Abstract
The focal adhesion kinase inhibitor, PF-562,271, is currently in clinical development for cancer, however it is not known how PF-562,271 affects T cell function. Here, we demonstrate inhibitory effects of PF-562,271 on the activation of primary human and mouse T cells. PF-562,271 inhibits T cell receptor signaling-induced T cell adhesion to intercellular adhesion molecule-1 and T cell interactions with antigen-presenting cells. An additional focal adhesion kinase inhibitor, PF-573,228, and genetic depletion of focal adhesion kinase also impair T cell conjugation with antigen-presenting cells. PF-562,271 blocks phosphorylation of the signaling molecules zeta chain associate protein of 70 kDa, linker of activated T cells, and extracellular signal-regulated kinase, and impairs T cell proliferation. The effects observed on T cell proliferation cannot solely be attributed to focal adhesion kinase inhibition, as genetic depletion did not alter proliferation. The effect of PF-562,271 on T cell proliferation is not rescued when proximal T cell receptor signaling is bypassed by stimulation with phorbol-12-myristate-13-acetate and ionomycin. Taken together, our findings demonstrate that focal adhesion kinase regulates integrin-mediated T cell adhesion following T cell receptor activation. Moreover, our findings suggest that PF-562,271 may have immunomodulatory effects that could impact its therapeutic applications.
Keywords: T cell receptor; integrin; focal adhesion kinase; RhoA; PF-562,271
1. Introduction
Focal adhesion kinase (FAK) plays a central role in cancer cell adhesion, migration, and cell cycle progression and represents an intriguing anti-cancer target (reviewed in [1]). Previous studies have demonstrated that FAK expression is amplified in human tumors, including breast cancer, and mammary specific deletion of FAK impairs tumor progression and metastasis in mouse models [2,3]. Therefore, there has been substantial interest in developing agents that block FAK signaling. At least two FAK inhibitors are currently in clinical trials for treatment of pancreatic, head and neck, and prostate cancers, including PF-562,271 [4]. PF-562,271 (Figure 1A) is an inhibitor of FAK and Pyk2, with a 4-fold increase in sensitivity for FAK over Pyk2 in cultured cells [5]. Animal studies with this compound show dose-dependent decreases in tumor volume for prostate, breast, pancreatic, colon, glioblastoma, lung, bone and hepatic tumors [5-8]. This compound has reached Phase II clinical trials and has shown promising effects for several tumor types with few adverse effects [9,10]. However, it is not known whether or not FAK inhibitors such as PF-562,271 affect T cell function.
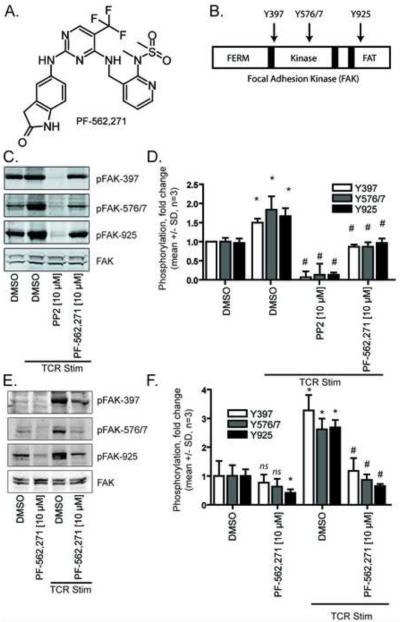
FIGURE 1.
PF-562,271 inhibits site-specific phosphorylation of FAK in human peripheral blood T cells A) Chemical structure of the FAK inhibitor PF-562,271. B) Domain architecture of FAK (FERM = 4.1 protein, ezrin, radixin and moesin, FAT = focal adhesion targeting). C) Western Blot analysis of FAK phosphorylation relative to total FAK in the presence or absence of 10 μM test compounds. Blots are representative of three independent experiments. D) Quantification of FAK phosphorylation (mean +/− SD, n=3, p < 0.05 relative to DMSO control (*) or relative to TCR control (#), ANOVA). E) Western Blot analysis of FAK phosphorylation relative to total FAK in the presence or absence of 10 μM PF-562,271. Blots are representative of three independent experiments. F) Quantification of FAK phosphorylation (mean +/− SD, n=3, p < 0.05 relative to DMSO control (*) or relative to TCR control (#), ANOVA).
T cells express both FAK and its homolog Pyk2, which are tyrosine phosphorylated in response to chemokines [11], signaling from integrins (including lymphocyte function-associated antigen 1 (LFA-1, αLβ2) [12], and ligation of the T cell receptor (TCR) [13,14]. Pyk2 knockout mice [15] are viable and fertile and demonstrate impaired macrophage [16] and CD8+ T cell function [17]. CD8+ T cells from Pyk2-deficient mice demonstrate a defect in LFA-1 mediated adhesion to intercellular adhesion molecule 1 (ICAM-1) and a decrease in effector but not memory T cell responses [17]. There is evidence that at least some patients with Systematic Lupus Erythematosus have increased Pyk2 expression and phosphorylation in circulating peripheral blood mononuclear cells [18]. However, because FAK knockout mice are not viable, studies on the functions of FAK in primary T cells have lagged those of its homolog.
Here, we sought to determine how the clinical FAK inhibitor PF-562,271 modulates T cell function. We demonstrate that PF-562,271 impairs primary CD4+ T cell activation by affecting both proximal and distal TCR signaling and disrupts interactions between T cells and antigen presenting cells. Impaired T cell conjugation was also seen with a second FAK inhibitor. To further validate FAK as a drug target, we generated a conditional FAK knock out mouse, which lacks FAK in CD4+ T cells, and used it to confirm that FAK depletion impairs T cell conjugation. We also show that PF-562,271 inhibits T cell proliferation, against which it is surprisingly more potent than the pan-Src kinase inhibitor PP2. However, in contrast to PP2, the effect of PF-562,271 on T cell proliferation is not rescued when proximal T cell receptor signaling is bypassed by stimulation with phorbol-12-myristate-13-acetate and ionomycin. These findings suggest that FAK inhibition may alter T cell conjugation, activation, and proliferation with potential implications for the treatment of autoimmune disease.
2. Materials and Methods
2.1. Reagents and Supplies
Lymphoprep, RPMI-1640, Fetal Bovine Serum, β-mercaptoethanol, Phosphate Buffered Saline, CellQuantiBlue, Triton X-100, PMSF, leupeptin, aprotinin, BD Optilux 384-well tissue culture treated plates, and Greiner 96-well high protein binding capacity plates were obtained from Thermo Fisher Scientific (Waltham, MA). PP2, bovine serum albumin, PKH-26, PMA, ionomycin, phytohemagglutinin, phosphatase inhibitor cocktail, protease inhibitor cocktail, and goat anti-mouse F(Ab)2 were obtained from Sigma-Aldrich (Saint Louis, MO). p-zap70-Y319, p-LAT-Y191, total ZAP-70, total LAT, p-FAK-Y397, p-FAK-Y576/7, p-FAK-Y925, total FAK, RhoA, Rac1/2/3, and tubulin antibodies were obtained from Cell Signaling (Danvers, MA). Calcein acetoxymethyl ester, carboxyfluorescein diacetate, succinimidyl ester, CD3/CD28-coated Dynabeads, and Alexa-Fluor 680 goat-anti-mouse IgG were obtained from Life Technologies (Carlsbad, CA). Biotinylated CD3 antibody (2C11) and anti-CD4 antibody were obtained from eBioscience (San Diego, CA). LB27.4 cells, CHO-ICAM-1 cells, and OKT3 hybridoma cells were obtained from ATCC (Manassas, VA). PF-562,271 was obtained from Symansis (Timaru, New Zealand). PF-573,228 was obtained from Tocris Bioscience (Bristol, United Kingdom). Interleukin 2 (IL-2) was obtained from (Chiron, Emeryville, CA). OVA peptide was obtained from AnaSpec (Fremont, CA). Streptavidin was obtained from New England Biolabs (Ipswich, MA). Agarose-conjugated GST-RalGDS-RBD, GST-Rhotekin-RBD, and PAK-1 PBD were obtained from Millipore (Billerica, MA). Anti-Rap1 was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). IRDye 800CW goat-anti-rabbit IgG was obtained from Rockland Immunochemicals, (Gilbertsville, PA). Protein G Sepharose was obtained from GE healthcare (Little Chalfont, United Kingdom).
2.2 T cell isolation and culture
CD4+ Human peripheral blood T cells were obtained from whole blood [19] using Lymphoprep and resuspended in fresh T cell media (RPMI-1640, 10% heat-inactivated FBS, 1x HEPES, pyruvate, non-essential amino acids, β-mercaptoethanol). Peripheral blood mononuclear cells were stimulated with phytohemagglutinin and expanded in the presence of interleukin 2 (IL-2) (50 U/mL) for 5-10 days. Floxed FAK mice [20] were crossed with C57BL/6 mice expressing Cre-recombinase driven by a CD4 promoter and the ovalbumin (OVA) peptide specific transgenic TCR OTII [21] and housed at the University of Wisconsin in accordance with IACUC. Single cell suspensions were made from lymph node and spleen, were stimulated with OVA peptide, and maintained in culture. LB27.4 cells were maintained in T cell media.
2.3. Generation of ICAM-1-coated plates
ICAM-1-Fc was purified from CHO-ICAM-1 cells by batch centrifugation with protein G sepharose according to manufacturer’s protocol. For imaging experiments, BD Optilux 384-well tissue culture treated plates were coated by addition of ICAM-1 (20 μL to each well) at 2.5 μg/mL in coating buffer (Tris pH 9.5). Plates were next incubated for 1 hour at 37° C. After the coating step, the plates were washed and blocked by addition of 50 μl of 2% BSA in 1x PBS for 1 hour. For adhesion experiments, 96-well high protein binding capacity plates were used.
2.4. Quantification of adhesion
The monoclonal antibody OKT3 was purified from a hybridoma line by batch centrifugation with protein G sepharose according to manufacturer’s protocol. Cells were suspended at 1 million/mL and labeled by addition of 1 μM calcein acetoxymethyl ester for 15 minutes [22]. Cells were collected by centrifugation and washed once with media. Cells were plated and centrifuged at 500g for 5 minutes, then incubated for 30 minutes at 37° C in the presence of inhibitors. For human cells, 1 μg/mL OKT3, a CD3 antibody known to stimulate TCR signaling [23], was added for 10 minutes. For mouse cells, cells were pre-coated for 15 minutes with 1 μg/mL of biotinylated CD3 antibody and stimulation was initiated by addition of 1 μg/mL streptavidin immediately before plating, followed by a 25 minute incubation. Cells were aspirated and washed 3-6 times with culture media. Adherent cells were quantified by plate reading and percent adhesion was determined as a ratio of remaining fluorescence intensity over baseline.
2.5. Live-cell imaging-based migration assay
50 μL of human peripheral blood T cells at 1 × 106/mL (following 5-14 days of expansion) were added to each well of a 384-well plate [24,25]. Cells were plated and centrifuged at 500g for 5 minutes, then incubated for 5 minutes at 37° C. Cells were washed twice with media and test compounds were added. The T cell stop signal was induced with soluble OKT3 (1 μg/mL) for 10 minutes after plating and washing the cells. Migration was monitored using a Nikon microscope. Twenty-one images were acquired under 10x magnification over a period of 15 minutes.
2.6. Cell-based conjugation assay
Conjugation assays were performed as described previously [26]. Briefly, OTII+ T cells at day 7-10 post-activation were stained with 0.5 μg/mL calcein acetoxymethyl ester and re-suspended in Hanks Buffered Salt Solution supplemented with 2 mg/mL bovine serum albumin and 1x HEPES. Cells were pretreated with 40 μM of test compound or DMSO control for 30 minutes before combining with equal numbers of LB27.4 cells labeled with 2.5 μM PKH-26 in the presence or absence of 2.5 μg/mL OVA peptide. Cells were pelleted by centrifugation at 960g for 3 minutes at 4° C and incubate at 37° C for 10 minutes. Non-specific conjugates were dissociated by vortexing and the percentage of OVA-dependent conjugation was determined by the percentage of double positive cells divided by the sum of the percentage of double and single positive cells of the OVA-treated cells minus the untreated cells.
2.7. T cell proliferation assay
To assess human T cell proliferation, T cells were obtained as described above. On day 6-20, 5000 cells/well were added to 384-well plates in 50 μL volume. Cells were stimulated by addition of an equivalent number of CD3/CD28-coated Dynabeads for 96 hours. Where described, cells were stimulated with a mixture of PMA (10 ng/mL) and ionomycin (1 μM). During the last two hours, cells were labeled with 5 μL of CellQuantiBlue reagent, and were scanned with a Victor plate reader. To assess mouse T cell proliferation, rested OTII+CD4+ T cells were stained with 0.25 μM carboxyfluorescein diacetate, succinimidyl ester according to manufacturer’s directions. Cells were left unstimulated or stimulated with anti-CD3/CD28 coated beads at a ratio of 1 bead per cell or 5 ng/mL PMA and 0.5 μg/mL ionomycin. Additionally, CD4+ T cells were stimulated with irradiated splenocytes (3000 gy) loaded with 0 or 2.5 μg/mL OVA peptide. Cells were treated with 0.2, 1 or 5 μM PP2, PF-562,271 or DMSO control through the 72 hour stimulation. Following activation, cells were stained with anti-CD4 and dye dilution in CD4+ T cells analyzed using a FACS Caliber. Histograms were produced using FlowJo software and the percentage of cells in each division was determined using ModFit 3.2.1.
2.8. Western Blot Analysis
T cells (Day 5-10) at 2 × 107/mL in 0.5-1 mL media were incubated with compounds for 30 minutes [27]. For zeta chain associate protein of 70 kDa (ZAP-70) and linker of activated T cells (LAT) analysis, cells were placed on ice for 5 minutes then coated with 1 μg/mL OKT3 on ice for 20 minutes. Cells were suspended in 100 μL of media containing goat anti-mouse F(Ab)2 at 37° C for 3 minutes. Cells were lysed with 500 μL ice cold lysing buffer (25 mM Tris·HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS). Lysing buffer contained freshly added phosphatase inhibitor cocktail (1:100 dilution) and protease inhibitor cocktail (1:100 dilution). Proteins were resolved by SDS-PAGE on 10% gels, transferred to nitrocellulose, and blotted with p-zap70-Y319, p-LAT-Y191, total ZAP-70 and/or total LAT.
For FAK analysis, cells that had been pre-treated with inhibitors were loaded onto 6 well plates that had been pre-coated with 2 μg/mL OKT3 for 1 hour and pre-blocked with 2% BSA for 1 hour. Cells were centrifuged at 500 rpm for 3 minutes and incubated at 37° C for 7 minutes. Floating cells were gently removed, pelleted, and placed on ice, while adherent cells were lysed with ice-cold lysing buffer containing protease and phosphatase inhibitors. Fractions were combined, and separated by SDS-PAGE on 7.5% gels, transferred to nitrocellulose, and blotted with p-FAK-Y397, p-FAK-Y576/7, p-FAK-Y925, or total FAK antibodies. Detection was performed using Alexa-Fluor 680 goat-anti-mouse IgG and IRDye 800CW goat-anti-rabbit IgG
2.9. Small GTPase activation assays.
T cells (2-4 × 107/mL) were suspended in PBS, divided into 1 mL aliquots, and stimulated as described above. Cells were lysed in 500 μL Rapl lysis buffer (1% Triton X-100; 50 mM Tris-HCl, pH 7.5; 100 mM NaCl; 10 mM MgCl2; 1 mM PMSF; 1 mM leupeptin; 0.5 mM aprotinin) [28]. Lysates were cleared by centrifugation (16000 RPM for 10 minutes) and incubated with either agarose-conjugated GST-RalGDS-RBD, GST-Rhotekin-RBD, or PAK-1 PBD agarose for 45 minutes at 4° C with rotation. Beads were washed three times with lysis buffer and subjected to Western Blot analysis with either anti-Rap1, anti-RhoA, or anti Rac1/2/3. 30 μL of lysate was reserved to use as a loading control. Western blot intensities were normalized to the mean value of all the lanes in each individual experiment, then data from three or more independent experiments was combined and analyzed.
3. Results
3.1. PF-562,271 inhibits TCR-induced FAK phosphorylation
FAK is phosphorylated in T cells following TCR engagement or integrin ligation [12,29]. In fibroblasts, integrin engagement induces autophosphorylation of FAK Y397 providing binding sites for Src family kinases and the subsequent phosphorylation of distal sites [30]. However, it has not been fully determined how TCR stimulation induces FAK phosphorylation [29]. We used site specific phospho-antibodies to characterize the effects of PF-562,271 on FAK phosphorylation in T cells following TCR stimulation (Figure 1B-D).
To test the effects of PF-562,271 on FAK phosphorylation in T cells, we pretreated T cells with PF-562,271, the vehicle DMSO as a negative control, or the pan-Src inhibitor PP2 to inhibit proximal TCR signaling (Figure 1C/D). We found that TCR stimulation resulted in an approximately 1.5 fold increase in Y397 autophosphorylation of FAK relative to control unstimulated T cells. Consistent with previous reports [29,31], treatment with PP2 reduced FAK Y397 phosphorylation. Likewise, we found that pretreatment with PF-562,271 impaired TCR-induced FAK phosphorylation at Y397. In contrast to PP2, PF-562,271 reduced phosphorylation at FAK Y397 only to levels similar to unstimulated control cells, suggesting that PF-562,271, and PP2 may be inhibiting FAK phosphorylation through different mechanisms. PF-562,271 also inhibited phosphorylation of FAK at the Y576/577 and Y925 sites with a similar pattern of activity. Treatment with PF-562,271 in the absence of TCR stimulation caused a perceptible decease in phosphorylation on all sites tested with a statistically significant decrease seen at the Y925 site (Figure 1E/F).
3.2. PF-562,271 impairs TCR-induced morphological changes and RhoA activation
The integrin LFA-1 is important for T cell adhesion to antigen presenting cells and the endothelium via its interaction with ICAM-1. FAK is critical for turnover of fibroblast adhesions, and fibroblasts deficient in FAK show reduced focal adhesion turnover and impaired migration [15]. To examine the effects of PF-562,271 on T cells, we analyzed the effects of PF-562,271 on human peripheral blood T cell spreading. When plated on ICAM-1 without inhibitor, T cells exhibited a polarized and migratory morphology, while TCR stimulation resulted in loss of polarity and migration arrest (Figure 2A). Interestingly, upon TCR engagement, PF-562,271 treated cells spread with an abnormal morphology characterized by many elongated stellate projections. Because RhoA is critical for the regulation of cytoskeletal organization and contributes to T cell migration and ICAM-1 binding [32], we tested whether FAK inhibition alters RhoA activation. Surprisingly, PF-562,271 caused a substantial increase in RhoA GTP binding (Figure 2B). TCR stimulation in the presence of PF-562,271 led to a decrease in GTP-bound RhoA, although the levels were still higher than in the absence of the compound with TCR stimulation. In contrast, there was no effect of PF-562,271 on levels of GTP-bound Rac (Figure 2C). These findings demonstrate that PF-562,271 impairs TCR-induced T cell morphological changes and alters activity of RhoA but not Rac.
FIGURE 2.
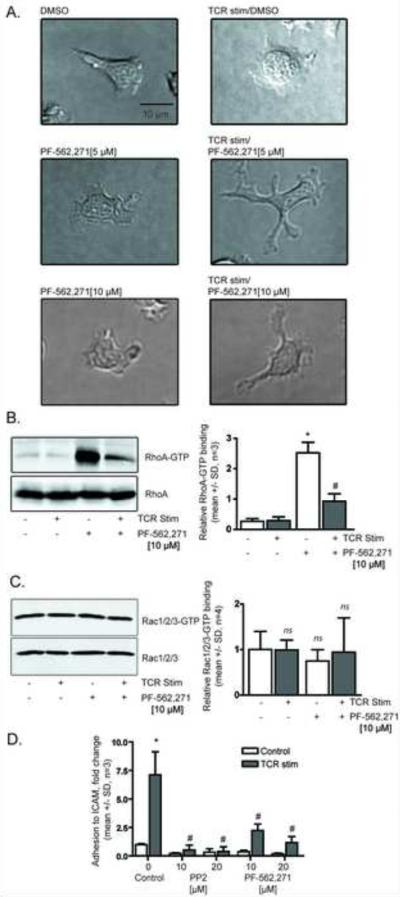
PF-562,271 alters human peripheral blood T cell morphology, RhoA GTP binding, and adhesion to ICAM-1. A) Morphology of T cells treated with PF-562,271 in the presence or absence of T cell receptor stimulation (representative of three independent experiments). Cells were plated, incubated in the presence or absence of the test compounds for 30 minutes, then stimulated for 10 minutes with OKT3. B) PF-562,271 increases levels of GTP-bound RhoA. Blots are representative of three independent experiments. Quantification of RhoA GTP binding (mean +/− SD, n=3, p < 0.05 relative to DMSO control (*) or relative to TCR control (#), ANOVA). C) PF-562,271 does not affect GTP-bound Rac. Blots are representative of four independent experiments. Quantification of Rac GTP binding (mean +/− SD, n=4, ns= so significant change, ANOVA). D) Adhesion to ICAM-1 (mean +/− SD, n=3, p < 0.05 relative to DMSO control (*) or relative to TCR control (#), ANOVA).
3.3. PF-562,271 impairs TCR-induced T cell adhesion to ICAM-1
In Pyk2 knockout mice, CD8+ T cells have decreased adhesion to ICAM-1 [17]. Here, we tested the ability of PF-562,271 to alter human peripheral blood T cell adhesion to ICAM-1 following TCR stimulation. Without TCR stimulation, control cells exhibited a low basal rate of adhesion to ICAM-1 (Figure 2D). However, following TCR stimulation, there was a 7 fold increase in ICAM-1 adhesion in DMSO treated cells. As expected, PP2 treatment abolished all TCR-mediated increase in T cell adhesion to ICAM-1. We found PF-562,271 also reduced TCR-induced T cell adhesion to ICAM-1.
3.4. PF-562,271 inhibits phosphorylation of ZAP-70 and LAT
Our findings of decreased T cell adhesion following treatment with PF-562,271 raised the question of whether PF-562,271 alters proximal signal transduction following TCR engagement. TCR engagement leads to rapid phosphorylation of Src family kinases Lck and Fyn, which phosphorylate ZAP-70 and LAT to form signaling platforms that promote T cell activation. Previous reports of Pyk2 activation in T cells, show that Pyk2 phosphorylation precedes ZAP-70 phosphorylation induced by TCR engagement [29].
We induced TCR signaling in DMSO-treated cells and there was a robust increase in total tyrosine phosphorylation (Figure 3A). As expected, treatment with PP2 dramatically reduced TCR-induced tyrosine phosphorylation. However, PF-562,271 treatment of T cells prior to TCR stimulation did not result in a general loss of tyrosine phosphorylation, indicating that this inhibitor is not broadly inhibiting Src kinase signaling.
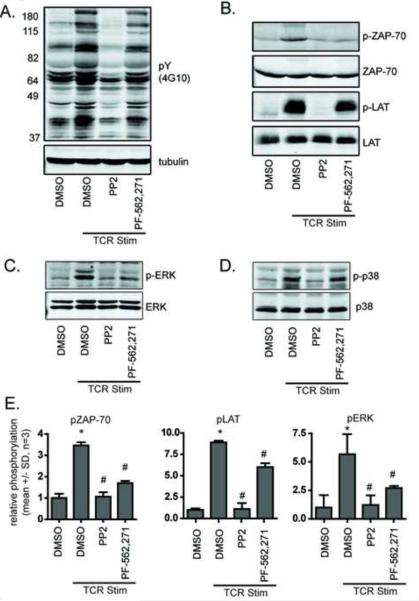
FIGURE 3.
Proximal TCR signaling through ZAP-70 and LAT is impaired by treatment with PF-562,271. A) Western Blot analysis of total phospho-tyrosine levels relative to tubulin levels in human peripheral blood T cells in the presence or absence of 10 μM test compounds. Blots are representative of three independent experiments. B) Western Blot analysis of ZAP-70 at Y319 and LAT phosphorylation at Y191 relative to total protein levels in human peripheral blood T cells. Blots are representative of three independent experiments. C) Western Blot analysis of ERK and D) p38 MAPK phosphorylation relative to total protein levels in human peripheral blood T cells. Blots are representative of three independent experiments. E) Quantification of ZAP-70, LAT, and ERK phosphorylation (mean +/− SD, n=3, p < 0.05 relative to DMSO control (*) or relative to TCR control (#), ANOVA).
Surprisingly however, PF-562,271 impaired TCR-induced phosphorylation of ZAP-70, similar to PP2 and also had modest effects on LAT phosphorylation (Figure 3B). These results suggest that PF-562,271 impairs proximal TCR signaling. To further characterize how PF-562,271 alters TCR signaling, we assessed how PF-562,271 affects TCR signaling to extracellular signal-regulated kinase (ERK) and p38 mitogen activated protein kinase (MAPK), which both contribute to activation-induced proliferation (Figure 3C-E). We performed Western Blot analysis for phosphorylation of ERK and p38 MAPK in the presence or absence of PP2 or PF-562,271. PP2 blocked phosphorylation of both ERK and p38 MAPK. In contrast, PF-562,271 significantly inhibited phosphorylation of ERK but not p38 MAPK.
3.5. Antigen-dependent T cell conjugation is impaired by PF-562,271
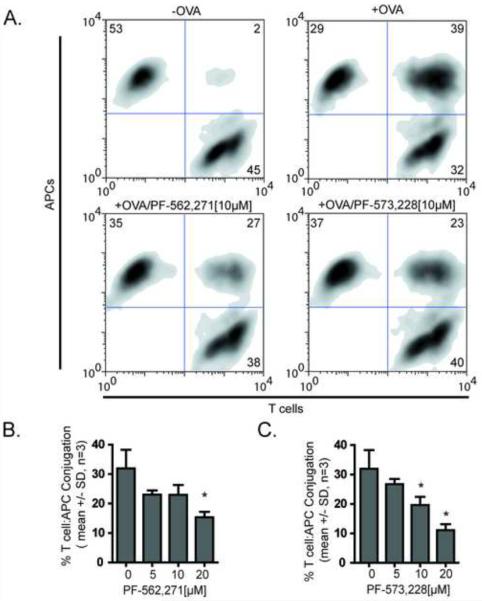
To determine if PF-562,271 alters conjugation between T cells and antigen presenting cells, we performed quantitative analysis of conjugation efficiency using flow cytometry of T cells from the TCR OTII transgenic mouse (Figure 4). Following TCR stimulation, T cells form stable, long-lasting interactions with antigen presenting cells [33] that depend on the integrin LFA-1. In the OTII transgenic mouse, all T cells express the OTII TCR, which recognizes an ovalbumin fragment (OVA peptide). T cells were treated with DMSO or various doses of PF-562,271 and tested for their ability to form stable conjugates with LB27.4 cells (Figure 4A). Treatment with either PF-562,271 (Figure 4B) or a second FAK inhibitor, PF-573,228 (Figure 4C) impaired T cell conjugation with antigen presenting cells.
FIGURE 4.
Antigen-dependent mouse T cell conjugation is impaired by PF-562,271 and PF-573,228. Green-labeled OVA peptide expanded mouse T cells were pretreated with test compounds for 30 minutes and allowed to interact with red-labeled LB27.4 B cell in the presence or absence of antigen and analyzed by flow cytometry. A) Representative density plots of conjugates following 10 μM drug treatment. B) Mean +/− SD of antigen dependent conjugation in the presence of varying doses of PF-562,271 from 3 independent experiments *=p<0.05. C) Mean +/− SD of antigen dependent conjugation in the presence of varying doses of PF-573,228 from 3 independent experiments *=p<0.05.
3.6. Generation of a genetic model for FAK depletion in T cells
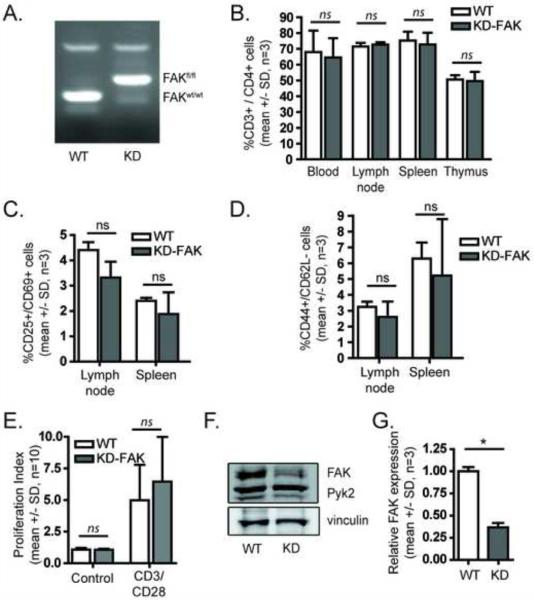
A key limitation of kinase inhibitor studies is the difficulty in differentiating between on-target and off-target effects. To investigate whether FAK itself is required for T cell adhesion and conjugation, we generated a T cell specific conditional FAK knockout mouse (see Methods). We used mice in which the FAK gene was flanked by two loxP sites (Figure 5A), and bred them with mice expressing a gene for Cre recombinase driven by a CD4 promoter, which expressed Cre in CD4+ cells. These mice also were bred to express the OTII transgenic TCR, to allow for OVA peptide based TCR stimulation as described above. These mice showed no differences in viability relative to their littermates, and developed CD4+ T cells similar to C57BL/6 controls (Figure 5B). Additionally, there was no difference in T cell activation as determined by expression of CD25 and CD69 (Figure 5C) or in memory cell development based on expression of CD44 and CD62L (Figure 5D). FAK deficient T cells expanded normally following OVA peptide stimulation, and there was no significant difference in proliferation upon re-stimulation (Figure 5E). We analyzed the expression of FAK in re-stimulated cells (Figure 5F,G) and observed about a 65% decrease in FAK expression, with the residual FAK expression likely due to contaminating cells or partial deletion. Taken together, these results show that FAK-deficient T cells do not show significant defects in their development or proliferation.
FIGURE 5.
Generation of a mouse with depletion of FAK in CD4+ T cells. A) Tail DNA from floxed FAK mice or C57BL/6 mice was analyzed by PCR. B) Expression of CD4+ T cells in whole blood, lymph nodes, spleen, or thymus as a percentage of total CD3+ cells in the respective organ was analyzed by flow cytometry (mean +/− SD, n=3). C) Expression of CD4+ T cells positive for CD25 and CD69 in lymph nodes or spleen was analyzed by flow cytometry (mean +/− SD, n=3). D) Expression of CD4+ T cells positive for CD44 and negative for CD62L in lymph nodes or spleen was analyzed by flow cytometry (mean +/− SD, n=3). E) CD4+ T cells were expanded with OVA peptide and restimulated with CD3/CD28 coated beads and the proliferative index was determined by ModFIT analysis (mean +/− SD, n=10). F) OVA peptide expanded cells were analyzed by Western Blot analysis for expression of FAK (one representative blot shown). G) Quantification of FAK expression (mean +/− SD, n=3, p < 0.05, ANOVA).
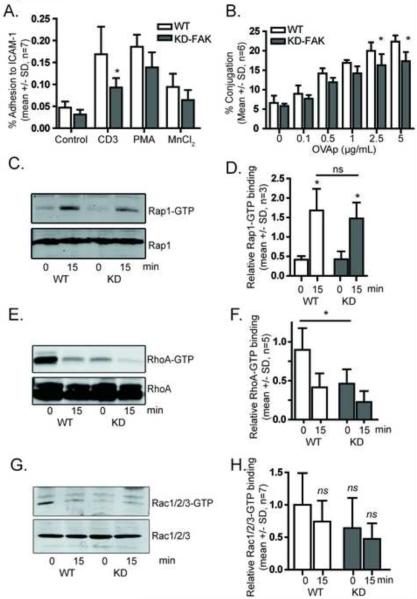
3.7. FAK depletion impairs adhesion to ICAM-1 and conjugation to antigen presenting cells
To determine if FAK is important for LFA-1 activation in response to TCR signaling, we performed an adhesion assay on ICAM-1 coated plates. We stimulated cells with a CD3 antibody, PMA, or MnCl2, which stabilizes the high affinity state (Figure 6A). As expected, TCR stimulation led to increased adhesion, which was significantly reduced in the FAK-depleted cells. In both PMA- and MnCl2-stimulated cells there was a slight reduction in adhesion in FAK-deficient cells that was not statistically significant. We next tested whether FAK was required for conjugation with antigen-presenting cells (Figure 6B). We observed dose-dependent increases in T cell conjugation with antigen presenting cells in the presence of OVA peptide. At higher concentrations of OVA there was a modest but statistically significant decrease in conjugation in FAK-deficient cells. Because Rap1 has previously been shown to contribute to T cell LFA-1 activation [28], we then tested if FAK depletion altered GTP binding of Rap1 and found that FAK depletion did not alter Rap1 GTP binding in the presence or absence of TCR stimulation (Figure 6C, D). However, because RhoA also contributes to LFA activation in T cells [34] and we had seen altered RhoA activity with FAK inhibitor treatment, we tested whether FAK depletion alters RhoA activation. FAK depletion resulted in a significant decrease in the levels of GTP-bound RhoA (Figure 6E, F), which was further decreased in the presence of TCR stimulation. We observed a similar trend in Rac GTP binding, however this trend was not statistically significant after seven independent experiments (Figure 6G, H). Taken together, FAK depletion impairs adhesion and conjugation of T cells and alters RhoA signaling.
FIGURE 6.
FAK-deficient mouse CD4+ T cells exhibit reduced adhesion to ICAM-1 and reduced conjugation with antigen-presenting cells. A) Adhesion to ICAM-1 (mean +/− SD, n=6, p < 0.05, ANOVA). B) Green-labeled OVA peptide expanded mouse T cells were allowed to interact with red-labeled LB27.4 B cells in the presence or absence of antigen and analyzed by flow cytometry. Mean +/− SD of antigen dependent conjugation from 6 independent experiments *=p<0.05. C) Western Blot analysis of Rap1 GTP binding (one representative blot shown) following 0 or 15 minutes of TCR stimulation as indicated. D) Quantification of Rap1 GTP binding (mean +/− SD, n=3, p < 0.05, ANOVA). E) Western Blot analysis of RhoA GTP binding (one representative blot shown) following 0 or 15 minutes of TCR stimulation. F) Quantification of RhoA GTP binding (mean +/− SD, n=3, p < 0.05, ANOVA). G) Western Blot analysis of Rac GTP binding (one representative blot shown) following 0 or 15 minutes of TCR stimulation. H) Quantification of Rac GTP binding (mean +/− SD, n=7, ns=no significant change, ANOVA).
3.8. PF-562,271 inhibits antigen presenting cell-induced T cell proliferation
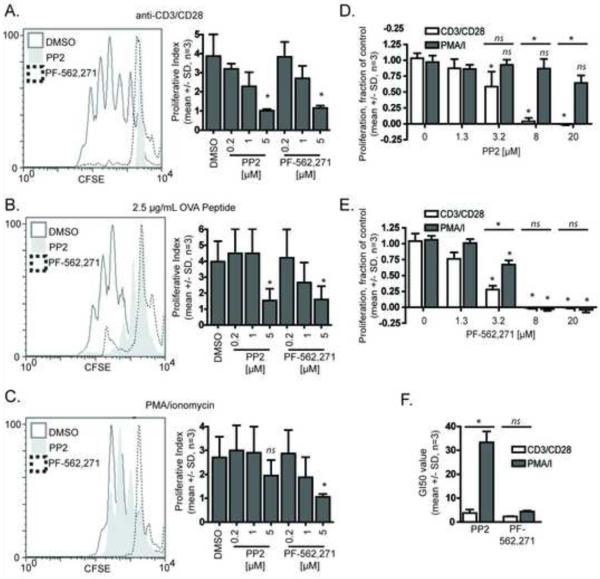
Because T cell conjugation was impaired by PF-562,271, we next wanted to determine if PF-562,271 affects antigen-dependent T cell proliferation. To test this, OTII+ TCR transgenic T cells were labeled with the cytoplasmic dye carboxyfluorescein diacetate, succinimidyl ester and left unstimulated or stimulated with anti-CD3/CD28 coated beads or irradiated splenocytes loaded with or without OVA peptide (Figure 7). Three days following stimulation, carboxyfluorescein diacetate, succinimidyl ester dye dilution was measured by flow cytometry. The degree of proliferation can be assessed by the proliferative index, with unstimulated cells having a proliferative index of one. This assay measures only viable cells which fall within forward and side scatter gating limits, and stain positive for CD4 expression. We observed a dose-dependent decrease in proliferation following bead (Figure 7A), and OVA peptide (Figure 7B) stimulation in both PP2 and PF-562,271 treated T cells compared to control. Surprisingly, the activity of PF-562,271 in this assay was similar to the pan-Src inhibitor PP2, suggesting that PF-562,271 is a potent inhibitor of antigen-induced T cell proliferation even though it is more selective in terms of inhibition of TCR induced tyrosine phosphorylation of downstream targets.
FIGURE 7.
PF-562,271 inhibits T cell proliferation. OVA peptide expanded mouse CD4+ cells were stained with 0.25 μM carboxyfluorescein diacetate, succinimidyl ester and stimulated with anti-CD3/CD28 coated beads (A), irradiated splenocytes loaded 2.5 μg/mL OVA peptide (B), or PMA/Ionomycin (C). Cells were incubated with vehicle control or indicated concentration of inhibitor. Carboxyfluorescein diacetate, succinimidyl ester dilution was measured 72 hours following stimulation and representative plots from 5 μM treatments (left panels) and data from dose response experiments (right panels) are shown. The proliferative index was determined by ModFIT analysis. Data represent averages +/− SD from 3 independent experiments *= p < 0.05. D) Dose-dependent growth inhibition of human CD4+ T cells by PP2 (mean +/− SD, n=3, p < 0.05, ANOVA). E) Growth inhibition of human CD4+ T cells by PF-562,271 (mean +/− SD, n=3, p < 0.05, ANOVA). F) Growth inhibitory 50 values (GI50) for PP2 and PF-562,271 following stimulation with either CD3/CD28 coated beads or the combination of PMA and ionomycin (mean +/− SD, n=3, p < 0.05, ANOVA).
3.9. PF-562,271 acts downstream of proximal signaling to inhibit mouse T cell proliferation
Because we demonstrated that PF-562,271 alters proximal TCR signaling, it was possible that the anti-proliferative effects of PF-562,271 were due to its effects on proximal TCR signaling. To test this possibility, we stimulated mouse T cells with phorbol-12-myristate-13-acetate (PMA) (a protein kinase C agonist) and ionomycin (a calcium ionophore), which together serve to activate T cells downstream of proximal TCR signaling. Proliferation of PP2-treated T cells was restored with PMA and ionomycin treatment (Figure 7C). This is consistent with the known effects of PP2 on proximal TCR signaling. In contrast, PMA and ionomycin stimulation did not restore proliferation in PF-562,271-treated T cells. This finding suggests that PF-562,271 may affect T cell proliferation by also acting downstream of proximal TCR signaling.
3.10. PF-562,271 inhibits human T cell proliferation induced by CD3/CD28 cross-linking
To determine if PF-562,271 also inhibits the proliferation of primary human T cells, we used anti-CD3/CD28 coated beads or PMA and ionomycin to stimulate phytohemagglutinin-activated resting T cells. We measured the number of viable cells using a fluorescent dye that requires activation by cellular esterases as described in Materials and Methods. At 96 hours following stimulation, we found that both the pan-Src inhibitor PP2 (Figure 7D) and PF-562,271 (Figure 7E) impaired anti-CD3/CD28 coated bead induced proliferation in a dose-dependent manner with full inhibition achieved at 8 μM for both PP2 and PF-562,271. Notably, PF-562,271 exhibited a lower IC50 value than PP2 (Figure 7F). Additionally, similar to the mouse T cells, stimulation with PMA/ionomycin was able to rescue the inhibition of PP2 on human T cell proliferation, but not inhibition caused by PF-562,271. Taken together, the findings indicate that PF-562,271 exhibits stronger anti-proliferative effects than PP2 and can act downstream of proximal TCR induced signaling.
4. Discussion
In this report, we provide the first evidence that the clinical FAK inhibitor PF-562,271 impairs primary mouse and human CD4+ T cell activation. PF-562,271 decreases adhesion to ICAM-1 and reduces T cell conjugation with antigen-presenting cells. PF-562,271 also impairs antigen-dependent and antigen-independent T cell proliferation by affecting both proximal and distal TCR signaling. For the first time, we have demonstrated that PF-562,271 increases activation of RhoA. Taken together, our findings suggest that inhibitors of FAK may have immunomodulatory effects. Therefore, it may be important to monitor for adverse immunological effects in patients taking PF-562,271 for solid tumor therapy. Moreover, compounds such as PF-562,271 may also represent an attractive strategy to treat autoimmune disease by affecting T cell activation.
We characterized several site-specific effects of TCR-induced phosphorylation of FAK in the presence or absence of PF-562,271. Previous reports were limited by use of a total phosphotyrosine antibody, which could not distinguish between the auto-activation site (Y397) and other phosphorylation sites [13,14]. We demonstrated that several important tyrosine residues of FAK, including the autophosphorylation site (Y397) and the Src dependent sites (Y576/7, Y925) are phosphorylated in response to TCR signaling, and that this phosphorylation is blocked by both PP2 and PF-562,271. The magnitude of this effect however was greater with PP2, than PF-562,271. The inhibition of Y925 is especially relevant because it has been reported that Src-dependent phosphorylation of Y925 promotes ERK/MAPK signaling and growth of a breast cancer cell line [35]. We observed both inhibition of Y925 and ERK phosphorylation in T cells upon treatment with PF-562,271.
Src family kinases have previously been shown to be potent inhibitors of T cell activation with therapeutic promise [36]. We now show that PF-562,271 inhibits T cell proliferation and TCR signaling by different mechanisms than Src inhibition. While PP2 is a potent inhibitor of all Src-dependent tyrosine phosphorylation in response to TCR stimulation, PF-562,271 has more specific effects. FAK inhibition specifically limits TCR-induced phosphorylation of ZAP-70, LAT and ERK, but not p38 or total phosphotyrosine. Despite having more limited effects on TCR signaling than PP2, PF-562,271 is a more potent inhibitor of T cell proliferation than PP2 likely through the modulation of other downstream signaling pathways.
As is common with inhibitors, the effects of PF-562,271 are likely due to both FAK inhibition and effects on other pathways including Pyk2. Based on initial in vitro kinase activity characterizations [5], PF-562,271 has an IC50 of 1.5 nM for FAK, 13 nM for Pyk2 and 277 nM for Fyn. Thus, at the concentrations we studied, it is possible that cellular Fyn inhibition also occurred. However, cellular Fyn inhibition cannot account for the decreased proliferation caused by PF-562,271, because proliferation in response to PMA and ionomycin (which bypass proximal TCR signaling) is restored in cells treated with PP2 but not PF-562,271. Additionally, the finding that PF-562,271 and PP2 have similar effects on ERK phosphorylation but different effects on p38 MAPK phosphorylation further supports a model in which PF-562,271 differentially affects signaling downstream of the TCR. Because genetic depletion of FAK had no significant effect on T cell proliferation, it is unlikely that the potent effects of PF-562,271 on T cell proliferation (Figure 7) are due to FAK inhibition, but rather due to a yet unidentified kinase, possibly a cyclin dependent kinase [5], that acts downstream of Src and FAK. A potential caveat is that the FAK knockout T cells may not be fully depleted of endogenous FAK.
We found that T cell conjugation with antigen presenting cells and TCR-induced T cell adhesion to ICAM-1 were both impaired in FAK-deficient T cells and in the presence of PF-562,271, supporting a role for FAK in T cell interactions with antigen presenting cells. However, the magnitude of the conjugation effects were more profound with PF-562,271 treatment, indicating that there are likely other effects of this compound in addition to FAK inhibition. In support of this idea, we found that PF-562,271 and FAK depletion had opposing effects on RhoA activation. PF-562,271 resulted in an over 10-fold increase in RhoA GTP binding while FAK depletion led to a 50% reduction in RhoA activation. We currently do not understand the basis for this differential regulation of RhoA activation but it may be due to off target effects of the inhibitor. To our knowledge, this is the first report that PF-562,271 affects RhoA activation, which merits future study in other cell types. However, because RhoA was differentially regulated in the two models, it is unlikely that RhoA activation contributes to the altered T cell adhesion and conjugation induced by FAK inhibition.
In summary, we find that PF-562,271 has inhibitory effects on T cell signaling and proliferation in response to TCR signaling, and that these effects are different than treatment with the Src inhibitor PP2. These findings suggest that there may be a potential clinical use for PF-562,271 or similar compounds in the treatment of autoimmune diseases. Indeed, the first phase of clinical trials for cancer suggest that PF-562,271 is relatively well-tolerated [9]. Ultimately, future studies of PF-562,271 in advanced models of autoimmunity are warranted to test its potential as an immunomodulatory agent.
Acknowledgements
We thank Miriam Shelef for phlebotomy assistance and Danny Grahf for genotyping assistance. This work was supported by National Institutes of Health Grant NIAID R01 to A.H. [AI068062] and NIH 1R56AI094923-01 to A.H. Postdoctoral support was provided to A.J.W. by the UW Institute on Aging Training Grant (NIH#T32AG000213-17), Sanjay Asthana PI and by Postdoctoral Fellowship #122088-PF-12-050-01-CSM from the American Cancer Society. Support was also provided to A.J.W. by internal funding from the University of Connecticut Department of Pharmaceutical Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FOOTNOTE
HEB has recently become affiliated with Pfizer. The experiments on PF-562,271 were performed prior to this affiliation and were performed solely at the University of Wisconsin-Madison by the other authors, who have no affiliation with Pfizer.
Bibliography
- 1.McLean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC. The role of focal-adhesion kinase in cancer - a new therapeutic opportunity. Nat Rev Cancer. 2005;5:505–15. doi: 10.1038/nrc1647. [DOI] [PubMed] [Google Scholar]
- 2.Provenzano PP, Inman DR, Eliceiri KW, Beggs HE, Keely PJ. Mammary epithelial-specific disruption of focal adhesion kinase retards tumor formation and metastasis in a transgenic mouse model of human breast cancer. Am.J.Pathol. 2008;173:1551–65. doi: 10.2353/ajpath.2008.080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pylayeva Y, Gillen KM, Gerald W, Beggs HE, Reichardt LF, Giancotti FG. Ras- and PI3K-dependent breast tumorigenesis in mice and humans requires focal adhesion kinase signaling. J.Clin.Invest. 2009;119:252–66. doi: 10.1172/JCI37160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hao H, Naomoto Y, Bao X, Watanabe N, Sakurama K, Noma K, et al. Focal adhesion kinase as potential target for cancer therapy (Review) Oncol.Rep. 2009;22:973–9. doi: 10.3892/or_00000524. [DOI] [PubMed] [Google Scholar]
- 5.Roberts WG, Ung E, Whalen P, Cooper B, Hulford C, Autry C, et al. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res. 2008;68:1935–44. doi: 10.1158/0008-5472.CAN-07-5155. [DOI] [PubMed] [Google Scholar]
- 6.Bagi CM, Christensen J, Cohen DP, Roberts WG, Wilkie D, Swanson T, et al. Sunitinib and PF-562,271 (FAK/Pyk2 inhibitor) effectively block growth and recovery of human hepatocellular carcinoma in a rat xenograft model. Cancer Biol Ther. 2009;8:856–65. doi: 10.4161/cbt.8.9.8246. [DOI] [PubMed] [Google Scholar]
- 7.Bagi CM, Roberts GW, Andresen CJ. Dual focal adhesion kinase/Pyk2 inhibitor has positive effects on bone tumors: implications for bone metastases. Cancer. 2008;112:2313–21. doi: 10.1002/cncr.23429. [DOI] [PubMed] [Google Scholar]
- 8.Schultze A, Fiedler W. Therapeutic potential and limitations of new FAK inhibitors in the treatment of cancer. Expert Opin.Investig.Drugs. 2010;19:777–88. doi: 10.1517/13543784.2010.489548. [DOI] [PubMed] [Google Scholar]
- 9.Siu LL, Burris HA, Mileshkin L, Camidge DR, Rischin D, Chen EX, et al. Phase 1 study of a focal adhesion kinase (FAK) inhibitor PF-00562271 in patients (pts) with advanced solid tumors. Journal of Clinical Oncology; 2007 ASCO Annual Meeting Proceedings Part I.2007. p. 3527. [Google Scholar]
- 10.Infante JR, Camidge DR, Mileshkin LR, Chen EX, Hicks RJ, Rischin D, et al. Safety, pharmacokinetic, and pharmacodynamic phase I dose-escalation trial of PF-00562271, an inhibitor of focal adhesion kinase, in advanced solid tumors. J.Clin.Oncol. 2012;30:1527–33. doi: 10.1200/JCO.2011.38.9346. [DOI] [PubMed] [Google Scholar]
- 11.Bacon KB, Szabo MC, Yssel H, Bolen JB, Schall TJ. RANTES induces tyrosine kinase activity of stably complexed p125(FAK) and ZAP-70 in human T cells. J.Exp.Med. 1996;184:873–82. doi: 10.1084/jem.184.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabassam FH, Umehara H, Huang JY, Gouda S, Kono T, Okazaki T, et al. Beta2-integrin, LFA-1, and TCR/CD3 synergistically induce tyrosine phosphorylation of focal adhesion kinase (pp125(FAK)) in PHA-activated T cells. Cell.Immunol. 1999;193:179–84. doi: 10.1006/cimm.1999.1472. [DOI] [PubMed] [Google Scholar]
- 13.Berg NN, Ostergaard HL. T cell receptor engagement induces tyrosine phosphorylation of FAK and Pyk2 and their association with Lck. J.Immunol. 1997;159:1753–7. [PubMed] [Google Scholar]
- 14.Maguire JE, Danahey KM, Burkly LC, van Seventer GA. T cell receptor- and beta 1 integrin-mediated signals synergize to induce tyrosine phosphorylation of focal adhesion kinase (pp125FAK) in human T cells. J.Exp.Med. 1995;182:2079–90. doi: 10.1084/jem.182.6.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ilic D, Furuta Y, Suda T, Atsumi T, Fujimoto J, Ikawa Y, et al. Focal adhesion kinase is not essential for in vitro and in vivo differentiation of ES cells. Biochem.Biophys.Res.Commun. 1995;209:300–9. doi: 10.1006/bbrc.1995.1503. [DOI] [PubMed] [Google Scholar]
- 16.Okigaki M, Davis C, Falasca M, Harroch S, Felsenfeld DP, Sheetz MP, et al. Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc.Natl.Acad.Sci.U.S.A. 2003;100:10740–5. doi: 10.1073/pnas.1834348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beinke S, Phee H, Clingan JM, Schlessinger J, Matloubian M, Weiss A. Proline-rich tyrosine kinase-2 is critical for CD8 T-cell short-lived effector fate. Proc.Natl.Acad.Sci.USA. 2010;107:16234–9. doi: 10.1073/pnas.1011556107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang M, Zhang W, Zhang Y. Activation signal transduction by proline-rich tyrosine kinase 2 (PYK2) in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Hybridoma (Larchmt) 2009;28:333–9. doi: 10.1089/hyb.2009.0023. [DOI] [PubMed] [Google Scholar]
- 19.Simonson WTN, Franco SJ, Huttenlocher A. Talin1 regulates TCR-mediated LFA-1 function. Journal of Immunology. 2006;177:7707–14. doi: 10.4049/jimmunol.177.11.7707. [DOI] [PubMed] [Google Scholar]
- 20.Beggs HE, Schahin-Reed D, Zang KL, Goebbels S, Nave KA, Gorski J, et al. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003;40:501–14. doi: 10.1016/s0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wernimont SA, Wiemer AJ, Bennin DA, Monkley SJ, Ludwig T, Critchley DR, et al. Contact-dependent T cell activation and T cell stopping require talin1. J.Immunol. 2011;187:6256–67. doi: 10.4049/jimmunol.1102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiemer AJ, Lokuta MA, Surfus JC, Wernimont SA, Huttenlocher A. Calpain inhibition impairs TNF-alpha-mediated neutrophil adhesion, arrest and oxidative burst. Mol.Immunol. 2010;47:894–902. doi: 10.1016/j.molimm.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Wauwe JP, De Mey JR, Goossens JG. OKT3: a monoclonal anti-human T lymphocyte antibody with potent mitogenic properties. J.Immunol. 1980;124:2708–13. [PubMed] [Google Scholar]
- 24.Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, et al. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–5. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 25.Wiemer AJ, Wernimont S, Huttenlocher A. Live imaging of LFA-1-dependent T-cell motility and stop signals. Methods Mol.Biol. 2012;757:191–204. doi: 10.1007/978-1-61779-166-6_13. [DOI] [PubMed] [Google Scholar]
- 26.Wernimont SA, Legate KR, Simonson WT, Fassler R, Huttenlocher A. PIPKI gamma 90 negatively regulates LFA-1-mediated adhesion and activation in antigen-induced CD4+ T cells. J.Immunol. 2010;185:4714–23. doi: 10.4049/jimmunol.1001445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiemer AJ, Hegde S, Gumperz JE, Huttenlocher A. A live imaging cell motility screen identifies prostaglandin E2 as a T cell stop signal antagonist. J.Immunol. 2011;187:3663–70. doi: 10.4049/jimmunol.1100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katagiri K, Hattori M, Minato N, Kinashi T. Rap1 functions as a key regulator of T-cell and antigen-presenting cell interactions and modulates T-cell responses. Mol.Cell.Biol. 2002;22:1001–15. doi: 10.1128/MCB.22.4.1001-1015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins M, Tremblay M, Chapman N, Curtiss M, Rothman PB, Houtman JCD. The T cell receptor-mediated phosphorylation of Pyk2 tyrosines 402 and 580 occurs via a distinct mechanism than other receptor systems. J.Leukoc.Biol. 2010;87:691–701. doi: 10.1189/jlb.0409227. [DOI] [PubMed] [Google Scholar]
- 30.Zhao J, Guan JL. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 2009;28:35–49. doi: 10.1007/s10555-008-9165-4. [DOI] [PubMed] [Google Scholar]
- 31.Qian D, Lev S, van Oers NS, Dikic I, Schlessinger J, Weiss A. Tyrosine phosphorylation of Pyk2 is selectively regulated by Fyn during TCR signaling. J.Exp.Med. 1997;185:1253–9. doi: 10.1084/jem.185.7.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heasman SJ, Carlin LM, Cox S, Ng T, Ridley AJ. Coordinated RhoA signaling at the leading edge and uropod is required for T cell transendothelial migration. J.Cell Biol. 2010;190:553–63. doi: 10.1083/jcb.201002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–9. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 34.Pasvolsky R, Grabovsky V, Giagulli C, Shulman Z, Shamri R, Feigelson SW, et al. RhoA is involved in LFA-1 extension triggered by CXCL12 but not in a novel outside-in LFA-1 activation facilitated by CXCL9. J.Immunol. 2008;180:2815–23. doi: 10.4049/jimmunol.180.5.2815. [DOI] [PubMed] [Google Scholar]
- 35.Mitra SK, Mikolon D, Molina JE, Hsia DA, Hanson DA, Chi A, et al. Intrinsic FAK activity and Y925 phosphorylation facilitate an angiogenic switch in tumors. Oncogene. 2006;25:5969–84. doi: 10.1038/sj.onc.1209588. [DOI] [PubMed] [Google Scholar]
- 36.Schade AE, Schieven GL, Townsend R, Jankowska AM, Susulic V, Zhang R, et al. Dasatinib, a small-molecule protein tyrosine kinase inhibitor, inhibits T-cell activation and proliferation. Blood. 2008;111:1366–77. doi: 10.1182/blood-2007-04-084814. [DOI] [PMC free article] [PubMed] [Google Scholar]