Abstract
In animal studies, brain-derived neurotrophic factor (BDNF) is an important regulator of central nervous system development and synaptic plasticity. WAGR (Wilms tumour, Aniridia, Genitourinary anomalies, and mental Retardation) syndrome is caused by 11p13 deletions of variable size near the BDNF locus and can serve as a model for studying human BDNF haploinsufficiency (+/−). We hypothesized that BDNF+/− would be associated with more severe cognitive impairment in subjects with WAGR syndrome. Twenty-eight subjects with WAGR syndrome (6–28y), 12 subjects with isolated aniridia due to PAX6 mutations/microdeletions (7–54y), and 20 healthy controls (4–32y) received neurocognitive assessments. Deletion boundaries for the subjects in the WAGR group were determined by high resolution oligonucleotide array comparative genomic hybridization. Within the WAGR group, BDNF+/− subjects (n=15), compared with BDNF intact (+/+) subjects (n=13), had lower adaptive behaviour (p=.02), reduced cognitive functioning (p=.04), higher levels of reported historical (p=.02) and current (p=.02) social impairment, and higher percentage meeting cut-off score for autism (p=.047) on Autism Diagnostic Interview-Revised. These differences remained nominally significant after adjusting for visual acuity. Using diagnostic measures and clinical judgment, 3 subjects (2 BDNF+/− and 1 BDNF+/+) in the WAGR group (10.7%) were classified with autism spectrum disorder. A comparison group of visually impaired subjects with isolated aniridia had cognitive functioning comparable to that of healthy controls. In summary, among subjects with WAGR syndrome, BDNF+/− subjects had a mean Vineland Adaptive Behaviour Compose score that was 14 points lower and a mean IQ that was 20 points lower than BDNF+/+ subjects. Our findings support the hypothesis that BDNF plays an important role in human neurocognitive development.
Keywords: brain-derived neurotrophic factor, WAGR syndrome, 11p deletion, IQ, autism
1. Introduction
Brain-derived neurotrophic factor (BDNF) is a 27 kDa homodimeric protein in the nerve growth factor family of peptides that is widely expressed throughout the central nervous system and, in animal studies, appears to play an important role in regulating neuronal development, synaptic plasticity, and energy balance (Cohen-Cory et al., 2010; Greenberg et al., 2009; Xu et al., 2003). Heterozygous Bdnf knockout mice exhibit hyperphagia, obesity, decreased nociception, and impaired learning and social behaviours (Kernie et al., 2000; Lyons et al., 1999; MacQueen et al., 2001). WAGR (Wilms tumour, Aniridia, Genitourinary anomalies, mental Retardation) syndrome is a rare genetic disorder caused by heterozygous chromosome 11p13 contiguous gene deletions of variable size. The genitourinary and ocular manifestations of the syndrome are attributed to hemizygosity for WT1 and PAX6, respectively, but the aetiology of cognitive impairment, which is quite variable among individuals with WAGR syndrome, has not been well elucidated (Clericuzio et al., 2011; Fischbach et al., 2005). The gene encoding BDNF is located at 11p14, only 4Mb distal to PAX6, and hemizygosity for BDNF frequently occurs in subjects with WAGR syndrome (Han et al., 2008). Subjects with WAGR syndrome and other 11p deletions can serve, therefore, as models for studying human BDNF haploinsufficiency.
We previously reported associations of BDNF haploinsufficiency with obesity and reduced responsiveness to pain in subjects with WAGR syndrome, findings that support the role of BDNF in human energy homeostasis and nociception (Han et al., 2008). Extant data to support the role of BDNF in human cognitive functioning have been limited to case series. One case report described obesity and cognitive impairment in a child with a paracentric 11p13p15.3 inversion and functional BDNF haploinsufficiency (Gray et al., 2006). Two case series described a total of 9 subjects with 11p14 deletions involving BDNF who displayed obesity and various neurodevelopmental abnormalities, including intellectual disability, attention deficit hyperactivity disorder, and autism (Ernst et al., 2012; Shinawi et al., 2011). However, no prior study has examined cognitive and behaviour phenotypes using standardized clinical assessments in 11p deletion subjects with BDNF haploinsufficiency versus those with both alleles for BDNF intact.
To determine if BDNF deletion status contributes to the neurocognitive abnormalities of subjects with WAGR syndrome, we examined the association of BDNF haploinsufficiency with cognitive functioning, adaptive and problematic behaviours, and symptoms of autism in a cohort of 28 subjects with WAGR syndrome. For comparison of neurocognition and brain magnetic resonance imaging (MRI) findings, we assessed subjects with visual impairments due to isolated aniridia, as well as healthy controls. We hypothesized that BDNF haploinsufficiency would be associated with more severe neurocognitive impairments in subjects with WAGR syndrome.
2. Methods
2.1 Participants
Three groups of subjects were recruited for this study using posted online and local advertisements: (1) individuals with WAGR/11p13 deletion syndrome with prior genetic testing showing karyotype with non-mosaic 11p13 deletion, (2) subjects with isolated aniridia who had prior genetic testing showing mutations of PAX6, serving as a contrast group; and (3) healthy control subjects who had no chronic medical conditions. The study was approved by the institutional review board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD and is registered at www.clinicaltrials.gov as NCT00758108. Written informed consent was obtained from adult subjects who were competent to provide consent and from the parents or legal guardians of children and adults with cognitive impairment. Twenty-two subjects in the WAGR group were described previously in a case series examining hyperphagia and body-mass index (Han et al., 2008). Thirteen of the subjects in the WAGR group also were described in a previous case series examining parent-reported history of intellectual disability and ASD diagnoses (Xu et al., 2008). The characteristics of these previously described subjects (along with the rest of the cohort) are shown in Supplementary Table 1.
2.2 Deletion mapping for subjects with WAGR syndrome
Deletion boundaries for each subject with WAGR syndrome were determined using oligonucleotide array comparative genomic hybridization using a custom-designed microarray platform (Agilent Technologies, Inc., Santa Clara, CA) containing 105,000 60-mer oligonucleotide probes using NCBI Build 36 (hg18) human reference sequence as previously described (Han et al., 2008). Probes for chromosome 11p were spaced at approximately 400 bp intervals (excluding repeat regions) using 57,925 probes; 121 probes were located within BDNF. Probes for the remainder of the genome were spaced at approximately 35 kb intervals. BDNF is comprised of 11 exons and 9 promoters that drive tissue and brain-region specific transcription of 17 different mRNA variants in which different combinations of the 5′ untranslated region (UTR) exons are variably spliced with a final coding exon (Pruunsild et al., 2007). Prior studies have shown that BDNF transcripts containing exons I and II are highly expressed in the hippocampus and amygdala, regions of the brain important for learning, memory, and emotion (Han et al., 2008; Pruunsild et al., 2007). Subjects with deletion of all or any portion of the BDNF gene were designated as BDNF+/− while subjects with deletions that did not involve BDNF 5′ UTR or coding sequence were designated as BDNF+/+. PHF21A (at 11p11.2) was recently identified as the gene responsible for severe intellectual disability and craniofacial anomalies in Potocki-Shaffer-Syndrome (Kim et al., 2012); therefore, two subjects with deletions involving PHF21A (at 11p11.2) were excluded from the current study due to possible confounding effects of PHF21A hemizygosity. Clinical assessments, conducted between December 2008 and August 2012 at the NIH Clinical Research Center, were performed by the ophthalmologist, neuroradiologist, and psychologist while unaware of BDNF deletion status.
2.3 Ophthalmologic examination
Best-corrected visual acuity was assessed by an ophthalmologist using one of the following methods adapted to age, visual function, and ability to cooperate and answer questions: Snellen acuity, HOTV/crowded single HOTV optotypes, Allen/picture projector, Teller acuity cards, counting fingers, detection of hand motion and light perception. Each subject was assigned a score based upon the function of the better eye (while wearing corrective lenses if routinely used by the subject) using a 7-point scale for ranges of visual acuity loss (International Council of Ophthalmology, 2002): 1=normal vision (20/25 or better), 2=near-normal vision (20/32 to 20/63), 3=moderate visual impairment (20/80 to 20/160), 4=severe visual impairment (20/200 to 20/400), 5=profound visual impairment (20/500 to 20/1000), 6=near blindness, and 7=no light perception. Visual acuity was used as a covariate in analyses of adaptive behaviour, cognitive functioning, and autism-related symptoms.
2.4 Brain imaging
Magnetic resonance imaging (MRI) of the brain was performed using an 8 channel 12 element head array coil on a 3 tesla system (Philips Achieva, Best, the Netherlands). Routine clinical MRI sequences (e.g. T1-weighted, T2-weighted, fluid attenuated inversion recovery [FLAIR]) and high resolution 3D T1 turbo field echo with approximately 1 mm isotropic voxels were obtained.
2.5 Neurocognitive assessment
Adaptive behaviour was assessed with the Vineland Adaptive Behaviour Scales, Second Edition (Sparrow et al., 2005) for all subjects with WAGR syndrome, and for subjects in both the aniridia and control groups who lived with a caregiver. The Vineland is a semi-structured caregiver interview designed to measure adaptive functioning in four domains: Communication, Daily Living Skills, Socialization, and Motor Skills. Standard scores are provided for each domain and for an overall score, Adaptive Behaviour Composite (ABC). The Vineland manual states that individuals with visual impairments were included in the standardization sample.
When possible, cognitive functioning was assessed for subjects with WAGR syndrome and isolated aniridia using standardized tests of development or intelligence, including Wechsler tests [Wechsler Adult Intelligence Scale, Third Edition: WAIS-III (Wechsler, 1997) or Wechsler Intelligence Scale for Children, Fourth Edition: WISC-IV (Wechsler, 2003)], Mullen Scales of Early Learning (Mullen, 1995), Stanford Binet Intelligence Scales, Fifth Edition (Roid, 2003), or the Differential Ability Scales, Second Edition (Elliott, 2007), depending on the subject’s age. Minor adjustments to these tests were sometimes employed to accommodate visual impairment (e.g., enlarging visual stimuli) (Storandt and Futterman, 1982). For individuals with significant visual impairments that prevented administration of other tests, the Cognitive Test for the Blind (Dial et al., 1991) (a test designed for individuals with significant visual impairments, with minimum mental age of 14) was administered if a “floor” (minimum number of items to score) could be achieved. The choice of test to estimate cognitive functioning was based on the visual and developmental abilities of the subject, with the test most appropriately age-normed and most generalisable to the general population always preferred. Among subjects in the BDNF+/− group who had cognitive functioning assessed (n=13), the tests used were: Cognitive Test for the Blind (n=1), Differential Ability Scales (n=5), Mullen Scales of Early Learning (n=2), and Wechsler Intelligence Scales (n=5). Among subjects in the BDNF+/+ group who had cognitive functioning assessed (n=11), the tests used were Cognitive Test for the Blind (n=1), Differential Ability Scales (n=5), Mullen Scales of Early Learning (n=1), Stanford Binet Intelligence Scales (n=1), and Wechsler Intelligence Scales (n=3). The distributions of tests used for the two WAGR groups were well-balanced (p=0.79). Cognitive functioning for healthy controls was assessed using the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999).
To obtain ratings of potentially clinically significant behavioural problems, one of the questionnaires from the Achenbach System of Empirically Based Assessment (ASEBA) was administered as follows: the Child Behaviour Checklist (CBCL, for all subjects <18y), the Adult Behaviour Checklist (ABCL, for all adults who lived with a caregiver), or the Adult Self Report (ASR, for all adults who lived independently) (Achenbach and Rescorla, 2000, 2001, 2003). These measures were completed either by a caregiver (CBCL, ABCL) or self-respondent (ASR). All ASEBA questionnaires are rated on a 3-point scale, with a score of “0” indicating “Not true,” and a score of “2” indicating the statement is “Very true” or “Often true.” Across all ASEBA questionnaires, test-retest reliability coefficients (r) ranged from .71 to .99. Each of these measures uses normative samples to generate scores for various behaviours that comprise “internalizing” and “externalizing” syndromes. The scales have been used for individuals with visual impairments (Alimovic, 2013), but it should be noted that norming samples for the measures excluded individuals with visual impairments.
For the assessment of autism symptoms, a modified DSM-IV autism checklist was used, which included specific considerations for individuals with visual impairments (Supplementary Table 2). The Social Responsiveness Scale (SRS) (Constantino and Gruber, 2005) and the Social Communication Questionnaire, Lifetime Form (SCQ) (Rutter et al., 2003) were used to rule out significant autism spectrum disorder (ASD) symptoms in the aniridia and control groups. The SRS is a 65-item parent report measure standardized on children ages 4–18, rated on a 4-point Likert-type scale, scored as “not true” to “almost always true.” Tests for internal consistency were reported to be high, .94 for males, and .93 for females (Constantino and Gruber, 2005). The SCQ is a 40-item parent response measure standardized on individuals ages 4–40 (appropriate for those with mental age of at least 2), scored dichotomously with “yes” if the behavior was ever present, and “no” if the behavior was absent; internal consistency ranged from .84 to .93 (Rutter et al., 2003). For subjects with WAGR syndrome, autism symptoms were also assessed using the Autism Diagnostic Interview-Revised (ADI-R) and the Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 2000), instruments designed to assist with the diagnosis of autism that were not specifically designed to assess severity of symptoms nor were developed to rate symptoms in individuals with significant sensory impairments. Specifically, the ADI-R allows for two different algorithms to be computed, a diagnostic algorithm, which utilizes “ever” scores that include historical symptoms from the age period of 4 to 5 years or “ever,” as well as a current algorithm, which quantifies current symptoms, defined as those present during the last 3 months. In addition, administration and scoring of both the ADI-R and the ADOS were sometimes altered to adjust for visual impairments. For instance, a “not applicable” code was used for eye contact, when reduced eye contact was reported to be due to visual impairment. A diagnosis of ASD was determined on the basis of these assessments in combination with the clinical judgment of a doctoral level clinical psychologist (American Psychiatric Association, 1994; Risi et al., 2006).
2.6 Statistical methods
Statistical analyses were performed using SPSS software (version 16, IBM Corp., Armnok, NY). The primary outcome measures were adaptive behaviour and cognitive functioning. Power analyses determined that a sample size of 12 BDNF+/− and 12 BDNF+/+ subjects would be sufficient for >80% power to detect a difference between groups of 1.25 standard deviations at a level of significance of p<.05. Secondary outcomes were ADI-R scores and autism diagnosis. Tertiary outcomes were ASEBA subscale scores and brain MRI findings. Parametric tests were used for normally distributed data, while skewed data were assessed using nonparametric tests. For comparison of WAGR, isolated aniridia, and control subjects, analyses of variance and Kruskal-Wallis tests were performed, with post-hoc Bonferroni correction for multiple pair-wise comparisons. For comparison of BDNF+/− and BDNF+/+ subjects with WAGR syndrome, Pearson and Spearman correlations were used to assess the associations between variables. Fisher’s exact tests assessed differences in percentages. Independent samples T tests and Mann-Whitney U tests were used to compare continuous variables. Verbal and nonverbal IQs were compared using paired T tests. Analyses of covariance, adjusting for visual acuity, assessed differences in cognitive functioning, adaptive behaviour, and ADI-R scores. Means ± standard deviations (SD) are shown unless otherwise specified. Nominal p-values are shown. P-values <.05 were considered nominally significant; p-values <.01 were considered significant when accounting for multiple comparisons.
3. Results
3.1 Subject characteristics
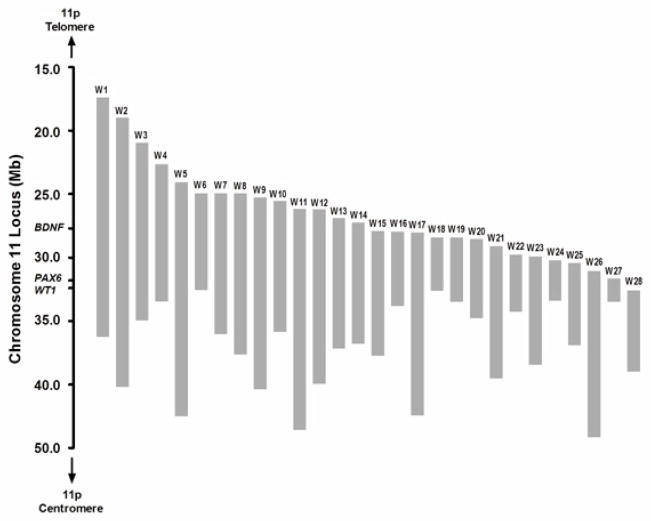
Subjects included 28 individuals with WAGR/11p13 deletion syndrome (6–28y), 12 subjects with aniridia (7–54y), and 20 healthy controls (4–32y). Subject characteristics are shown in Table 1. Deletion sizes for the subjects with WAGR syndrome ranged from 2.22 to 21.62 Mb, and spanned the region of chr11:17,181,487 to 44,329,536 (hg18) (Figure 1 and Supplementary Table 1). One BDNF+/− subject (W15 in Supplementary Table 1) had a deletion involving only the first 3 exons in the 5′ UTR of BDNF, sparing the final coding exon for the protein; the remainder of the BDNF+/− subjects had deletions that included the entire BDNF gene. The 15 (53.6%) BDNF+/− subjects and 13 (46.4%) BDNF+/+ subjects were not significantly different in age, sex, race, history of Wilms tumour, age of Wilms tumour, and visual acuity (Table 2). All subjects with aniridia had heterozygous point mutations or small deletions within PAX6 (Supplementary Table 3). Visual acuity scores were similar for subjects with aniridia compared to subjects with WAGR syndrome (Table 1).
Table 1.
Characteristics of subjects with WAGR Syndrome, isolated aniridia, and healthy controls. Mean ± standard deviation (range) or percentage shown.
| WAGR Syndrome (n=28) | Isolated Aniridia (n=12) | Healthy Controls (n=20) | P-Values | ||||
|---|---|---|---|---|---|---|---|
| Overall | WAGR vs. Aniridia | WAGR vs. Healthy | Aniridia vs. Healthy | ||||
| Age (y) | 14.8 ± 7.5 (6.1–28.3) | 28.9 ± 13.4 (7.3–54.0) | 15.3 ± 7.3 (4.1–32.8) | <.001 | <.001 | 1.00 | <.001 |
| Sex (% Female) | 60.7 | 50.0 | 60.0 | .81 | .73 | 1.00 | .72 |
| Race/Ethnicity (%) | .26 | .74 | .15 | .32 | |||
| Non-Hispanic Caucasian | 89.2 | 91.7 | 60.0 | ||||
| Hispanic Caucasian | 3.6 | 0 | 5.0 | ||||
| African American | 0 | 0 | 10.0 | ||||
| Asian | 3.6 | 0 | 15.0 | ||||
| Mixed Race | 3.6 | 8.3 | 10.0 | ||||
| Visual Acuity Score | 4.3 ± 1.3 (2–7) | 4.0 ± 1.4 (2–7) | all = 1.0 | <.001 | .53 | <.001 | <.001 |
| Vineland Adaptive Behaviour Composite Score | 62 ± 16 (20–90) (n=28) |
81 ± 19 (37–99) (n=8) |
105 ± 7 (91–117) (n=13) |
<.001 | .009 | <.001 | .002 |
| Composite IQ/DQ | 58 ± 24 (16–121) (n=24) |
101 ± 11 (86–117) (n=10) |
113 ± 13 (92–138) (n=20) |
<.001 | <.001 | <.001 | .29 |
Figure 1.
Chromosome 11p13 deletions for subjects with WAGR syndrome. Deletion boundaries were determined using high-resolution oligonucleotide array comparative genomic hybridization (NCBI Build 36, hg 18). Deletions for each subject are shown as gray bars. Heterozygous deletions of WT1 and PAX6 are responsible for the core features of this syndrome: Wilms tumour, aniridia, and genitourinary abnormalities. Heterozygous deletion of BDNF, which is only 4Mb telomeric to PAX6, was found in approximately one-half subjects with WAGR syndrome in this study.
Table 2.
Comparison of BDNF+/− and BDNF+/+ subjects with WAGR syndrome. Mean ± standard deviation (range) or percentage shown.
| BDNF+/− (n=15) | BDNF+/+ (n=13) | P-Value | |
|---|---|---|---|
| Age (y) | 15.3 ± 7.4 (6.2–28.3) | 14.2 ± 7.9 (6.1–28.3) | .71 |
| Sex (% Female) | 53.3 | 69.2 | .46 |
| Race/Ethnicity (% Non-Hispanic Caucasian) | 93.3 | 84.6 | .36 |
| History of Wilms Tumour (%) | 66.7 | 53.8 | .70 |
| Age of Wilms Tumour (y) | 2.4 ± 2.1 (.3–7) | 2.6 ± 1.6 (1.2–5.5) | .88 |
| Visual Acuity Score | 4.3 ± 1.4 (3–7) | 4.2 ± 1.4 (2–7) | .95 |
| Vineland Adaptive Behaviour Composite Score | 55 ± 18 (20–82) (n=15) |
69 ± 10 (46–90) (n=13) |
.02 |
| Composite IQ/DQ | 49 ± 20 (16–84) (n=13) |
69 ± 25 (16–121) (n=11) |
.04 |
3.2 BDNF haploinsufficiency is associated with lower adaptive behaviour and reduced cognitive functioning
The Vineland Adaptive Behaviour Scales, Second Edition was administered for all 28 subjects with WAGR syndrome, and as expected, the Vineland Adaptive Behaviour Composite (ABC) score was negatively correlated with visual acuity score (r= −.41, p=.03). Cognitive functioning was assessed in 24 subjects with WAGR syndrome (13 BDNF+/− and 11 BDNF+/+), 10 subjects with isolated aniridia, and 20 healthy controls. As expected, cognitive functioning was not correlated with visual acuity (p=.57 for WAGR group and p=.76 for aniridia group). Given the negative correlation of adaptive behaviour with visual acuity, however, we chose to include visual acuity in secondary analyses of our outcome measures because of the potential contributions that visual acuity might have to the assessment of cognition and behaviour. Because of the wide range in ages and different cognitive functioning measures used, we examined the associations between age and intelligence/developmental quotient (IQ/DQ) scores and observed no significant correlations (p=.26 for all subjects; p=.49 for WAGR group; p=.64 for BDNF+/− subgroup of WAGR; p=.36 for BDNF+/+ subgroup of WAGR; p=0.97 for aniridia group; p=0.72 for control group).
The deletion boundaries for each subject in the WAGR group are shown in Figure 1 and Supplementary Table 1. The telomeric boundary indicates how far the deletion extends away from the WT1-PAX6 region (centromeric side of deletion boundary) toward BDNF, with higher numbers indicating greater distance from BDNF. Within the WAGR group, telomeric deletion boundary was associated (while adjusting for visual acuity) with the Vineland ABC score (r=.54, p=.004), as well as all of the specific domains of communication (r=.52, p=.006), daily living (r=.52, p=.005), and socialization (r=.49, p=.009). Nonverbal (r=.46, p=.03), verbal (r=.47, p=.02), and composite (r=.52, p=.01) intelligence/developmental quotient (IQ/DQ) scores were also associated with telomeric deletion boundary. Conversely, the centromeric deletion boundary was not correlated with either adaptive behaviour (p=.54 for Vineland ABC) or cognitive functioning (p=.31 for composite IQ/DQ). By inclusion criteria for the study, subjects with WAGR syndrome had deletions anchored at the centromeric end to encompass WT1 and PAX6. As a consequence, deletion size was nearly 2-fold larger in BDNF+/− subjects (13.8±4.0 Mb) versus BDNF+/+ subjects (7.3±3.7 Mb, p<.001). Deletion size was negatively correlated with adaptive behaviour (r= −.47, p=.01) and cognitive functioning (r= −.55, p=.007) adjusted for visual acuity. However, when BDNF+/− status was also included as a covariate, deletion size was no longer significantly associated with adaptive behaviour (p=.23) or cognitive functioning (p=.07). Furthermore, within each BDNF subgroup, there was no statistically significant correlation between deletion size and adaptive behaviour (p=.47 within BDNF+/− and p=.56 within BDNF+/+) or cognitive functioning (p=.12 within BDNF+/− and p=.48 within BDNF+/+) adjusted for visual acuity. Although sample size limits interpretation, these results suggest that the location of the telomeric deletion boundary (which determines whether BDNF is encompassed by the deletion) rather than the general size of deletion in this region may have a greater impact on neurodevelopment.
Furthermore, in group comparisons, it was found that BDNF+/− subjects had lower adaptive behaviour (p=.02) and cognitive functioning (p=.04) compared with BDNF+/+ subjects, with a mean difference of 14-points on Vineland ABC score and 20-points on IQ (Table 2). After adjustment for visual acuity, these differences remained statistically significant (p=.01 for adaptive behaviour and p=.04 for cognitive functioning; Figure 2). The differences between BDNF+/− and BDNF+/+ subjects persisted when age was included as a covariate (p=.02 for Vineland ABC and p=.045 for IQ/DQ). Also as expected, cognitive functioning was lower in the subjects with WAGR syndrome compared to subjects with isolated aniridia and healthy controls, who had similar cognitive functioning scores (Table 1).
Figure 2.
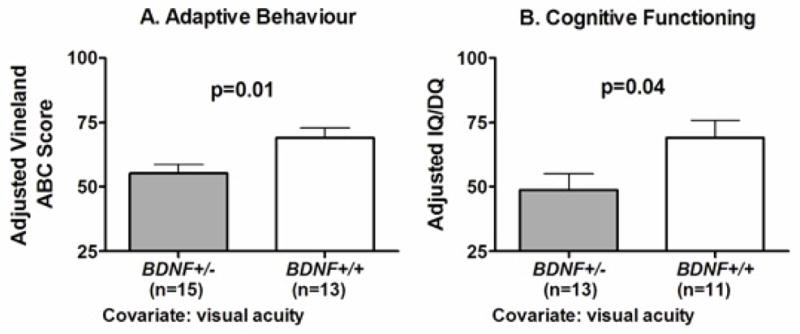
Association of BDNF haploinsufficiency with lower adaptive behavior and reduced cognitive functioning. (A) Vineland-II adaptive behavior composite (ABC) score adjusted for visual acuity. (B) Cognitive functioning [estimated full scale intelligence or developmental quotient (IQ/DQ)] adjusted for visual acuity. Cognitive functioning was assessed using a full scale equivalent for either one of the Wechsler tests, Mullen Scales of Early Learning, Differential Ability Scales-II, or the Cognitive Test for the Blind. Adjusted means ± SEM shown.
3.3 Hypoplastic corpus callosum is observed in ~31% of subjects with WAGR syndrome, but is not associated with BDNF haploinsufficiency
Brain MRI was performed in 26 subjects with WAGR syndrome (13 BDNF+/− and 13 BDNF+/+ subjects), 12 subjects with aniridia, and 19 healthy controls. There were no gross morphologic brain abnormalities detected for the subjects with aniridia or the healthy controls compared to 38.5% (5 BDNF+/− and 5 BDNF+/+; specific individuals and findings indicated in Supplementary Table 4) for subjects with WAGR syndrome (p<.001). In the WAGR group, hypoplastic corpus callosum was observed in 8 subjects (30.8%; 4 BDNF+/− and 4 BDNF+/+), enlarged ventricles in 3 subjects (11.5%; 2 BDNF+/− and 1 BDNF+/+), and gray matter heterotopia in 2 subjects (7.7%; 1 BDNF+/− and 1 BDNF+/+), but none of these findings was associated with BDNF haploinsufficiency (p’s=1.00). Among the 2 subjects with heterotopia, one subject (W14 in Supplementary Table 4) had a history of seizure disorder. The 10 subjects with WAGR syndrome who had structural brain abnormalities had similar adaptive behaviour (p=.91) and cognitive functioning (p=.78) scores compared to the rest of the WAGR cohort.
3.4 Behaviour problems are frequently reported in subjects with WAGR syndrome, but are not associated with BDNF haploinsufficiency
Age appropriate rating scales of behaviour problems (CBCL, ABCL, or ASR) were obtained for 21 subjects with WAGR syndrome (10 BDNF+/− and 11 BDNF+/+ subjects). Within the WAGR group, the number of subjects with T scores in the clinical range (≥70; >97th percentile) was 3/21 (14.3%) for somatic complaints, 1/17 (5.9%) for social problems, 6/21 (28.6%) for thought problems, 6/20 (30%) for attention problems, 1/21 (4.8%) for rule-breaking, and 2/21 (9.5%) for aggressive behaviour; no subjects scored in the clinical range on the anxious/depressed or withdrawn/depressed subscales. There were no significant differences between BDNF+/− and BDNF+/+ subjects in mean T-scores (all p’s >.40) or percentage falling in the clinical range for any of the subscales (all p’s >.21). Cognitive functioning (adjusted for visual acuity) was negatively correlated with social problems T score (r= −.55, p=.03) and thought problems T score (r= −.50, p=.03)
3.5 BDNF haploinsufficiency is associated with greater social impairments on ADI-R, but is not associated with ASD diagnosis
Based upon review of medical records, 6 (21.4%) of the 28 subjects in the WAGR group [4 of 15 BDNF+/− subjects (26.7%) and 2 of 13 BDNF+/+ subjects (15.4%)] had been previously diagnosed with an ASD by their local providers, with no statistically significant difference between BDNF+/− versus BDNF+/+ subgroups (p=.65).
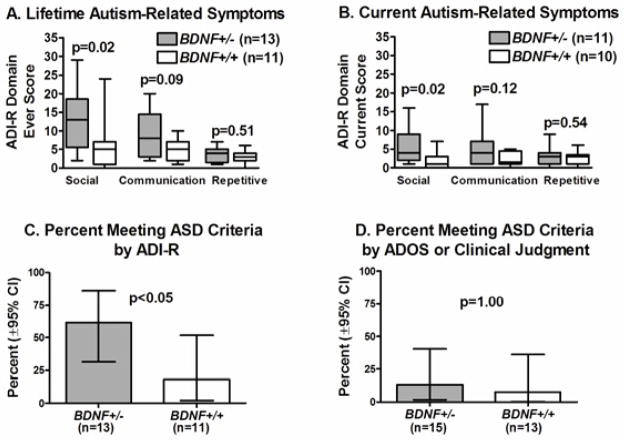
ASD was ruled out in all of the aniridia and healthy control subjects, based on observation and scores on screening questionnaires (SCQ, SRS). ADI-R was conducted for 13 BDNF+/− and 11 BDNF+/+ subjects in the WAGR group. Three of these subjects were nonverbal at age 10 years; therefore, since the current algorithm was not established for nonverbal children in this age-range, only “ever” scores were obtained for these individuals. Within the WAGR group, ADI-R social domain ever and current scores were higher (indicating more impairment) in BDNF+/− vs. BDNF+/+ subjects (p’s=.02), but communication and repetitive behaviour domains ever and current scores were not statistically different between groups (Figure 3). Visual acuity was not associated with any of the ADI-R ever or current scores (all p’s >.60); thus, as expected, after including visual acuity as a covariate, the differences between BDNF+/− and BDNF+/+ remained significant for ADI-R social domain ever (p=.03) and current (p=.04) scores. Cognitive functioning was negatively correlated with all ADI-R ever social (rho= −.51, p=.02) and communication (rho= −.52, p=.02) domain scores; after adjustment for cognitive functioning, there were no significant differences between BDNF+/− and BDNF+/+ for ADI-R scores (all p’s >.43). Ten (41.7%) of 24 subjects in the WAGR group met ADI-R diagnostic algorithm cut-off criteria (which uses ever scores), with a higher percentage (p=.047) in BDNF+/− versus BDNF+/+ subjects (Figure 3). ADOS was performed for 21 WAGR subjects: 11 BDNF+/− and 10 BDNF+/+. Three (14.3%) met the ADOS algorithm cutoff for ASD with no difference in percentage within BDNF+/− versus BDNF+/+ (Figure 3). Based on clinical judgment and all assessment measures, 3 of 28 subjects (2 BDNF+/− and 1 BDNF+/+) in the WAGR group were classified with ASD, for an overall percentage of 10.7% (95%CI: 2.3–28.2). These 3 subjects had a previous diagnosis of ASD based on medical chart review. Of note, one of these 3 subjects was the subject with partial BDNF deletion (W15 in Supplementary Table 1), who had severe cognitive impairment, with a Vineland ABC of 20.
Figure 3.
Autism-related symptoms in subjects with WAGR syndrome. (A) Impairment scores using ADI-R ever codes. (B) Impairment scores using ADI-R current codes. (C) Percentage meeting ASD cut-off criteria using ADI-R ever codes. (D) Percentage meeting ASD cut-off criteria using ADOS or clinical judgment. For the box-and-whiskers plots, central line is the median, box indicates 25th–75th percentile, and whiskers show the range (minimum and maximum). Percentages meeting ASD criteria are shown with ± 95% confidence intervals.
4. Discussion
Prior to this current study, the two largest case series describing the neurocognitive phenotype of subjects with WAGR syndrome relied solely on medical record review or parent-completed health questionnaires and telephone interviews (Fischbach et al., 2005; Xu et al., 2008). In the current study, we performed standard diagnostic neurocognitive testing in a cross-sectional cohort of 28 children and adults with WAGR syndrome and observed that BDNF haploinsufficiency was associated with lower adaptive behaviour and cognitive functioning, even after accounting for visual impairment, which can be an important confounding factor (Hill-Briggs et al., 2007). While previous case series have described cognitive impairment in individuals with BDNF haploinsufficiency, our study is the first to compare subjects having 11p13 deletions, with and without BDNF involvement, using a systematic diagnostic approach to correlate genotype with neurocognitive phenotype. Our findings provide strong evidence to support the hypothesis that BDNF plays an important role in human neurocognitive development.
In cross-sectional studies of post-mortem human brains, an age-dependent pattern of BDNF expression which varies by brain region has been previously described. In the dorsolateral prefrontal cortex, BDNF mRNA levels rise from infancy to adulthood, peak during early adulthood, and remain fairly constant throughout adulthood and aging (Webster et al., 2002). In contrast, within the hippocampus, BDNF mRNA levels do not change significantly with age, while in the temporal cortex, BDNF mRNA levels are highest in neonates and then decrease with age (Webster et al., 2006). Thus, BDNF appears to have multiple roles during different stages of neurocognitive development, maturation, and sustainment throughout life. In our cohort, the ages of our subjects with WAGR syndrome spanned childhood, adolescence, and adulthood, and we observed that BDNF haploinsufficiency was associated, independent of age, with lower adaptive behaviour and cognitive functioning.
BDNF haploinsufficiency was also associated with higher ADI-R scores for lifetime problems in social interaction and higher percentage meeting the diagnostic algorithm cut-off for autism. However, when using screening and diagnostic tests combined with the clinical judgment of a doctoral level clinical psychologist, only 3/28 (10.7%) were diagnosed with ASD, with no significant difference between BDNF+/− and BDNF+/+. Thus, contrary to a previous study (Fischbach et al., 2005), in which, based solely on medical record review, 24.1% of subjects with WAGR syndrome were diagnosed with ASD, and also contrary to the percentage based on the current subjects’ prior medical records (21.4%; 6/28), the actual percentage of subjects with ASD in the current study was considerably lower. In fact, the rate of autism observed in the current study is consistent with one of the only other studies that assessed for ASD using direct observation in individuals with visual impairments; that study (Mukaddes et al., 2007) found a rate of 11.7% and also found that intellectual disability was associated with increased risk for ASD diagnosis among the visually impaired. In contrast, a study (Parr et al., 2010) which used retrospective medical chart review to assign diagnosis of ASD in a cohort of visually impaired children yielded a much higher rate of 31%. Furthermore, one longitudinal study (Hobson and Lee, 2010) showed that 8 of 9 congenitally blind children compared with 0 of 7 sighted children had “reversal” of ASD diagnosis in adolescence, suggesting that blindness may cause ASD-like behaviours that resolve as compensatory skills are acquired.
Conclusions regarding behavioural problems measured via self or others’ reports of dimensional symptoms and syndromes are limited since reported symptoms are difficult to interpret due to inadequate norming and multiple potential confounds. However, the findings of the current study are consistent with prior literature indicating significant behaviour problems in individuals with chronic medical impairments, visual impairments, and/or intellectual disability (Alimovic, 2013; Oeseburg et al., 2010a; Oeseburg et al., 2010b).
Our data support findings from studies of subjects with genetic disorders (Moss et al., 2011) and individuals with intellectual disability (de Bildt et al., 2004) that cognitive impairments can confound the assessment for autism symptoms. In particular, we observed that lower cognitive functioning was associated with higher ADI-R social and communication domain scores, and after adjustment for cognitive functioning, there were no significant differences between BDNF+/− and BDNF+/+ subjects for any ADI-R domain. Early childhood illness with conditions such as Wilms tumour (which occurred in 61% of our cohort) might also contribute to higher “ever” scores when parents are interviewed about their children’s behaviour as toddlers and preschoolers (Kurtz and Abrams, 2010). Furthermore, the degree of visual impairment in many individuals with WAGR syndrome rendered some of the ADI-R questions difficult to answer and precluded administration of the ADOS for more severely impaired subjects. As has been found with other studies, the ADI-R has lower specificity in individuals with significant cognitive deficits (Risi et al., 2006). The increased social deficits among BDNF+/− subjects is nonetheless intriguing and corroborate rodent data demonstrating that Bdnf+/− mice display abnormal social behaviours (Kernie et al., 2000; Lyons et al., 1999).
One significant limitation of this study is the lack of existing measures suitable for assessing cognitive functioning, behaviour, and autism symptoms in subjects with combined visual and intellectual impairments. Because of the wide range of age, developmental abilities, and visual acuity of the subjects in the WAGR group, multiple cognitive tests were administered within this group, limiting the validity of score comparisons. Other measures used, including the ADI-R and ADOS have not been normed or tested extensively among visually-impaired populations; thus, modifications to their use and the diagnostic process were required (Johansson et al., 2010). Visual acuity was not significantly associated with cognitive functioning or ADI-R scores in our cohort, suggesting that the modifications used in our assessments adequately accounted for visual impairment. We also observed that IQ scores for subjects with aniridia (who had similar visual impairment as the subjects in the WAGR group) were within the normal range and comparable to a contemporary cohort of healthy controls, confirming the expected lack of association between visual acuity and cognitive functioning. Within the WAGR group, we observed that in addition to the social and communication impairment scores on ADI-R, social and thought problems on ASEBA behaviour assessments were also negatively associated with cognitive functioning, suggesting that at least some of these autism-related symptoms and behaviour problems may be attributed to intellectual disability.
Another limitation of this study is that 11p deletion size was greater in BDNF+/− subjects (potentially confounding whether BDNF+/− status or deletion size was more important) because participants were recruited based upon having WAGR syndrome and, therefore, had deletions anchored around WT1. However, cognitive functioning was correlated only with telomeric deletion boundary (which determines whether or not BDNF is affected) and not the centromeric boundary. Furthermore, there was no correlation between deletion size within the separate BDNF+/− and BDNF+/− groups. Our findings are consistent with reported case series (Ernst et al., 2012; Shinawi et al., 2011) which together described a total of 9 non-WAGR subjects with 11p14 deletions that spared WT1 and PAX6, who all displayed neurodevelopmental abnormalities; the shared minimal critical region of overlap for these 9 subjects included BDNF, BDNFOS, LIN7C, LGR4, and CCDC34. In our cohort, one subject (W15 in Figure 1 and Table S1) with a deletion involving the first 3 exons of BDNF (while sparing BDNFOS, LIN7C, LGR4, and CCDC34) had severe cognitive impairment and ASD, thereby further narrowing the critical region to the BDNF locus. Although the coding exon for the BDNF protein was intact in this subject, haploinsufficiency for BDNF exons I-III appeared to be sufficient to cause significant neurodevelopmental problems, suggesting an important role of the 5′ UTR exons, as supported by the observation that BDNF transcripts containing exons I and II are highly expressed in regions of the brain critical for learning and memory (Han et al., 2008; Pruunsild et al., 2007).
Hypoplastic corpus callosum (HCC) was observed in 8/26 (30.8%) subjects with WAGR syndrome (4 BDNF+/− and 4 BDNF+/+ subjects). The corpus callosum and interhemispheric connectivity are believed to play important roles in language and emotion processing (Booth et al., 2011; Friederici, 2011). While it appears that BDNF haploinsufficiency is not associated with HCC or other gross morphologic abnormalities, additional genes within the 11p13 region may be candidates, and further analyses using quantitative morphologic methods could reveal subtle anomalies not detected in this study.
5. Conclusions
In summary, BDNF haploinsufficiency appears to be a modulating factor for more severe impairments in adaptive behaviour and cognitive functioning in subjects with 11p deletions. BDNF haploinsufficiency in our study was associated with a 14-point lower mean Vineland ABC score and a 20-point lower mean IQ in subjects with WAGR syndrome. Social deficits associated with BDNF haploinsufficiency appear to be mediated through impaired cognitive function. These phenotype-genotype associations have important clinical implications for the anticipatory guidance and care of patients with WAGR syndrome based on individual BDNF deletion status. With the increased availability of molecular genetic testing, defining the deletion boundaries of patients with WAGR syndrome should be considered as an important diagnostic and prognostic tool. We previously showed that BDNF haploinsufficiency is associated with the development of childhood obesity (Han et al., 2008), and currently advise that BDNF+/− patients receive more vigilant weight monitoring and that their families receive early preventive guidance regarding dietary intake and physical activity. Based on the findings of this present study, knowledge of a patient’s BDNF status can better inform the genetic counselling provided to families. It should be noted that the range in cognitive functioning varied widely within the BDNF+/− and BDNF+/+ WAGR subgroups, but overall, those with BDNF haploinsufficiency had lower adaptive behaviour and cognitive functioning in a cross-sectional sample that included children, adolescents, and adults. Further studies of individuals with deletions spanning other portions of 11p may be beneficial in elucidating the role of additional genes besides BDNF and PHF21A (Kim et al., 2012) in neurocognitive development, and in parsing the cognitive and behavioural deficits that may be unique to WAGR syndrome or attributable to significant medical, visual, and intellectual disabilities.
WAGR syndrome can serve as a model for gaining insights on the role of BDNF in more common conditions. Reduced BDNF levels have been associated with various psychiatric and neurodegenerative disorders (Autry and Monteggia; Zuccato and Cattaneo, 2009), and in the general population, the common BDNF Val66Met polymorphism appears to be associated with diminished cognitive performance, possibly due to decreased activity-dependent BDNF secretion (Egan et al., 2003). Thus, pursuit of further studies in subjects with WAGR syndrome to examine the therapeutic potential of increasing BDNF receptor signalling through pharmacotherapy or behavioural interventions (e.g., dietary restriction, exercise, and environmental enrichments may each increase endogenous BDNF levels (Autry and Monteggia, 2012; Schmidt-Kassow et al., 2012; Zuccato and Cattaneo, 2009)) could benefit patients with BDNF haploinsufficiency as well as individuals with other conditions associated with relative BDNF insufficiency.
Supplementary Material
Acknowledgments
This study was funded by the Intramural Research Programs of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (ZIAHD008898 and Z1AHD00641) and the National Institute of Mental Health (ZIAMH002868) of the National Institutes of Health (NIH), and by the NIH Bench-to-Bedside Program. We thank the members of International WAGR Syndrome Association for assistance in identifying families who were willing to participate in this study. We thank Jack Yanovski and Daniel Weinberger for helpful discussions. We thank Margaret Pekar, Miriya Tune, Mark Lee, Matthew Tsang, Tanvee Singh, Emily Yin, Jamila Crossman, and Rachel Kim for assistance with psychological testing and administrative support.
Footnotes
Supplementary material available on-line includes four tables.
Disclaimer: the opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Navy or the Department of Defense.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM, Rescorla LA. Manual for ASEBA Preschool Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2000. [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for ASEBA Adult Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2003. [Google Scholar]
- Alimovic S. Emotional and behavioural problems in children with visual impairment, intellectual and multiple disabilities. Journal of Intellectual Disability Research. 2013;57 (2):153–160. doi: 10.1111/j.1365-2788.2012.01562.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV. 4. Arlington, VA: American Psychiatric Association; 1994. [Google Scholar]
- Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 64(2):238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacological Reviews. 2012;64 (2):238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth R, Wallace GL, Happe F. Connectivity and the corpus callosum in autism spectrum conditions: insights from comparison of autism and callosal agenesis. Progress in Brain Research. 2011;189:303–317. doi: 10.1016/B978-0-444-53884-0.00031-2. [DOI] [PubMed] [Google Scholar]
- Clericuzio C, Hingorani M, Crolla JA, van Heyningen V, Verloes A. Clinical utility gene card for: WAGR syndrome. European Journal of Human Genetics. 2011;19(4) doi: 10.1038/ejhg.2010.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Developmental Neurobiology. 2010;70 (5):271–288. doi: 10.1002/dneu.20774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. Social Responsiveness Scale (SRS) Los Angeles, CA: Western Psychological Services; 2005. [Google Scholar]
- de Bildt A, Sytema S, Ketelaars C, Kraijer D, Mulder E, Volkmar F, Minderaa R. Interrelationship between Autism Diagnostic Observation Schedule-Generic (ADOS-G), Autism Diagnostic Interview-Revised (ADI-R), and the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) classification in children and adolescents with mental retardation. Journal of Autism and Developmental Disorders. 2004;34 (2):129–137. doi: 10.1023/b:jadd.0000022604.22374.5f. [DOI] [PubMed] [Google Scholar]
- Dial JG, Chan F, Mezger C, Parker HJ, Zangia K, Wong DW, Gray S. Comprehensive Evaluation System (CVES) for visually impaired/blind individuals: A validation study. Journal of Visual Impairments and Blindness. 1991;85:153–157. [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112 (2):257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Elliott CD. Manual for the Differential Ability Scales. 2. Harcourt Assessment; San Antonio, TX: 2007. [Google Scholar]
- Ernst C, Marshall CR, Shen Y, Metcalfe K, Rosenfeld J, Hodge JC, Torres A, Blumenthal I, Chiang C, Pillalamarri V, Crapper L, Diallo AB, Ruderfer D, Pereira S, Sklar P, Purcell S, Wildin RS, Spencer AC, Quade BF, Harris DJ, Lemyre E, Wu BL, Stavropoulos DJ, Geraghty MT, Shaffer LG, Morton CC, Scherer SW, Gusella JF, Talkowski ME. Highly Penetrant Alterations of a Critical Region Including BDNF in Human Psychopathology and Obesity. Archives of General Psychiatry. 2012:1–9. doi: 10.1001/archgenpsychiatry.2012.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach BV, Trout KL, Lewis J, Luis CA, Sika M. WAGR syndrome: a clinical review of 54 cases. Pediatrics. 2005;116 (4):984–988. doi: 10.1542/peds.2004-0467. [DOI] [PubMed] [Google Scholar]
- Friederici AD. The brain basis of language processing: from structure to function. Physiological Reviews. 2011;91 (4):1357–1392. doi: 10.1152/physrev.00006.2011. [DOI] [PubMed] [Google Scholar]
- Gray J, Yeo GS, Cox JJ, Morton J, Adlam AL, Keogh JM, Yanovski JA, El Gharbawy A, Han JC, Tung YC, Hodges JR, Raymond FL, O’Rahilly S, Farooqi IS. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes. 2006;55 (12):3366–3371. doi: 10.2337/db06-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: implications in CNS function. Journal of Neuroscience. 2009;29 (41):12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JC, Liu QR, Jones M, Levinn RL, Menzie CM, Jefferson-George KS, Adler-Wailes DC, Sanford EL, Lacbawan FL, Uhl GR, Rennert OM, Yanovski JA. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. New England Journal of Medicine. 2008;359 (9):918–927. doi: 10.1056/NEJMoa0801119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill-Briggs F, Dial JG, Morere DA, Joyce A. Neuropsychological assessment of persons with physical disability, visual impairment or blindness, and hearing impairment or deafness. Archives of Clinical Neuropsychology. 2007;22 (3):389–404. doi: 10.1016/j.acn.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Hobson RP, Lee A. Reversible autism among congenitally blind children? A controlled follow-up study. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2010;51 (11):1235–1241. doi: 10.1111/j.1469-7610.2010.02274.x. [DOI] [PubMed] [Google Scholar]
- International Council of Ophthalmology. Report of the International Council of Ophthalmology for the 29th International Congress of Ophthalmology: Visual Standards - Aspects and Ranges of Vision Loss. http://www.icoph.org/downloads/visualstandardsreport.pdf2002.
- Johansson M, Gillberg C, Rastam M. Autism spectrum conditions in individuals with Mobius sequence, CHARGE syndrome and oculo-auriculo-vertebral spectrum: diagnostic aspects. Research in Developmental Disabilities. 2010;31 (1):9–24. doi: 10.1016/j.ridd.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO Journal. 2000;19 (6):1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HG, Kim HT, Leach NT, Lan F, Ullmann R, Silahtaroglu A, Kurth I, Nowka A, Seong IS, Shen Y, Talkowski ME, Ruderfer D, Lee JH, Glotzbach C, Ha K, Kjaergaard S, Levin AV, Romeike BF, Kleefstra T, Bartsch O, Elsea SH, Jabs EW, MacDonald ME, Harris DJ, Quade BJ, Ropers HH, Shaffer LG, Kutsche K, Layman LC, Tommerup N, Kalscheuer VM, Shi Y, Morton CC, Kim CH, Gusella JF. Translocations disrupting PHF21A in the Potocki-Shaffer-syndrome region are associated with intellectual disability and craniofacial anomalies. American Journal of Human Genetics. 2012;91 (1):56–72. doi: 10.1016/j.ajhg.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz BP, Abrams AN. Psychiatric aspects of pediatric cancer. Child and Adolescent Psychiatric Clinics of North America. 2010;19(2):401–421. x–xi. doi: 10.1016/j.chc.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30 (3):205–223. [PubMed] [Google Scholar]
- Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proceedings of the National Academy of Sciences of the United States of America. 1999;96 (26):15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen GM, Ramakrishnan K, Croll SD, Siuciak JA, Yu G, Young LT, Fahnestock M. Performance of heterozygous brain-derived neurotrophic factor knockout mice on behavioral analogues of anxiety, nociception, and depression. Behavioral Neuroscience. 2001;115 (5):1145–1153. doi: 10.1037//0735-7044.115.5.1145. [DOI] [PubMed] [Google Scholar]
- Moss J, Howlin P, Oliver C. The assessment and presentation of autism spectrum disorder and associated characteristics in individuals with severe intellectual disability and genetic syndromes. In: Burack J, Hodapp R, Iarocci G, Zigler E, editors. The Oxford Handbook of Intellectual Disablity and Development. Oxford University Press; New York, NY: 2011. pp. 1–57. [Google Scholar]
- Mukaddes NM, Kilincaslan A, Kucukyazici G, Sevketoglu T, Tuncer S. Autism in visually impaired individuals. Psychiatry and Clinical Neurosciences. 2007;61 (1):39–44. doi: 10.1111/j.1440-1819.2007.01608.x. [DOI] [PubMed] [Google Scholar]
- Mullen E. Mullen Scales of Early Learning – AGS edition. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- Oeseburg B, Groothoff JW, Dijkstra GJ, Reijneveld SA, Jansen DE. Pervasive developmental disorder behavior in adolescents with intellectual disability and co-occurring somatic chronic diseases. Research in Developmental Disabilities. 2010a;31 (2):496–501. doi: 10.1016/j.ridd.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Oeseburg B, Jansen DE, Groothoff JW, Dijkstra GJ, Reijneveld SA. Emotional and behavioural problems in adolescents with intellectual disability with and without chronic diseases. Journal of Intellectual Disability Research. 2010b;54 (1):81–89. doi: 10.1111/j.1365-2788.2009.01231.x. [DOI] [PubMed] [Google Scholar]
- Parr JR, Dale NJ, Shaffer LM, Salt AT. Social communication difficulties and autism spectrum disorder in young children with optic nerve hypoplasia and/or septo-optic dysplasia. Developmental Medicine and Child Neurology. 2010;52 (10):917–921. doi: 10.1111/j.1469-8749.2010.03664.x. [DOI] [PubMed] [Google Scholar]
- Pruunsild P, Kazantseva A, Aid T, Palm K, Timmusk T. Dissecting the human BDNF locus: bidirectional transcription, complex splicing, and multiple promoters. Genomics. 2007;90 (3):397–406. doi: 10.1016/j.ygeno.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risi S, Lord C, Gotham K, Corsello C, Chrysler C, Szatmari P, Cook EH, Jr, Leventhal BL, Pickles A. Combining information from multiple sources in the diagnosis of autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45 (9):1094–1103. doi: 10.1097/01.chi.0000227880.42780.0e. [DOI] [PubMed] [Google Scholar]
- Roid GH. Stanford-Binet Intelligence Scales. 5. Itasca, IL: Riverside Publishing; 2003. [Google Scholar]
- Rutter M, Bailey A, Lord C. The Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Schmidt-Kassow M, Schadle S, Otterbein S, Thiel C, Doehring A, Lotsch J, Kaiser J. Kinetics of serum brain-derived neurotrophic factor following low-intensity versus high-intensity exercise in men and women. Neuroreport. 2012;23 (15):889–893. doi: 10.1097/WNR.0b013e32835946ca. [DOI] [PubMed] [Google Scholar]
- Shinawi M, Sahoo T, Maranda B, Skinner SA, Skinner C, Chinault C, Zascavage R, Peters SU, Patel A, Stevenson RE, Beaudet AL. 11p14.1 microdeletions associated with ADHD, autism, developmental delay, and obesity. American Journal of Medical Genetetics Part A. 2011;155A (6):1272–1280. doi: 10.1002/ajmg.a.33878. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales. 2. Circle Pines, MN: AGS Publishing; 2005. [Google Scholar]
- Storandt M, Futterman A. Stimulus size and performance on two subtests of the Wechsler Adult Intelligence Scale by younger and older adults. Journal of Gerontology. 1982;37 (5):602–603. doi: 10.1093/geronj/37.5.602. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Herman MM, Kleinman JE, Shannon Weickert C. BDNF and trkB mRNA expression in the hippocampus and temporal cortex during the human lifespan. Gene Expr Patterns. 2006;6 (8):941–951. doi: 10.1016/j.modgep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Weickert CS, Herman MM, Kleinman JE. BDNF mRNA expression during postnatal development, maturation and aging of the human prefrontal cortex. Brain Research. Developmental Brain Research. 2002;139 (2):139–150. doi: 10.1016/s0165-3806(02)00540-0. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III: Wechsler Adult Intelligence Scale. 3. San Antonio: Harcourt Assessment, Inc; 1997. [Google Scholar]
- Wechsler D. WASI: Wechsler Abbreviated Scale of Intelligence. San Antonio: Harcourt Assessment, Inc; 1999. [Google Scholar]
- Wechsler D. WISC-IV: Wechsler Intelligence Scale for Children. 4. San Antonio: Harcourt Assessment, Inc; 2003. [Google Scholar]
- Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nature Neuroscience. 2003;6 (7):736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Han JC, Morales A, Menzie CM, Williams K, Fan YS. Characterization of 11p14-p12 deletion in WAGR syndrome by array CGH for identifying genes contributing to mental retardation and autism. Cytogenetics and Genome Research. 2008;122(2):181–187. doi: 10.1159/000172086. erratum in: Cytogenetetics and Genome Research 124 (1), 112, 2009. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nature Reviews Neurology. 2009;5 (6):311–322. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.