Abstract
Previous research has revealed a significant bivariate relationship between anabolic androgenic steroid (AAS) use and reduced condom use among adolescent boys. However, to date, no known studies have explored the psychological mechanisms that may explain this relationship. Thus, the current study sought to examine two possible mediators in the association between AAS and condom use—depressive symptoms and substance use. Data were extracted from a nationally representative sample of U.S. adolescents. Participants were 3,780 U.S. high school boys who responded to self-report items assessing a number of health behaviors, including symptoms of depression, substance use, AAS use, and use of condoms during their most recent act of intercourse. Both depression and substance use were significant mediators in the relationship between AAS and condom use. However, when these effects were contrasted, the indirect effect of substance use was significantly stronger in magnitude than the effect of depression. Although AAS use is associated with sexual risk behaviors among adolescent boys, significant variance in this relationship is accounted for by elevated levels of depression and substance use, with substance use demonstrating a particularly salient pathway.
Keywords: condom use, sexual risk, steroids, depression, substance use, adolescence, boys
Adolescents and young adults account for a disproportionate number of new sexually transmitted infections (STIs) each year (Weinstock, Berman, & Cates, 2004). Although STI prevalence is highest among females, a nationally representative sample of more than 6,500 males in grades 7–12 found that 5.3% tested positive for chlamydia, gonorrhea, or trichomonas vaginalis (Ford et al., 2005). Safer sexual practices, such as condom use, are important preventative measures against the spread and transmission of STIs, yet a third of sexually active U.S. high school boys report engaging in unprotected sex(Eaton et al., 2012) and STI prevalence rates continue to increase among this population (CDC, 2011).
The use of condoms during sexual activity occurs within a psychosocial context; thus, to properly illuminate points of prevention intervention, risk factors and pathways to sexual risk must be identified. Although a host of psychosocial variables have been revealed to be associated with condom use (e.g., parental communication, perceived risk, peer norms; see Kaplan et al., 2001) there is relatively scant literature regarding the role of anabolic androgenic steroid (AAS) use.
AAS (e.g., testosterone or synthetically derived testosterone) support protein synthesis and the development of secondary male sex characteristics(Pope & Brower, 2009) and are often initiated to enhance muscularity, athletic performance, and physical appearance (Ip, Barnett, Tenerowicz, & Perry, 2011). Adolescent boys are exposed to various sociocultural pressures to be muscular, including the popular media as well as peer and parental attitudes (Irving, Wall, Neumark-Sztainer, & Story, 2002; Ricciardelli & McCabe, 2004; Smolak, Murnen, & Thompson, 2005). These influences may make AAS particularly attractive to adolescent boys as a means of attaining a more ideal body shape (Labre, 2002). Indeed, rates of AAS use within this population have been found to be up to 5.9% (Eisenberg, Wall, & Neumark– Sztainer, 2012; Irving et al., 2002; Miller, Barnes, Sabo, Melnick & Farrell, 2002b; Miller et al., 2005). Importantly, a bivariate relationship has been established between condom and AAS use in adolescent boys, with AAS users reporting more unprotected intercourse than non-users (Middleman, Faulkner, Woods, Emans, DuRant, 1995; Miller, Barnes, Sabo, Melnick & Farrell, 2002a). Studies have hypothesized that AAS use and unprotected sex fall under a “risk behavior syndrome,” a cluster of related problem behaviors engendered by genetic and environmental influences (Jessor, 1991). Although AAS use and sexual risk behaviors have been found to co-occur, the literature to date has not yet thoroughly examined this association and little is known about its underlying mechanisms.
Depressive symptoms may constitute one potential mechanism between AAS use and unprotected sex. AAS use has been linked with depressive symptoms in adolescent boys (Irving et al., 2002; Smolak et al., 2005). This relationship is likely bidirectional; depressive symptoms may result from AAS use and withdrawal (Kanayama, Hudson, & Pope, 2008), and AAS use has been associated with body image disturbances (Smolak et al., 2005), which have been linked to depression (Cafri, Strauss, & Thompson, 2002; Ricciardelli & McCabe, 2004). Moreover, depressive symptoms in young people have been associated with sexual risk behaviors (Brown et al., 2006; Shrier et al., 2001); specifically, depression was found to inhibit safer sex practices, such as condom use, in a longitudinal study (Lehrer, Shrier, Gortmaker, & Buka, 2006). Depressed adolescents may have a diminished sense of self-efficacy, or beliefs in their own competence to successfully engage in a particular behavior (Bandura, 1986; Ehrenberg, Cox, & Koopman, 1991), and low self-efficacy has been found to negatively impact condom use among adolescents (Sieving et al., 1997). In addition, core depressive symptoms such as hopelessness or worthlessness may promote decreased self-care activities, including sexual health behaviors (Allgower, Wardle, & Steptoe, 2001; Broccoli & Sanchez, 2009; DiMatteo, Lepper, & Croghan, 2000; Katon et al., 2010).
Another possible mechanism in the link between AAS and sexual risk is substance use. Illicit substance use figures as part of a conceptual framework of Jessor’s “problem behavior syndrome” (Jessor, 1991), which includes concurrent risk behaviors. In support of this model, the association between AAS and substance use in adolescent boys has been robustly established, with those using AAS reporting significantly more tobacco, alcohol, marijuana, cocaine, injection drug use, and other illicit drug use compared to those not using AAS (DuRant et al., 1995; Irving et al., 2002;Middleman et al., 1995; Miller et al., 2005; Miller et al., 2002a). Further, substance use has consistently been linked with risky sexual behavior, with heavier substance users reporting a greater number of unprotected sexual encounters (e.g., Bryan, Schmiege, Magnan, 2012; Levy, Sherritt, Gabrielli, Shrier, & Knight, 2009; Parkes, Wight, Henderson, & Hart, 2009). In addition to increasing disinhibition and impairing decision-making, substance use may also pose a logistical barrier to safer sex, as adolescents under the influence of drugs or alcohol report an increased number of unplanned sexual encounters in which neither party has a condom available (Poulin & Graham, 2001).
Unprotected sex places adolescents at risk of receiving and transmitting STIs, a public health concern that persists despite intervention efforts. Given the established relationship between AAS and condom use among adolescents, it is important to discern the possible mechanisms behind this relationship, with aims of informing future intervention development. Although there is abundant research to suggest that substance use and depressive symptoms may feature significantly in this association, no studies to date have explicitly examined these mechanisms. The current study sought to contribute to the literature by empirically examining the role of depression and substance abuse as possible mediators of the relationship between AAS and condom use.
Method
Participants and Procedure
We used data from the 2009 national Youth Risk Behavior Survey (Centers for Disease Control; CDC, 2004; Eaton et al., 2010), a nationally-representative survey assessing the health behaviors among U.S. high school students. The YRBS used a three-stage cluster sample design that yielded a representative sample of 9th through 12th grade students. In 2009, the CDC’s Institutional Review Board (IRB) approved the 98-item YRBS. Student participation in the study was voluntary and anonymous, and included all public, Catholic, and other private school students. A total of 16,410 students responded to the survey (88% return rate), from 158 schools (81% return rate). For the purposes of the current study, only male participants who reported being sexually active were included (N = 3,780). Please refer to CDC (2004) for additional information regarding the methodology of the YRBS.
Measures
AAS use
Lifetime AAS use was assessed with the following item, “During your life, how many times have you taken steroid pills or shots without a doctor’s prescription?” The response options for this item included, “0 times,” “1 or 2 times”, “3 to 9 times,” “10 to 19 times,” “20 to 39 times,” or “40 or more times.” A dichotomous score was created by coding no use as “0” and responses greater than or equal to “1 or 2 times” as “1.”
Depressive symptoms
Symptoms of depression were assessed via four individual items, which included feeling sad or hopeless nearly every day for a two-week period, as well as items assessing suicidal ideation, plans, and attempts over the previous 12 months. All but the latter item (regarding suicide attempts) were responded to via yes/no. The suicide attempts item was dichotomized into “none/1 or more”, and a total depression score was calculated on the sum of the four individual items, resulting in a possible range of scores of 0 to 4, with higher scores denoting increased depressive symptoms. Internal consistency for the current sample was KR-20 = .76.
Substance use
Substance use was evaluated with six items. Three items asked how many days in the last 30 days participants had drank alcohol, consumed five or more alcoholic beverages, and used cocaine. Three additional items assessed lifetime use of methamphetamine, cocaine, and MDMA (i.e., ecstasy). Because questions used different response scales, z-scores were calculated for each item and a composite z-score was created to obtain a global substance use score, with higher scores denoting increased use. Internal consistency for the current sample was α = .88.
Condom use
Condom use was assessed with the following item: “The last time you had sexual intercourse, did you or your partner use a condom?” Responses were coded 1 for “no” and 0 for “yes.”
Statistical analyses
Simultaneous multiple mediation (SMM) was conducted according to the bootstrapping strategy recommended by Preacher and Hayes (2008). SMM allows researchers to determine not only whether an individual variable meets criteria for mediation conditionally on the presence of other variables in the model, but also whether the combination of two or more variables meets criteria for mediation. One can also determine the relative magnitude of the indirect effects, in essence, comparing mediator variables’ unique ability to mediate, above and beyond other mediators in the model (Preacher & Hayes, 2008).
Bootstrapping is a non-parametric statistical approach in which cases from the original data set are randomly re-sampled (n = 2,000) with replacement, to re-estimate the sampling distribution. An indirect effect is considered to be “significant” if zero is not contained between the lower and upper 95% confidence intervals. Bootstrapping is generally preferred over traditional methods of studying mediation (i.e., the Causal Steps Approach and the Product-of-Coefficients Approach; Shrout & Bolger, 2002). One important advantage of this approach is that it does not require variables to conform to normal distributions. In the current study, SMM with bias-corrected 95% confidence intervals (BC 95% CI) was conducted via PROCESS, a statistical program compatible with SPSS (Hayes, 2012).
Results
Descriptive analyses
The demographic characteristics of the sample (N = 3,780) included: 59.2% non-Hispanic white, 13.6% non-Hispanic black, 17.7% Hispanic, and 9.5% other race/ethnicity. There were fewer participants in higher grades than lower (28.6% in 9th grade, 26.3% in 10th grade, 23.1% in 11th grade, and 21.6% in 12th grade), and participant grade was controlled for in the SMM model. Roughly 25% of participants experienced at least some depressive symptoms. Substance use varied by type of substance, with any cocaine use being the lowest (4%) and any alcohol use being the highest (41%). The prevalence of lifetime AAS use was 4.3%, whereas unprotected sex during the last sexual encounter was 12.4% (see Table 1).
Table 1.
Descriptive statistics.
| Variable | M | SD | |
|---|---|---|---|
| Age | 16.1 | 1.2 | |
| Substance Use | .26 | 1.0 | |
| Depressive Symptoms | .55 | 1.0 | |
|
|
|||
| N | % | ||
|
|
|||
| Condom Use | 2510 | 70.4 | |
| AAS Use | 255 | 7.2 | |
Note: Substance use was converted to a z-score. The possible range for depressive symptoms was 0 to 4.
Mediational analyses
Using AAS was associated with an increase of 2 standard deviations in substance use (B= 2.0, SE = .06, t(3776) = 34.3, p < .00001) and a .81 increase in depressive symptoms (on a scale ranging from 0 to 4; B = .81, SE = .07, t(3777) = 12.3, p < .00001). Grade was associated with an increase of .03 standard deviations in substance use (B = .03, SE = .01, t(3776) = 2.0, p = .05, and a .03 decrease in depressive symptoms (B = −.03, SE = .02, t(3777) = −2.0, p = .04.
Depressive symptoms were significantly related to substance use (B = .22, SE = .01, t(3776) = 16.0, p < .00001). Substance use, B = .26, SE .04, z(3778) = 6.4, Odds Ratio = 1.3, p < .00001, depressive symptoms, B = .22, SE = .04, z(3778) = 6.4, Odds Ratio = 1.2, p < .00001, and grade, B= .13, SE = .03, z(3778) = 3.8, Odds Ratio = 1.1, p = .0001, were related to condom use, with one-point increases associated with 30%, 20%, and 10% reductions in condom use, respectively.
The total effect of AAS use on condom use also emerged as significant, B = .77, SE =.13, z(3778) = 5.4, Odds Ratio = 2.2, p < .00001, with AAS nonusers at two times increased odds to use condoms compared to AAS users. While controlling for depressive symptoms, substance use, and grade, the direct effect of AAS use on condom use became non-significant, B = .04, SE = .17, z(3778) = .23, Odds Ratio = 1.3, p = .82.
To quantify the difference between the total and direct effects, the global indirect effect (i.e., the combined mediational effect of substance use and depressive symptoms), was assessed and emerged as significant, B = .75, SE = .10, 95% BC CI [.56, .98]. Both individual indirect effects were also significant: depression, B = .18, SE = .04, 95% BC CI [.12, .27], and substance use, B = .52, SE = .09, 95% BC CI [.35, .71], revealing that depression and substance use independently mediated the association between AAS and condom use. However, when these effects were contrasted, the indirect effect of substance use was significantly stronger in magnitude, Bdep-sub = −.34, SE = .10, 95% BC CI [−.54, −.13].
Discussion
The current study sought to test potential psychological mechanisms accounting for the relationship between AAS and condom use and explored how AAS use is associated with sexual risk among adolescent boys. Results indicated that overall, depressive symptoms and substance use served as significant mediators of this relationship. However, when these two independent pathways were contrasted, substance use exhibited a significantly stronger effect.
Those who engaged in unprotected sex were, in isolation, at two times the increased odds of using AAS. Interestingly, when the effects of depression and substance use were controlled for, this effect became non-significant. However, it would not be expected that AAS use would occur in isolation, given the high levels of psychological distress and substance use that are often associated with this behavior (Irving et al., 2002; Miller et al., 2005; Smolak et al., 2005).
Mediational models revealed that AAS use was negatively correlated with condom use through elevated levels of depression and substance use. In other words, boys who engage in AAS use are more likely to experience symptoms of depression and substance use. It is these factors that, in turn, place boys at risk for engaging in unprotected sex. Perhaps boys who use AAS also have decreased condom self-efficacy and inhibition in the context of elevated depressive symptoms and substance use. When the effects of depression and substance use were contrasted, substance use contributed more significantly to condom use than depression. Thus, although depression is one pathway from AAS to condom use, substance use appears to be a more salient mechanism.
Despite the additions to the literature the current study presents, it is not without limitations. Of note, the assessment of depressive symptoms was limited in measuring the full range of possible symptoms, and may be more reflective of severe depression, given the over-representation of suicide items. The measurement of AAS use is also limited in that it is possible that participants interpreted this item to include non-anabolic/androgenic steroids; however, this concern may be diminished given that the AAS use item was included in the section of the YRBS with other illicit substances (e.g., cocaine, MDMA, heroin, LSD). Although limited depth of assessment is common among large epidemiological datasets, future research would benefit from a more sensitive approach to the measurement of relevant variables. The study was also cross-sectional, which precludes temporal inference. To properly address the question of temporal prediction among the variables of interest, longitudinal designs are needed. For example, it is possible that both depression and substance use have bidirectional effects with AAS use. Although the current study is limited in addressing causality, given the use of a nationally-representative sample, the findings do generalize to the entire population of sexually active adolescent boys in the United States. Further, there are likely additional pathways from AAS to condom use that should be explored, in addition to searching for moderator variables of this relationship. Illuminating protective factors would yield potential areas of targeted intervention.
One variable that may be important for future research to examine is sexual orientation. Gay men report higher levels of body dissatisfaction compared to their heterosexual counterparts (Morrison, Morrison & Sager, 2004) and body dissatisfaction is associated with AAS use (Parent & Moradi, 2011; Ricciardelli & McCabe, 2004). Theoretically, then, AAS use may be more prevalent among gay young men. This is particularly important in the context of the current study’s findings. In the U.S., gay and bisexual men are, by far, the most at risk group for acquiring and transmitting HIV (Prejean et al., 2011). Thus, the role of AAS, substance use, and sexual risk is salient for these individuals.
The results also have the potential to inform clinical interventions. Given that AAS use is associated with substance use, which in turn is highly associated with sexual risk, interventions with the aim of decreasing unprotected sex may potentially be enhanced by addressing co-occuring substance use. Although the role of depression did not have the same magnitude as substance use, it still accounted for unique and significant variance in the model. Thus, perhaps addressing multiple health behaviors—depression, substance use, and AAS use—would be a particularly effective intervention approach. To date there have been several school/group based programs that have targeted multiple health behaviors, such as substance use, sexual risk behaviors, and negative affect (e.g., Flay & Allred, 2003; Flay, Graumlich, Segawa, Burns, & Holliday, 2004); however, to our knowledge, no known individually-based interventions exist which target AAS use, substance use, depression, and sexual risk behaviors.
In summary, adolescent boys who use AAS are more likely to engage in unprotected sex. Two mechanisms of this relationship are elevated levels of depressive symptoms and substance use. Of the two, substance use proved to be of stronger magnitude. Integrative sexual risk reduction interventions addressing AAS use, depression, and substance use may be a particularly effective treatment strategy.
Figure 1.
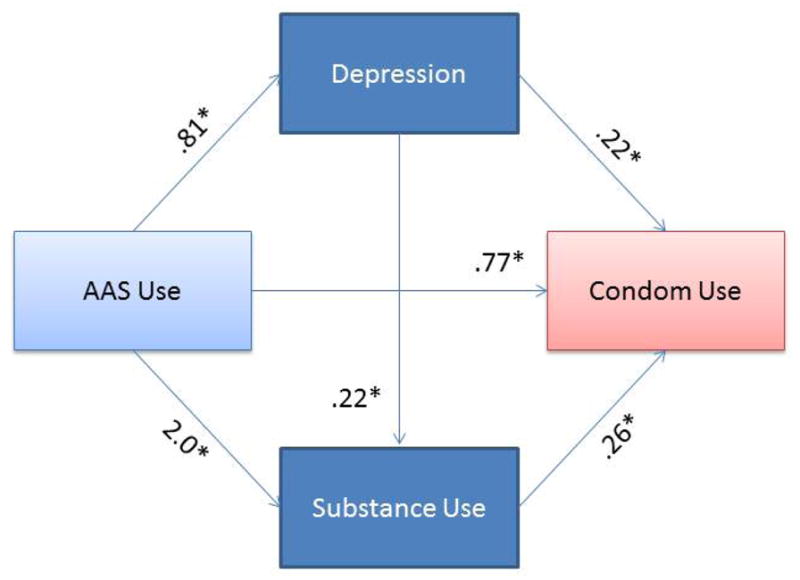
Mediational Model of AAS to Condom Use.
AAS Use = Anabolic androgenic steroid use. Unstandardized regression coefficients. Direct effect of AAS to condom use: B = .04, SE = .17, z(3778) = .23, Odds Ratio = 1.3, p = .82. Global indirect effect: B = .75, SE = .10, 95% BC CI [.56, .98]. Substance use indirect effect: B = .52, SE = .09, 95% BC CI [.35, .71]. Depression indirect effect: B = .18, SE = .04, 95% BC CI [.12, .27]. Contrast of depression-substance use indirect effect: Bdep-sub = −.34, SE = .10, 95% BC CI [−.54, −.13].
Acknowledgments
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number K23MH096647, awarded to AJB and K24MH094214 to SAS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Allgöwer A, Wardle J, Steptoe A. Depressive symptoms, social support, and personal health behaviors in young men and women. Health Psychology. 2001;20:223–227. [PubMed] [Google Scholar]
- Bandura A. Social foundations of thought and action: A social cognitive theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- Broccoli TL, Sanchez DT. Implicit hopelessness and condom use frequency: Exploring nonconscious predictors of sexual risk behavior. Journal of Applied Social Psychology. 2009;39:430–448. [Google Scholar]
- Brown LK, Tolou-Shams M, Lescano C, Houck C, Zeidman J, Pugatch D Project Shield Study Group. Depressive symptoms as a predictor of sexual risk among African American adolescents and young adults. Journal of Adolescent Health. 2006;39:444.e1–444.e8. doi: 10.1016/j.jadohealth.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Bryan AD, Schmiege SJ, Magnan RE. Marijuana use and risky sexual behavior among high-risk adolescents: Trajectories, risk factors, and event-level relationships. Developmental Psychology. 2012;48:1429–1442. doi: 10.1037/a0027547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafri G, Strauss J, Thompson J. Male body image: Satisfaction and its relationship to well-being using the Somatomorphic Matrix. International Journal of Men’s Health. 2002;1:215–231. [Google Scholar]
- Centers for Disease Control and Prevention. Methodology of the Youth Risk Behavior Surveillance System. MMWR. 2004;53:RR-12. [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. STD Surveillance, 2010-STDs in Adolescents and Young Adults. 2011 Retrieved from http://www.cdc.gov/std/stats10/adol.htm#foot2.
- DiMatteo M, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Archives of Internal Medicine. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- DuRant RH, Escobedo LG, Heath GW. Anabolic-steroid use, strength training, and multiple drug use among adolescents in the United States. Pediatrics. 1995;96:23–28. [PubMed] [Google Scholar]
- Eaton DK, Kann L, Kinchen S, Shanklin S, Ross J, Hawkins J, Weschler H. Youth risk behavior surveillance-United States, 2009. MMWR Surveillance Summary. 2010;59:1–142. [PubMed] [Google Scholar]
- Eaton DK, Kann L, Kinchen S, Shanklin S, Flint KH, Hawkins J, Weschler H. Youth risk behavior surveillance-United States, 2011. MMWR Surveillance Summary. 2012;61:1–162. [PubMed] [Google Scholar]
- Ehrenberg MF, Cox DN, Koopman RF. The relationship between self-efficacy and depression in adolescents. Adolescence. 1991;26:361–374. [PubMed] [Google Scholar]
- Eisenberg ME, Wall M, Neumark-Sztainer D. Muscle-enhancing behaviors among adolescent girls and boys. Pediatrics. 2012;130:1019–1026. doi: 10.1542/peds.2012-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flay BR, Allred CG. Long-term effects of the Positive Action program. American Journal of Health Behavior. 2003;27:S6–S21. [PubMed] [Google Scholar]
- Flay BR, Graumlich S, Segawa E, Burns JL, Holliday MY. Effects of 2 prevention programs on high-risk behaviors among African American Youth. Archives of Pediatrics and Adolescent Medicine. 2004;158:377–384. doi: 10.1001/archpedi.158.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CA, Pence B, Miller WC, Resnick MD, Bearinger MS, Pettingell S, Cohen M. Predicting adolescents’ longitudinal risk for sexually transmitted infection: Results from the National Longitudinal Study of Adolescent Health. Archives of Pediatrics &Adolescent Medicine. 2005;159:657–664. doi: 10.1001/archpedi.159.7.657. [DOI] [PubMed] [Google Scholar]
- Hayes AF. PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling. 2012 [White paper]. Retrieved at: http://www.afhayes.com/public/process2012.pdf.
- Ip EJ, Barnett MJ, Tenerowicz MJ, Perry PJ. The Anabolic 500 survey: Characteristics of male users versus nonusers of anabolic-androgenic steroids for strength training. Pharmacotherapy. 2011;31:757–766. doi: 10.1592/phco.31.8.757. [DOI] [PubMed] [Google Scholar]
- Irving LM, Wall M, Neumark-Sztainer D, Story M. Steroid use among adolescents: Findings from Project EAT. Journal of Adolescent Health. 2002;30:243–252. doi: 10.1016/s1054-139x(01)00414-1. [DOI] [PubMed] [Google Scholar]
- Jessor R. Risk behavior in adolescence: A psychosocial framework for understanding and action. Journal of Adolescent Health. 1991;12:597–605. doi: 10.1016/1054-139x(91)90007-k. [DOI] [PubMed] [Google Scholar]
- Kanayama G, Hudson JI, Pope HG., Jr Long-term psychiatric and medical consequences of anabolic-androgenic steroid abuse: A looming public health concern? Drug and alcohol dependence. 2008;98:1–12. doi: 10.1016/j.drugalcdep.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DW, Feinstein RA, Fisher MM, Klein JD, Olmedo LF, Rome ES Committee on Adolescence. Condom use by adolescents. Pediatrics. 2001;107:1463–1469. doi: 10.1542/peds.107.6.1463. [DOI] [PubMed] [Google Scholar]
- Katon W, Richardson L, Russo J, McCarty CA, Rockhill C, McCauley E, Grossman DC. Depressive symptoms in adolescence: The association with multiple health risk behaviors. General Hospital Psychiatry. 2010;32:233–239. doi: 10.1016/j.genhosppsych.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labre MP. Adolescent boys and the muscular male body ideal. Journal of Adolescent Health. 2002;30:233–42. doi: 10.1016/s1054-139x(01)00413-x. [DOI] [PubMed] [Google Scholar]
- Lehrer JA, Shrier LA, Gortmaker S, Buka S. Depressive symptoms as a longitudinal predictor of sexual risk behaviors among US middle and high school students. Pediatrics. 2006;118:189–200. doi: 10.1542/peds.2005-1320. [DOI] [PubMed] [Google Scholar]
- Levy S, Sherritt L, Gabrielli J, Shrier LA, Knight JR. Screening adolescents for substance use-related high-risk sexual behaviors. Journal of Adolescent Health. 2009;45:473–477. doi: 10.1016/j.jadohealth.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison MA, Morrison TG, Sager CL. Does body satisfaction differ between gay men and lesbian women and heterosexual men and women: A meta-analytic review. Body Image. 2004;1:127–138. doi: 10.1016/j.bodyim.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Middleman AB, Faulkner AH, Woods ER, Emans SJ, DuRant RH. High-risk behaviors among high school students in Massachusetts who use anabolic steroids. Pediatrics. 1995;96:268–272. [PubMed] [Google Scholar]
- Miller KE, Barnes GM, Sabo DF, Melnick MJ, Farrell MP. A comparison of health risk behavior in adolescent users of anabolic-androgenic steroids, by gender and athlete status. Sociology of Sport Journal. 2002a;19:385–402. [Google Scholar]
- Miller KE, Barnes GM, Sabo DF, Melnick MJ, Farrell MP. Anabolic-androgenic steroid use and other adolescent problem behaviors: Rethinking the male athlete assumption. Sociological Perspectives. 2002b;45:467–489. [Google Scholar]
- Miller KE, Hoffman JH, Barnes GM, Sabo D, Melnick MJ, Farrell MP. Adolescent anabolic steroid use, gender, physical activity, and other problem behaviors. Substance Use and Misuse. 2005;40:1637–1657. doi: 10.1080/10826080500222727. [DOI] [PubMed] [Google Scholar]
- Parent MC, Moradi B. His biceps become him: An application of objectification theory to understanding drive for muscularity and propensity for steroid use in college men. Journal of Counseling Psychology. 2011;58:246–256. doi: 10.1037/a0021398. [DOI] [PubMed] [Google Scholar]
- Parkes A, Wight D, Henderson M, Hart G. Explaining associations between adolescent substance use and condom use. Journal of Adolescent Health. 2007;40:180.e1–180.e18. doi: 10.1016/j.jadohealth.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Poulin C, Graham L. The association between substance use, unplanned sexual intercourse and other sexual behaviours among adolescent students. Addiction. 2001;96:607–621. doi: 10.1046/j.1360-0443.2001.9646079.x. [DOI] [PubMed] [Google Scholar]
- Pope HG, Brower KJ. Anabolic-Androgenic Steroid-Related Disorders. In: Sadock B, Sadock V, editors. Comprehensive Textbook of Psychiatry. 9. Philadelphia, PA: Lippincott Williams & Wilkins; 2009. pp. 1419–1431. [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavioral Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Prejean J, Song R, Hernandez A, Ziebell R, Green T, Walker F, Hall H. Estimated HIV Incidence in the United States, 2006–2009. PLoS ONE. 2011;6:e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardelli LA, McCabe MP. A biopsychosocial model of disordered eating and the pursuit of muscularity in adolescent boys. Psychological Bulletin. 2004;130:179–205. doi: 10.1037/0033-2909.130.2.179. [DOI] [PubMed] [Google Scholar]
- Shrier LA, Harris SK, Sternberg M, Beardslee WR. Associations of depression, self-esteem, and substance use with sexual risk among adolescents. Preventative Medicine. 2001;33:179–189. doi: 10.1006/pmed.2001.0869. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychological Methods. 2002;7:422–45. [PubMed] [Google Scholar]
- Sieving R, Resnick MD, Bearinger L, Remafedi G, Taylor BA, Harmon B. Cognitive and behavioral predictors of sexually transmitted disease risk behavior among sexually active adolescents. Archives of Pediatrics & Adolescent Medicine. 1997;151:243–251. doi: 10.1001/archpedi.1997.02170400029006. [DOI] [PubMed] [Google Scholar]
- Smolak L, Murnen S, Thompson JK. Sociocultural influences and muscle building in adolescent boys. Psychology of Men and Masculinity. 2005;6:227–239. [Google Scholar]
- Weinstock H, Berman S, Cates W. Sexually transmitted diseases among American youth: Incidence and prevalence estimates, 2000. Perspectives on Sexual and Reproductive Health. 2004;36:6–10. doi: 10.1363/psrh.36.6.04. [DOI] [PubMed] [Google Scholar]


