrections and further removal of undersampling artifact will likely serve to improve the diagnostic potential of VIPR-IDEAL.
Conclusion
VIPR-IDEAL provides the very high isotropic resolution available with 3D radial techniques along with robust fat-water separation including in challenging B0 environments that are difficult to shim. The dual-pass, dual half-echo 3D radial trajectory accommodates the selection of echo spacing amenable to IDEAL, as well as to the optimal placement of nulls in the response spectrum. The sampling of a unique set of radial lines at each echo time reduces artifact and improves fat suppression for on-resonant spins, and may provide an option to address the redundancy of data collection in this radial mulit-echo technique. In vivo experiments in the breast and knee demonstrate the diagnostic potential of VIPR-IDEAL and warrant further investigation of this technique, in particular with regards to the effect of undersampling artifacts on the depiction of fine morphology and quantitative mapping.
Figure 10.
Fat-water separation and hence depiction of structural detail is compromised by regions of B0 inhomogeneity with the previously described VIPR-SSFP method (a). The B0 inhomogeneity leads to shading across the center of the breast (solid arrow), drop out of signal from the nipple (dashed arrow) and fat-water swap in regions of large inhomogeneity in the superior region of the breast (dotted arrow). VIPR-IDEAL better accounts for B0 inhomogeneity (b) and thus resolves signal in these regions, preventing swaps and signal drop out and improving contrast between fibroglandular tissue and fat-suppressed regions, without loss of resolution or increase in scan time.
Acknowledgements
We thank Brian Hargreaves for his support and useful discussions and Elizabeth Nett for assistance with phantom experiments. We also gratefully acknowledge the support of GE Healthcare and NIH T32 CA009206.
References
- 1.Vasanawala SS, Pauly JM, Nishimura DG. Fluctuating equilibrium MRI. Magn Reson Med. 1999;42(5):876–883. doi: 10.1002/(sici)1522-2594(199911)42:5<876::aid-mrm6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Nayak KS, Lee HL, Hargreaves BA, Hu BS. Wideband SSFP: alternating repetition time balanced steady state free precession with increased band spacing. Magn Reson Med. 2007;58(5):931–938. doi: 10.1002/mrm.21296. [DOI] [PubMed] [Google Scholar]
- 3.Lu A, Brodsky E, Grist TM, Block WF. Rapid fat-suppressed isotropic steady-state free precession imaging using true 3D multiple-half-echo projection reconstruction. Magn Reson Med. 2005;53(3):692–699. doi: 10.1002/mrm.20389. [DOI] [PubMed] [Google Scholar]
- 4.Lu A, Barger AV, Grist TM, Block WF. Improved spectral selectivity and reduced susceptibility in SSFP using a near zero TE undersampled three-dimensional PR sequence. J Magn Reson Imaging. 2004;19(1):117–123. doi: 10.1002/jmri.10435. [DOI] [PubMed] [Google Scholar]
- 5.Vasanawala SS, Pauly JM, Nishimura DG. Linear combination steady-state free precession MRI. Magn Reson Med. 2000;43(1):82–90. doi: 10.1002/(sici)1522-2594(200001)43:1<82::aid-mrm10>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 6.Leupold J, Hennig J, Scheffler K. Alternating repetition time balanced steady state free precession. Magn Reson Med. 2006;55(3):557–565. doi: 10.1002/mrm.20790. [DOI] [PubMed] [Google Scholar]
- 7.Cukur T, Lee JH, Bangerter NK, Hargreaves BA, Nishimura DG. Non-contrast-enhanced flow-independent peripheral MR angiography with balanced SSFP. Magn Reson Med. 2009;61(6):1533–1539. doi: 10.1002/mrm.21921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gold GE, Hargreaves BA, Vasanawala SS, Webb JD, Shimakawa AS, Brittain JH, Beaulieu CF. Articular cartilage of the knee: evaluation with fluctuating equilibrium MR imaging--initial experience in healthy volunteers. Radiology. 2006;238(2):712–718. doi: 10.1148/radiol.2381042183. [DOI] [PubMed] [Google Scholar]
- 9.Lee HL, Shankaranarayanan A, Pohost GM, Nayak KS. Improved coronary MR angiography using wideband steady state free precession at 3 tesla with sub-millimeter resolution. J Magn Reson Imaging. 2010;31(5):1224–1229. doi: 10.1002/jmri.22150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie J, Lai P, Bhat H, Li D. Whole-heart coronary magnetic resonance angiography at 3.0T using short-TR steady-state free precession, vastly undersampled isotropic projection reconstruction. J Magn Reson Imaging. 2010;31(5):1230–1235. doi: 10.1002/jmri.22140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larson AC, Simonetti OP. Real-time cardiac cine imaging with SPIDER: steady-state projection imaging with dynamic echo-train readout. Magn Reson Med. 2001;46(6):1059–1066. doi: 10.1002/mrm.1299. [DOI] [PubMed] [Google Scholar]
- 12.Moran CJ, Kelcz F, Jung Y, Brodsky EK, Fain SB, Block WF. Pilot study of improved lesion characterization in breast MRI using a 3D radial balanced SSFP technique with isotropic resolution and efficient fat-water separation. J Magn Reson Imaging. 2009;30(1):135–144. doi: 10.1002/jmri.21807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kijowski R, Lu A, Block W, Grist T. Evaluation of the articular cartilage of the knee joint with vastly undersampled isotropic projection reconstruction steady-state free precession imaging. J Magn Reson Imaging. 2006;24(1):168–175. doi: 10.1002/jmri.20596. [DOI] [PubMed] [Google Scholar]
- 14.Klaers J, Jashnani Y, Jung Y, Brodsky E, Jacobson J, Kijowski R, Block WF. Dual half-echo phase correction for implementation of 3D radial SSFP at 3.0 T. Magn Reson Med. 2010;63(2):282–289. doi: 10.1002/mrm.22284. [DOI] [PubMed] [Google Scholar]
- 15.Kijowski R, Blankenbaker DG, Klaers JL, Shinki K, De Smet AA, Block WF. Vastly undersampled isotropic projection steady-state free precession imaging of the knee: diagnostic performance compared with conventional MR. Radiology. 2009;251(1):185–194. doi: 10.1148/radiol.2511081133. [DOI] [PubMed] [Google Scholar]
- 16.Reeder SB, Wen Z, Yu H, Pineda AR, Gold GE, Markl M, Pelc NJ. Multicoil Dixon chemical species separation with an iterative least-squares estimation method. Magn Reson Med. 2004;51(1):35–45. doi: 10.1002/mrm.10675. [DOI] [PubMed] [Google Scholar]
- 17.Pineda AR, Reeder SB, Wen Z, Pelc NJ. Cramer-Rao bounds for three-point decomposition of water and fat. Magn Reson Med. 2005;54(3):625–635. doi: 10.1002/mrm.20623. [DOI] [PubMed] [Google Scholar]
- 18.Reeder SB, Pineda AR, Wen Z, Shimakawa A, Yu H, Brittain JH, Gold GE, Beaulieu CH, Pelc NJ. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): application with fast spin-echo imaging. Magn Reson Med. 2005;54(3):636–644. doi: 10.1002/mrm.20624. [DOI] [PubMed] [Google Scholar]
- 19.Reeder SB, McKenzie CA, Pineda AR, Yu H, Shimakawa A, Brau AC, Hargreaves BA, Gold GE, Brittain JH. Water-fat separation with IDEAL gradient-echo imaging. J Magn Reson Imaging. 2007;25(3):644–652. doi: 10.1002/jmri.20831. [DOI] [PubMed] [Google Scholar]
- 20.Gold GE, Reeder SB, Yu H, Kornaat P, Shimakawa AS, Johnson JW, Pelc NJ, Beaulieu CF, Brittain JH. Articular cartilage of the knee: rapid three-dimensional MR imaging at 3.0 T with IDEAL balanced steady-state free precession--initial experience. Radiology. 2006;240(2):546–551. doi: 10.1148/radiol.2402050288. [DOI] [PubMed] [Google Scholar]
- 21.Reeder SB, Pelc NJ, Alley MT, Gold GE. Rapid MR imaging of articular cartilage with steady-state free precession and multipoint fat-water separation. AJR Am J Roentgenol. 2003;180(2):357–362. doi: 10.2214/ajr.180.2.1800357. [DOI] [PubMed] [Google Scholar]
- 22.Reeder SB, Markl M, Yu H, Hellinger JC, Herfkens RJ, Pelc NJ. Cardiac CINE imaging with IDEAL water-fat separation and steady-state free precession. J Magn Reson Imaging. 2005;22(1):44–52. doi: 10.1002/jmri.20327. [DOI] [PubMed] [Google Scholar]
- 23.Madhuranthakam AJ, Yu H, Shimakawa A, Busse RF, Smith MP, Reeder SB, Rofsky NM, Brittain JH, McKenzie CA. T(2)-weighted 3D fast spin echo imaging with water-fat separation in a single acquisition. J Magn Reson Imaging. 2010;32(3):745–751. doi: 10.1002/jmri.22282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z, Graff C, Gmitro AF, Squire SW, Bilgin A, Outwater EK, Altbach MI. Rapid water and lipid imaging with T2 mapping using a radial IDEAL-GRASE technique. Magn Reson Med. 2009;61(6):1415–1424. doi: 10.1002/mrm.21918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flask CA, Dale B, Lewin JS, Duerk JL. Radial alternating TE sequence for faster fat suppression. Magn Reson Med. 2003;50(5):1095–1099. doi: 10.1002/mrm.10615. [DOI] [PubMed] [Google Scholar]
- 26.Barger AV, Block WF, Toropov Y, Grist TM, Mistretta CA. Time-resolved contrast-enhanced imaging with isotropic resolution and broad coverage using an undersampled 3D projection trajectory. Magn Reson Med. 2002;48(2):297–305. doi: 10.1002/mrm.10212. [DOI] [PubMed] [Google Scholar]
- 27.Madhuranthakam AJ, Yu H, Shimakawa A, Smith MP, Reeder SB, Rofsky NM, McKenzie CA, Brittain JH. Joint Annual Meeting ISMRM-ESMRMB. Stockholm, Sweden: 2010. Flexible and Efficient Data Acquisition Technique for 3D-FSE-IDEAL; p. 2906. [Google Scholar]
- 28.Bieri O, Scheffler K. Flow compensation in balanced SSFP sequences. Magn Reson Med. 2005;54(4):901–907. doi: 10.1002/mrm.20619. [DOI] [PubMed] [Google Scholar]
- 29.Bieri O, Markl M, Scheffler K. Analysis and compensation of eddy currents in balanced SSFP. Magn Reson Med. 2005;54(1):129–137. doi: 10.1002/mrm.20527. [DOI] [PubMed] [Google Scholar]
- 30.Glover GH. Multipoint Dixon technique for water and fat proton and susceptibility imaging. J Magn Reson Imaging. 1991;1(5):521–530. doi: 10.1002/jmri.1880010504. [DOI] [PubMed] [Google Scholar]
- 31.Kijowski R, Woods MA, Lee KS, Takimi K, Yu H, Shimakawa A, Brittain JH, Reeder SB. Improved fat suppression using multipeak reconstruction for IDEAL chemical shift fat-water separation: application with fast spin echo imaging. J Magn Reson Imaging. 2009;29(2):436–442. doi: 10.1002/jmri.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henze-Bancroft LC, Moran CJ, Reeder SB, Kelcz F, Block WF. Bilateral Breast Imaging Using IDEAL Fat-Water Separation and an Undersampled 3D Radial bSSFP Acquisition. Montreal, Quebec, Canada: 2011. p. 1009. [Google Scholar]
- 33.Larson PZ, Gurney PT, Nishimura DG. Anisotropic field-of-views in radial imaging. IEEE Trans Med Imaging. 2008;27(1):47–57. doi: 10.1109/TMI.2007.902799. [DOI] [PubMed] [Google Scholar]
- 34.Nayak KS, Nishimura DG. Automatic field map generation and off-resonance correction for projection reconstruction imaging. Magn Reson Med. 2000;43(1):151–154. doi: 10.1002/(sici)1522-2594(200001)43:1<151::aid-mrm19>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 35.Lu W, Yu H, Shimakawa A, Alley M, Reeder SB, Hargreaves BA. Water-fat separation with bipolar multiecho sequences. Magn Reson Med. 2008;60(1):198–209. doi: 10.1002/mrm.21583. [DOI] [PubMed] [Google Scholar]
- 36.Duyn JH, Yang Y, Frank JA, van der Veen JW. Simple correction method for k-space trajectory deviations in MRI. J Magn Reson. 1998;132(1):150–153. doi: 10.1006/jmre.1998.1396. [DOI] [PubMed] [Google Scholar]
- 37.Jung Y, Jashnani Y, Kijowski R, Block WF. Consistent non-cartesian off-axis MRI quality: calibrating and removing multiple sources of demodulation phase errors. Magn Reson Med. 2007;57(1):206–212. doi: 10.1002/mrm.21092. [DOI] [PubMed] [Google Scholar]
- 38.Brodsky EK, Holmes JH, Yu H, Reeder SB. Generalized k-space decomposition with chemical shift correction for non-Cartesian water-fat imaging. Magn Reson Med. 2008;59(5):1151–1164. doi: 10.1002/mrm.21580. [DOI] [PubMed] [Google Scholar]
- 39.Deppe MH, Parra-Robles J, Ajraoui S, Parnell SR, Clemence M, Schulte RF, Wild JM. Susceptibility effects in hyperpolarized (3)He lung MRI at 1.5T and 3T. J Magn Reson Imaging. 2009;30(2):418–423. doi: 10.1002/jmri.21852. [DOI] [PubMed] [Google Scholar]