Abstract
Cocaine addiction is a chronic, relapsing disease characterized by an inability to regulate drug-seeking behavior. Here we investigated the role of mGluR5 in the ventral and dorsal striatum in regulating cocaine-seeking following both abstinence and extinction. Animals underwent 2 weeks of cocaine self-administration followed by 3 weeks of home-cage abstinence. Animals were then reintroduced to the operant chamber for a context-induced relapse test, followed by 7–10 days of extinction training. Once responding was extinguished, cue-primed reinstatement test was conducted. Both drug-seeking tests were conducted in the presence of either the mGluR5 negative allosteric modulator, MTEP or vehicle infused into either the nucleus accumbens (NA) core or dorsolateral striatum (dlSTR). We found that MTEP infused in the NA core attenuated both context-induced relapse following abstinence and cue-primed reinstatement following extinction training. Blocking dlSTR mGluR5 had no effect on context- or cue-induced cocaine-seeking. However, the intra-dlSTR MTEP infusion on the context-induced relapse test day attenuated extinction learning for 4 days after the infusion. Furthermore, mGluR5 surface expression was reduced and LTD was absent in dlSTR slices of animals undergoing 3 weeks of abstinence from cocaine but not sucrose self-administration. LTD was restored by bath application of VU-29, a positive allosteric modulator of mGluR5. Bath application of MTEP prevented the induction of LTD in dSTR slices from sucrose animals. Taken together, this data indicates that dlSTR mGluR5 plays an essential role in extinction learning but not cocaine relapse, while NA core mGluR5 modulates drug-seeking following both extinction and abstinence from cocaine self-administration.
Keywords: mGluR5, dorsal striatum, nucleus accumbens, cocaine, abstinence, extinction, long-term depression, surface expression
INTRODUCTION
Cocaine addiction is a compulsive and chronically-relapsing disorder (McLellan et al. 2000; O’Brien 2001). The risk of relapse remains high even after months or years of abstinence and represents a major challenge in the successful treatment of drug addiction. Animal models of relapse have been developed to study the neural circuitry and molecular substrates underlying persistent drug-seeking and ultimately to screen targeted pharmacological treatments to prevent relapse. In these models, animals do not relapse to drug-taking (e.g. intravenous drug delivery) but instead relapse is considered to be a resumption of the drug-seeking response (e.g. lever pressing). One such model is the extinction-reinstatement paradigm, in which animals are trained to self-administer drug in an operant chamber and then undergo extinction training during which previously reinforced behaviors no longer result in drug infusion and said behavior decreases (de Wit & Stewart 1981). Once behavioral responding is low, the drug-seeking response is reinstated with stimuli known to cause relapse in humans, including stress, discrete and contextual cues previously associated with drug delivery, and/or the drug itself (for review see Epstein et al. 2006). A second animal model is the abstinent-relapse model in which animals do not undergo extinction training following self-administration but instead experience abstinence in the home cage with daily handling. Animals are then re-exposed to the drug-taking environment (operant chamber) for a context-induced relapse test, which is also Day 1 of extinction training (for review see Reichel & Bevins 2009). Both models have been judged to possess face validity for different facets of addiction and are valuable tools for screening potential pharmacotherapies for their ability to attenuate drug-craving and relapse (Epstein et al. 2006; Reichel & Bevins 2009).
The extinction-reinstatement model has been extensively used to identify the neural circuitry involved in relapse, with the ventral striatum (in particular nucleus accumbens core) being identified as a key structure in mediating stress- and drug-primed reinstatement (McFarland and Kalivas 2001; McFarland et al. 2003; McFarland et al. 2004). Reversible inactivation of both the nucleus accumbens (NA) core and the dorsal medial prefrontal cortex (dmPFC) projection to the NA core attenuate drug-primed reinstatement following extinction training (McFarland & Kalivas 2001). Furthermore, stress and cocaine-primed reinstatement are driven by a release of glutamate along this pathway (McFarland et al. 2003; McFarland et al. 2004).
Using the abstinent-relapse model, it has been found that inactivation of the lateral subregion of dorsal striatum (or dorsolateral striatum - dlSTR) attenuates context-induced drug-seeking following 2–3 weeks of abstinence (Fuchs et al. 2006). Interestingly, neither the dmPFC nor the NA are necessary for context-induced relapse following abstinence (Fuchs et al. 2006; See et al. 2007), although both have previously been shown to be necessary for explicit cue-induced reinstatement of extinguished cocaine-seeking (Fuchs et al. 2004; McLaughlin & See 2003). It has been suggested that both the dmPFC and NA are incorporated into the reinstatement neurocircuitry through the process of extinction learning (Peters et al. 2008). Furthermore, See and colleagues (2007) determined that while reversible inactivation of the NA core did not affect abstinent-relapse, extinction learning was attenuated on subsequent days following the inactivation. Conversely, reversible inactivation of the dlSTR significantly attenuated abstinent-relapse but did not affect subsequent extinction learning.
As evidenced by a number of studies using the extinction-reinstatement model, dysregulation of glutamate homeostasis in the NA is the primary driver of drug-seeking behavior during reinstatement (see Knackstedt & Kalivas 2009 for review). Metabotropic glutamate receptors of subtype 5 (mGluR5) are highly enriched in the striatum and mediate long-term synaptic plasticity, such as long-term depression (LTD; Sung et al. 2001, Forgeaud et al. 2004, Moussawi et al. 2009). Systemic pharmacological or genetic disruption of mGluR5 function attenuates the reinstatement of extinguished cocaine-seeking (Chiamulera et al. 2001; Bäckström & Hyytiä 2006; Kumaresan et al. 2009; Martin-Fardon et al. 2009). Specific blockade of NA core (Wang et al. 2013) and NAc shell (Kumaresan et al. 2009) mGluR5 receptors also attenuates cocaine reinstatement. Moreover, in rats with a history of cocaine self-administration followed by 2–3 weeks of extinction training, the ability to induce mGluR5-dependent LTD in the NA core is lost (Knackstedt et al. 2010). The loss of LTD is accompanied by a decrease in mGluR5 surface expression and changes in the mGluR5 signaling complex in this brain region (Knackstedt et al. 2010). Interestingly, mGluR5 surface levels as well as mGluR5-dependent LTD in the NA core are not significantly altered in animals which underwent cocaine self-administration and extended abstinence but not extinction training (Knackstedt et al. 2010; McCutcheon et al. 2011a). In general, there is a distinctive pattern of glutamatergic neuroadaptations found in the striatum, depending on post-cocaine experience (abstinence vs. extinction) and the length of drug-free interval (days vs. weeks; Sutton et al. 2003; Ghasemzadeh et al. 2009; Knackstedt et al. 2010; Edwards et al. 2011; McCutcheon et al. 2011b). While there is strong support for the theory that glutamate (including mGluR5-dependent) signaling in the NA core is essential for reinstatement following extinction training (Cornish & Kalivas 2000; McFarland et al. 2003; Wang et al. 2013), the identity of the neurotransmitters and receptors in the dorsal and ventral STR involved in context-induced relapse following abstinence remain poorly understood. Our initial studies have indicated that in the dSTR, several proteins within the mGluR5 receptor signaling complex (such as RGS4, Gαq subunit and PLCβ1) are dysregulated following cocaine self-administration and prolonged (3 week) abstinence, (Schwendt et al. 2007a; Schwendt et al. 2007b). However, it remains unclear whether mGluR5 function in the dSTR is altered following abstinence from cocaine self-administration, and whether manipulation of mGluR5 receptor in this brain region would interfere with relapse and or extinction learning.
Here we investigated the role of mGluR5 in relapse following both abstinence (without extinction) and extinction training. We utilized an animal model which places animals into forced abstinence followed by reintroduction to the self-administration environment for a context-induced relapse test. This test day is also Day 1 of extinction training as presses on the previously active lever do not yield drug or presentation of drug-paired cues. Extinction training continues until responding is reduced and animals are then tested for cue-primed reinstatement. Using this model, we tested the ability of MTEP, a highly specific negative allosteric modulator (NAM) of mGluR5 (Anderson et al. 2002 & 2003; Cosford et al. 2003), to attenuate both context- and cue-induced relapse when infused into either the dlSTR or NA core. We also assessed potential changes in mGluR5 function as measured by slice electrophysiology and surface biotinylation in dlSTR slices.
MATERIALS AND METHODS
Drugs
Cocaine hydrochloride (Coc) was acquired from the NIDA Controlled Substances Program (Research Triangle Institute, NC). The selective mGluR5 negative allosteric modulator,3-[(2- methyl-1, 3-thiazol-4-yl) ethynyl] pyridine (MTEP) hydrochloride, was purchased from Abcam Biochemicals (Cambridge, MA, USA). The selective mGluR5 positive allosteric modulator 4-nitro-N-(1,3-diphenyl-1H-pyrazol-5-yl) benzamide (VU-29) was obtained from Dr. Foster Olive (Arizona State University, Tempe, AZ). The cocaine used for i.v. self-administration was dissolved in 0.9% physiological saline. MTEP and VU-29 were dissolved in 1% Tween-80 in artificial cerebrospinal fluid (aCSF; in mM: 125 NaCl, 1 CaCl2, 2.5 KCl, 4 MgCl2, 25 NaHCO3, 1.25 NaH2PO4, 0.4 ascorbic acid and 10 D-glucose). Tween-80 (1%) in combination with aCSF was used as vehicle control for these agents. Solutions of all drugs were neutralized to pH 7.2–7.4 with 1N NaOH, if necessary. The concentrations of the drugs used were determined based on the results of previously published studies (Ayala et al. 2009; Gass & Olive 2009b; Sinclair et al. 2012; Schwendt et al. 2012; Wang et al. 2013), as well as on our preliminary behavioral experiments (Hearing, Knackstedt, Schwendt - unpublished data).
Animals
Adult male Sprague–Dawley rats (Charles River Laboratories, Wilmington, MA), weighing 275–300 g were single-housed in a temperature- and humidity-controlled vivarium on a reversed 12-h light/dark cycle with water available ad libitum. Animals were food-restricted to 85% of free-feeding weight. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina and were performed in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Academy Press 1996).
Catheter and stereotaxic surgery
Rats were anesthetized with ketamine HCl (87.5 mg/kg, IM) and xylazine (5 mg/kg, IM). Ketorolac (3 mg/kg, IP) was administered pre-operatively to provide analgesia. SILASTIC tubing (0.51 mm inner diameter, 0.94 mm outer diameter; Dow Corning, Midland, MI) was implanted into the jugular vein. The tubing exited the vein and traveled subcutaneously between the shoulder blades to exit from the skin on the back. The tubing was attached to a stainless-steel cannulae which was held within a harness (Instech, Plymouth Meeting, PA). Animals were then placed into a small animal stereotaxic instrument (Kopf Instruments, Tujunga, CA) and guide cannulas were implanted and secured with dental cement. Cannulas (20 gauge; Plastics One) were implanted 2 mm above the infusion target according to following coordinates: dlSTR (+1.2 mm AP, ±3.4 mm ML and −3.4 mm DV), NA core (+1.8 mm AP, ±1.6 mm ML and −5.5 mm DV) relative to bregma according to (Paxinos and Watson 2007). Animals were allowed to recover for 5 days prior to the initiation of self-administration. Catheters were flushed with 100 U/ml heparinized saline (Elkins-Sinn, Cherry Hill, NJ) daily throughout self-administration.
Self-administration and abstinence
Rats were trained to press a lever to receive an intravenous infusion of cocaine in operant chambers (30 × 24 × 30 cm; Med Associates, St. Albans, VT) under an FR1 schedule of reinforcement for 2 h daily during the dark cycle for a minimum of 12 days. Responses on the active lever resulted in the delivery of cocaine (0.2 mg in 50 µl saline) accompanied by a 5-s presentation of a light + tone conditioned cue. Each cocaine infusion was followed by a 20 s ‘time-out’ period during which presses on the drug-paired lever did not yield drug or cue presentation. Responses on the inactive lever had no programmed consequences but were recorded. Catheter patency was periodically verified with methohexital sodium (10 mg/ml; Eli Lilly, Indianapolis, IN), a short-acting barbiturate that produces a rapid loss of muscle tone when administered intravenously. Control animals received sucrose pellets and cue presentations on an FR-1 schedule of reinforcement. Data collection and reinforcer delivery were controlled using Med PC software version IV (Med Associates). Following the completion of 12-day self-administration regimen (minimum of 12 infusions or 50 sucrose pellets / day) all animals underwent 20 days of home cage abstinence. During the last 10 days of abstinence, rats were handled and habituated for 2 h/day to another procedure room. At the end of abstinence, animals were assigned to one of three cohorts receiving the following procedures: 1) animals were euthanized and the dlSTR slices were collected for surface biotinylation and western blot analysis; 2) animals were euthanized and slices of the dlSTR were made for electrophysiology, or 3) animals were tested to evaluate the effects of intra-NA core or intra-dlSTR vehicle vs. MTEP microinjections on drug-seeking and extinction.
Intracranial microinjections
Two 33 gauge microinjectors that extended 2 mm beyond the tip of the guide cannula were bilaterally inserted into the NA core or dlSTR. Vehicle (VEH) or MTEP was infused at a rate of 0.5 µl/min in a total volume of 0.5 µl/side (NA core) or 1µl/side (dlSTR) using an infusion pump (Harvard Apparatus, Holliston, MA). After the infusion was completed, the microinjectors were left in place for an additional 1 min to allow for diffusion of the drugs. Only rats with correct cannula placements were included in the data analysis.
Relapse, extinction and reinstatement procedures
On day 21 of abstinence, animals received a single bilateral microinjection of VEH or MTEP into the dlSTR (5 µg/side) or NA core (1 µg/side) using the procedure described above and were placed into the operant chambers for a 2-h context-induced relapse test during which lever presses were recorded but had no programmed consequences. After the test, rats underwent daily 2-h extinction sessions until extinction criterion (a minimum of seven extinction sessions and ≤25 active lever responses per session for the last two consecutive days) was reached. Upon completion of extinction training, animals underwent a cue-induced relapse test in the same manner as described above. A cross-over design was used so that animals received infusion of one substance (MTEP or VEH) prior to the context-induced relapse test and the other substance prior to the cue-primed reinstatement test. During the cue-primed reinstatement test, active lever presses resulted in discrete cue presentation (light + tone) in the absence of cocaine delivery. At the end of this final test, animals were euthanized and the brains harvested for histological verification of cannulae sites. Animals received vehicle and MTEP infusions in a counterbalanced manner and at least 1 week separated two consecutive tests.
Locomotor Testing
We quantified locomotor behavior (total distance traveled) after infusion of MTEP and VEH into the dlSTR and NA core. To do so we used a subset of the animals which underwent cocaine and sucrose relapse testing; locomotor testing was completed 5–7 days following the last reinstatement test. Animals were placed into the locomotor apparatus (Accuscan Instruments, Columbus, OH) and baseline locomotion was recorded for one hour, at which point animals received microinjection of either VEH or MTEP and behavior was recorded for 2 hours. Animals receiving intra-dlSTR infusions were also tested in the same manner but without intra-dlSTR infusions on the 2 days following the first test.
Histology
In some experiments, following the last reinstatement or locomotor testing, animals were rapidly euthanized and brains collected, frozen and stored until analysis of microinjection placements. Brains were blocked and sliced in coronal sections (50 µm) through the striatal subregions analyzed. Sections were mounted on positively charged Superfrost slides (Fisher Scientific Co., Houston, TX), stained with cresyl violet, and examined for cannula placement by an individual unaware of each subject’s behavior. All placements were determined using the rat brain atlas by Paxinos and Watson (2007).
Slice electrophysiology
Brain slices were prepared from cocaine and sucrose self-administering animals. Coronal slices (350 µm) were cut in ice-cold modified artificial cerebrospinal fluid (aCSF) containing the following (in mM): 200 sucrose, 1.9 KCl, 6 MgCl2, 33 NaHCO3, 1.2 Na2HPO4, 0.5 CaCl2, 0.4 ascorbic acid, 10 D-glucose and adjusted to pH 7.4 by bubbling with 95% O2/5% CO2. Slices containing the cortex and the anterior striatum were completely submerged and continuously bathed in incubation aCSF (in mM: 125 NaCl, 1 CaCl2, 2.5 KCl, 4 MgCl2, 25 NaHCO3, 1.25 NaH2PO4, 0.4 ascorbic acid and 10 D-glucose). Recording aCSF contained (in mM): 125 NaCl, 2.5 KCl, 2 CaCl2, 1.2 MgCl2, 25 NaHCO3, 0.4 ascorbic acid, 10 D-glucose. Recording aCSF and drugs (MTEP 1µM, VU-29 1µM) were bath applied to slices by gravity flow. Superfusate was passed through an in-line heater at a flow rate of 1.5 –2.5 ml/min and temperature was held constant at 34°C.
Extracellular recordings were obtained using pipettes made from borosilicate glass. Pipette resistance ranged from 0.9 to 1.1 MΩ when filled with recording aCSF solution. Signals were amplified using an Axon 700B (Molecular Devices, Sunnyvale, CA) recorded using a Windows PC running Axograph X and Multiclamp Commander. The stimulus intensity was set to the level at which an evoked population spike (PS) was approximately one half of the amplitude of the maximal obtainable response prior to HFS. Stimulus intensity was adjusted to a level evoking a maximal response during HFS. Stimulus intensity ranged from 15 to 120 V while the stimulus duration was set to 0.1 and delivered using a Grass S-88 Stimulator. The majority of the slices were also subjected to maximal stimulation at the end of an experiment to ensure that no significant deterioration of the slices occurred during the experiment, as determined by the presence of a maximal PS in response to such stimulation. LTD was induced by the following HFS protocol: four 1-s duration, 100-Hz trains, delivered at a frequency of one train every 10 s (Partridge et al. 2000).
Slice biotinylation
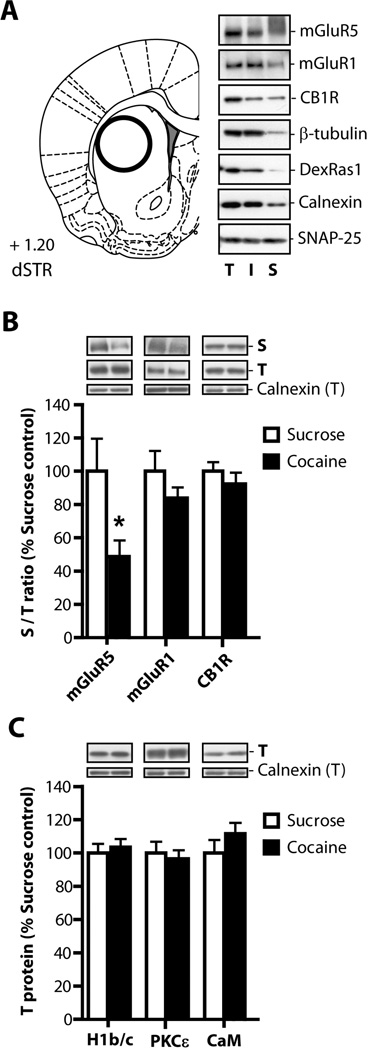
Slice preparation and surface biotinylation was conducted as described in our previous studies (Hearing et al. 2011; Knackstedt et al. 2010) with few modifications. The dlSTR was punched from a 2-mm coronal section (Figure 6) and cut into 250-µm slices using a McIlwain Tissue Chopper (Stoelting, Wood Dale, IL). Slices were immediately biotinylated with EZlink NHS-SS-Biotin (1 mg/ml in aCSF; Thermo Fisher Scientific, Rockford, IL) for 1 h at 4°C. Next, biotinylation reaction was quenched with 100 mM glycine/aCSF buffer followed by two washes with aCSF. Biotinylated slices were homogenized by a brief sonication in a HEPES-Triton-SDS lysis buffer [25 mM HEPES, 150 mM NaCl, 1% Triton X-100, 0.1% SDS] supplemented with protease inhibitors and phosphatase inhibitors. Following incubation for 1 h at 4°C, insoluble debris was removed by centrifugation. An equal aliquot of precleared solubilizate (total protein fraction, ‘T’) was saved and stored at −80°C. Biotinylated proteins were captured by incubating with streptavidin agarose beads (Thermo Fisher Scientific) overnight at 4°C. After a brief centrifugation, supernatants (non-biotinylated, intracellular proteins, ‘I’) were saved and beads were washed three times with lysis buffer. Biotinylated (surface, ‘S’) proteins were eluted off the beads with Laemmli sample buffer [62.5 mM Tris-HCl, pH 6.8, 20% glycerol, 2% SDS, and 50 mM DTT] and incubation at 95°C for 10 min.
Figure 6. Cocaine self-administration followed by 3 weeks of abstinence reduces surface expression of mGluR5 receptors in the dlSTR.
(A) The rat brain atlas coordinates according to Paxinos and Watson (2007) used to conduct tissue dissections. Representative immunoblot analysis of biotinylated slices isolated from the dlSTR. (B) The ratio of protein density in the surface (biotinylated) fraction to that in the total fraction was analyzed. We found a significant decrease in surface mGluR5s in cocaine relative to sucrose animals but no change in mGluR1 or CB1 receptor surface expression. (C) In the total fraction we found no differences in expression of mGluR5-associated proteins Homer 1b/c (H1b/c), calmodulin (CaM), or protein kinase C isoform ε (PKCε). * = p<0.05 in comparison to sucrose. N= 6–8/group. T - total lysate, I - intracellular fraction, S - surface fraction.
Immunoblotting
Proteins of interest in total (T), intracellular (I) and surface (S) fractions were analyzed by immunoblotting. Equal aliquots from each fraction separated by SDS-PAGE (4–15%) and transferred to PVDF membrane. Membranes were blocked for 1 h in 5% milk/Tris-buffered saline and probed overnight at 4°C with primary antibodies diluted in 5% milk/Tris-buffered saline with 0.1% Tween 20. The following primary antisera were used: anti-calnexin (Enzo Life Sciences, Farmingdale, NY), anti-mGluR1 and anti-mGluR5 (Millipore, Billerica, MA), anti-cannabinoid CB1 receptor, anti-β-tubulin and anti-SNAP25 (all Abcam, Cambridge, MA). Anti-DexRas1 antibody, a gift from Dr. Stephen Lanier (MUSC, Charleston, SC), was characterized previously (Vaidyanathan et al. 2004). After incubation with an appropriate HRP-conjugated secondary antiserum (all from Jackson Immuno Research, West Grove, PA), immunoreactive bands on the membranes were detected by ECL+ chemiluminescence reagents on an X-ray film (GE Healthcare, Piscataway, NJ). Subsequently, the blots were stripped and re-probed with anti-DexRas1 antibody to monitor biotinylation of intracellular proteins and with anti-calnexin antibody to normalize for unequal loading and/or transfer of proteins. The integrated band density of each protein sample was measured using NIH Image J software version 1.32j (http://rsb.info.nih.gov/ij/).
Statistical Analysis
For all statistical analyses used, the alpha level was set at p < 0.05. Relapse tests were analyzed with independent sample t-tests. Both extinction training and locomotor data were analyzed with mixed-factorial 2-way ANOVAs, with time as the repeated measure. Independent sample t-tests were used to examine group differences only if a significant Group × Time interaction was obtained. Immunoblotting data, represented by integrated density of individual protein bands, were normalized for the density of calnexin immunoreactivity within the same sample and the two groups (sucrose and cocaine) were analyzed by t-test.
Electrophysiology data were analyzed as follows: PS amplitude was measured using Axograph X using methods of Partridge et al. (2000). The magnitude of LTD was normalized to baseline on a single-recording basis. The data was analyzed with independent sample t-tests and the time-course data was analyzed with a mixed-factorial 2-way ANOVA with repeated measures on time. LSD post-hoc tests were used when appropriate.
RESULTS
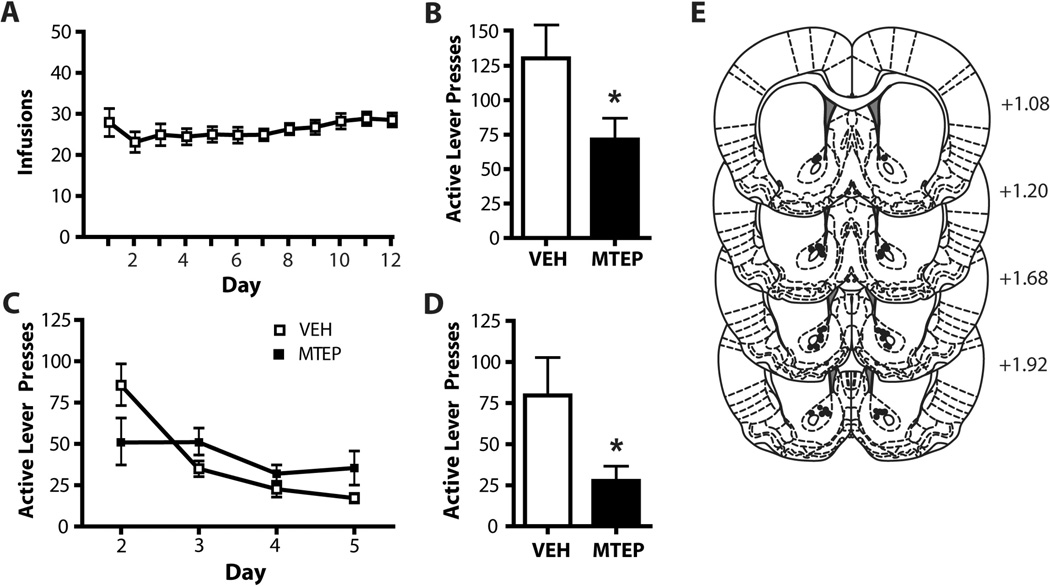
Animals self-administered cocaine (Figure 1A, 2A) or sucrose (Figure 3A) for 12 days on an FR-1 schedule of reinforcement. Following three weeks of abstinence in the home cage (without extinction training), animals were placed into the operant boxes for a 2 h context-induced relapse test following infusion of MTEP or vehicle (VEH) into either the NA core or dlSTR. Animals which received intra-accumbens MTEP (1 µg/side) displayed significantly less presses on the previously active lever (t(1,10) = 5.597, p<0.05; Figure 1B). There was no effect of intra-accumbens MTEP during the abstinent relapse test on subsequent extinction learning (Figure 1C). A two-way ANOVA conducted on these data revealed no effect of Group (F(1,10)= 1.121, n.s.) or Group × Day interaction (F(1,10)= 1.959, n.s.), however there was a significant effect of Day (F(3,30)=7.360, p<0.05), as both groups decreased lever presses over days. Intra-accumbens MTEP significantly attenuated cue-primed reinstatement following extinction training (t(1,13)=7.040, p<0.05; Figure 1D). Histological analysis of microinjection placements confirmed, that microinjection sites clustered within the core subregion or on the border with NA shell (Figure 1E). We found no effect of MTEP on inactive lever pressing during either the abstinent-relapse test (t(1,10)=0.218, n.s.) or cue-primed reinstatement (t(1,13)=0.87, n.s.). Mean inactive lever presses were as follows, abstinent-relapse test: MTEP: 24.8 ± 7.6, VEH: 31.1 ± 9.3; cue-primed reinstatement: MTEP: 1.6 ± 0.9, VEH: 3.43 ± 1.0.
Figure 1. MTEP infused into the NA core attenuates relapse after both abstinence and extinction.
(A) Mean number infusions attained during cocaine self-administration. (B) Intra-NA core MTEP attenuates context-induced relapse following abstinence. (C) Intra-NA core MTEP on test day has no lasting effects on extinction learning. (D) Intra-NA core MTEP significantly attenuates cue-primed reinstatement following extinction. N = 4–8/group. (E) Diagrams of the rat brain coronal sections, adapted from Paxinos and Watson (2007), show the distribution microinjection sites in the NA core. Numbers indicate distance from bregma in the anteroposterior plane. * = p<0.05 in comparison to vehicle.
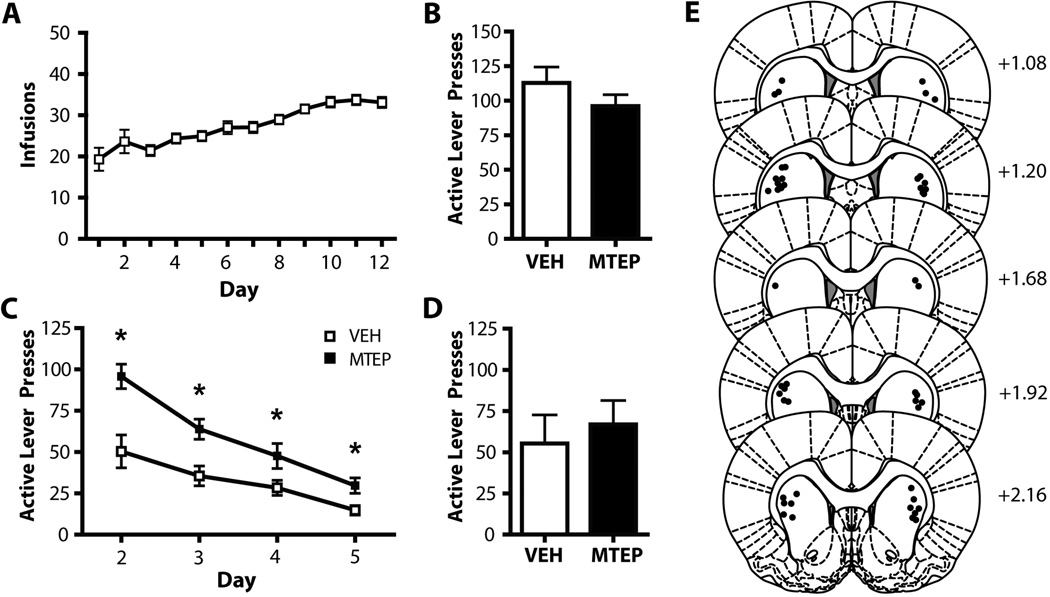
Figure 2. MTEP infused into the dlSTR does not affect relapse but has lasting effects on extinction learning.
A) Mean number infusions attained during cocaine self-administration. (B) Intra-dlSTR MTEP does not affect context-induced relapse following abstinence. (C) Intra-dlSTR MTEP prior to the context-induced relapse test (extinction Day 1) has lasting effects on extinction learning in subsequent days. (D) Intra-dlSTR MTEP does not affect cue-primed reinstatement following extinction. (E) Diagrams of the rat brain coronal sections, adapted from Paxinos and Watson (2007), show the distribution microinjection sites in the dlSTR. Numbers indicate distance from bregma in the anteroposterior plane. * = p<0.05 in comparison to vehicle. N = 10–12/group.
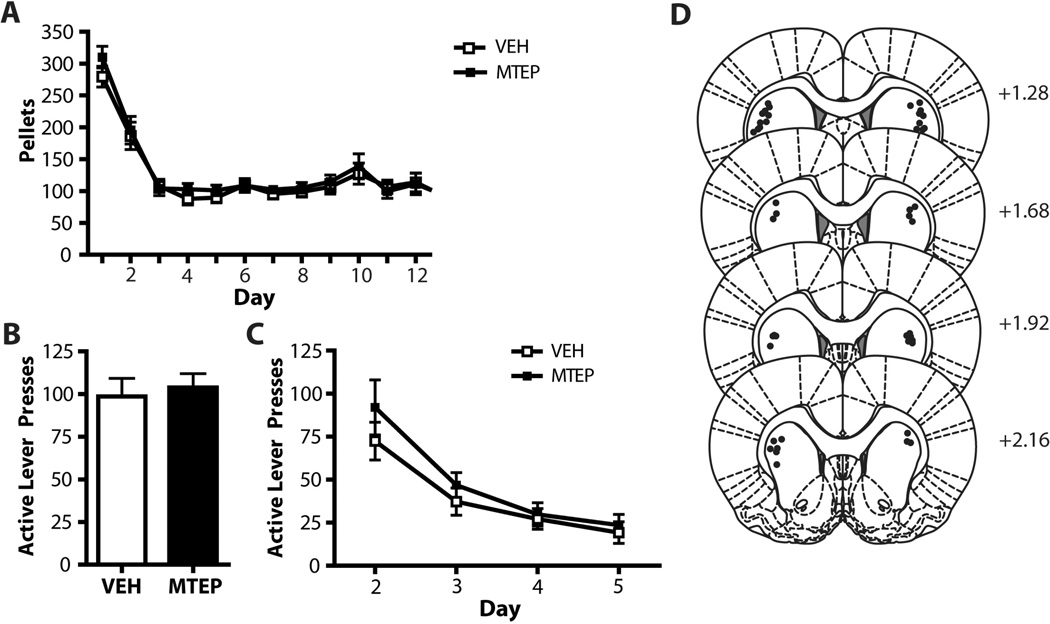
Figure 3. MTEP infused into the dlSTR does not affect relapse or extinction learning in sucrose self-administering animals.
(A) Mean number pellets attained during sucrose self-administration. (B) Intra-dlSTR MTEP does not affect context-induced relapse following abstinence from sucrose self-administration. (C) Intra-dlSTR MTEP prior to the context-induced relapse test (extinction Day 1) has no effect on extinction learning in subsequent days. (D) Diagrams of the rat brain coronal sections, adapted from Paxinos and Watson (2007), show the distribution microinjection sites in the dlSTR. Numbers indicate distance from bregma in the anteroposterior plane. N = 11–12/group.
Intra-dlSTR MTEP (5 µg/side) had no effect on context-induced relapse following 3 weeks of abstinence (t(1,21)=0.834, n.s.; Figure 2B). However, animals receiving MTEP infusions only on the day of the context-induced relapse test subsequently displayed attenuated extinction learning (Figure 2C). A two-way ANOVA revealed significant effects of Day (F(3,60)=37.632, p<0.001) and Group (F(1,20)= 5.569, p<0.05) and a significant Group × Day interaction (F(1,20)=2.948, p<0.05). Intra-dlSTR MTEP had no effect on cue-induced reinstatement following extinction training (t(1,21)=0.254, n.s; Figure 2D). Histological analysis of microinjection placements confirmed, that microinjection sites were located within the dlSTR subregion (Figure 2E). We found no effect of intra-dlSTR MTEP on inactive lever pressing during either the abstinent-relapse test (t(1,21)=1.643, n.s.) or cue-primed reinstatement (t(1,21)=1.794, n.s.). Mean inactive lever presses were as follows, abstinent-relapse test: MTEP: 14.7 ± 3.4, VEH: 22.9 ± 8.1; cue-primed reinstatement: MTEP: 5.33 ± 2.1, VEH: 3.08 ± 0.7.
An alternate explanation for the elevation in active lever pressing observed following MTEP infusions in the dlSTR (relative to VEH-infused animals) is that extinction learning occurred at an enhanced rate in the VEH-infused animals. Comparing active lever pressing between animals infused with VEH in NA core (Figure 1C) vs. the dlSTR (Figure 2C), it is evident that dlSTR-infused animals show less absolute active lever pressing on Day 2. However, it is important to consider Day 2 behavior in relation to Day 1 behavior for each cohort. In order to investigate this further, we calculated the ratio of Day 2 lever pressing to Day 1 lever pressing and found no significant difference in extinction learning between VEH-infused dlSTR and NA core animals (t(1, 14)) = 0.05, n.s.; Supp. Fig. 1). In agreement with our conclusion that MTEP infusion in the dlSTR depresses extinction learning, we found a significant difference in the rate of extinction when comparing animals infused in the dlSTR with VEH vs. MTEP (t(1,18) = 5.718, p<0.05; Supplementary Figure 1).
Intra-dlSTR MTEP had no effect on context-induced relapse of sucrose-seeking (t(1,22)=2.037; Figure 3B) and no effect on subsequent extinction learning (Figure 3C). A two-way ANOVA revealed a significant effect of Day (F(3,63)= 69.830, p<0.001), but no effect of Group (F(1,21)=2.133, n.s.) and no Group × Day interaction (F(1,21)= 0.159, n.s.). Histological analysis of microinjection placements confirmed, that microinjection sites were located within the dlSTR subregion (Figure 3D). There was no effect of MTEP on inactive lever presses (t(1,22)=2.463). Mean inactive lever presses for the MTEP-treated animals was 20.3 ± 6.7 while the mean for the VEH-treated animals was 9.1 ± 1.7.
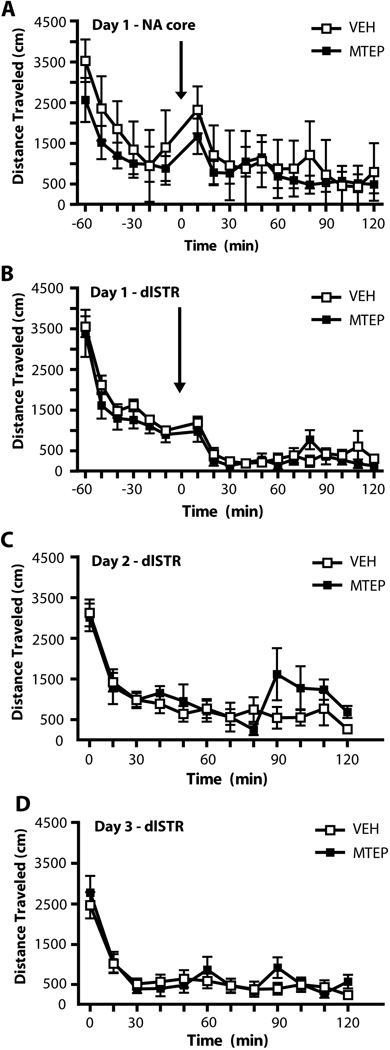
To investigate whether the significant attenuation of both context- and cue-induced relapse occurred due to locomotor-suppressive effects of intra-accumbens MTEP, animals were placed into a locomotor chamber for a 60 min habituation period, followed by infusion of either MTEP or vehicle and a 2 h test (Figure 4A). A two-way ANOVA conducted on this data revealed a significant effect of Time (F(17,170)=12.545, p<0.001) but no effect of Group (F(1,10)=0.244, n.s.) and no Group × Time interaction (F(1,10)=0.881, n.s.). Because the context-induced relapse and cue-primed reinstatement tests were conducted using a counterbalanced, cross-over design all NA core animals had a prior history of both MTEP and VEH infusions prior to locomotor testing. To investigate whether attenuated extinction learning in the days subsequent to MTEP infusion into the dlSTR was an artifact of increased locomotion, animals were placed into a locomotor chamber for a 60 min habituation period, followed by intra-dlSTR infusion of either MTEP or vehicle and a 2 h test (Day 1; Figure 4B). A two-way ANOVA conducted on this data revealed a significant effect of Time (F(17,170)=48.826, p<0.001) but no effect of Group (F(1,10)=0.855, n.s.) and no Group × Time interaction (F(1,10)=0.810, n.s.). Locomotor activity was also recorded for 2 h (without prior infusion) on the two days following Day 1 and there were no changes in locomotion in animals which had received MTEP infusions on Day 1 (Figure 4C,D). A two-way ANOVA conducted on Day 2 data revealed a significant effect of Time (F(11,110)=15.953, p<0.001) but no effect of Group (F(1,11)=0.611, n.s.) and no Group × Time interaction (F(1,11)=1.516, n.s.). A two-way ANOVA conducted on Day 3 data revealed a significant effect of Time (F(11,110)=22.125, p<0.001) but no effect of Group (F(1,11)=0.014, n.s.) and no Group × Time interaction (F(1,11)=0.788, n.s.). A subset of cocaine- and sucrose-self-administering animals with dlSTR cannulae were used to investigate the effects of MTEP on locomotor activity: the cocaine animals all had a history of both MTEP and VEH injections; however, the sucrose animals were only tested once with either MTEP or VEH prior to locomotor testing. We observed no effect of prior treatment on locomotor activity of the sucrose self-administering animals.
Figure 4. Locomotion was not affected by MTEP infused into the NA core or the dlSTR.
Infusion occurred at Time 0 as indicated by arrows. (A) Intra-accumbens MTEP infusion did not affect locomotion (total distance traveled) immediately following infusion. N= 6/group. (B) Intra dlSTR MTEP infusion did not affect locomotion immediately following infusion or on the days following infusion (C,D). N = 5–7/group.
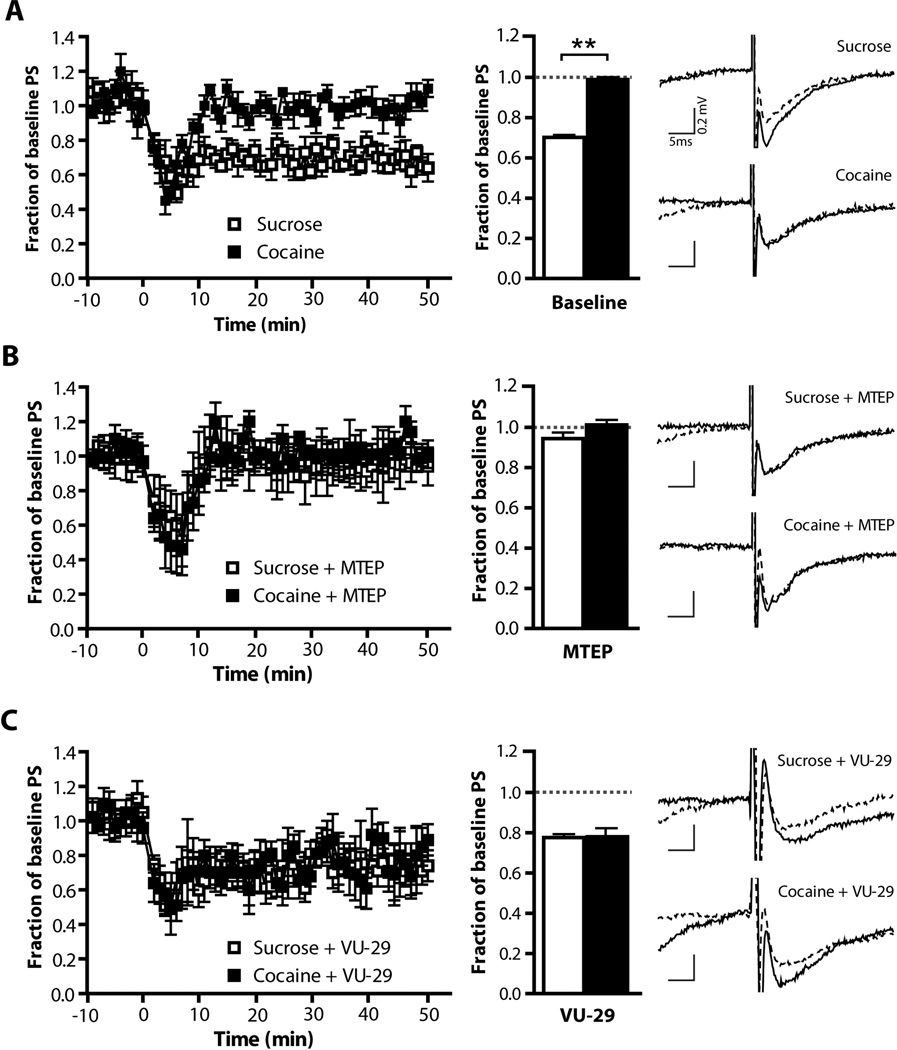
We have previously shown that LTD, a form of mGluR5-dependent plasticity, cannot be induced in the NA core of animals with a history of cocaine self-administration and extinction training (Knackstedt et al., 2010). Here we wanted to investigate whether disrupted LTD in the dlSTR could account for the effects of MTEP on extinction learning. LTD was induced in slices taken from the dlSTR following 3 weeks of abstinence from either sucrose or cocaine self-administration. Figure 5 shows the field amplitudes (normalized to baseline). We found a loss of the ability to induce LTD in animals with a history of cocaine self-administration (Figure 5A). A 2-way ANOVA revealed significant effects of Time (F(59,826)=10.04,p<0.001), Group (F(1,826)=13.89, p<0.01) and a significant Time × Group interaction (F(59,826)=4.37, p<0.001). An independent-sample t-test conducted on the field amplitudes recorded 30 to 45 min post-LTD induction (normalized to baseline) revealed a significant difference between sucrose and cocaine self-administering animals (t(1,14)=16.88, p<0.001; Figure 5A) indicating a loss of LTD in the cocaine group. Applying MTEP (1µM) to the recording bath abolished LTD in sucrose animals while having no additional effect in the cocaine group (Figure 5B; Group: F(1,826)=0.29, n.s.; Time × Group : F(59, 826)=0.37, n.s.). Thus, after MTEP field amplitudes did not differ between sucrose and cocaine groups (t(1,14)= 1.761). On the other hand, treating slices with the mGluR5 PAM VU-29 (1µM) restored LTD in slices taken from animals with a history of cocaine self-administration.(Figure 5C; t(1,12)=0.1337). Comparing the field potentials over time following VU-29 application, a two-way ANOVA revealed no differences between groups (Figure 5C left panel; Group: F(1,590)=0.0, n.s.; Time × Group interaction: F(59,590)=0.4, n.s.).
Figure 5. Cocaine self-administration followed by 3 weeks of abstinence impairs mGluR5-dependent LTD in the dlSTR slices.
Field amplitudes normalized to baseline; LTD was induced at Time 0. The left panels show field amplitudes across time while the middle panels show the change in average amplitude during the 30 – 45 minutes after LTD induction. The right panels show representative traces both pre- (dashed line) and post-LTD induction (solid line). (A) LTD could be induced only in slices taken from animals with a history of sucrose self-administration but not in cocaine. (B) Applying MTEP (1µM) to the recording bath abolished LTD in sucrose animals while having no additional effect in the cocaine group. (C) Treating slices with the mGluR5 PAM VU-29 (1 µM) restored LTD in slices taken from animals with a history of cocaine. N = 3–4/experiment. **=p<0.01 in comparison to sucrose.
In order to assess whether a loss of LTD in the dlSTR observed in animals with a cocaine history was due to a loss of mGluR5 availability, surface expression of mGluR5 receptors was analyzed by biotinylation of slices obtained from this brain region. Figure 6A (left panel) shows the rat brain atlas coordinates used as guidelines for preparation of dlSTR slices. A representative immunoblot analysis of biotinylated slices isolated from the dlSTR is depicted in Figure 6A (right panel). Signal corresponding to mGluR5 was detected in all three (total – T, intracellular - I and surface - S) fractions. Under the low reducing conditions used in this study (5–10 mM DTT), mGluR5 were detected almost exclusively in a dimer form (~250kDa, see also (Romano et al. 1996). Since dimerization/oligomerization of G-protein coupled receptors is an essential step in the generation of “mature” functional complexes (Milligan et al. 2006) only dimeric form of mGluR5 receptor was analyzed in the present study. Two additional receptors participating in LTD induction and maintenance, mGluR1 and cannabinoid receptor 1 (CB1R; for review see Lovinger 2012), were also detected in all three fractions. Control intracellular proteins β-tubulin and DexRas1 displayed only minimal or no presence in the surface fraction (Fang et al. 2000; Holman and Henley 2007), suggesting that non-specific biotinylation of intracellular proteins was limited. On the other hand, loading control calnexin and synaptosome-associated protein SNAP-25 were present across all fractions in agreement with previous findings (Oyler et al. 1989; Okazaki et al. 2000). It should be noted, that while calnexin is traditionally believed to be a resident endoplasmic reticulum protein, it could be recruited to the plasma membrane of neuronal and non-neuronal cells (Chang et al. 1997; Tsujimura et al. 2002; Manganas and Trimmer 2004; Zhang et al. 2010).
Western blot analysis of total and biotinylated fractions was conducted on dlSTR tissue of rats which had self-administered cocaine or sucrose and underwent 3 weeks of abstinence. The ratio of protein density in the surface (biotinylated) fraction to that in the total fraction was compared between cocaine and sucrose animals for mGluR5, mGluR1 and CB1 receptors. There was a significant decrease in surface mGluR5 in following cocaine relative to sucrose (t(1,13)=2.453, p< 0.05; Figure 6B). Figure 6B also shows no significant differences in surface/total ratio for mGluR1 (t(1,13)=1.366, n.s.) or CB1 (t(1,13)=0.688, n.s.). In addition, we examined total fraction for levels of several intracellular proteins previously shown to regulate mGluR5 surface expression (Ango et al. 2002; Lee et al. 2008; Figure 6C). However, there were no differences in the expression of Homer 1b/c (t(1,14)=0.233, n.s.), calmodulin (CaM, t(1,14)=1.323, n.s.) and ε subtype of the protein kinase C (PKCε, t(1,14)=0.169).
DISCUSSION
Here we sought to investigate the role of striatal mGluR5 receptors in mediating context- and cue-induced relapse to cocaine-seeking after both abstinence and extinction. We used MTEP to target mGluR5 as it has been shown to be highly selective for mGluR5 (vs. mGluR1) and has negligible off target effects, as compared to older mGluR5 blockers, such as MPEP (Anderson et al. 2002 & 2003; Cosford et al. 2003). See et al. (2007) determined that temporary inactivation of the NA core did not attenuate context-induced relapse after abstinence, but had lasting negative effects on extinction learning. Conversely, they found that inactivation of the dlSTR attenuated context-induced relapse but had no effect on subsequent extinction learning. We hypothesized that intra-NA core and dlSTR infusions of MTEP would provide similar effects on behavior. Interestingly, we found the opposite. Blockade of mGluR5 in the NA core significantly attenuated context-induced relapse after abstinence (Figure 1B), with no observed change in subsequent extinction learning (Figure 1C). Intra-NA core MTEP also significantly attenuated cue-primed reinstatement after extinction training (Figure 1D), in agreement with the results of Wang et al., (2013). Conversely, intra-dlSTR MTEP had no effect on context-induced relapse but did significantly attenuate extinction learning on the days immediately following MTEP infusion (Figure 2B, C). There was also no observed effect of intra-dlSTR MTEP on cue-primed reinstatement following extinction training (Figure 2D). MTEP infused into the dlSTR of rats trained to self-administer sucrose pellets did not affect either context-induced relapse after abstinence (Figure 3A) or extinction learning following MTEP infusion (Figure 3B). These results imply that mGluR5 blockade is not sufficient to mimic the effects of inactivation of the same brain regions and thus a lack of signaling through these receptors may not mediate the effects observed by See et al., (2007). An alternate explanation for the discrepancy in results is that See et al. (2007) trained animals to self-administer cocaine in the absence of discrete cues while we paired both cocaine and sucrose delivery with cues so that we could later test for cue-primed reinstatement. Indeed, two studies have found that training animals to self-administer in the presence of discrete cues recruits dopamine and glutamate signaling in the dlSTR (Ito et al. 2002; Vanderschuren et al. 2005). This supports the idea that our choice to train animals to self-administer in the presence of discrete cues altered the neurocircuitry involved in context-induced relapse and extinction training. However, further investigation is needed since (1) involvement of the dlSTR in drug-cue associative processing clearly depends on the extent of training (Gabriele and See 2010), and (2) only some (AMPA but not NMDA) glutamate receptors in the dlSTR seem to regulate drug-seeking controlled by discrete cues (Vanderschuren et al. 2005). The present study is also limited in that only one dose of MTEP was infused into both the NA core and dlSTR.
MTEP infused into the NA core did not affect locomotor activity (Figure 4A), indicating that the reduction in lever pressing during testing was not due to a general suppression of locomotion. Sinclair et al. (2012) showed that a higher dose of MTEP (3 ug/side) infused in this brain region did not affect sucrose-seeking, providing further support for the specificity of the effect of mGluR5 blockade on the attenuation of cocaine-seeking. It should be noted that systemic MTEP has been found to attenuate the cue-primed reinstatement of sweetened condensed milk (Martin-Fardon et al., 2009) but not food pellets (Gass et al., 2009). We also determined that intra-dlSTR MTEP infusion did not affect locomotor activity immediately after infusion (Figure 4B) or 24 and 48 h post-infusion (Figure 4C,D). Thus, the attenuation of extinction learning (higher levels of lever responding) observed in the days subsequent to MTEP infusion in the dlSTR was not a result of an increase in locomotor behavior.
We hypothesized that blocking mGluR5 function with intra-dlSTR MTEP impaired synaptic plasticity during the context-induced relapse test (Day 1 of extinction) which prevented animals from consolidating the memory of the non-reinforced session and thus attenuating extinction learning on subsequent days. The role of group I mGluRs in regulating striatal synaptic plasticity is well-documented. Either repeated paired-pulse stimulation or short-term treatment with an mGluR1/5 agonist (such as DHPG) reliably induces cortico-striatal LTD (for review see Lovinger 2012). A number of studies have shown that chronic non-contingent or contingent cocaine exposure alters LTD in the NA (Thomas et al. 2001; Knackstedt et al. 2010; McCutcheon et al. 2011a; Huang et al. 2012), but regulation of LTD by cocaine or other drugs of abuse in the dSTR has not been investigated. While Centonze et al. (2006) described depotentiation of cortico-striatal synapses in the dSTR following non-contingent cocaine administration, these findings only suggest that cocaine can block the reversal of LTP rather induce LTD. Here we show for the first time that cocaine-self-administration impairs LTD in dlSTR, demonstrated as a difference in baseline LTD between cocaine and sucrose self-administering animals (Figure 5A). We also found that MTEP only significantly impaired the ability to induce LTD in slices from animals which self-administered sucrose and not cocaine (Figure 5B), likely because MTEP could not further impair LTD in slices from cocaine self-administering animals. Bath application of VU-29, a positive allosteric modulator of mGluR5, restored LTD in slices from cocaine self-administering animals (Figure 5C), indicating that the loss of ability to induce LTD stemmed from impairment of mGluR5 function rather than from a general cocaine-induced disruption of electrophysiological properties of neurons in the dlSTR. In this paper we attribute the impairment of mGluR5-dependent LTD in the dSTR to a reduced cell surface expression mGluR5 after cocaine (but no sucrose) self-administration. We have previously shown a similar reduction in surface mGluR5 in the NA core following extinction from cocaine self-administration which was accompanied by an increase in Homer 1b/c expression. Based on work showing that Homer 1 b/c can internalize mGluR5 as well as serve as a component of the signaling scaffold (Ango et al. 2002), we hypothesized that the upregulation in Homer 1b/c levels caused a decrease in surface mGluR5. Here we did not observe a similar increase in Homer 1b/c (Figure 6B), nor did we observe any changes in several other intracellular proteins previously described to regulate mGluR5 trafficking such as PKCε and CaM (Figure 6C, Roche et al. 1999; Lee et al. 2008). However, the mGluR5 intracellular tail interacts with a host of scaffolding and signaling proteins (Mao et al. 2008; Enz 2012), and some these proteins could enhance or negate Homer-dependent regulation of mGluR5 trafficking (Hu et al. 2012). The cellular mechanism regulating mGluR5 surface expression in animals with a cocaine history will be evaluated in future studies.
mGluR1/5 work in concert with the endocanabinoid system to regulate synaptic depression that underlies some forms of learning and memory, including extinction. Thus systemic administration of a CB1 receptor antagonist or conditional deletion of mGluR5 attenuates the extinction of fear conditioning (Marsicano et al. 2002; Xu et al. 2009). Blockade of CB1 receptors in the dSTR interferes with the extinction of a striatal-based response-learning task (Rueda-Orozco et al. 2008). Because we found a decrease in surface mGluR5 (Figure 6B) accompanying a loss of the ability to induce LTD, we suggest that the integrity of the mGluR5-dependent LTD in the dSTR is critical for post-cocaine extinction learning. In support, we have previously shown that knockdown of the immediate early gene Arc in the dlSTR does not alter context-induce relapse, but attenuates extinction learning in a manner very similar to the present study (Hearing et al. 2011). Arc protein is a regulator of synaptic and experience-dependent plasticity and is typically activated during mGluR5-dependent LTD events (Waung et al. 2008). Interestingly, there seem to be a critical time period when inhibition of mGluR5 function exerts lasting effects on extinction learning, as a single infusion of MTEP into dSTR attenuated extinction on the following 4 days. As the identity of exact molecular mechanisms remains unknown, we can only speculate to the causes of the duration of this effect. It has been shown that in addition to LTD, stimulation of striatal mGluR1/5 receptors produces an insertion of GluR2-containing AMPA receptors into the membrane (McCutcheon et al. 2011a). Perhaps a long-lasting loss of mGluR5-AMPA functional synergism contributes to a persistent behavioral impairment.
The electrophysiological data also indicate that MTEP infusion into the dlSTR likely impaired LTD in sucrose animals during the context-induced relapse test, and yet this group exhibited normal extinction learning on subsequent days (Figure 3B). Thus, a loss of LTD in this region is not the sole cause for the attenuation of extinction learning observed in animals with a history of cocaine self-administration. Instead, it is possible that animals with a history of cocaine self-administration possess deficits in additional brain regions involved in extinction learning, while those regions remain ‘healthy’ in sucrose animals. For example, the inframlimbic cortex, NA shell, and basolateral amygdala (BLA) are regions which regulate extinction learning following both drug self-administration and fear conditioning (Peters et al. 2008; Peters et al. 2009). It is possible that cocaine induces pathologies in these regions which then result in the dlSTR exerting more control over extinction learning than in sucrose animals which have intact extinction learning circuitry (for review see Kalivas 2008). Importantly, the inability to recruit other circuits to compensate for striatal dysfunction has been also observed in cocaine addicts (Hanlon et al. 2009).
The context-primed relapse test likely engages extinction learning as it is the first occasion on which animals are placed into the self-administration environment and presses on the previously active lever are not reinforced. Thus, a reduction in responding on the previously reinforced lever during that test could be viewed as facilitated extinction learning and account for the reduction in lever presses following MTEP infusion into the NA core (Figure 1B). However, that is unlikely to have occurred following MTEP infusion, as it is positive, not negative, allosteric modulation of mGluR5 that has repeatedly been shown to enhance multiple types of learning, including extinction learning (Ayala et al., 2009; Gass and Olive 2009a; Kufahl et al., 2012; Uslaner et al., 2009).
In conclusion, the present study suggests an important role for NA core mGluR5 in relapse following both abstinence and extinction training and for dSTR mGluR5 receptors in regulating extinction learning. In rats, the administration of mGluR5 PAMs facilitate the extinction of a cocaine-associated contextual memory and cocaine-seeking (Gass & Olive 2009a; Cleva et al. 2011). Therefore either pharmacological enhancement of mGluR5 function or increasing population of functional (surface) mGluR5 receptors may represent a novel approach toward enhancing extinction learning in the context of drug addiction and reducing the risk of relapse.
Supplementary Material
Acknowledgments
The authors thank Rebecca Madell, Phong Do, Ilan Sondheimer and Stacey Sigmon for excellent technical assistance. We also thank Dr. Stephen Lanier (MUSC, Charleston, SC) for kindly providing RasD1/AGS1 antiserum and Dr. Foster Olive (ASU, Tempe, AZ) for providing VU-29. This work was supported by the National Institutes of Health Grant R21 DA025846 (M.S.), R21 DA026010 (L.A.K), R21 MH080774 (H.T.D.), C06 RR015455, and P50 DA015369 (MUSC Neurobiology of Addiction Research Center Pilot Project, M.S.).
Footnotes
Authors Contribution
MS and LAK were responsible for the study concept and design. MS and LAK (with the assistance of technical personnel acknowledged above) conducted behavioral experiments, as well as intracranial drug administration and surface expression experiments. HTD performed electrophysiological experiments. LAK, HTD and MS analyzed the data. MS and LAK provided funds to support animal studies and wrote the manuscript. All authors have critically reviewed content and approved final version submitted for publication.
REFERENCES
- Anderson JJ, Bradbury MJ, Giracello DR, Chapman DF, Holtz G, Roppe J, King C, Cosford ND, Varney MA. In vivo receptor occupancy of mGlu5 receptor antagonists using the novel radioligand [3H]3-methoxy-5-(pyridine-2-ylethynyl)pyridine) Eur J Pharmacol. 2003;473:35–40. doi: 10.1016/s0014-2999(03)01935-6. [DOI] [PubMed] [Google Scholar]
- Anderson JJ, Rao SP, Rowe B, Giracello DR, Holtz G, Chapman DF, Tehrani L, Bradbury MJ, Cosford ND, Varney MA. [3H]Methoxymethyl-3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine binding to metabotropic glutamate receptor subtype 5 in rodent brain: in vitro and in vivo characterization. J Pharmacol Exp Ther. 2002;303:1044–1051. doi: 10.1124/jpet.102.040618. [DOI] [PubMed] [Google Scholar]
- Ango F, Robbe D, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L. Homer-dependent cell surface expression of metabotropic glutamate receptor type 5 in neurons. Mol Cell Neurosci. 2002;20:323–329. doi: 10.1006/mcne.2002.1100. [DOI] [PubMed] [Google Scholar]
- Ayala JE, Chen Y, Banko JL, Sheffler DJ, Williams R, Telk AN, Watson NL, Xiang Z, Zhang Y, Jones PJ, Lindsley CW, Olive MF, Conn PJ. mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology. 2009;34:2057–2071. doi: 10.1038/npp.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology. 2006;31:778–786. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- Centonze D, Costa C, Rossi S, Prosperetti C, Pisani A, Usiello A, Bernardi G, Mercuri NB, Calabresi P. Chronic cocaine prevents depotentiation at corticostriatal synapses. Biol Psychiatry. 2006;60:436–443. doi: 10.1016/j.biopsych.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- Cleva RM, Hicks MP, Gass JT, Wischerath KC, Plasters ET, Widholm JJ, Olive MF. mGluR5 positive allosteric modulation enhances extinction learning following cocaine self-administration. Behav Neurosci. 2011;125:10–19. doi: 10.1037/a0022339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. Journal Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosford ND, Roppe J, Tehrani L, Schweiger EJ, Seiders TJ, Chaudary A, Rao S, Varney MA. [3H]-methoxymethyl-MTEP and [3H]-methoxy-PEPy: potent and selective radioligands for the metabotropic glutamate subtype 5 (mGlu5) receptor. Bioorg Med Chem Lett. 2003;13:351–354. doi: 10.1016/s0960-894x(02)00997-6. [DOI] [PubMed] [Google Scholar]
- deWit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Edwards S, Bachtell RK, Guzman D, Whisler KN, Self DW. Emergence of context-associated GluR1) and ERK phosphorylation in the nucleus accumbens core during withdrawal from cocaine self-administration. Addict Biol. 2011;16:450–457. doi: 10.1111/j.1369-1600.2010.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enz R. Metabotropic glutamate receptors and interacting proteins: evolving drug targets. Curr Drug Targets. 2012;13:145–156. doi: 10.2174/138945012798868452. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Jaffrey SR, Sawa A, Ye K, Luo X, Snyder SH. Dexras1: a G protein specifically coupled to neuronal nitric oxide synthase via CAPON. Neuron. 2000;28:183–193. doi: 10.1016/s0896-6273(00)00095-7. [DOI] [PubMed] [Google Scholar]
- Fourgeaud L, Mato S, Bouchet D, Hemar A, Worley PF, Manzoni OJ. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci. 2004;24:6939–6945. doi: 10.1523/JNEUROSCI.0671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2004;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Gabriele A, See RE. Reversible inactivation of the basolateral amygdala, but not the dorsolateral caudate putamen, attenuates consolidation of cocaine-cue associative learning in a reinstatement model of drug-seeking. Eur J Neurosci. 2010:1024–1029. doi: 10.1111/j.1460-9568.2010.07394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Positive allosteric modulation of mGluR5 receptors facilitates extinction of a cocaine contextual memory. Biol Psychiatry. 2009a;65:717–720. doi: 10.1016/j.biopsych.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Role of protein kinase C epsilon (PKCvarepsilon) in the reduction of ethanol reinforcement due to mGluR5 antagonism in the nucleus accumbens shell. Psychopharmacology. 2009b;204:587–597. doi: 10.1007/s00213-009-1490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Osborne MPH, Watson NL, Brown JL, Olive MF. mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats. Neuropsychopharmacology. 2009;34(4):820–833. doi: 10.1038/npp.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Vasudevan P, Mueller C, Seubert C, Mantsch JR. Neuroadaptations in the cellular and postsynaptic group 1 metabotropic glutamate receptor mGluR5 and Homer proteins following extinction of cocaine self-administration. Neurosci Let. 2009;452:167–171. doi: 10.1016/j.neulet.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Wesley MJ, Porrino LJ. Loss of functional specificity in the dorsal striatum of chronic cocaine users. Drug and alcohol dependence. 2009;102:88–94. doi: 10.1016/j.drugalcdep.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing MC, Schwendt M, McGinty JF. Suppression of activity-regulated cytoskeleton-associated gene expression in the dorsal striatum attenuates extinction of cocaine-seeking. Int J Neuropsychopharmacology. 2011;14:784–795. doi: 10.1017/S1461145710001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman D, Henley JM. A novel method for monitoring the cell surface expression of heteromeric protein complexes in dispersed neurons and acute hippocampal slices. J Neurosci Methods. 2007;160:302–308. doi: 10.1016/j.jneumeth.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JH, Yang L, Kammermeier PJ, Moore CG, Brakeman PR, Tu J, Yu S, Petralia RS, Li Z, Zhang PW, Park JM, Dong X, Xiao B, Worley PF. Preso1 dynamically regulates group I metabotropic glutamate receptors. Nat Neurosci. 2012;15:836–844. doi: 10.1038/nn.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Hsu KS. Activation of NMDA receptors reduces metabotropic glutamate receptor-induced long-term depression in the nucleus accumbens via a CaMKII-dependent mechanism. Neuropharmacology. 2012;63:1298–1307. doi: 10.1016/j.neuropharm.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Huang CC, Yeh CM, Wu MY, Chang AY, Chan JY, Chan SH, Hsu KS. Cocaine withdrawal impairs metabotropic glutamate receptor-dependent long-term depression in the nucleus accumbens. J Neurosci. 31:4194–4203. doi: 10.1523/JNEUROSCI.5239-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci. 2002;22:6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox Res. 2008;14:185–189. doi: 10.1007/BF03033809. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Glutamate and reinstatement. Curr Opin Pharmacol. 2009;9:59–64. doi: 10.1016/j.coph.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci. 2010;30:7984–7992. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, Pierce RC. Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behav Brain Res. 2009;202:238–244. doi: 10.1016/j.bbr.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn Mem. 2010;17:168–175. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Lee J, Choi KY, Hepp R, Lee JY, Lim MK, Chatani-Hinze M, Roche PA, Kim DG, Ahn YS, Kim CH, Roche KW. Calmodulin dynamically regulates the trafficking of the metabotropic glutamate receptor mGluR5. Proc Natl Acad Sci U S A. 2008;105:12575–12580. doi: 10.1073/pnas.0712033105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology. 2012;58:951–961. doi: 10.1016/j.neuropharm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao LM, Liu XY, Zhang GC, Chu XP, Fibuch EE, Wang LS, Liu Z, Wang JQ. Phosphorylation of group I metabotropic glutamate receptors (mGluR1/5) in vitro and in vivo. Neuropharmacology. 2008;55:403–408. doi: 10.1016/j.neuropharm.2008.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgänsberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Baptista MA, Dayas CV, Weiss F. Dissociation of the effects of MTEP [3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine] on conditioned reinstatement and reinforcement: comparison between cocaine and a conventional reinforcer. J Pharmacol Exp Ther. 2009;329:1084–1090. doi: 10.1124/jpet.109.151357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Loweth JA, Ford KA, Marinelli M, Wolf ME, Tseng KY. Group I mGluR activation reverses cocaine-induced accumulation of calcium-permeable AMPA receptors in nucleus accumbens synapses via a protein kinase C-dependent mechanism. J Neurosci. 2011a;31:14536–14541. doi: 10.1523/JNEUROSCI.3625-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2011b;31:5737–5743. doi: 10.1523/JNEUROSCI.0350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology. 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Milligan G, Canals M, Pediani JD, Ellis J, Lopez-Gimenez JF. The role of GPCR dimerisation/oligomerisation in receptor signalling. Ernst Schering Found Symp Proc. 2006;2:145–161. doi: 10.1007/2789_2006_007. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nature Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien C. Drug addiction and drug abuse. In: Hardman JLL, Gilman AG, editors. The Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 2001. pp. 621–642. [Google Scholar]
- Okazaki Y, Ohno H, Takase K, Ochiai T, Saito T. Cell surface expression of calnexin, a molecular chaperone in the endoplasmic reticulum. J Biol Chem. 2000;275:35751–35758. doi: 10.1074/jbc.M007476200. [DOI] [PubMed] [Google Scholar]
- Oyler GA, Higgins GA, Hart RA, Battenberg E, Billingsley M, Bloom FE, Wilson MC. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol. 1989;109:3039–3052. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge JG, Tang KC, Lovinger DM. Regional and postnatal heterogeneity of activity-dependent long-term changes in synaptic efficacy in the dorsal striatum. J Neurophysiol. 2000;84:1422–1429. doi: 10.1152/jn.2000.84.3.1422. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Sterotaxic Coordinates. 6th edition ed. San Diego: Academic press; 2007. [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Bevins RA. Forced abstinence model of relapse to study pharmacological treatments of substance use disorder. Curr Drug Abuse Rev. 2009;2:184–194. doi: 10.2174/1874473710902020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, Tu JC, Petralia RS, Xiao B, Wenthold RJ, Worley PF. Homer 1b regulates the trafficking of group I metabotropic glutamate receptors. J Biol Chem. 1999;274:25953–25957. doi: 10.1074/jbc.274.36.25953. [DOI] [PubMed] [Google Scholar]
- Romano C, Yang WL, O'Malley KL. Metabotropic glutamate receptor 5 is a disulfide-linked dimer. J Biol Chem. 1996;271:28612–28616. doi: 10.1074/jbc.271.45.28612. [DOI] [PubMed] [Google Scholar]
- Rueda-Orozco PE, Montes-Rodriguez CJ, Soria-Gomez E, Mendez-Diaz M, Prospero-Garcia O. Impairment of endocannabinoids activity in the dorsolateral striatum delays extinction of behavior in a procedural memory task in rats. Neuropharmacology. 2008;55:55–62. doi: 10.1016/j.neuropharm.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Schwendt M, Hearing MC, See RE, McGinty JF. Chronic cocaine reduces RGS4 mRNA in rat prefrontal cortex and dorsal striatum. Neuroreport. 2007a;18:1261–1265. doi: 10.1097/WNR.0b013e328240507a. [DOI] [PubMed] [Google Scholar]
- Schwendt M, Knackstedt L, McGinty JF. Neuroscience Meeting Planner. San Diego, CA, USA: Society for Neuroscience; 2007b. Cocaine self-administration followed by prolonged abstinence or extinction downregulates RGS4 protein in rat forebrain: comparison with Gi/Go/Gq-proteins. p Program No. 815.810. [Google Scholar]
- See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology. 2007;194:321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]
- Sinclair CM, Cleva RM, Hood LE, Olive MF, Gass JT. mGluR5 receptors in the basolateral amygdala and nucleus accumbens regulate cue-induced reinstatement of ethanol-seeking behavior. Pharmacol Biochem Behav. 2012;101:329–335. doi: 10.1016/j.pbb.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung KW, Choi S, Lovinger DM. Activation of group I mGluRs is necessary for induction of long-term depression at striatal synapses. J Neurophys. 2001;86:2405–2412. doi: 10.1152/jn.2001.86.5.2405. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Parmentier-Batteur S, Flick RB, Surles NO, Lam JS, McNaughton CH, et al. Dose-dependent effect of CDPPB, the mGluR5 positive allosteric modulator, on recognition memory is associated with GluR1 and CREB phosphorylation in the prefrontal cortex and hippocampus. Neuropharmacology. 2009;57:531–538. doi: 10.1016/j.neuropharm.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan G, Cismowski MJ, Wang G, Vincent TS, Brown KD, Lanier SM. The Ras-related protein AGS1/RASD1 suppresses cell growth. Oncogene. 2004;23:5858–5863. doi: 10.1038/sj.onc.1207774. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; Xu J, Zhu Y, Contractor A, Heinemann SF. mGluR5 has a critical role in inhibitory learning. J Neurosci. 2009:3676–3684. doi: 10.1523/JNEUROSCI.5716-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Moussawi K, Knackstedt L, Shen H, Kalivas PW. Role of mGluR5 neurotransmission in reinstated cocaine-seeking. Addict Biol. 2013;18:40–49. doi: 10.1111/j.1369-1600.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59:84–97. doi: 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhu Y, Contractor A, Heinemann SF. mGluR5 has a critical role in inhibitory learning. J Neurosci. 2009;29:3676–3684. doi: 10.1523/JNEUROSCI.5716-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.