Abstract
Objective
Self-medication models of smoking posit that the emotional benefits of smoking reinforce and maintain cigarette use, yet research demonstrates both positive and adverse affective consequences of smoking. The current study examined longitudinal changes in adolescent mood variability and overall negative mood at various stages of smoking behavior to inform understanding of the etiology of adolescent smoking.
Method
Participants included 461 adolescents (mean age = 15.67 years, SD = 0.61; 55% girls; 56.8% white) drawn from a longitudinal study of adolescent smoking. Youth provided data on smoking behavior at baseline and a 15-month follow-up wave. Ecological momentary assessments (EMA) were used to measure overall levels of negative mood as well as within-person mood fluctuations (i.e., negative mood variability) at each wave.
Results
Findings revealed that smoking-mood relations vary across different stages of smoking behavior. Youth who rapidly escalated in their smoking during the study experienced improved mood regulation (for girls) and improved overall mood (for boys) as smoking increased. However, mood improvements were not observed among youth with sustained heavy use and symptoms of dependence.
Conclusions
The current data argue for a model of smoking that accounts for changes in risk and maintenance factors at different points along the developmental trajectory of smoking, involving elements of both self-medication and dependence.
Keywords: adolescent smoking, mood variability, Ecological Momentary Assessments (EMA)
Adolescence is a critical period of vulnerability for both the initiation of smoking as well as for the development of emotional difficulties (Jamner et al., 2003). As such, much research has examined the link between emotional distress and smoking to enhance understanding of the etiology of cigarette use. Increasingly, researchers have focused on the role of emotion regulatory processes in the development and progression of youth smoking. Emotion regulation refers to a collection of involuntary and effortful processes responsible for guiding and managing affective responses (Forbes & Dahl, 2005; Thompson, 1994). Findings from this body of literature suggest that emotional dysregulation is related to the frequency of adolescent substance use (e.g. Simons & Carey, 2002; Wills, Walker, Mendoza, & Ainette, 2006) as well as the progression to more frequent smoking among youth (Novak & Clayton, 2001; Weinstein, Mermelstein, Shiffman, & Flay, 2008). Such findings support a self-medication model of smoking (e.g., Khantzian, 1997), suggesting that youth may smoke to regulate emotional distress. Yet, few longitudinal studies have examined how smoking may actually influence adolescent emotion regulation over time. Mood regulatory benefits of smoking may reinforce and maintain smoking behavior, and thus examining how mood may change via smoking is central to understanding the development of problematic patterns of smoking in adolescence.
Research suggests that cigarette use (particularly nicotine) may indeed modulate affect in the short term. Using real-time ambulatory methodology to assess self-reported mood states prior to and immediately following cigarette use, Delfino, Jamner, and Whalen (2001) found that smoking was associated with subsequent decreases in anger and sadness and an increase in happiness among adult men, as well as subsequent reductions in anxiety among adult women. In a study employing similar real-time methods among adolescents, Mermelstein and colleagues (Mermelstein, Hedeker, Flay, & Shiffman, 2003) found that youth who were characterized as regular smokers or who escalated in their smoking over time reported enhanced positive moods and reduced negative moods immediately following cigarette use. However, smoking was not associated with mood changes in adolescents who never progressed beyond initial experimentation with cigarettes. Hedeker et al. (Hedeker, Mermelstein, Berbaum, & Campbell, 2009) demonstrated further evidence of acute mood enhancement post-smoking among adolescents, such that youth reported better moods immediately following smoking as compared to their moods during non-smoking times. Longitudinal studies suggest that smoking may also have lasting effects on adolescent mood. Examining relationships between smoking and mood lability among eighth and 10th grade students early in their smoking careers (i.e., nonsmokers who were susceptible to smoking initiation based on reported intentions, recent smoking initiators, and infrequent smokers), we found that adolescents with steep increases in smoking behavior over time experienced concurrent improvements in mood stability compared to their non-escalating peers (Weinstein et al., 2008). Similar mood-regulatory outcomes have been found for depressive symptoms, such that increased smoking predicted decreased depressive symptoms from mid- to late-adolescence (Audrain-McGovern, Rodriguez, & Kassel, 2009).
Findings from brain imaging research highlight possible neurobiological bases for the affective benefits of smoking (Brody, 2006). Such work suggests that acute and chronic nicotine exposure may contribute to mood enhancement via increased dopamine concentration in the ventral striatum/nucleus accumbens as well as inhibited monoamine oxidase (MAO) activity in the basal ganglia. These findings are consistent with animal research indicating antidepressant effects of acute and chronic nicotine administration in rats (Tizabi et al., 1999). Moreover, individuals with affect regulation difficulties may have heightened sensitivity to the therapeutic effects of nicotine (Choi, Patten, Gillin, Kaplan, & Pierce, 1997). The positive neural consequences of nicotine administration are posited to reinforce substance use (Frawley, 1988) and thus may explain the progression to, and maintenance of, regular smoking.
In sum, promising evidence lends support to the self-medication model of cigarette use, suggesting that smoking may have acute mood regulatory benefits that reinforce such behavior (Shadel, Shiffman, Niaura, Nichter, & Abrams, 2000). However, a competing hypothesis posits a use-to-distress relationship such that smoking adversely influences mood regulation over time, due to alterations in the neural circuitry associated with emotional functioning (Munafò, Hitsman, Rende, Metcalfe, & Niaura, 2007). Indeed, research with adolescents has demonstrated that chronic smoking prospectively predicts increases in depressive symptomatology (e.g., Choi et al., 1997; Munafò et al., 2007) and is associated with more negative moods overall (Hedeker et al., 2009). Mixed findings regarding the affective consequences of smoking point to the possibility of bidirectional influences between emotional functioning and adolescent smoking. Bidirectional relationships have been supported in past research, with findings indicating that emotional distress (Orlando et al., 2001) and depressive symptoms (Windle & Windle, 2001) predicted growth in smoking and, likewise, heavier smoking predicted increases in emotional distress and depressive symptoms.
Thus, the relationship between smoking and emotional functioning may change across the development of smoking behavior. Yet, few studies have investigated such reciprocal mood-smoking relations among youth using indices of mood regulation. Moreover, little attention has been paid to how mood dysregulation may differentially relate to smoking at various developmental stages of smoking behavior (i.e., nonsmoking, experimentation, regular smoking, daily smoking, and dependence). Rather, past investigations have primarily examined cross-sectional relations between mood dysregulation and continuous measures of cigarette use frequency (e.g., Wills et al., 2006; Simons & Carey, 2002; Simons, Carey, & Gaher, 2004; for an exception, see Novak & Clayton, 2001). To clarify the dynamic associations of mood regulation and smoking across critical transition points in the development of smoking, the current study examined prospective changes in negative mood variability along with overall negative mood level as a function of change in smoking level over time. Negative mood variability reflects the intensity and frequency of within-individual fluctuations in negative emotional states (Eid & Diener, 1999). In the present study as well as past work (Weinstein & Mermelstein, under review; Weinstein et al., 2008) we conceptualize mood variability as a byproduct of affect regulation, with high levels of negative mood variability reflecting maladaptive regulatory processes or affect dysregulation. Specifically, we examined changes in moods vis-à-vis changes in smoking patterns to identify how mood variability may change or improve with increased smoking, including the potential to develop nicotine dependence, over time. This study extends our prior work (Weinstein et al., 2008) by following a sample of youth further along in the progression of smoking and thus at-risk for further progression to advanced stages of cigarette use. The current sample comprised youth currently experimenting with smoking or engaging in frequent cigarette use at baseline, in an attempt to prospectively capture changes in mood across escalation to more problematic levels of use and dependence during the study. Thus, we examined youth at various stages across the trajectory of smoking experience, including abstinence, experimentation, escalation from lower use to frequent smoking, and chronic frequent smoking (i.e., youth likely to develop nicotine dependence) during a 15-month period.
In previous work on this sample (Weinstein & Mermelstein, under review), findings supported a complex self-medication model of smoking escalation, whereby adolescents with diverse affect vulnerabilities – manifesting as labile moods for girls and high negative mood for boys – may use cigarettes to stabilize mood volatility or to relieve negative moods. Specifically, girls’ with higher levels of negative mood variability and boys’ with greater negative moods were more likely to rapidly escalate in their smoking as compared to youth with more stable moods. We build on these findings in the current work by examining the reverse relationships: the longitudinal changes in mood that occurred among these youth as they progress to various stages of smoking. We used real-time methods of data assessment (i.e., ecological momentary assessments, EMA: Stone & Shiffman, 1994) to objectively assess adolescent mood as it actually occurs in daily experience. The random and frequent assessment of mood via EMA provides a finer-grained and more objective index of intraindividual mood fluctuations than can be captured using global, retrospective self-reports of trait-like mood lability. As predicted by the self-medication model of cigarette use, we hypothesized that increased levels of smoking over time (i.e., escalation) would be associated with improved affect regulation (i.e., a reduction in negative mood variability) and negative moods, whereas mood regulatory effects would lessen or worsen with chronic smoking over time. Additionally, in light of our previous findings suggesting sex differences in mood-smoking relations (Weinstein & Mermelstein, under review), exploratory analyses examined sex differences in these relationships.
Method
Design Overview
Data for this study come from a longitudinal, multi-method, natural history study of smoking among adolescents. For this study, participants completed self-report questionnaires and in-depth interviews, in addition to week-long time/event sampling via palmtop computers, at baseline and a 15-month follow-up wave.
Participants
The sample for the longitudinal study included 1,263 9th and 10th grade students from 16 Chicago-area high-schools recruited through a multi-stage process. All 9th and 10th graders at each school completed a brief screener survey (N = 12,970). Students were eligible to participate in the longitudinal study if they fell into one of four levels of smoking experience: 1) never smokers; 2) former experimenters (smoked in the last 12 months, but not in the last 90 days, and smoked fewer than 100 cigarettes in their lifetime); 3) current experimenters (smoked in the past 90 days but smoked fewer than 100 lifetime cigarettes); and 4) regular smokers (smoked in the past 30 days and have smoked more than 100 cigarettes in their lifetime). Invitation/recruitment packets were mailed to eligible students and their parents, including a random sample of the never smokers and former experimenters, and all current and regular smokers (N = 3,654). Youth were enrolled after written parental consent and student assent was obtained. Of those invited, 1,344 agreed to participate (36.8%), and 1,263 (94.0%) completed the baseline measurement wave. Agreement to participate did not vary by smoking history, race/ethnicity, or parental smoking, but girls were slightly more likely to agree to participate than boys.
The sample for the current study included a subset of the 1,263 students from the overall longitudinal study who provided EMA data at baseline (N = 461). Students were invited to carry palm-top computers if they were former experimenters (n = 112), current experimenters (n = 249), or regular smokers (n = 100); thus, all participants in the current study had previous or current smoking experience. Participants ranged in age from 13.85 years to 17.29 years (M = 15.67 years, SD = 0.61), 50.7% were 9th graders, 55% were girls, and racial/ethnic composition was as follows: 56.8% White; 15.8% African American; 20% Latino; 2.8% Asian/Pacific Islander; and 4.6% Other/Bi-racial. Average parental education for the sample was as follows: 32.3% completed high school or less; 19.5% completed some college; and 36.2% completed college or more; the remaining 12% was reported as “don’t know/not applicable” by the youth. The demographic characteristics of the participants enrolled in the EMA study were representative of the 1,263 students in the total study; no differences were found between the adolescents who did and did not participate in the EMA substudy for grade (χ2 = 3.66, p = .16), sex (χ2 = 0.54, p = .46), race/ethnicity (χ2 = 7.01, p = .32), or age (t (1261) = -1.63, p = .10).
Procedures
All procedures received approval from the Institutional Review Board at the University of Illinois at Chicago. Data collection modalities included self-report questionnaires on smoking and psychosocial functioning and EMA interviews via hand-held computers. The questionnaires were mailed to the students two weeks prior to each data collection wave, and students were instructed to bring the completed questionnaire to their in-depth interview session that occurred at their schools. Students were paid $20 upon receipt of the completed questionnaire.
The EMAs were used to assess daily mood states via hand-held palmtop computers programmed specifically for our data collection needs, with all other programs disabled. Data were stored on the device using a Microsoft Access database; at the end of the data collection week, data files were uploaded to an internal server and read into a SAS database using a complex computing routine. All participants were trained on the EMA device at the beginning of the data week and carried the device for seven consecutive days at each wave. Students completed three types of EMA interviews: in response to random prompts (“random prompt” interviews) and to actively event record every situation when they decided to smoke (“smoke” interviews) or when they decided not to smoke because of an internal decision or external restrictions (e.g., in school, lack of availability; “no smoke” interviews). Participants were allowed to carry the device at school, although were trained to temporarily disable the random prompt function during situations when they could not use a device (e.g., during a test, in response to a teacher’s requests). The device randomly prompted the adolescents approximately 5 times per day; in response to each signal, participants were trained to complete a brief (i.e., 60 to 90 second) interview about their activity, situation, and mood. Each random prompt was date- and time-stamped and recorded whether the interview was completed, missed, delayed, or disbanded. The smoke and no smoke interviews contained all of the questions from the random prompt interview, as well as the participants’ mood both before and after the event.
For all interviews, participants responded to questions on a series of sequential screens. Youth were asked to rate their mood just prior to the prompt (random prompts) or initiating the interview (smoke/no smoke interviews), with one screen for each mood adjective (i.e., “Before the signal, or smoking/choosing not to smoke, I felt sad”). Participants rated their mood according to a 10-point Likert-type scale that was presented graphically with anchors at the 1-point (Not at all), 5- (Somewhat), and 10-point (Very), and responded by moving their stylus along the scale. The screen did not advance until the student moved the stylus to indicate a response. The current study utilized the EMA mood data from the random prompt interviews as well as pre-event mood data from the smoke and no smoke interviews. Participants received a payment of $40 at the end of the baseline wave, and $50 at the 15-month wave.
Measures
Demographic information was assessed via questionnaire and included the adolescent’s age, grade, sex, race (Hispanic/Latino or not), ethnicity (White, African American, American Indian/Alaska Native, Asian, or Native Hawaiian/Other Pacific Islander), and parental education.
Smoking behavior was assessed with several items: 1) the number of days smoked in the past 30 days, with response categories ranging from 1 (none) to 9 (all 30 days), referred to as “smoking frequency”; 2) the number of cigarettes per day on days smoked in the past 30 days, with response categories ranging from 1 (none) to 11 (more than 20 per day), referred to as “smoking quantity”; 3) lifetime number of cigarettes, with response categories ranging from 1 (I have never smoked) to 9 (500 or more); and 4) seven-day smoking rate (cigarettes/day at end of week). Additionally, continuous measures of monthly smoking frequency and quantity were constructed by computing the mid-point of each response category (e.g., computing response “9 = all 30 days”, as “30”). The reliability of these retrospective self-reports of smoking behavior is supported by the strong correspondence with both daily diary reports of smoking episodes as well as interview-obtained reports of smoking behavior in our past work (Diviak, Kohler, Mermelstein, & Flay, 2001). In the current sample, agreement between EMA smoke reports and end-of-week reports of smoking history was higher than 80%; number of EMA smoke reports at baseline was also significantly and highly correlated with smoking frequency (r = .70), quantity (r = .66), and seven-day smoking rate (r = .65), ps < .0001.
Smoking Identity
To assess subjective identification with being a smoker, youth were asked two items that comprised a Smoker Identity scale: how much is being a smoker part of who you are, and how important are cigarettes in your life. Responses ranged from 1 (not at all) to 5 (very) and were averaged to form the scale. Coefficient alpha for this scale = .70.
Nicotine Dependence was measured using the seven-item adolescent version of the Fagerstrom Tolerance Questionnaire (mFTQ; Prokhorov, Pallonen, Fava, Ding, & Niaura, 1996; Prokhorov et al., 2000). Coefficient alpha for the total mFTQ score = .66. An mFTQ score of 6 or more is considered to represent a high level of nicotine dependence (Prokhorov et al., 1996).
Daily Affect (EMA)
Participants were asked on each EMA interview to rate their mood according to mood adjectives using a 10-point Likert-type scale. The adjectives were selected based on prior qualitative (focus groups and in-depth interviews) and quantitative data collection with adolescents. Consistent with factor analyses on the current data set, the following adjectives formed a strong “Negative Affect” (NA) scale: angry, frustrated, irritable, sad, and stressed, all with loadings greater than .79 (Coefficient alphas = .86 to .89 across waves). In the current sample, greater NA at baseline was related to poorer concurrent clinical outcomes, including greater symptoms of depression (r = .47) and anxiety (r = .44), trait measures of negative emotionality (r = .34), and worse negative mood regulation skills (r = -.38), ps < .0001. All correlations remained significant when examining baseline NA and follow-up outcomes.
Mood Variability (EMA)
An index of negative mood variability was constructed from EMA daily mood ratings by computing mean standard deviation scores for the NA scale for each participant across the measurement week at each wave. Thus, this index of mood variability quantifies the tendency to experience frequently varying and intense levels (i.e., dysregulation) of negative affect within a typical week. This approach has the same, if not fewer, limitations than alternate measurement approaches (e.g., spectral analysis; Eid & Diener, 1999); moreover, standard deviations have been used to quantify mood variability in the majority of EMA studies, with supported reliability and validity (Eid & Diener, 1999). Research supports relations between negative mood variability (assessed via standard deviations) and clinical outcomes in adolescents, including depressive symptoms (Silk, Steinberg, & Morris, 2003) and behavior problems (Silk et al., 2003). In the current sample, negative mood variability was positively correlated with depressive symptoms (r = .30), anxiety (r = .21), and negative emotionality (r = .27), and was inversely associated with negative mood regulation (r = -.22), ps < .001. Of note, correlations remained significant when examining baseline variability and clinical measures at the follow-up wave. Variability was also related to worse psychosocial functioning, including greater loneliness (r =.12, p = .01) and perceived stress (r = .17, p < .0001), and lower self-esteem (r = -.19, p < .001) and grade point average (r = .10, p = .03; higher scores denote worse performance), Thus, findings suggest meaningful relationships between mood variability and clinical and psychosocial outcomes. Despite increasing use of the standard deviation, few cutoffs have been established to denote maladaptive thresholds. Without guidelines, a wider range of fluctuation in negative affect was conceptualized as the result of dysfunctional mood regulation abilities (i.e., an adolescent with functional mood regulation would have less variability in daily negative moods) and thus considered to reflect problematic degrees of variability.
Results
Analytic Approach
To examine change in smoking patterns over time, longitudinal smoking groups were created based on observed change in monthly smoking frequency across baseline to the follow-up 15 month wave. Operational definitions of smoking change were created according to a priori potential points of escalation (e.g. monthly, weekly, and daily smoking) based on standardized definitions of the stages of smoking behavior (ie., nonsmoking, experimentation, regular smoking, daily smoking), resulting in the identification of seven groups of longitudinal smoking patterns. These included: nonsmokers, who did not smoke at baseline and remained abstinent at 15 months (n = 129, 32%); triers, with low levels of experimentation, as defined by zero to three days smoked in the past month at baseline and 15 months (n = 109, 27%); experimenters, who escalated from low levels of use at baseline (i.e., zero to five days of smoking in the past month) to weekly smoking at 15 months (i.e., five to 10 days smoking in the past 30 days; n = 32, 8%); rapid escalators, who escalated from low levels of use at baseline (i.e., monthly smoking of zero to five days) to near-daily or daily use at 15 months (i.e., smoking on 11 to 30 or more days in the past month; n = 34, 8%); infrequent stables, who maintained a stable level of approximately weekly smoking at baseline and 15 months (n = 37, 9%); smokers, who engaged in near daily to daily smoking at baseline and 15 months (n = 43, 11%); and quitters, who reported smoking at baseline but reported zero days of smoking at 15 months (n = 21, 5%). Given that we were examining smoking and mood at two waves, this approach captured the critical transition point (e.g., experimentation to regular use) that was the key focus of this study. Figure 1 displays the changes in smoking over time among the longitudinal smoking groups, and Table 1 displays smoking data for all groups at baseline and 15 months, including monthly smoking frequency and quantity, seven-day smoking rate, number of EMA smoke reports, nicotine dependence scores, and subjective smoking identity.
Figure 1.
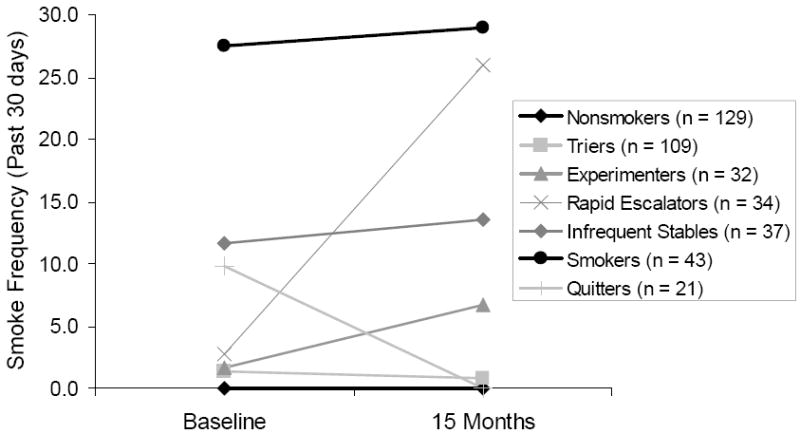
Monthly smoking frequency (number of days smoked in the past 30 days), from baseline to follow-up, by longitudinal smoking group.
Table 1.
Descriptive Statistics and Pairwise Comparisons for Smoking Measures, at Baseline and Follow-up, by Longitudinal Smoking Group
| BASELINE
|
15 MONTHS
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Smoke Frequency
|
Smoke Quantity
|
mFTQ
|
Smoke Frequency
|
Smoke Quantity
|
mFTQ
|
|||||||||
| N | M | SD | M | SD | M | SD | N | M | SD | M | SD | M | SD | |
|
|
|
|||||||||||||
| Nonsmokers | 129 | 0.00a | 0.00 | 0.00a | 0.00 | 0.79a | 0.85 | 110 | 0.00a | 0.00 | 0.00a | 0.00 | 0.77a | 0.85 |
| Triers | 109 | 1.40b | 0.95 | 0.81b | 0.83 | 1.30b | 0.85 | 105 | 0.85a | 1.03 | 0.60a,b | 0.99 | 1.26b | 0.70 |
| Experimenters | 32 | 1.69a,b | 2.12 | 0.48a,b | 0.60 | 1.16a,b | 0.88 | 31 | 6.69 | 2.02 | 177b,c,d | 1.48 | 1.55b | 0.72 |
| Rapid Escalators | 34 | 2.79b | 2.39 | 1.35b,c | 1.87 | 1.68b,c | 0.88 | 34 | 25.99 | 5.77 | 7.06e | 5.85 | 3.27 | 1.84 |
| Infrequent Stables | 37 | 11.62c | 5.32 | 2.18c,d | 1.28 | 2.08c,d | 0.83 | 37 | 13.58 | 10.86 | 2.95c | 3.08 | 2.43 | 1.29 |
| Smokers | 43 | 27.56 | 2.53 | 4.99 | 3.44 | 3.51 | 1.22 | 43 | 28.95 | 2.06 | 8.95e | 5.68 | 4.05 | 1.43 |
| Quitters | 21 | 9.70c | 8.27 | 1.79b,d | 2.18 | 1.96b,d | 1.32 | 21 | 0.00a | 0.00 | 0.00a,d | 0.00 | 1.33a,b | 1.02 |
|
|
|
|
|
|
|
|||||||||
| 7-day Smoke Rate
|
EMA Smoking
|
Smoking Identity
|
7-day Smoke Rate
|
EMA Smoking
|
Smoking Identity
|
|||||||||
| N | M | SD | M | SD | M | SD | N | M | SD | M | SD | M | SD | |
|
|
|
|||||||||||||
| Nonsmokers | 129 | 0.00a | 0.01 | 0.36a | 1.47 | 1.29a | 0.59 | 110 | 0.00a | 0.00 | 0.65 | 0.41 | 1.07a | 0.27 |
| Triers | 109 | 0.10a | 0.19 | 0.95a,b | 1.52 | 1.43a | 0.57 | 105 | 0.06a,b | 0.26 | 0.28a | 0.65 | 1.28a,b | 0.57 |
| Experimenters | 32 | 0.10a | 0.19 | 0.91a,b | 1.77 | 1.58a | 0.70 | 31 | 0.45a,b | 0.35 | 0.78a,b | 1.41 | 1.68b,c | 0.76 |
| Rapid Escalators | 34 | 0.22a,b | 0.44 | 2.71b,c | 3.74 | 1.61a | 0.67 | 34 | 4.70 | 5.56 | 8.26c | 11.65 | 3.05d | 1.15 |
| Infrequent Stables | 37 | 1.02b,c | 0.76 | 3.49c | 2.29 | 2.39b | 0.64 | 37 | 1.46b,c | 2.06 | 3.89b | 5.75 | 2.12c | 1.03 |
| Smokers | 43 | 4.08 | 3.34 | 11.49 | 9.60 | 3.24 | 1.05 | 43 | 7.24 | 4.99 | 11.14c | 10.49 | 3.33d | 0.86 |
| Quitters | 21 | 1.17b,c | 2.11 | 3.95c | 4.30 | 2.48b | 0.85 | 21 | 0.00a,c | 0.00 | 0.19a,b | 0.68 | 1.20 | 0.5 |
Note. Smoke frequency = # days smoked in the past month; Quantity = # cigarettes smoking on days smoked in the past month; mFTQ= Modified Fagerstrom Tolerance Questionnaire; 7-day Smoke Rate = #cigarettes/day during assessment week; EMA Smoking = # smoke reports recorded on EMA device; Smoking Identity = Smoking Identity Scale.
Within each smoking measure at each time point, mean values with shared alphabetic superscripts signify nonsignificant pairwise comparisons; all other pairwise comparisons were significant at p < .05.
A series of one-way between-subjects analyses of variance (ANOVAs) examined group differences in smoking variables at each wave. Note that, with the exception of smoking frequency, these smoking measures were not used to calculate group status and thus provide validity data on the groupings. As expected, analyses confirmed significant differences among the groups for smoking frequency at baseline, F (6, 384) = 599.74, p < .0001, η2partial = .90, and at 15 months F (6, 374) = 485.04, p < .0001, η2partial = .89. Additionally, significant group differences were found for all measures of smoking behavior at each wave, including: smoking quantity at baseline (F (6, 384) = 65.39, p < .0001, η2partial = .51) and 15-months (F (6, 374) = 76.82, p < .0001, η2partial = .55); seven-day smoking rate at baseline (F (6, 379) = 65.59, p < .0001, η2partial = .51) and 15-months (F (6, 375) = 64.84, p < .0001, η2partial = .51); number of EMA smoke reports at baseline (F (6, 398) = 53.98, p < .0001, η2partial = .45) and 15-months (F (6, 398) = 37.50, p < .0001, η2partial = .36); and smoking identity at baseline (F (6, 379) = 56.21, p < .0001, η2partial = .47) and 15-months (F (6, 375) = 88.51, p < .0001, η2partial = .59). Levels of nicotine dependence also significantly differed among groups at baseline, F (6, 384) = 49.78, p < .0001, η2partial = .44, and at 15 months, F (6, 374) = 67.36, p < .0001, η2partial = .51. As Table 1 reveals, mFTQ scores at baseline suggest low levels of dependence among all groups except the Smokers; however, by follow-up, Rapid Escalators and Smokers show moderate levels of dependence. Post-hoc Tukey tests examined pairwise comparisons among smoking groups, and findings are displayed in Table 1. As shown in Table 1, results at each wave for questionnaire and EMA-reports of smoking behavior, identity, and nicotine dependence scores were consistent with smoking group status; as expected, the nonsmokers, triers, and experimenters all report similar levels of smoking behavior, dependence, and identification as a smoker, and smokers are significantly different from other groups at each wave. Interestingly, the rapid escalators are similar to non/low-level smokers at baseline on all measures, but by follow-up resemble the smokers in terms of smoking behavior and identity. Thus, analyses suggest meaningful differences between smoking groups.
In addition, paired t-tests examined changes in all smoking variables between baseline and follow-up among the primary groups of interest. Consistent with their group status, rapid escalators significantly increased on all smoking measures over time (frequency t(32) = -23.50; quantity t(33) = -6.35; seven-day rate t(31) = -4.46; EMA smoke reports t(33) = -2.73; identity t(33) = -6.73; and dependence t(33) = -6.06, all ps < .01). For the triers, differences in smoking quantity, seven-day rate, and dependence between waves were not significant (ts(108) = 1.64, 0.90, and 0.67, respectively, ns), although they experienced slight but significant increases in smoking frequency (t(108) = 3.61, p < .001) and identity (t(108) = 2.61, p = .01) and a decrease in EMA smoke reports (t(108) = 4.50, p <. 001). Last, the smokers slightly but significantly escalated in several measures of smoking behavior over time (frequency t(42) = -3.63; quantity t(42) = -4.30; seven-day rate t(42) = -4.40; and dependence t(42) = -2.31, ps = .001 to .03). Smokers’ EMA smoke reports and identity did not change (t(42) = .22, -0.72, respectively, ns).
To evaluate dynamic mood-smoking patterns, random intercept mixed-effects regression models for continuous outcomes (MRMs; Laird & Ware, 1982) via SAS PROC MIXED examined change in mood variability across baseline to the 15-month wave as a function of change in smoking patterns over time. Mixed effects regression models are well-suited for the analysis of longitudinal data: these models are robust to the data dependency that occurs with the repeated assessments of individuals over time, and also can handle missing data. Additionally, MRMs account for each individual’s distinct initial level of mood variability and can accommodate the random variance across subjects. All analyses were conducted for the total sample and also stratified by sex. Given our interest in both mood variability and average mood level, all analyses examining mood variability (i.e., the standard deviations) were repeated using overall mood (i.e., mean levels of mood) as the predictor variable. Separate analyses allowed for comparisons between the mood variability-smoking and overall mood-smoking relationships.
Compliance and Attrition
Participants provided mood reports for a mean of 29.75 (SD = 7.16) random EMA prompts per person per wave. Across baseline and 15 months, participants responded to 73% of all random prompts, and missed a mean of 11.30 (SD = 7.25) random prompts. Participants provided a mean of 2.61 (SD = 5.12) smoke interviews and 2.11 (SD = 3.09) no smoke interviews across waves. Across waves, 27,324 observations of mood were analyzed.
Attrition in the current study was as follows. At the 15 month wave, 411 adolescents participated in data collection (89.2%) and 360 adolescents provided EMA data (78.1%). Analyses verified that there were no significant differences in retention for grade, sex, and race/ethnicity, nor for baseline reports of daily negative mood, negative mood variability, and monthly smoking quantity (effect sizes η2partial ranging from .00 to .01). However, participants who did not complete the 15 month wave reported significantly greater smoking frequency at baseline (i.e., number of days smoked in the past month; M = 8.53, SD = 10.63) than did those with complete data (M = 5.14, SD = 8.89), F (1, 458) = 6.14, p = .01, η2partial = .01.
Descriptive Analyses
Descriptive statistics for the main smoking and mood variables at both waves for the total sample and by gender are shown in Table 2. Table 2 also displays the outcomes of independent t-tests examining gender differences in the variables. Findings revealed that girls reported higher levels of negative affect (indicating worse mood) and negative mood variability than did boys at baseline and at 15 months. However, gender differences among smoking behaviors and nicotine dependence were not significant. Analyses also examined correlations between average negative mood and negative mood variability. Findings revealed that these constructs are significantly related (r’s range from .28 to .47 over time, ps < .001), but still distinct, dimensions of affect.
Table 2.
Descriptive Statistics for Mood and Smoking Measures at Baseline, for the Total Sample and by Gender
| Total Sample
|
Girls
|
Boys
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Range | M | SD | N | Range | M | SD | N | Range | M | SD | |
| BASELINE | ||||||||||||
| Smoking Frequency | 458 | 0.00 - 30.00 | 5.55 | 9.16 | 252 | 0.00 - 30.00 | 5.50 | 9.13 | 206 | 0.00 - 30.00 | 5.61 | 9.22 |
| Smoking Quantity | 461 | 0.00 - 15.00 | 1.22 | 2.07 | 254 | 0.00 - 15.00 | 1.19 | 1.99 | 207 | 0.00 - 15.00 | 1.24 | 2.17 |
| mFTQ | 447 | 0.00 - 6.00 | 1.57 | 1.30 | 248 | 0.00 - 6.00 | 1.54 | 1.33 | 199 | 0.00 - 6.00 | 1.60 | 1.28 |
| Overall NA | 461 | 1.02 - 8.16 | 3.53 | 1.51 | 254 | 1.12 - 8.16 | 3.79 | 1.59 | 207 | 1.02 - 7.39 | 3.21 | 1.35 ** |
| NA Variability | 461 | 0.07 - 3.41 | 1.63 | 0.62 | 254 | 0.27 - 3.18 | 1.73 | 0.58 | 207 | 0.07 - 3.41 | 1.50 | 0.64 *** |
| 15 MONTHS | ||||||||||||
| Smoking Frequency | 408 | 0.00 - 30.00 | 7.24 | 11.30 | 232 | 0.00 - 30.00 | 7.27 | 11.33 | 176 | 0.00 - 30.00 | 7.22 | 11.29 |
| Smoking Quantity | 409 | 0.00 - 25.00 | 2.10 | 4.09 | 232 | 0.00 - 25.00 | 1.77 | 3.47 | 177 | 0.00 - 20.00 | 2.53 | 4.75 |
| mFTQ | 387 | 0.00 - 7.00 | 1.76 | 1.54 | 221 | 0.00 - 7.00 | 1.69 | 1.53 | 166 | 0.00 - 7.00 | 1.86 | 1.55 |
| Overall NA | 360 | 1.00 - 9.97 | 3.32 | 1.53 | 206 | 1.05 - 9.97 | 3.50 | 1.61 | 154 | 1.00 - 8.11 | 3.08 | 1.39 * |
| NA Variability | 360 | 0.00 - 3.10 | 1.48 | 0.59 | 206 | 0.10 - 3.10 | 1.55 | 0.58 | 154 | 0.00 - 2.81 | 1.38 | 0.59 ** |
Note. NA: Negative Affect Scale - EMA; NA Mood Variability: Intraindividual standard deviations of Negative Affect Scale; Smoking Frequency: # Days smoked (past 30 days) - continuous measure; Smoking Quantity: # cigarettes on days smoked (past 30 days)-continuous measure. mFTQ: Modified Fagerstrom Tolerance Questionnaire.
For comparison of Girls versus Boys,
p < .05;
p < .01,
p < .001
Longitudinal Smoking-Mood Relationships
We hypothesized that mood variability and overall mood would change as a function of changes in smoking patterns over time, with mood becoming more stable and less negative among the adolescents with increased smoking (i.e., smoking escalators) versus those at the lower and upper ends of the smoking continuum. To test hypotheses, three planned contrast models were evaluated: (1) the triers/experimenters versus the rapid escalators; (2) the smokers versus the rapid escalators; and (3) the nonsmokers versus the smokers. Each contrast MRM included the effects of Smoking Group (Specific contrast), Time (Baseline, 15-months), and Smoking Group × Time on mood variability. Separate MRMs were conducted for the total sample and by gender. Identical contrast models were then evaluated for mean negative mood. For inclusion in these analyses, participants had to provide EMA data at both waves (Ns and number of mood observations fir each model are listed in the tables and described below).
Negative Mood Variability
Results of all MRMs for negative mood variability on the total sample and by gender, including effect sizes for allinteractions, are presented in Table 3; given the significant gender differences in longitudinal patterns, only analyses stratified by gender are discussed. In addition, Figure 2 displays estimated negative mood variability over time as a function of smoking group for girls (upper portion) and boys (lower portion).
Table 3.
Mixed Effects Regression Contrast Models on Negative Mood Variability, for the Total Sample and by Gender
| TOTAL
|
GIRLS
|
BOYS
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Estimate | SE | t | p | N (observations) | Estimate | SE | t | p | d | N (observations) | Estimate | SE | t | p | d | |
| Contrast 1 | 148 | 87 (174) | 61 (122) | ||||||||||||||
| Intercept | 1.64 | .05 | 30.96 | .000 | 1.70 | .07 | 25.20 | .000 | 1.56 | .08 | 19.02 | .000 | |||||
| Time | -0.13 | .05 | -2.53 | .012 | -0.18 | .07 | -2.47 | .015 | -0.07 | .07 | -0.92 | ns | |||||
| Group | 0.09 | .12 | 0.72 | ns | 0.24 | .15 | 1.58 | ns | -0.16 | .19 | -0.81 | ns | |||||
| Group × Time | -0.06 | .12 | -0.53 | ns | -0.09 | .17 | -0.53 | ns | 0.14 | -0.02 | .17 | -0.13 | ns | 0.04 | |||
| Contrast 2 | 66 | 39 (78) | 27 (54) | ||||||||||||||
| Intercept | 1.60 | .09 | 17.27 | .000 | 1.67 | .11 | 15.41 | .000 | 1.52 | .15 | 10.13 | .000 | |||||
| Time | 0.08 | .09 | 0.86 | ns | 0.14 | .13 | 1.07 | ns | 0.00 | .13 | -0.03 | ns | |||||
| Group | 0.13 | .14 | 0.88 | ns | 0.28 | .16 | 1.69 | ns | -0.12 | .24 | -0.50 | ns | |||||
| Group × Time | -0.28 | .15 | -1.92 | .059 | -0.41 | .20 | -2.04 | .048 | -0.66 | -0.09 | .20 | -0.44 | ns | 0.2 | |||
| Contrast 3 | 154 | 87 (174) | 67 (134) | ||||||||||||||
| Intercept | 1.52 | .06 | 26.75 | .000 | 1.72 | .07 | 23.13 | .000 | 1.27 | .08 | 16.02 | .000 | |||||
| Time | -0.14 | .06 | -2.26 | .025 | -0.18 | .09 | -1.97 | .052 | -0.09 | .08 | -1.13 | ns | |||||
| Group | 0.08 | .11 | 0.71 | ns | -0.05 | .15 | -0.36 | ns | 0.25 | .16 | 1.52 | ns | |||||
| Group × Time | 0.22 | .12 | 1.78 | .077 | 0.32 | .18 | 1.80 | .076 | 0.44 | 0.08 | .16 | 0.53 | ns | 0.2 | |||
Note. Contrast 1: Triers/Experimenters v. Rapid Escalators; Contrast 2: Smokers v. Rapid Escalators; Contrast 3: Nonsmokers v. Smokers. Observations = number of mood reports examined.
Effect sizes for Group × Time effects calculated via Cohen’s d.
Figure 2.
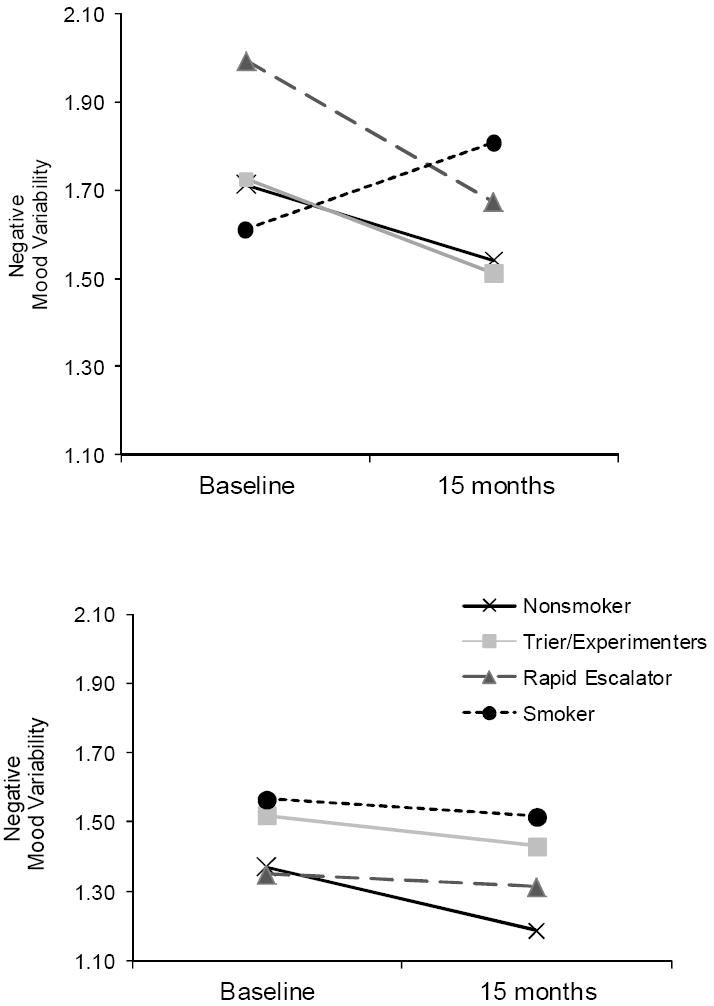
Estimated negative mood variability across baseline to 15 months as a function of longitudinal smoking pattern, for girls (upper portion) and boys (lower portion). Contrast 1: Trier/Experimenter v. Rapid Escalator; 2: Smokers v. Rapid Escalators; 3: Nonsmoker v. Smoker.
Contrast 1: Rapid Escalators v. Triers/Experimenters
Results for girls revealed a significant time effect but neither a Smoking Group nor Group × Time effect. As Figure 2 reveals, girls who escalated in their smoking (n = 17) as well as girl triers/experimenters (n = 70) experienced a significant reduction in mood variability over time. For boys, no effects were found to be significant. As Figure 2 reveals, boy rapid escalators (n = 11) and triers/experimenters (n = 50) exhibited similar and stable levels of variability over time.
Contrast 2: Smokers v. Rapid Escalators
Results of the second model among girls revealed a significant Smoking Group × Time interaction, with a medium-large effect size. To identify the source of this effect, follow-up MRMs were conducted by smoking group. As Figure 2 illustrates, for rapid escalators (n = 17), negative mood variability decreased as they increased in smoking over time (Estimate = -0.31, SE = .15, p < .05), whereas increases over time for the smokers (n = 22) were not significant; Estimate = 0.18, SE = .14, ns. Again, findings for boys were not significant: rapid escalators (n = 11) and smokers (n = 16) had similar patterns of negative mood variability.
Contrast 3: Nonsmokers v. Smokers
Results for the girls revealed a time effect and a trend for Smoking Group × Time; effects of smoking status on changes in mood variability approached a medium effect size. Follow-up analyses indicated that girl nonsmokers (n = 65) experienced a decrease in mood variability that approached significance (Estimate = -0.17, SE = .09, p = .06) whereas changes for the girl smokers (n = 22) were not significant. In contrast, boy smokers (n = 16) and nonsmokers (n = 51) exhibited similar mood variability patterns overall and over time; no effects were significant.
Overall Negative Mood
Findings for models examining overall negative mood are presented in Table 4; results for gender-stratified analyses are discussed below. In addition, Figure 3 displays overall negative mood over time as a function of smoking group for girls (upper portion) and boys (lower portion). Gender-specific ns are identical to those listed above.
Table 4.
Mixed Effects Regression Contrast Models on Overall Negative Mood, for the Total Sample and by Gender
| TOTAL
|
GIRLS
|
BOYS
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Estimate | SE | t | p | N (observations) | Estimate | SE | t | p | d | N (observations) | Estimate | SE | t | p | d | |
| Contrast 1 | 148 | 87 (5,963) | 61 (2,256) | ||||||||||||||
| Intercept | 3.69 | .13 | 28.15 | .000 | 3.87 | .18 | 21.80 | .000 | 3.42 | .18 | 19.14 | .000 | |||||
| Time | -0.16 | .04 | -3.95 | .000 | -0.23 | .05 | -4.18 | .000 | -0.07 | .06 | -1.11 | ns | |||||
| Group | 0.41 | .30 | 1.37 | ns | 0.62 | .40 | 1.54 | ns | 0.08 | .42 | 0.18 | ns | |||||
| Group × Time | -0.52 | .09 | -5.94 | .000 | -0.20 | .12 | -1.71 | .088 | -0.46 | -0.99 | .13 | -7.50 | .000 | -2.50 | |||
| Contrast 2 | 66 | 39 (3,105) | 27 (2,269) | ||||||||||||||
| Intercept | 3.67 | .22 | 16.48 | .000 | 3.85 | .31 | 12.53 | .000 | 3.42 | .27 | 12.53 | .000 | |||||
| Time | -0.07 | .06 | -1.04 | ns | -0.18 | .09 | -2.08 | .037 | 0.08 | .09 | 0.87 | ns | |||||
| Group | 0.43 | .34 | 1.25 | ns | 0.64 | .47 | 1.37 | ns | 0.08 | .43 | 0.19 | ns | |||||
| Group × Time | -0.62 | .10 | -6.10 | .000 | -0.24 | .13 | -1.82 | .069 | -0.59 | -1.14 | .15 | -7.53 | .000 | -2.95 | |||
| Contrast 3 | 154 | 87 (6,070) | 67 (4,750) | ||||||||||||||
| Intercept | 3.14 | .12 | 25.71 | .000 | 3.37 | .17 | 19.51 | .000 | 2.83 | .16 | 17.29 | .000 | |||||
| Time | -0.20 | .04 | -4.95 | .000 | -0.33 | .06 | -5.77 | .000 | -0.03 | .05 | -0.59 | ns | |||||
| Group | 0.54 | .24 | 2.19 | .030 | 0.48 | .34 | 1.40 | ns | 0.59 | .33 | 1.76 | .083 | |||||
| Group × Time | 0.13 | .07 | 1.75 | .081 | 0.14 | .11 | 1.35 | ns | 0.33 | 0.11 | .10 | 1.12 | ns | 0.32 | |||
Note. Contrast 1: Triers/Experimenters v. Rapid Escalators; Contrast 2: Smokers v. Rapid Escalators; Contrast 3: Nonsmokers v. Smokers. Observations = number of mood reports examined.
Effect sizes for Group × Time effects calculated via Cohen’s d.
Figure 3.
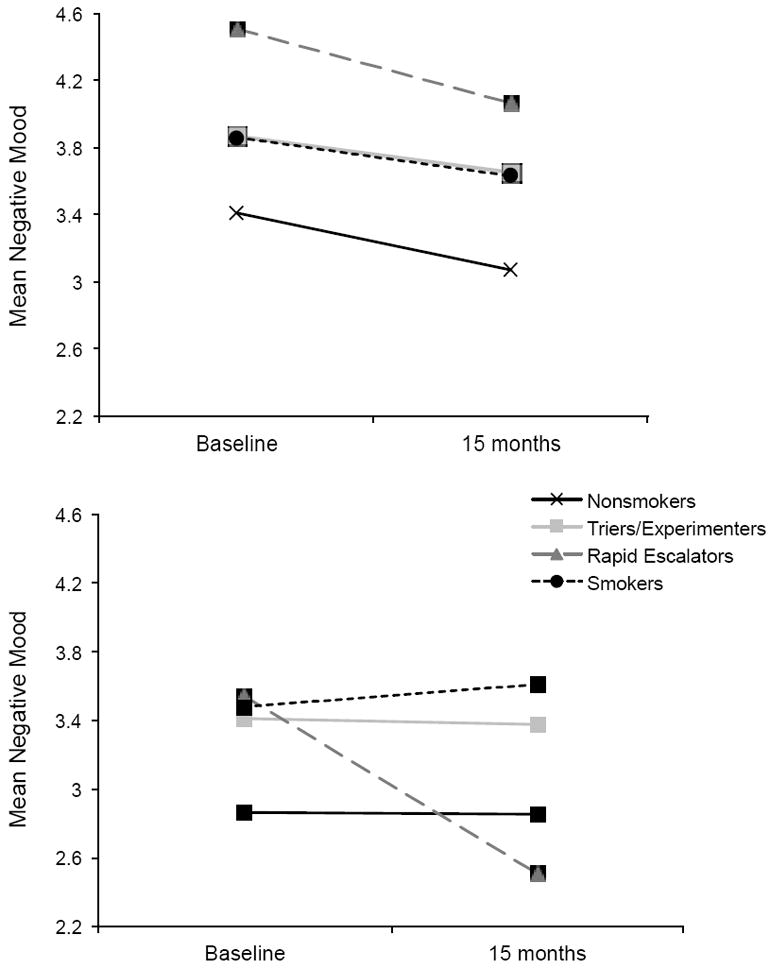
Estimated mean negative mood across baseline to 15 months as a function of longitudinal smoking pattern, for girls (upper portion) and boys (lower portion). Contrast 1: Trier/Experimenter v. Rapid Escalator; 2: Smokers v. Rapid Escalators; 3: Nonsmoker v. Smoker.
Contrast 1: Triers/Experimenters v. Rapid Escalators
Findings for boys revealed significant Time and Group × Time effects. Follow-up analyses revealed that, as shown in Figure 3, boy rapid escalators improved in negative mood over time (Estimate = -1.10, SE = .11, p < .001) whereas the triers/experimenters remained stable over time (Estimate = -0.07, SE = .06, ns). The magnitude of changes in mood over time for triers/experimenters versus rapid escalators was very large (d = 2.50). Analyses among girls revealed a significant Time and marginal Group × Time effect. As Figure 3 indicates, girl escalators improved in their negative mood over time (Estimate = -0.43, SE = .10, p < .001) as did the triers/experimenters (Estimate= -0.22, SE = .05, p < .001), with slightly greater changes for the rapid escalators. The magnitude of changes for escalators versus triers/experimenters approached a medium effect size.
Contrast 2: Smokers v. Rapid Escalators
Findings revealed a significant and large group by time interaction for boys, indicating that changes in negative mood over time varied as a function of smoking pattern. Contrary to the improvements in negative mood among the rapid escalators over time, the boy smokers remained stable in their negative mood across baseline to follow-up (Estimate = 0.08, SE = .10, ns; see Figure 3). Additionally, findings revealed a significant effect for time and a marginal, yet moderately-sized group by time interaction among girls. Rapid escalators and smokers both experienced significant reductions in negative mood over time (Smokers Estimate = -0.18, SE = .09, p < .05), but changes were slightly larger for the girl escalators (see Estimate above).
Contrast 3: Nonsmokers v. Smokers
Among girls, only the Time effect was significant: nonsmokers and smokers exhibited similar reductions in negative mood over time. Findings for boys revealed a marginal Smoking Group effect only. Thus, boy nonsmokers experienced slightly lower (i.e., better) levels of negative mood at baseline and over time versus the smokers.
Discussion
To inform understanding of the mood-smoking relationships involved in the progression to, and maintenance of, smoking, we examined the relationships between affect dysregulation – operationalized as negative mood variability – and developmental patterns of smoking behavior among a diverse sample of adolescents. Our central finding was that smoking-mood relations varied by gender and by stages of smoking. Findings, although preliminary and limited by small group sizes, suggest a possible self-medication function of smoking during the initial rise of smoking: the rapidly escalating youth showed mood improvements over time, as smoking increased from less than weekly use at baseline to near-daily use by follow-up. Results are consistent with our prior work among youth early in their smoking careers (Weinstein et al., 2008). Moreover, in parallel to previous findings with this sample (Weinstein & Mermelstein, under review), longitudinal changes in the specific dimensions of negative affect varied by gender. Findings point to the salience of mood variability for girls but overall mood level for boys. Specifically, in contrast to the smokers, girl escalators experienced a stabilization of negative mood variability with increased smoking over time; girls whose smoking escalated also experienced trends in improved negative affect. The effects of changes in girls’ smoking behavior (i.e., escalating versus consistent frequent smoking) on longitudinal mood change was moderately-sized for both mood variability and overall negative mood, suggesting meaningful differences between these smoking groups in their mood patterns over time. Boys whose smoking escalated also demonstrated mood improvements, with overall negative moods improving with increased cigarette use. Moreover, the effect of smoking status on longitudinal mood changes for boys was extremely robust, indicating meaningful differences between boys who escalate in their smoking versus those who only experiment or who maintain frequent use.
In contrast to escalators, girls who smoked at consistently higher levels did not experience improvement in mood across the study. Although interpretations must be made cautiously given that the same youth were not followed from low to frequent use to sustained daily smoking, findings suggest that the relationship between moods and cigarette use among girls differs by stage of smoking. Specifically, labile girls may escalate in their smoking over time to level their fluctuating negative moods, and initially may experience a stabilization of moods (i.e., improved affect regulation) with increased use. However, these patterns were not observed among youth who maintain high levels of use. The current findings extend our prior work by examining emotional outcomes at more advanced stages of smoking. Bi-directional smoking-mood relations were found in previous longitudinal work with adolescents (e.g., Orlando et al., 2001; Windle & Windle, 2001) and, thus, past and present work point to the possibility of transactional relationships between smoking and mood with progression in smoking. Such dynamic associations may help reconcile conflicting findings in the extant literature regarding unidirectional distress-to-use (e.g., Choi et al., 1997; Wu & Anthony, 1999) and use-to-distress relationships (e.g., Repetto, Caldwell, & Zimmerman, 2005; Duncan & Rees, 2005), as unidirectional models capture only a static portion of the shifting risk and maintenance processes involved in adolescent smoking.
Mood benefits experienced during the progression of smoking may play an important role in the development of regular smoking and dependence. Learning models of addiction posit that smoking-related mood improvement perpetuates smoking through negative reinforcement (Shadel et al., 2000). Moreover, cigarette use may enhance a girl’s sense of emotional control by modulating the frequency and/or intensity of their affective changes (i.e., reducing the swings between emotional extremes), and thus serves an important mood regulatory function that increases the likelihood of future smoking (Khantzian, 1990). Several mechanisms may account for the observed mood regulatory trends accompanying increased smoking levels. Cigarette use may initiate or influence key emotion regulation processes, including neurophysiological responses, attentional processes, and behavioral responses (Thompson, 1994). Nicotine exposure may directly enhance or stabilize moods via effects on dopaminergic and monoamine oxidase activity (Brody, 2006) or the desensitization of neuronal nicotinic acetylcholine receptors (nAChR; Brody et al., 2006; Quick & Lester, 2002; Shytle et al., 2002). In addition, the act of smoking, by way of a physical relocation or a narrowing of attention, may offer a reprieve from distressing situations or cognitions (Byrne & Mazanov, 1999; Kassel, Stroud, & Paronis, 2003). Such processes may elevate mood or enhance emotional control in the short-term.
Yet with maintained habitual smoking, dependence may develop (USDHHS, 1998) and smoking-related mood benefits may cease or reverse. Past longitudinal work suggests that cigarette use prospectively predicts increased depressive symptoms among youth with heavy levels of use (e.g., Choi et al., 1997; Munafò et al., 2008; Windle & Windle, 2001) and among nicotine-dependent young adults specifically (Breslau, Kilbey, & Andreski, 1991), but less work has focused on adolescent mood variability. Future research should investigate whether frequent cigarette use that is sustained over time adversely affects mood stability, given suggestive trends observed in the current study. Changes in mood variability with prolonged smoking may stem from the interplay of withdrawal-related mood changes and distress that comes from smoking for a variety of reasons. Withdrawal models assert that nicotine dependency exacerbates emotional distress via nicotine withdrawal (Parrott, 1999), with symptoms worsening at heavier levels of use (Colby, Tiffany, Shiffman, & Niaura, 2000; Parrott, 2006). Thus, with sustained frequent smoking, girl smokers may have experienced withdrawal symptoms during periods of abstinence, followed by only short-term mood improvements subsequent to nicotine administration. This explanation is consistent with findings that mood improvements were not seen among youth with sustained frequent use and higher dependence symptoms, versus the stabilization of mood observed among rapid escalators (who had lower symptoms of dependence even at follow-up). Importantly, addiction models posit that the alleviation of withdrawal symptoms may maintain regular cigarette use and nicotine dependence via negative reinforcement (Baker, Piper, McCarthy, Majeski, & Fiore, 2004).
In addition to the effects of nicotine withdrawal, alternate biological mechanisms proposed to account for a smoking-to-distress relationship include the neurochemical effects of nicotine on mood regulation (Kendler et al., 1993) and thyroid functioning (Joffe & Levitt, 1988). Further, chronic smoking may render youth vulnerable to emotional distress through the dysregulation of stress response systems (e.g., the hypothalamic-pituitary-adrenal system; Koob & Le Moal, 2001), effects which may be amplified in girls (Munafò et al., 2008). Last, but likely in concert with withdrawal effects and biological stress response systems, the consistent use of cigarettes to self-regulate may exacerbate mood lability over time as youth do not develop more adaptive strategies for coping with stress. Alternately, different mood patterns for persistent smokers may be explained by a third factor that was not examined in the current study. For example, girl smokers may be more likely to develop other deviant behaviors (e.g., alcohol and drug use) or family discord that counteract any smoking-related mood improvement or heighten emotional volatility (Escobedo, Reddy, &Giovino, 1998). Nevertheless, previous adolescent research has found that the tobacco use-distress link was maintained despite controlling for similar potential confounds (e.g., Orlando et al., 2001).
It is important to consider findings against the backdrop of normative trends in adolescent emotional experience. Interestingly, levels of negative mood and variability were largely found to improve or remain stable across time and smoking groups. Although these patterns stand in contrast to the documented rise in depressive symptoms and negative mood during adolescence (e.g., Garber, Keiley, & Martin, 2002; Larson et al., 1990), our results are similar to past EMA findings that the mood deteriorations experienced in early adolescence stabilized or reversed by mid-high school (Larson, Moneta, Richards, & Wilson, 2002; Moneta, Schneider, & Csikszentmihalyi, 2001; Weinstein, Mermelstein, Hankin, Hedeker, & Flay, 2007). It has been posited that mood states level out in mid-adolescence following the reduction in intrapersonal and environmental changes accompanying early adolescence (e.g., puberty, transition to high school), resulting in better and more stable moods (Larson et al., 2002). The timing of our study – following youth in mid-ninth and tenth grades across a 15-month period – is consistent with this explanation. It is important to note that the smokers in the present study experienced the worst mood overall, suggesting that these youth may reflect a subset with greater mood difficulties. However, among less experienced smokers, this study may be capturing a window of normative decelerations in mood changes among youth.
Study limitations should be noted. This study captured only one component of the complex construct of affect regulation via intraindividual mood variability. Although the standard deviation has been used in past work to index mood variability, this approach is limited by potential floor effects as well as the inability to distinguish between extremity versus frequency of mood changes. Second, the use of observed classifications to create longitudinal smoking groups, rather than statistical methods to derive groups (e.g., latent trajectory analyses), may limit the generalizability of findings. As such, conclusions concerning differences between the smoking groups must be viewed as preliminary and warrant replication with smoking classes derived via a latent trajectory approach. Third, interpretations regarding the influences of smoking escalation and habitual use on mood must be made cautiously, as the methods used did not directly examine causal relations between smoking and mood. Similarly, although smoking-mood relations were examined across rapid escalation to daily use and sustained smoking, the same adolescents were not followed for equivalent lengths of time in a sustained smoking phase. As such, we cannot rule out group differences in the effects of smoking on mood for the rapid escalators versus smokers. Indeed, findings beg the question: what happens to the escalators’ mood variability during subsequent years of smoking? Inferences regarding the sequencing of effects are strengthened by the fact that, at baseline, smokers have levels of mood variability as low as the rapid escalators at follow-up. Moreover, the rapid escalators nicotine dependence scores at follow-up approximated those of the smokers at baseline, further supporting the similar characteristics of the smoking group at baseline and the escalating group at follow-up. Nonetheless, future research must follow the same cohort of adolescents across experimentation through extended periods of habitual use.
A fourth limitation concerns the small sample sizes in the gender-stratified analyses; given the small group sizes, further investigation with larger samples is needed to validate the current findings. In addition, youth with the highest levels of baseline mood variability were more likely not to participate at follow-up, and hence findings may underestimate mood changes over time. Generalizability of findings is also limited by the participation rates for the larger study. Finally, given the small sample sizes in the gender-stratified analyses, we chose to limit the number of predictor variables under investigation and thus did not control for potential confounds related to smoking and mood (e.g., genetics, family factors, or alcohol/drug use). However, findings are strengthened by the similar patterns found in both the present results and previous studies that did control for potential confounds (e.g., Orlando et al., 2001; Wills & Stoolmiller, 2002; Windle & Windle, 2001).
In sum, results – albeit preliminary given the limitations of the sample size and analytic approach employed – suggest that complex mood-smoking relationships may comprise part of the etiology of regular smoking among youth. The current data argue for a model of smoking that accounts for changes in risk and maintenance factors at different points along the developmental trajectory of smoking, involving elements of both self-medication and dependence. Findings provide insight into the evolving and dynamic nature of smoking-mood relationships, relations that may have been masked in previous work that failed to differentiate among developmental patterns of cigarette use.
Acknowledgments
This work was supported by National Cancer Institute Grant 5 P01 CA099262
References
- Audrain-McGovern J, Rodriguez D, Kassel JD. Adolescent smoking and depression: Evidence for self-medication and peer smoking mediation. Addiction. 2009;104:1743–1756. doi: 10.1111/j.1360-0443.2009.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kilbey MM, Andreski P. Nicotine dependence, major depression, and anxiety in young adults. Archives of General Psychiatry. 1991;48:1–69. 1074. doi: 10.1001/archpsyc.1991.01810360033005. [DOI] [PubMed] [Google Scholar]
- Brody AL. Functional brain imaging of tobacco use and dependence. Journal of Psychiatric Research. 2006;40:404–418. doi: 10.1016/j.jpsychires.2005.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, et al. Cigarette smoking saturates brain α4β2 nicotinic acetylcholine receptors. Archives of General Psychiatry. 2006;63:907–915. doi: 10.1001/archpsyc.63.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne DG, Mazanov J. Sources of adolescent stress, smoking and the use of other drugs. Stress medicine. 1999;15:215–227. [Google Scholar]
- Choi WS, Patten CA, Gillin JC, Kaplan RM, Pierce JP. Cigarette smoking predicts development of depressive symptoms among U.S. adolescents. Annals of Behavioral Medicine. 1997;19:42–50. doi: 10.1007/BF02883426. [DOI] [PubMed] [Google Scholar]
- Colby SM, Tiffany ST, Shiffman S, Niaura RS. Are adolescent smokers dependent on nicotine? A review of the evidence. Drug and Alcohol Dependence. 2000;59:S83–S95. doi: 10.1016/s0376-8716(99)00166-0. [DOI] [PubMed] [Google Scholar]
- Delfino RJ, Jamner LD, Whalen CK. Temporal analysis of the relationship of smoking behavior and urges to mood sates in men versus women. Nicotine & Tobacco Research. 2001;3:235–248. doi: 10.1080/14622200110050466. [DOI] [PubMed] [Google Scholar]
- Diviak KR, Kohler S, Mermelstein RJ, Flay B. Reliability of adolescents’ self-reports across data collection modalities. Poster presented at the annual meeting of the Society for Research on Nicotine and Tobacco; Seattle, WA. 2001. [Google Scholar]
- Escobedo LG, Reddy M, Giovino GA. The relationship between cigarette smoking and depressive symptoms in US adolescents. Addiction. 1998;93:433–440. doi: 10.1046/j.1360-0443.1998.93343311.x. [DOI] [PubMed] [Google Scholar]
- Eid M, Diener E. Intraindividual variability in affect: Reliability, validity, and personality correlates. Journal of Personality and Social Psychology. 1999;76:662–676. [Google Scholar]
- Forbes EE, Dahl RE. Neural systems of positive affect: Relevance to understanding child and adolescent depression? Development and Psychopathology. 2005;17:827–850. doi: 10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frawley PJ. Neurobehavioral model of addiction: addiction as a primary disease. In: Peele S, editor. Visions of addiction: Major contemporary perspectives on addiction and alcoholism. Lexington, MA: DC Health; 1988. pp. 25–44. [Google Scholar]
- Garber J, Keiley MK, Martin N. Developmental trajectories of adolescents’ depressive symptoms: Predictors of change. Journal of Consulting and Clinical Psychology. 2002;70:79–95. doi: 10.1037//0022-006x.70.1.79. [DOI] [PubMed] [Google Scholar]
- Hedeker D, Mermelstein RJ, Berbaum ML, Campbell RT. Modeling mood variation association with smoking: An application of a heterogeneous mixed-effects model for analysis of Ecological Momentary Assessment (EMA) data. Addiction. 2009;104:297–307. doi: 10.1111/j.1360-0443.2008.02435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe RT, Levitt AJ. Effects of cigarette smoking on thyroid function in depressed patients. Neuropsychology. 1988;20:12–14. doi: 10.1159/000118466. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychological Bulletin. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, MacLean CJ, Heath AC, Eaves LJ, Kessler RC. Smoking and major depression: A causal analysis. Archives of General Psychia1try. 1993;50:36–43. doi: 10.1001/archpsyc.1993.01820130038007. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: A reconsideration and recent applications. Harvard Review of Psychiatry. 1997;4:231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiological similarities in depression nand drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Larson RW, Raffaelli M, Richards MH, Ham M, Jewell L. Ecology of depression in late childhood and early adolescence: A profile of daily states and activities. Journal of Abnormal Psychology. 1990;99:92–102. doi: 10.1037//0021-843x.99.1.92. [DOI] [PubMed] [Google Scholar]
- Larson RW, Moneta G, Richards M, Wilson S. Continuity, stability and change in daily emotional experience across adolescence. Child Development. 2002;73:1151–1165. doi: 10.1111/1467-8624.00464. [DOI] [PubMed] [Google Scholar]
- Mermelstein R, Hedeker D, Flay B, Shiffman S. Do changes in mood following smoking predict longitudinal changes in adolescent smoking patterns?; Paper presented at the Annual Meeting of the Society for Research on Nicotine and Tobacco; New Orleans. 2003. Feb, [Google Scholar]
- Moneta GB, Schneider B, Csikszentmilhalyi M. A longitudinal study of self-concept and experiential components of self-worth and affect across adolescence. Applied Developmental Science. 2001;5:125–157. [Google Scholar]
- Munafò MR, Hitsman B, Rende R, Metcalfe C, Niaura R. Effects of progression to cigarette smoking on depressed mood in adolescents: evidence from the National Longitudinal Study of Adolescent Health. Addiction. 2008;103:162–171. doi: 10.1111/j.1360-0443.2007.02052.x. [DOI] [PubMed] [Google Scholar]
- Novak SP, Clayton RR. Influence of school environment and self-regulation on transitions between stages of cigarette smoking. Health Psychology. 2001;20:196–207. [PubMed] [Google Scholar]
- Orlando M, Ellickson PL, Jinnett K. The temporal relationship between emotional distress and cigarette smoking during adolescence and young adulthood. Journal of Consulting and Clinical Psychology. 2001;69:959–970. doi: 10.1037//0022-006x.69.6.959. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Does cigarette smoking cause stress? American Psychologist. 1999;54:817–820. doi: 10.1037//0003-066x.54.10.817. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Nicotine psychobiology: how chronic-dose prospective studies can illuminate some of the theoretical issues from acute-dose research. Psychopharmacology. 2006;184:567–576. doi: 10.1007/s00213-005-0294-y. [DOI] [PubMed] [Google Scholar]
- Prokhorov AV, Pallonen UE, Fava JL, Ding L, Niaura R. Measuring nicotine dependence among high-risk adolescent smokers. Journal of Addictive Behaviors. 1996;21:117–127. doi: 10.1016/0306-4603(96)00048-2. [DOI] [PubMed] [Google Scholar]
- Prokhorov AV, de Moor C, Pallonen UE, Hudmon KS, Koehly L, Hu S. Validation of the modified Fagerstrom Tolerance Questionnaire with salivary cotinine in adolescents. Addictive Behaviors. 2000;25:429–433. doi: 10.1016/s0306-4603(98)00132-4. [DOI] [PubMed] [Google Scholar]
- Quick MW, Lester AJ. Desensitization of neuronal nicotinic receptors. Journal of Neurobiology. 2002;53:457–478. doi: 10.1002/neu.10109. [DOI] [PubMed] [Google Scholar]
- Repetto PB, Caldwell CH, Zimmerman MA. A longitudinal study of the relationship between depressive symptoms and cigarette use among African American adolescents. Health Psychology. 2005;24:209–219. doi: 10.1037/0278-6133.24.2.209. [DOI] [PubMed] [Google Scholar]
- Shadel WG, Shiffman S, Niaura R, Nichter M, Abrams DB. Current models of nicotine dependence: What is known and what is needed to advance understanding of tobacco etiology among youth. Drug and Alcohol Dependence. 2000;59:S9–S21. doi: 10.1016/s0376-8716(99)00162-3. [DOI] [PubMed] [Google Scholar]
- Shytle RD, Silver AA, Lukas RJ, Newman MB, Sheehan DV, Sanberg PR. Nicotinic acetylcholine receptors as targets for antidepressants. Molecular Psychiatry. 2002;7:525–535. doi: 10.1038/sj.mp.4001035. [DOI] [PubMed] [Google Scholar]
- Silk JS, Steinberg L, Morris AS. Adolescents’ emotion regulation in daily life: Links to depressive symptoms and problem behavior. Child Development. 2003;74:1869–80. doi: 10.1046/j.1467-8624.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- Simons JS, Carey KB. Risk and vulnerability for marijuana use problems: The role of affect dysregulation. Psychology of Addictive Behaviors. 2002;16:72–75. [PubMed] [Google Scholar]
- Simons JS, Carey KB, Gaher RM. Lability and impulsivity synergistically increase risk for alcohol-related problems. The American Journal of Drug and Alcohol Abuse. 2004;30:685–694. doi: 10.1081/ada-200032338. [DOI] [PubMed] [Google Scholar]
- Stone AA, Shiffman S. Ecological momentary assessment (EMA) in behavioral medicine. Annals of Behavioral Medicine. 1994;16:199–202. [Google Scholar]
- Thompson RA. Emotion regulation: A theme in search of definition. Monographs of the Society for Research in Child Development. 1994;59(2 – 3, Serial No. 240):25–52. [PubMed] [Google Scholar]
- Tizabi Y, Overstreet DH, Rezvani AH, Louis VA, Clark E, Janowsky DS, Kling MA. Antidepressant effects of nicotine in an animal model of depression. Psychopharmacology. 1999;142:193–199. doi: 10.1007/s002130050879. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (USDHHS) Tobacco use among high school students – U.S., 1997. Morbidity and Mortality Weekly Report. 1998;47:229–33. [PubMed] [Google Scholar]
- Weinstein SM, Mermelstein R. Influences of mood variability, negative moods, and depression on adolescent cigarette smoking. Paper submitted for publication. 2012 doi: 10.1037/a0031488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein SM, Mermelstein R, Hankin B, Hedeker D, Flay B. Longitudinal patterns of daily affect and global mood during adolescence. Journal of Research on Adolescence. 2007;17:587–600. doi: 10.1111/j.1532-7795.2007.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein SM, Mermelstein R, Shiffman S, Flay B. Mood variability and cigarette smoking escalation among adolescents. Psychology of Addictive Behavior. 2008;22:504–13. doi: 10.1037/0893-164X.22.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Stoolmiller M. The role of self-control in early escalation of substance use: a time-varying analysis. Journal of Consulting and Clinical Psychology. 2002;70:986–997. doi: 10.1037//0022-006x.70.4.986. [DOI] [PubMed] [Google Scholar]
- Wills TA, Walker C, Mendoza D, Ainette MG. Behavioral and emotional self-control: Relations to substance use in samples of middle and high school students. Psychology of Addictive Behaviors. 2006;20:265–278. doi: 10.1037/0893-164X.20.3.265. [DOI] [PubMed] [Google Scholar]
- Windle M, Windle RC. Depressive symptoms and cigarette smoking among middle adolescents: Prospective associations and intrapersonal and interpersonal influences. Journal of Consulting and Clinical Psychology. 2001;69:215–226. [PubMed] [Google Scholar]
- Wu LT, Anthony JC. Tobacco smoking and depressed mood in late childhood and early adolescence. American Journal of Public Health. 1999;89:1837–1840. doi: 10.2105/ajph.89.12.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]


