Summary
Background
Diagnosis of phaeochromocytoma is commonly performed by measurements of plasma free normetanephrine and metanephrine. Plasma deconjugated normetanephrine and metanephrine have been proposed as alternative equivalent, but easier to measure biomarkers.
Objective
The aim of this study was to compare the diagnostic performances of plasma free versus deconjugated normetanephrine and metanephrine in patients tested for phaeochromocytoma.
Methods
The study population included a reference group of 262 normotensive and hypertensive volunteers, 198 patients with phaeochromocytoma and 528 patients initially suspected of having the tumour, but with negative investigations after at least 2 years of follow up. Measurements were performed using liquid chromatography with electrochemical detection.
Results
Median plasma concentrations of free normetanephrine were 17-fold higher in patients with phaeochromocytoma than in the reference population, a 72% larger (p<0.001) difference than that for the 10-fold higher levels of plasma deconjugated normetanephrine. In contrast, relative increases of plasma concentrations of free and deconjugated metanephrine were similar. Using upper cut-offs established in the reference population, measurements of plasma free metabolites provided superior diagnostic performance than deconjugated metabolites according to measures of both sensitivity (97% vs 92%, p=0.002) and specificity (93 vs 89%, p=0.012). The area under the receiver operating characteristic curve for the free metabolites was larger than that for the deconjugated metabolites (0.986 vs 0.965, p<0.001).
Conclusion
Measurements of plasma free normetanephrine and metanephrine are superior to the deconjugated metabolites for diagnosis of phaeochromocytoma.
Keywords: Phaeochromocytoma, diagnostic tests, free metanephrines, deconjugated metanephrines, normetanephrine, metanephrine
Introduction
Phaeochromocytomas are catecholamine-producing tumours arising from chromaffin cells of the adrenal medulla or extra-adrenal paraganglionic tissue and represent rare but important usually curable causes of hypertension (1). Clinical presentation of the tumours can be varied with nonspecific signs and symptoms, rendering the diagnosis crucially dependent on demonstration of excessive production of catecholamines (2–5). An undiagnosed phaeochromocytoma can result in life threatening cardiovascular consequences (6). Thus, maximally sensitive and specific biochemical assays to reliably exclude or confirm phaeochromocytoma are crucial.
Measurements of plasma or urinary metanephrines (metanephrine and normetanephrine), the O-methylated extraneuronal metabolites of catecholamines, provide superior tests for diagnosis of phaeochromocytoma than other tests and are currently recommended for initial screening (7–13). Metanephrines in plasma or urine can be measured as free metabolites or after a deconjugation step involving either acid hydrolysis or enzyme catalyzed conversion of the sulphate conjugated to the free metabolites.
The free fractions of metanephrines are formed in considerable amounts in adrenal medullary chromaffin cells whereas the sulphate-conjugated metabolites are formed from the free amines by the actions of sulphate transferase 1A3 (SULT1A3), an enzyme located primarily in the gastrointestinal tract (14–16). The free metanephrines are cleared rapidly from the circulation so that their concentrations are low (17). In contrast, deconjugated metanephrines are cleared slowly. Therefore, plasma levels of the deconjugated metabolites are 15- to 30-fold higher than the free metabolites and much easier to measure. However, whether determination of the deconjugated metanephrines offers any diagnostic advantage over the free metabolites is not established. Recently Grouzmann et al., reported that plasma free and deconjugated metanephrines are equivalent biomarkers for diagnosing phaeochromocytoma (18).
The aim of the present study was to compare the diagnostic performances of plasma deconjugated with plasma free metanephrines in patients tested for phaeochromocytoma. The analysis took advantage of dataset from a previous cohort study of 1211 subjects (19), in which measurements of both plasma free and deconjugated metabolites were performed in a subset of patients, but in which up until this report there was no comparison of diagnostic performance.
Materials and Methods
Study design and participants
The essential criterion for inclusion of subjects into the analysis was that the same plasma sample analysed for concentrations of free metanephrines was also analysed for deconjugated metanephrines. The study population therefore, consisted of 726 patients tested for phaeochromocytoma. The analysis also included 262 normotensive and hypertensive volunteers included as a reference population. Patients were investigated under multicentre National Institute of Health (NIH) based protocols and all provided informed consent.
In order to avoid biasing the analyses of test performance, the results of biochemical tests were not used to exclude or confirm phaeochromocytoma or for the purposes of selection of patients into the study. Confirmation of phaeochromocytoma required histopathological examination of surgically resected or biopsied tumor tissue or a diagnosis of inoperable malignant disease based on functional imaging evidence of metastatic lesions. Exclusion of phaeochromocytoma required either one or the combination of three criteria: 1. lack of radiological evidence of a tumour by computed tomography or magnetic resonance imaging; 2. pathological examination of a surgically resected or biopsied adrenal mass; and 3. lack of phaeochromocytoma on patient follow-up two or more years after initial testing. Follow-up consisted of clinical evaluation and when indicated, repeated biochemical tests. Using the above criteria, phaeochromocytoma was confirmed in 198 patients and excluded in 528, all of whom were included in the final analyses (Table 1).
Table 1.
Patient Characteristics
| Reference Population | phaeochromocytoma Excluded | phaeochromocytoma Confirmed | |
|---|---|---|---|
| No. of patients | 262 | 528 | 198 |
| Age (mean ±SD) | 41±19 | 41±14 | 40±17 |
| Gender | |||
| Females | 134 | 305 | 96 |
| Males | 128 | 223 | 102 |
Collections of blood samples
Blood samples from subjects were collected using a forearm venous cannula after 30 minutes of supine rest. Subjects were instructed to fast and abstain from caffeinated and decaffeinated beverages overnight and to avoid taking acetaminophen for 5 days before blood sampling. Samples of blood were transferred into heparinised tubes and placed on ice until centrifuged (4°C) to separate plasma, which was stored at −80°C until assayed.
Analyses of metanephrines
Measurements of plasma free and deconjugated normetanephrine and metanephrine concentrations were performed using liquid chromatography with electrochemical detection (LC-ECD) as described elsewhere (20). For determination of plasma deconjugated metanephrines 200 μl samples of plasma were incubated for 30 minutes at 37°C with 0.5 units of sulphatase (Sigma, St. Louis, MO, USA). Samples of enzyme-processed plasma for measurements of deconjugated metanephrines were then subjected to the same solid phase extraction and LC-ECD procedures as samples of plasma free metanephrines.
Data analysis
Statistical analyses were carried out using R Version 2.15.2 (The R Foundation for Statistical Computing, Vienna, Austria). For all differences a p<0.05 was considered significant. Because plasma concentrations of metanephrines were non-normally distributed, results for these parameters were presented as medians with reference intervals established using the 2.5 and 97.5 percentiles of distributions (21). Comparisons of increases median plasma concentrations of free versus deconjugated metanephrines among patients with phaeochromocytoma were assessed with the non-parametric Wilcoxon related sample test. A false negative result in a patient with phaeochromocytoma or a true negative result in a patient without phaeochromocytoma was defined as a value for each measurement in the pair (i.e., normetanephrine and metanephrine) lower than the upper cut-off of reference intervals. A true positive result for pairs of measurements in a patient with phaeochromocytoma or a false positive result in a patient without phaeochromocytoma was defined as a value for either or both measurements higher than the upper cut-off of reference intervals. Sensitivity was calculated from the percentage of true positive over the total of true positive plus false negative test results in patients with phaeochromocytoma. Specificity was calculated from the percentage of true negative over the total of true negative plus false positive test results in patients without phaeochromocytoma. Differences in sensitivity and specificity were examined using the McNemar test. From each parameter, using logistic regression analysis, a receiver operating characteristic (ROC) curve was constructed from the relationship between true positive and false positive results (that is sensitivity compared with 1-specificity) for a diagnosis of phaeochromocytoma, based on different upper reference limits for each variable (22). As summary measures of the diagnostic utility of each test independent of upper reference limits, areas under the ROC curves were calculated and differences among tests examined according to the method of Hanley (23).
Results
Biochemical Results
Median plasma concentrations of deconjugated normetanephrine were increased by 10-fold in patients with phaeochromocytoma relative to the reference population. This increase was 72% lower (p<0.001) than the 17-fold increase of plasma free normetanephrine (Table 2). In contrast, the increase of median plasma concentrations of deconjugated metanephrine in patients with phaeochromocytoma did not differ from that of plasma concentrations of free metanephrine (2.3 vs 2.6-fold increase, p=0.190)
Table 2.
Summary data of the plasma outputs of the various analytes for both groups of patients with and without phaeochromocytoma
| Reference population n=262 |
Patients without phaeochromocytoma n=528 |
Patients with phaeochromocytoma n=198 |
||||
|---|---|---|---|---|---|---|
|
| ||||||
| Median | 2.5–97.5% | Median | Range | Median | Range | |
| Deconjugated NMN (nmol/L) | 8.42 | 3.71–25.37 | 10.76 | 3.31–394.55 | 83.92 | 6.66–2699.68 |
| Free NMN (nmol/L) | 0.26 | 0.11–0.65 | 0.31 | 0.08–1.57 | 4.48 | 0.16–172.30 |
| Deconjugated MN (nmol/L) | 4.24 | 1.38–9.18 | 4.28 | 0.05–184.94 | 9.67 | 0.99–2019.97 |
| Free MN (nmol/L) | 0.15 | 0.05–0.33 | 0.14 | 0.01–0.72 | 0.39 | 0.01–22.20 |
All results are medians with 2.5 and 97.5 percentiles for the reference population and ranges for patients groups tested for phaeochromocytoma.
Diagnostic Efficacy
Using upper cut-offs determined from the 97.5 percentiles of distributions of metabolite concentrations in the reference population (Table 2), plasma concentrations of deconjugated normetanephrine were falsely negative in 29 patients with phaeochromocytoma (Table 3). In contrast, plasma concentrations of free normetanephrine were falsely negative in only 10 patients with the tumour. Measurements of plasma free normetanephrine thus provided higher diagnostic sensitivity than measurements of plasma deconjugated normetanephrine (95% vs 85%, p<0.001). In contrast, respective diagnostic specificities for plasma deconjugated and free normetanephrine were similar (93% vs 95%, p=0.154). Diagnostic sensitivities for plasma concentrations of deconjugated and free metanephrine were also similar (51% vs 54%; p=0.405). However, 32 patients without phaeochromocytoma had elevated plasma concentrations of deconjugated metanephrine compared to only 12 patients with elevated plasma concentrations of free metanephrine. Thus, diagnostic specificity of plasma concentrations of free metanephrine was higher than for deconjugated metanephrine (98% vs 94%, p<0.001). Combinations of measurements of plasma free normetanephrine and metanephrine provided both higher diagnostic sensitivity (97% vs 92%, p=0.002) and specificity (93% vs 89%, p=0.012) than combinations of deconjugated metabolites.
Table 3.
Diagnostic sensitivities and specificities for plasma free and deconjugated normetanephrine (NMN) and metanephrine (MN) measured alone and in combination.
| Upper reference limits | Sensitivities | Specificities |
|---|---|---|
| Deconjugated NMN | 85% (169/198) | 93% (493/528) |
| Free NMN | 95% (188/198) | 95% (503/528) |
| Deconjugated MN | 51% (100/198) | 94% (496/528) |
| Free MN | 54% (107/198) | 98% (516/528) |
| Deconjugated (NMN+MN) | 92% (179/198) | 89% (472/528) |
| Free (NMN+MN) | 97% (193/198) | 93% (493/528) |
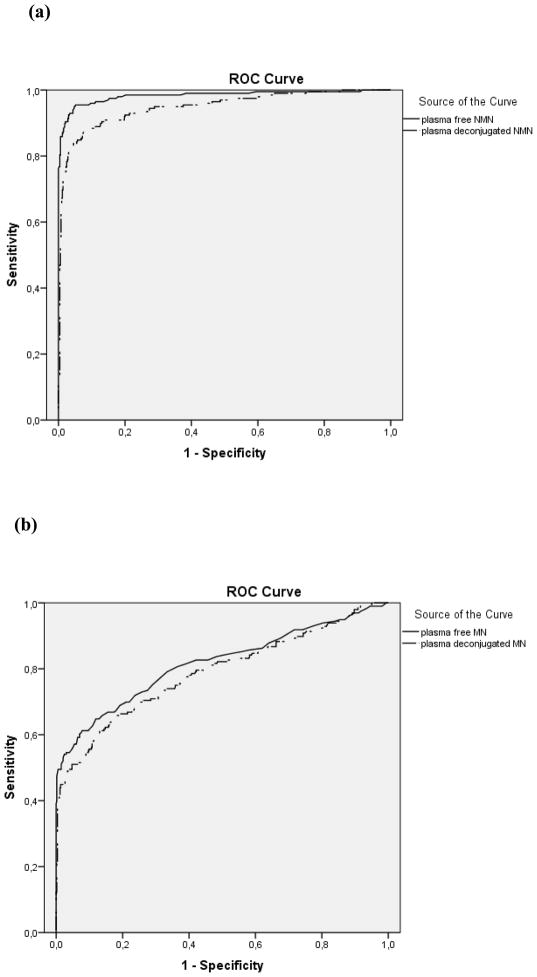
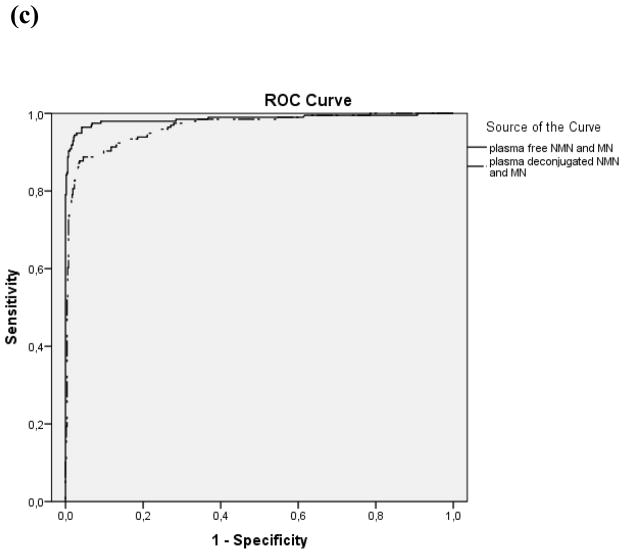
Paired wise comparisons showed that the area under the ROC curve for plasma free normetanephrine (0.983, CI 0.971–0.995), was higher (p<0.001) than that for plasma deconjugated normetanephrine (0.949, CI 0.929–0.969), whereas areas under ROC curves for plasma free metanephrine (0.816, CI 0.775–0.856) and deconjugated metanephrine (0.789, CI 0.746–.0.831) did not differ (Fig. 1). For the combinations of measurements of normetanephrine and metanephrine areas under the curve were higher (p<0.001) for free (0.986, CI 0.974–0.998) than for the deconjugated metabolites (0.965, CI 0.951–0.980).
Figure 1.
Individual comparison of ROC curves using all available data: plasma free normetanephrine (NMN) vs. plasma deconjugated NMN, p<0.001 (a), plasma free metanephrine (MN) vs. plasma deconjugated MN, p=0.188 (b), paired plasma free NMN and MN vs. paired plasma deconjugated NMN and MN p<0.001(c)
Discussion
The present study establishes that measurements of plasma free normetanephrine and metanephrine provide a superior test for diagnosis of phaeochromocytoma than measurements of the deconjugated metabolites. Furthermore our analysis shows that this almost entirely reflects a larger diagnostic signal for free than deconjugated normetanephrine.
The significantly larger diagnostic signal provided by free than by deconjugated normetanephrine, but similar signals for free and deconjugated metanephrine can be explained by different sources of the O-methylated metabolites. Free normetanephrine and metanephrine are formed within chromaffin cells and other extraneuronal tissues by catechol-O-methyltransferase while the sulphate-conjugated metabolites are formed from the free metabolites by the action of a specific sulphotransferase enzyme, SULT1A3, located principally in the gastrointestinal tract (15, 16).
Although sulphated forms of both normetanephrine and metanephrine are formed by the same enzyme, the sources of free metabolite precursors for each of the two sulphate-conjugated products differ. For metanephrine, almost all the conjugated metabolite produced by SULT1A3 is derived from free metanephrine produced in chromaffin cells of the adrenal medulla or tumours derived from these cells. Only a small proportion is derived from locally O-methylated adrenaline and of this almost all represents adrenaline extracted from blood passing through mesenteric organs (15, 24). Thus, the diagnostic signal for free and deconjugated metanephrine differs little in patients with adrenaline-producing phaeochromocytomas.
In contrast to metanephrine, for sulphate-conjugated normetanephrine, substantial amounts are derived from noradrenaline synthesised and released locally within mesenteric organs, these tissues contributing to nearly 50% of all the noradrenaline produced and metabolised within the human body (15, 25). While the free normetanephrine produced from mesenteric sources and entering the portal circulation is almost entirely extracted and metabolised within the liver (ultimately to vanillylmandelic acid), the sulphate conjugates escape hepatic extraction and enter the systemic circulation from where they are removed by the kidneys for excretion in urine (15). Thus, plasma concentrations of sulphate-conjugated normetanephrine reflect disproportionately more mesenteric organ metabolism of noradrenaline compared to free normetanephrine. As a consequence, the substantial mesenteric source of sulphate-conjugated normetanephrine acts to dilute any diagnostic signal compared to free normetanephrine. Since normetanephrine represents the most important metabolite for diagnosis, the overall lower diagnostic accuracy of the combination of deconjugated compared to free normetanephrine and metanephrine can be accounted for by the substantial mesenteric source of sulphate-conjugated normetanephrine from locally-produced noradrenaline.
Higher diagnostic sensitivity and specificity for measurements of free versus deconjugated metanephrines provides improved confidence using free than deconjugated metabolites that a negative result excludes disease and that a positive result confirms disease. This conclusion is at variance with the recent study of Grouzmann et al. (18) in which equivalent diagnostic performance was reported for deconjugated and free metanephrines for the diagnosis of phaeochromocytoma. The discrepancy between our findings and the above study could be partially explained by the differences in approaches in the analysis of data. In our study, upper cut-offs were established from a separate reference population, whereas in the study by Grouzmann et al. cut-offs were established from the same population used for estimation of diagnostic specificity. Furthermore, in our analysis diagnostic sensitivity and specificity were defined according to whether either or both measurements of normetanephrine returned positive or negative results according to the appropriate upper cut-offs of reference intervals. In the study of Grouzmann et al., comparisons of sensitivity and specificity values for the various markers were performed after expressing each value as ratio over its upper reference limit, adding the ratios for normetanephrine and metanephrine and defining cut-off values of 1 or 2 for this sum.
Although plasma concentrations of deconjugated metanephrine are much higher and thus easier to measure than concentrations of the free metabolites, this technical advantage is overridden by the lower diagnostic efficacy of the deconjugated than of the free metabolites. An additional contributing factor to the limited diagnostic utility of the deconjugated metabolites may relate to the different mechanisms in their circulatory clearance and elimination from the body. In particular, as metabolic end-products the sulphate-conjugated metabolites are cleared by renal elimination (25, 26). Therefore, plasma concentrations of the deconjugated metabolites depend on clearance by the kidneys and are consequently increased in patients with renal insufficiency (27). Furthermore, the much slower circulatory clearance of the conjugated than the free metabolites, although resulting in much higher easier to measure concentrations of the former than the latter, also means that procedures for minimising false positive results, such as sampling in the supine position, cannot be reliably used for measurements of plasma deconjugated metabolites (28). Presumably the above factors contribute to the lower diagnostic specificity of measurements of deconjugated than free metanephrines.
In conclusion, the present study establishes that measurements of plasma concentrations of free normetanephrine and metanephrine are superior to the deconjugated metabolites for the diagnosis of phaeochromocytoma. Given the additional technical requirements of a deconjugation step and the lack of commercially available sulphate-conjugated metabolites for both calibration and quality control purposes, we see no overall benefit in measurements of deconjugated over free metanephrines.
Acknowledgments
C.P. is supported under an Erasmus fellowship. This work was supported by the Deutsche Forschungsgesellschaft (EI855/1-1).
Footnotes
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- 1.Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–675. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 2.Bravo EL. Evolving concepts in the pathophysiology, diagnosis, and treatment of pheochromocytoma. Endocrine Reviews. 1994;15:356–368. doi: 10.1210/edrv-15-3-356. [DOI] [PubMed] [Google Scholar]
- 3.Manger WM, Gifford RW., Jr Pheochromocytoma: current diagnosis and management. Cleveland Clinic Journal of Medicine. 1993;60:365–378. doi: 10.3949/ccjm.60.5.365. [DOI] [PubMed] [Google Scholar]
- 4.Mannelli M. Diagnostic problems in pheochromocytoma. Journal of Endocrinological Investigation. 1989;12:739–757. doi: 10.1007/BF03350050. [DOI] [PubMed] [Google Scholar]
- 5.Bravo EL, Tarazi RC, Gifford RW, Stewart BH. Circulating and urinary catecholamines in pheochromocytoma. New England Journal of Medicine. 1979;301:682–686. doi: 10.1056/NEJM197909273011302. [DOI] [PubMed] [Google Scholar]
- 6.Prejbisz A, Lenders JW, Eisenhofer G, Januszewicz A. Cardiovascular Manifestations of Phaeochromocytoma. Journal of Hypertension. 2011;29:2049–2060. doi: 10.1097/HJH.0b013e32834a4ce9. [DOI] [PubMed] [Google Scholar]
- 7.Lenders JW, Pacak K, Walther MM, Linehan WM, Mannelli M, Friberg P, Kreiser HR, Goldstein DS, Eisenhofer G. Biochemical diagnosis of pheochromocytoma: which test is best? Journal of the American Medical Association. 2002;287:1427–1434. doi: 10.1001/jama.287.11.1427. [DOI] [PubMed] [Google Scholar]
- 8.Pacak K, Linehan WM, Eisenhofer G, Walther MM, Goldstein DS. Recent advances in genetics, diagnosis, localization, and treatment of pheochromocytoma. Annals of Internal Medicine. 2001;134:315–329. doi: 10.7326/0003-4819-134-4-200102200-00016. [DOI] [PubMed] [Google Scholar]
- 9.Mundschenk J, Lehnert H. Malignant pheochromocytoma. Experimental and Clinical Endocrinology and Diabetes. 1998;106:373–376. doi: 10.1055/s-0029-1212001. [DOI] [PubMed] [Google Scholar]
- 10.Raber W, Raffesberg W, Bischof M, Scheuba C, Niederle B, Gasic S, Waldhäusl W, Roden M. Diagnostic efficacy of unconjugated plasma metanephrines for the detection of pheochromocytoma. Archives of Internal Medicine. 2000;160:2957–2963. doi: 10.1001/archinte.160.19.2957. [DOI] [PubMed] [Google Scholar]
- 11.Perry CG, Sawka AM, Singh R, Thabane L, Bajnarek J, Young WF., Jr The diagnostic efficacy of urinary fractionated metanephrines measured by tandem mass spectrometry in detection of pheochromocytoma. Clinical Endocrinology. 2007;66:703–708. doi: 10.1111/j.1365-2265.2007.02805.x. [DOI] [PubMed] [Google Scholar]
- 12.Václavík J, Stejskal D, Lacnák B, Lazárová M, Jedelský L, Kadalová L, Janosová M, Frysák Z, Vlce P. Free plasma metanephrines as a screening test for pheochromocytoma in low-risk patients. Journal of Hypertension. 2007;25:1427–1431. doi: 10.1097/HJH.0b013e32813aeb5a. [DOI] [PubMed] [Google Scholar]
- 13.Sawka AM, Jaeschke R, Singh RJ, Young WF., Jr A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. Journal of Clinical Endocrinology & Metabolism. 2003;88:553–558. doi: 10.1210/jc.2002-021251. [DOI] [PubMed] [Google Scholar]
- 14.Dajani R, Cleasby A, Neu M, Wonacott AJ, Jhoti H, Hood AM, Modi S, Hersey A, Taskinen J, Cooke RM, Manchee GR, Goughtrie MW. X-ray crystal structure of human dopamine sulfotransferase, SULT1A3. Molecular modeling and quantitative structure-activity relationship analysis demonstrate a molecular basis for sulfotransferase substrate specificity. Journal of Biological Chemistry. 1999;274:37862–37868. doi: 10.1074/jbc.274.53.37862. [DOI] [PubMed] [Google Scholar]
- 15.Eisenhofer G, Aneman A, Hooper D, Rundqvist B, Friberg P. Mesenteric organ production, hepatic metabolism, and renal elimination of norepinephrine and its metabolites in humans. Journal of Neurochemistry. 1996;66:1565–1573. doi: 10.1046/j.1471-4159.1996.66041565.x. [DOI] [PubMed] [Google Scholar]
- 16.Eisenhofer G, Coughtrie MW, Goldstein DS. Dopamine sulphate: an enigma resolved. Clinical and Experimental Pharmacology & Physiology. 1999;26:41–53. [PubMed] [Google Scholar]
- 17.Eisenhofer G, Lenders J. Rapid circulatory clearances and half-lives of plasma free metanephrines. Clinical Endocrinology. 2012;77:484–485. doi: 10.1111/j.1365-2265.2012.04340.x. [DOI] [PubMed] [Google Scholar]
- 18.Grouzmann E, Drouard-Troalen L, Baudin E, Plouin PF, Muller B, Grand D, Buclin T. Diagnostic accuracy of free and total metanephrines in plasma and fractionated metanephrines in urine of patients with pheochromocytoma. European Journal of Endocrinology. 2010;162:951–960. doi: 10.1530/EJE-09-0996. [DOI] [PubMed] [Google Scholar]
- 19.Eisenhofer G, Pacak K, Huynh TT, Qin N, Bratslavsky G, Linehan WM, Mannelli M, Friberg P, Grebe SK, Timmers HJ, Bornstein SR, Lenders JW. Catecholamine metabolomic and secretory phenotypes in phaeochromocytoma. Endocrine Related Cancer. 2010;18:97–111. doi: 10.1677/ERC-10-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenhofer G, Goldstein DS, Stull R, Keiser HR, Sunderland T, Murphy DL, Kopin IJ. Simultaneous liquid-chromatographic determination of 3,4-dihydroxyphenylglycol, catecholamines, and 3,4-dihydroxyphenylalanine in plasma, and their responses to inhibition of monoamine oxidase. Clinical Chemistry. 1986;32:2030–2033. [PubMed] [Google Scholar]
- 21.Ceriotti F, Hinzmann R, Panteghini M. Reference intervals: the way forward. Annals of Clinical Biochemistry. 2009;46:8–17. doi: 10.1258/acb.2008.008170. [DOI] [PubMed] [Google Scholar]
- 22.Beck JR, Shultz EK. The use of relative operating characteristic (ROC) curves in test performance evaluation. Archives of Pathology & Laboratory Medicine. 1986;110:13–20. [PubMed] [Google Scholar]
- 23.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 24.de Jong WH, Eisenhofer G, Post WJ, Muskiet FA, de Vries EG, Kema IP. Dietary influences on plasma and urinary metanephrines: implications for diagnosis of catecholamine-producing tumors. Journal of Clinical Endocrinology & Metabolism. 2009;94:2841–2849. doi: 10.1210/jc.2009-0303. [DOI] [PubMed] [Google Scholar]
- 25.Eisenhofer G, Rundquist B, Aneman A, Friberg P, Dakak N, Kopin IJ, Jacobs MC, Lenders JW. Regional release and removal of catecholamines and extraneuronal metabolism to metanephrines. Journal of Clinical Endocrinology & Metabolism. 1995;80:3009–3017. doi: 10.1210/jcem.80.10.7559889. [DOI] [PubMed] [Google Scholar]
- 26.Eisenhofer G, Huynh TT, Hiroi M, Pacak K. Understanding catecholamine metabolism as a guide to the biochemical diagnosis of pheochromocytoma. Reviews in Endocrine & Metabolic Disorders. 2001;2:297–311. doi: 10.1023/a:1011572617314. [DOI] [PubMed] [Google Scholar]
- 27.Eisenhofer G, Huysmans F, Pacak K, Walther MM, Sweep FC, Lenders JW. Plasma metanephrines in renal failure. Kidney International. 2005;67:668–677. doi: 10.1111/j.1523-1755.2005.67123.x. [DOI] [PubMed] [Google Scholar]
- 28.Deutschbein T, Unger N, Jaeger A, Broecker-Preuss M, Mann K, Petersenn S. Influence of various confounding variables and storage conditions on metanephrine and normetanephrine levels in plasma. Clinical Endocrinology. 2010;73:153–160. doi: 10.1111/j.1365-2265.2009.03761.x. [DOI] [PubMed] [Google Scholar]