Abstract
Background
Our previous data have indicated that nerve injury-induced upregulation of thrombospondin-4 (TSP4) proteins in dorsal spinal cord plays a causal role in neuropathic pain state development in a spinal nerve ligation model. To investigate whether TSP4 proteins also contribute to the development of centrally mediated changes in nociception after spinal cord injury (SCI), we investigated whether SCI injury induced TSP4 dysregulation, and if so, whether this change correlated with changes in nociception in a T9 spinal cord contusion injury model.
Methods
Behavioral sensitivity to mechanical, thermal stimuli and locomotor function recovery were tested blindly in SCI or sham rats post injury. Intrathecal antisense or mismatch control oligodeoxynucleotides were used to treat SCI rats with nociceptive hyperreflexia and Western blots were used to measure TSP4 protein levels in dorsal spinal cord samples.
Results
SCI induced below-level hindpaw hypersensitivity to stimuli. TSP4 protein levels are upregulated in dorsal spinal cord of SCI rats with nociceptive hyperreflexia, but not in SCI rats without nociceptive hyperreflexia. There was no significant difference in motor function recovery post injury between SCI rats with or without nociceptive hyperreflexia. Intrathecal treatment with TSP4 antisense, but not mismatch control, oligodeoxynucleotides led to reversal of injury-induced TSP4 upregulation and nociceptive hyperreflexia in SCI rats.
Conclusions
SCI leads to TSP4 upregulation in lumbar spinal cord that may play a critical role in mediating centrally mediated behavioral hypersensitivity. Blocking this pathway may be helpful in management of SCI induced changes in nociception.
Keywords: spinal cord injury, thrombospondin-4, nociception
Introduction
Spinal cord injury (SCI) is a devastating event that result in motor dysfunction as well as development of chronic pain syndromes. There are approximately 600,000 individuals in the United States suffering from SCI, and about 60-80% SCI patients experience significant chronic pain (Ataoglu et al., 2012; Celik et al., 2012; Finnerup et al., 2001; Jensen et al., 2009; Yezierski, 1996), which could be detected extensively as reflex hypersensitivity in animal models in the pain research field, possibly related to spinally mediated spasticity (Baastrup et al., 2010). SCI-induced changes in nociception can reduce the quality of life of patients to a greater extent than motor impairment (Finnerup et al., 2001; Yezierski, 1996). It tends to be long-term and difficult to manage, and often results in development of depression. The majority (83.2%) of the chronic pain is below the level of injury (Turner et al., 2001). Although pharmacological agents or non-pharmacological treatments could be used to relieve pain, none of them is highly specific and effective. Understanding the mechanism of post-SCI chronic pain therefore is critical for the development of next generation of target specific medications for SCI pain management.
Thrombospondin proteins (TSPs) are a family of large oligomeric, extracellular matrix glycoproteins that play important roles in cell attachment, migration and cytoskeletal dynamics (Bornstein et al., 2004). Recently, it has been shown that TSPs induce synapse formation by interacting with its neuronal receptor, the calcium channel alpha-2-delta-1 subunit proteins (Eroglu et al., 2009). Previous studies indicated that spinal cord contusion injury causes upregulation of the calcium channel alpha-2-delta-1 proteins at below-level in lumbar dorsal spinal cord (Boroujerdi et al., 2011) that leads to the development of reduced paw withdrawal thresholds to mechanical stimuli, a widely accepted method for measuring nociceptive responses in animal models (Boroujerdi et al., 2011; Mills et al., 2001; Yoon et al., 2004). In addition, peripheral nerve injury induces upregulation of thrombospondin-4 (TSP4) proteins in dorsal spinal cord that plays a causal role in the development of neuropathic pain states in a peripheral nerve injury model (Kim et al., 2012). However, it is not know if SCI also causes TSP4 upregulation below-level in dorsal spinal cord that could play a critical role in mediating SCI-induced changes in nociception. In this study, we examined whether altered TSP4 expression in a rat SCI model plays an important role in mediating behavioral hypersensitivity.
Materials and Methods
All the experiments were performed with protocols approved by the Institutional Animal Care and Usage Committee of the University of California Irvine.
Spinal cord injury
Adult female Sprague Dawley rats (180-200g) were anesthetized with ketamine/xylazine (90:10 mg/kg). After the T8-11 region in the back was shaved and sterilized with Betadine followed by 75% ethanol, a midline incision was made and then T9 spinal cord was exposed by laminectomy. The contusion injury was generated by a 200-kilodyne weight-drop force onto the spinal cord from the distance of 3-4 mm using a Infinite Horizon device (Precision Systems and Instrumentation, LLC, Fairfax Station, VA) (Scheff et al., 2003). Sham rats were generated similarly as the SCI rats but without the spinal cord contusion. After the surgery, the incision was closed in two layers and the rats were placed on the heating pads for recovery. Postoperative care included daily subcutaneous injection of saline (10 mL) and Baytril (2.5 mg/kg/d) for 7 days to prevent dehydration and infection, respectively, as well as manual bladder expression for 10-14 days until the urination function was fully recovered, which varied among SCI rats but was not correlated with the development of abnormal behavioral sensitivity.
Locomotor function recovery test
The locomotor function was monitored using the Basso, Beattie and Bresnahan (BBB) locomotor rating scales (Basso et al., 1995). Before surgery, the rats were acclimated to the environment by handing and patting for 10 minutes and exposing them to the testing apparatus (circular metal enclosure) for 20 minutes daily for 7 days. The baseline test was performed 3 days prior to the surgery. The post-operation testing began as soon as the rats could support their body weight. The locomotor function test scale ranged from 0 to 21, which focuses on the paw position, toe clearance, trunk stability and tail position as described before (Basso et al., 1995).
Behavioral testing
The 50% hindpaw withdraw thresholds were measured blindly up to 40 days post surgery, starting about 10 days after the surgery when the rats regained the ability to support their body weight, using a modified up-down method of Dixon (1980). Baseline data were collected one day before the surgeries. Briefly, rats were randomly placed in the plastic enclosures on the top of an elevated wire mesh floor for at least 30 minute acclimation. A series of von Frey filaments (Stoelting, Wood Dale, IL), starting with the one providing a buckling force of 2.0 gm, were then used to measure the degree of mechanical sensitivity. Each filament was applied in a perpendicular fashion to the plantar surface of each hind paw. A sharp withdrawal or paw licking within 6 seconds was considered a positive response that led to the use of the next weaker filament. On the other hand, a negative response led to the application of the next larger filament. This paradigm was continued until 6 measurements were obtained starting from the one before the first change in response, or until four consecutive positive (scored 0.25g) or five consecutive negative (scored 15g) response were observed (Higuera and Luo, 2004).
Radiant heat plantar test was used to assess thermal sensitivity using a Hargreaves apparatus (Hargreaves et al., 1988), and thermal hyperalgesia was considered as reduced hindpaw withdrawal latency (PWL) to radiant heat stimulation. Briefly, individual free moving animals were acclimated for at least 30 minutes on the glass top of a hot box maintained at 30 ± 0.1 °C. Radiant heat from a high intensity light bulb was projected through a small aperture below the glass surface to the plantar surface of the hindpaw. The time between the application of heat stimulus and withdrawal of the targeted hindpaw was recorded (paw withdrawal latency). A 20 second cutoff time was set to prevent thermal injury of the skin. Two readings per paw were averaged, and the values were used for statistical analysis.
Intrathecal injection
The sequences of TSP4 antisense and mismatch oligodeoxynucleotides used were derived from the rat TSP4 cDNA sequence (Genbank accession number: X89963). Antisense: CCATCATTGTTGCTATCTTCC and mismatch: ACCATCGTTGTTACTTTCTCC oligodeoxynucleotides were ordered from GeneLink (Hawthome, NY) with modifications in both ends as described previously (Boroujerdi et al., 2008; Kim et al., 2009; Li et al., 2004). The oligodeoxynucleotides were precipitated and washed with 75% ethanol, then dissolved in sterile saline before use. Rats with hyperreflexic hindpaws (paw withdrawal threshold around or below 5 g) 40 days after SCI were anesthetized with 2-3% isoflurane and a 25 gauge (1/2 inch) needle was inserted between lumbar 5-6 vertebrae. The solution (10 μL) was injected intrathecally (0.4 sec/μL) using the microinjector (MINJ-PD micro-INJECTOR ALL-Digital Positive Dis-placement System, Tritech Research, Inc, Los Angeles, CA). After each injection, the needle was held for 10-20 seconds to avoid the outflow of drug solutions. The daily injection was performed after daily behavioral testing for four consecutive days.
Western Blot
Spinal cord samples were collected by hydraulic exclusion with ice-cold saline from deeply anesthetized rats after decapitation at time points when some SCI rats had developed behavioral hypersensitivity (30-40 days post SCI varying among different SCI groups), or one-day after intrathecal treatments that correlated with the peak anti-nociceptive effects of TSP4 antisense oligodeoxynucleotide treatment. L4-6 dorsal spinal cord from both sides was dissected with a razor blade and stored at -80 °C until use. Frozen L4-6 dorsal spinal cords were pulverized and total proteins were extracted in 50mM Tris buffer, PH 7.5, containing 0.5% Triton X-100, 150 mM NaCl, 1mM EDTA and protease inhibitors. Protein concentrations were calculated using the bicinchoninic acid protein assay kit (Pierce, Rockford, IL). Equal amounts of proteins were loaded into a NuPAGE 3-8% Tris-acetate gel (Invitrogen, Calsbad, CA). The protein bands were separated by electrophoresis and transferred onto a nitrocellulose membrane. After blocking nonspecific binding sites with 5% low-fat milk in PBS containing 0.1% Tween 20 (PBS-T), the primary polyclonal antibody for TSP4 (1:750) (Kim et al., 2012) or monoclonal antibody for β-actin (1:10,000; Novus, Littleton, CO) were used to probe the membranes in PBS-T for 1 hr at room temperature or overnight at 4°C. Then the membranes were washed three times in PBS-T, and incubated with secondary antibody for one hour in room temperature. The antibody-protein complexes were detected using a chemiluminescence reagent (Thermo Scientific, Rockford, IL) followed by X-ray film exposure within the linear range of the X-ray films. The band densities were quantified using Kodak Image Analysis version 4.0. The ratio of TSP4 band density to that of β-actin within each sample was calculated for normalization of sample loading before cross-sample comparison analyses.
Statistic analysis
Data were presented as the means ± SEM and analyzed by Two–way ANOVA analysis for multi-group comparisons, or Students’ t test for two group comparisons as indicated. P value < 0.05 was considered a significant difference.
Results
SCI induced reduction of hindpaw reflex thresholds
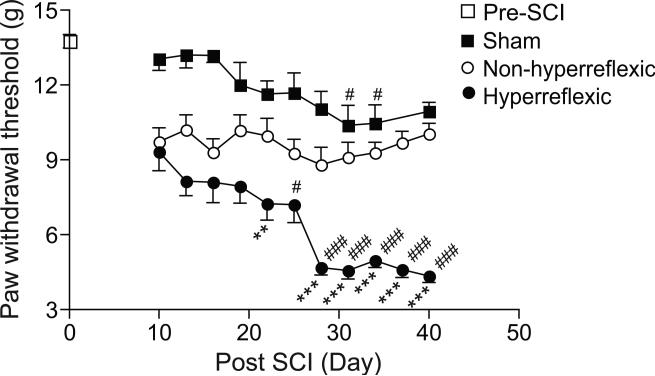
As shown in Figure 1, SCI induced a gradual reduction in reflex thresholds below the injury level in the hindpaw of roughly half of the SCI rats after 10 days post injury. This is consistent with reported observations in this model by different laboratories (Boroujerdi et al., 2011; Yoon et al., 2004). The peak reduction in hindpaw reflex thresholds occurred approximately 4 weeks post SCI. The slight reduction in reflex thresholds in the sham rats at some late time points may be due to increased stress level of these rats influenced by discomfort and ultrasonic vocalization of SCI rats tested blindly in the same room. Thermal hyperalgesia was also observed in the hindpaws of the SCI rats around the peak time point of hyperreflexic response (Mean ± SEM in paw withdrawal latency from Hot Box test: 10.32 ± 0.10 s for naïve rats, n = 4; 5.54 ± 0.25 s for SCI rats, n = 15. p < 0.001 by two-tailed Student's t test). The locomotor functional recovery scores indicated that there was no significant difference in the recovery of motor functions between SCI rats with or without reflex hypersensitivity at the peak time point of behavioral hypersensitivity (Mean ± SEM of BBB scores: 11.55 ± 0.25 from n = 11 in non-hyperreflexic group; 11.75 ± 0.22 from n = 12 in hyperreflexic group at 31 days post SCI. p = 0.5398 by two-tailed Student's t test). SCI did not cause behavioral hypersensitivity at- (thoracic) or above- (front paw) injury levels in this model when these rats were tested similarly as described but at different dermatomes (data not shown).
Figure 1. Development of below-level hypersensitivity to mechanical stimulation in some SCI rats.
The 50% paw withdrawal thresholds to von Frey filament stimulation were examined at the hind paws of SCI rats at designated time points. In sham rats, the hindpaw thresholds were between 10-15 grams, similar to the pre-surgery level of naïve rats. Behavioral hyperreflexia as indicated by reduced paw withdrawal thresholds to von Frey filament stimulation (around or below 5 g) was developed in the hindpaws of approximately half of the SCI rats. Another half of the SCI rats had hindpaw sensitivity similar to that from the sham control. Data presented are the means ± SEM from 34 rats in the pre-SCI groups, 15 rats in the sham group, 23 and 25 rats each in the hyperreflexic or non-hyperreflexic groups, respectively. **p < 0.01, ***p < 0.001 compared with the non-hyperreflexic group; #p < 0.05, ###p < 0.001 compared with day 10 post injury as determined by two-way ANOVA analyses.
Upregulated TSP4 proteins in SCI rats with reflex hypersensitivity
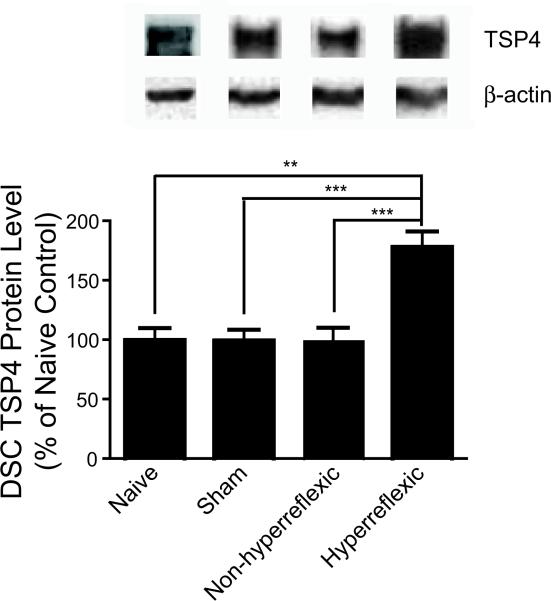
To determine whether SCI induced TSP4 upregulation in SCI rats that might have contributed to the changes in nociception, we examined the levels of TSP4 proteins in L4-6 dorsal spinal cord samples (DSC) from sham and SCI rats with or without hyperreflexic hindpaws 30-40 days after surgery when reflex hypersensitivity was fully developed in about 50% of the SCI rats. As indicated in Figure 2, TSP4 protein levels were similar between naïve, sham and SCI rats without reflex hypersensitivity. Importantly, TSP4 protein levels were significantly increased in SCI rats with reflex hypersensitivity compared with that from naïve, sham and SCI rats without reflex hypersensitivity. The data indicated that TSP4 protein levels were significantly upregulated in the DSC of SCI rats that correlated with reflex hypersensitivity development.
Fig. 2. Upregulation of TSP4 levels in L4-6 dorsal spinal cord of SCI rats with behavioral hypersensitivity.
Total proteins were extracted from L4-6 dorsal spinal cord after behavioral hypersensitivity was fully developed in some SCI rats (30 - 40 days after surgery varying among different SCI groups), and subjected to Western blot analysis for TSP4 protein levels. Representative Western blots were shown on top of each summarized bar graph (means ± SEM) from three naïve rats, six each of the sham, SCI group with (hyperreflexic) or without (non-hyperreflexic) behavioral hypersensitivity. The ratio of TSP4 band density to that of β-actin within each sample was calculated for normalization of sample loading before cross-sample comparison analyses. **p < 0.01, ***p < 0.001 determined by Students’ t test.
Intrathecal treatment with TSP4 antisense oligodeoxynucleotides in SCI rats resulted in a dose-dependent reversal of reflex hypersensitivity
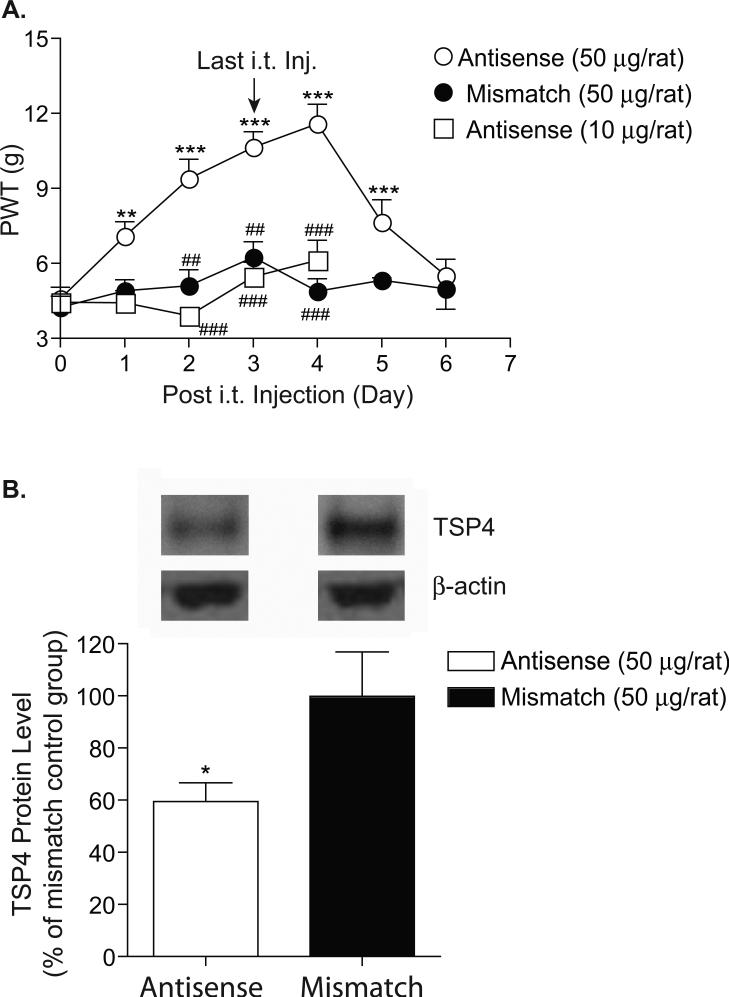
To determine whether TSP4 proteins contributed to the development of reflex hypersensitivity after SCI, we injected TSP4 antisense oligodeoxynucleotides intrathecally (between L5/6) into SCI rats with reflex hypersensitivity daily for 4 days to see whether intrathecal TSP4 antisense oligodeoxynucleotide treatment could block SCI-induced changes in nociception. This treatment has been shown to block TSP4 expression and behavioral hypersensitivity in a peripheral nerve injury model (Kim et al., 2012). Mismatch control oligodeoxynucleotides were used as controls. As shown in Figure 3A, intrathecal treatment with 50 μg/rat of TSP4 antisense oligodeoxynucleotides for four days led to a significant reversal of reflex hypersensitivity. The antisense effects had an onset time of 24 hrs, peaked approximately one day, and lasted for over two days after the last injection. Neither treatment with 10 μg/rat of TSP4 antisense oligodeoxynucleotides nor that with 50 μg/rat of mismatch oligodeoxynucleotides could reverse SCI-induce reflex hypersensitivity significantly.
Fig. 3. Intrathecal treatments with TSP4 antisense oligodeoxynucleotides reversed upregulation of TSP4 proteins in dorsal spinal cord and behavioral hypersensitivity in SCI rats.
A. SCI rats with hyperreflexic hindpaws 40 days post SCI were treated daily for 4 days with TSP4 antisense or mismatch oligodeoxynucleotides (50 μg/rat/day) via direct intrathecal injection into the L5-6 region. Paw withdrawal thresholds (PWT) to von Frey filament stimulation were measured daily before the injection, and continued after the last injection as indicated. Data presented are the means ± SEM from at least seven rats from each group except that 3 rats were in the mismatch oligodeoxynucleotide treatment group for day five and six after treatment initiation. **p < 0.01, ***p < 0.001 compared with pre-treatment levels; and ##p < 0.01, ###p < 0.001 compared with the 50 μg/rat/day antisense oligodeoxynucleotide treated group as determined by two-way ANOVA analyses. B. Western blot analysis was used to measure TSP4 levels in dorsal spinal cord collected approximately 24 hrs after the last injection that correlated with the peak anti-nociceptive effects of TSP4 antisense oligodeoxynucleotide treatment. Representative Western blots were shown on top of each summarized bar graph presenting the means ± SEM from five rats in each group. The ratio of TSP4 band density to that of β-actin within each sample was calculated for normalization of sample loading before cross-sample comparison analyses. *p < 0.05 compared with the mismatch oligodeoxynucleotide control treatment as determined by Student's t test.
Intrathecal treatment with TSP4 antisense oligodeoxynucleotides in behaviorally hypersensitive SCI rats resulted in diminished TSP4 protein level in DSC
To confirm that the effects of intrathecal antisense oligodeoxynucleotide treatments were mediated through the antisense mechanism, we examined TSP4 protein levels in DSC after the four-day antisense oligodeoxynucleotide treatment. As indicated in Figure 3B, data from Western blot analysis indicated that DSC TSP4 protein levels were reduced about 40 percent after TSP4 antisense oligodeoxynucleotide treatment compared with mismatch oligodeoxynucleotide control treatment. This percentage reduction was similar to the percentage increase of TSP4 in DSC of SCI rats with reflex hypersensitivity shown in Figure 2.
Discussion
Over the last decades, several mechanisms have been proposed to explain the condition of pain following SCI, including loss of spinal inhibitory mechanisms (Shapiro, 1997; Siddall and Loeser, 2001), synaptic plasticity (Shapiro, 1997; Yezierski, 2000), astrocyte and microglia activation and changes in cell-signaling pathways at spinal and supraspinal sites (Crown et al., 2006; Gwak et al., 2011; Hains and Waxman, 2006; Yu and Yezierski, 2005). We report here that SCI-induced elevation of thrombospondin-4 in dorsal spinal cord correlates with the development of behavioral hypersensitivity. Intrathecal treatment with TSP4 antisense oligodeoxynucleotides could reduce TSP4 expression and reverse SCI-induced behavioral hypersensitivity dose-dependently. These findings together suggest that SCI-induced TSP4 proteins in dorsal spinal cord may play a critical role in mediating SCI-induced changes in nociception.
In our study, approximately 50 percents of SCI rats developed below-level behavioral hyperreflexia post injury, similar to clinical observations that approximately a little more than half of SCI patients develop neuropathic pain and most of which is below the injury level (Siddall and Loeser, 2001; Turner et al., 2001). As there is no apparent difference in motor function recovery between SCI rats with or without behavioral hypersensitivity, and we only test behavioral hypersensitivity after the recovery of SCI animals to a level in which they can support their body weights and walk several weeks post injury (Fig. 1), the development of below-level behavioral hypersensitivity is not likely due to changes in motor functions on SCI rats, in agreement with our previous findings (Boroujerdi et al., 2011). Since intrathecal treatments with antisense, but not mismatch, oligodeoxynucleotides can reduce dorsal spinal cord TSP4 levels and reverse the established below-level behavioral hyperreflexia in a dose-dependent manner, our data support that SCI injury at the thoracic level leads to the development of below level neuroplasticity, including TSP4 overexpression, that contributes to the below-level SCI induced changes in nociception.
The mechanism underlying TSP4-mediated changes in nociception remains elusive. Data from recent studies have indicated that TSP proteins secreted from astrocytes play a critical role in inducing excitatory synapse formation in the central nervous system (Christopherson et al., 2005) by interacting with its receptor, the voltage gated calcium channel alpha-2-delta-1 subunit proteins (Eroglu et al., 2009). Interestingly, data from a peripheral nerve injury model have indicated that nerve injury increases TSP4 expression in activated dorsal spinal cord astrocytes that leads to the development of neuropathic pain states (Kim et al., 2012). In addition, SCI also causes upregulation of the calcium channel alpha-2-delta-1 subunit protein in lumbar dorsal spinal cord that plays a critical role in the development of centrally mediated below-level neuropathic pain states (Boroujerdi et al., 2011). Taken together, it is possible that SCI-induced TSP4 interacts with the calcium channel alpha-2-delta-1 subunit protein, leading to abnormal excitatory synaptogenesis and behavioral hypersensitivity. Further investigations to reveal the detail mechanism related to the role of TSP4 induction in the development of SCI induced behavioral hypersensitivity are warranted.
Acknowledgements
This study was supported in part by grants from the National Institutes of Health (NS064341 and DE021847) and California Spinal Cord Injury Research Funds (Z.D. Luo).
Footnotes
- It is known that peripheral nerve injury leads to TSP4 upregulation in spinal astrocytes that plays a critical role in the development of neuropathic pain states.
- Data from this study add that TSP4 is also upregulated in spinal cord after spinal cord injury, which may contribute to centrally mediated spinal cord injury induced changes in nociception.
Reference
- Ataoglu E, Tiftik T, Kara M, Tunc H, Ersoz M, Akkus S. Effects of chronic pain on quality of life and depression in patients with spinal cord injury. Spinal Cord. 2012;51:23–26. doi: 10.1038/sc.2012.51. [DOI] [PubMed] [Google Scholar]
- Baastrup C, Maersk-Moller CC, Nyengaard JR, Jensen TS, Finnerup NB. Spinal-, brainstem- and cerebrally mediated responses at- and below-level of a spinal cord contusion in rats: evaluation of pain-like behavior. Pain. 2010;151:670–679. doi: 10.1016/j.pain.2010.08.024. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Bornstein P, Agah A, Kyriakides TR. The role of thrombospondins 1 and 2 in the regulation of cell-matrix interactions, collagen fibril formation, and the response to injury. Int J Biochem Cell Biol. 2004;36:1115–1125. doi: 10.1016/j.biocel.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Boroujerdi A, Kim HK, Lyu YS, Kim DS, Figueroa KW, Chung JM, Luo ZD. Injury discharges regulate calcium channel alpha-2-delta-1 subunit upregulation in the dorsal horn that contributes to initiation of neuropathic pain. Pain. 2008;139:358–366. doi: 10.1016/j.pain.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroujerdi A, Zeng J, Sharp K, Kim D, Steward O, Luo ZD. Calcium channel alpha-2-delta-1 protein upregulation in dorsal spinal cord mediates spinal cord injury-induced neuropathic pain states. Pain. 2011;152:649–655. doi: 10.1016/j.pain.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik EC, Erhan B, Lakse E. The clinical characteristics of neuropathic pain in patients with spinal cord injury. Spinal Cord. 2012;50:585–589. doi: 10.1038/sc.2012.26. [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Crown ED, Ye Z, Johnson KM, Xu GY, McAdoo DJ, Hulsebosch CE. Increases in the activated forms of ERK 1/2, p38 MAPK, and CREB are correlated with the expression of at-level mechanical allodynia following spinal cord injury. Exp Neurol. 2006;199:397–407. doi: 10.1016/j.expneurol.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Annual Review of Pharmacology and Toxicology. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Eroglu C, Allen NJ, Susman MW, O'Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerup NB, Johannesen IL, Sindrup SH, Bach FW, Jensen TS. Pain and dysesthesia in patients with spinal cord injury: A postal survey. Spinal Cord. 2001;39:256–262. doi: 10.1038/sj.sc.3101161. [DOI] [PubMed] [Google Scholar]
- Gwak YS, Kang J, Unabia GC, Hulsebosch CE. Spatial and temporal activation of spinal glial cells: role of gliopathy in central neuropathic pain following spinal cord injury in rats. Exp Neurol. 2011;234:362–372. doi: 10.1016/j.expneurol.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, Waxman SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci. 2006;26:4308–4317. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Higuera ES, Luo ZD. A rat pain model of vincristine-induced neuropathy. Methods Mol Med. 2004;99:91–98. doi: 10.1385/1-59259-770-x:255. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Widerstrom-Noga E, Richards JS, Finnerup NB, Biering-Sorensen F, Cardenas DD. Reliability and validity of the International Spinal Cord Injury Basic Pain Data Set items as self-report measures. Spinal Cord. 2009;48:230–238. doi: 10.1038/sc.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Figueroa KW, Li KW, Boroujerdi A, Yolo T, Luo ZD. Profiling of dynamically changed gene expression in dorsal root ganglia post peripheral nerve injury and a critical role of injury-induced glial fibrillary acidic protein in maintenance of pain behaviors [corrected]. Pain. 2009;143:114–122. doi: 10.1016/j.pain.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Li KW, Boroujerdi A, Peter Yu Y, Zhou CY, Deng P, Park J, Zhang X, Lee J, Corpe M, et al. Thrombospondin-4 Contributes to Spinal Sensitization and Neuropathic Pain States. J Neurosci. 2012;32:8977–8987. doi: 10.1523/JNEUROSCI.6494-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Song YH, Higuera ES, Luo ZD. Spinal dorsal horn calcium channel alpha2delta-1 subunit upregulation contributes to peripheral nerve injury-induced tactile allodynia. J Neurosci. 2004;24:8494–8499. doi: 10.1523/JNEUROSCI.2982-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills CD, Hains BC, Johnson KM, Hulsebosch CE. Strain and model differences in behavioral outcomes after spinal cord injury in rat. J Neurotrauma. 2001;18:743–756. doi: 10.1089/089771501316919111. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE., Jr. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma. 2003;20:179–193. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- Shapiro S. Neurotransmission by neurons that use serotonin, noradrenaline, glutamate, glycine, and gamma-aminobutyric acid in the normal and injured spinal cord. Neurosurgery. 1997;40:168–176. doi: 10.1097/00006123-199701000-00037. discussion 177. [DOI] [PubMed] [Google Scholar]
- Siddall PJ, Loeser JD. Pain following spinal cord injury. Spinal Cord. 2001;39:63–73. doi: 10.1038/sj.sc.3101116. [DOI] [PubMed] [Google Scholar]
- Turner JA, Cardenas DD, Warms CA, McClellan CB. Chronic pain associated with spinal cord injuries: a community survey. Arch Phys Med Rehabil. 2001;82:501–509. doi: 10.1053/apmr.2001.21855. [DOI] [PubMed] [Google Scholar]
- Yezierski RP. Pain following spinal cord injury: the clinical problem and experimental studies. Pain. 1996;68:185–194. doi: 10.1016/s0304-3959(96)03178-8. [DOI] [PubMed] [Google Scholar]
- Yezierski RP. Pain following spinal cord injury: pathophysiology and central mechanisms. Prog Brain Res. 2000;129:429–449. doi: 10.1016/S0079-6123(00)29033-X. [DOI] [PubMed] [Google Scholar]
- Yoon YW, Dong H, Arends JJ, Jacquin MF. Mechanical and cold allodynia in a rat spinal cord contusion model. Somatosens Mot Res. 2004;21:25–31. doi: 10.1080/0899022042000201272. [DOI] [PubMed] [Google Scholar]
- Yu CG, Yezierski RP. Activation of the ERK1/2 signaling cascade by excitotoxic spinal cord injury. Brain Res Mol Brain Res. 2005;138:244–255. doi: 10.1016/j.molbrainres.2005.04.013. [DOI] [PubMed] [Google Scholar]