Abstract
Endothelial functions are highly regulated by imposed shear stress in vivo. The characteristics of shear stress determine mechanotransduction events that regulate phenotypic outcomes including redox and inflammatory states. Recent data indicates that microRNAs (miRs) in vascular endothelial cells (ECs) play an essential role in shear stress-regulated endothelial responses. More specifically, athero-protective pulsatile flow (PS) induces miRs that inhibit mediators of oxidative stress and inflammation while promoting those involved in maintaining vascular homeostasis. Conversely, oscillatory flow (OS) elicits the opposing networks. This is exemplified by the PS-responsive transcription factor, krueppel-like factor 2 (KLF2), which regulates miR expression but is also regulated by OS-sensitive miRs to ultimately regulate the oxidative and inflammatory state of the endothelium. In this review, we outline important findings demonstrating the multifaceted roles of shear stress-regulated miRs in endothelial redox and inflammatory balance. Furthermore, we discuss the use of algorithms in deciphering signaling networks differentially regulated by PS and OS.
Keywords: Endothelium, Shear Stress, miR, KLF2, Oxidative Stress, Inflammation
Introduction
Established evidence indicates that vascular endothelium in the arteries is not merely a tissue layer separating the circulating blood and the vascular wall. Rather, the endothelium plays a crucial role in maintaining vascular homeostasis, largely through its regulatory functions of anti-coagulation, anti-inflammation, and anti-oxidative stress [1, 2]. In vivo, the endothelium of the arterial tree is constantly exposed to shear stress (the tangential component of the hemodynamic forces due to blood flow), which plays an important role in endothelial homeostasis [3]. The athero-protective flow pattern in the straight parts of the vascular tree not only causes an elevated expression of anti-oxidative and anti-inflammatory genes, but also suppresses pro-oxidative and pro-inflammatory genes. In contrast, the athero-prone flow pattern in arterial curvatures and bifurcations results in functionally disturbed endothelium as manifested by the elevated expression of pro-oxidative and pro-inflammatory genes, and the down-regulation of anti-oxidative and anti-inflammatory genes [4]. The distinct phenotypes of vascular endothelial cells (ECs) under different blood flow patterns lead to the topographic distribution of atherosclerosis in the arterial tree of humans and many experimental animal models. The pulsatile shear stress (PS) and oscillatory shear stress (OS) applied to cultured ECs in vitro provide useful models for the study of athero- protective and athero-prone flow patterns in vivo, respectively [5].
microRNAs (miR) constitute an important part of gene regulation in species ranging from C. elegans to humans [6]. Instead of regulating target-gene expression at the levels of transcription and translation, miRs associate with their target mRNAs to form miR-induced silencing complex (miRISC), causing the degradation of the target mRNAs or suppression of their translation [7]. The key genes involved in the core functions of ECs such as angiogenesis, vascular tone, and leukocyte-endothelium interaction have been reported to be regulated by miRs, as in many other types of cells in the body [8, 9]. Because shear stresses are major physiological and pathophysiological stimuli that induce or suppress an array of genes in ECs, it is not surprising that miRs are involved in the shear stress regulation of endothelial biology and functions. Indeed, we and others have reported miR expression profiles in ECs responding to different flow patterns [10, 11].
Conceptually, shear stress, similar to other environmental cues, elicits mechanotransduction pathways to induce or silence the transcription of miR genes or miRs along with their parental genes [10, 11]. Because of the diversity of miRs that can be modulated by shear stress, their diverse targets can affect all aspects of endothelial biology. For example, the transcripts of a key EC transcriptional factor [e.g., Krüppel-like factor 2 and 4 (KLF2, KLF4)] can be regulated by shear stress-induced miRs [12, 13]. The gene products of this transcription factor may further regulate the expression of a panel of miRs [14]. In addition, the miR-involved signaling and gene expression networks are governed by temporal factors. The analysis of such complex and comprehensive system requires bioinformatics and systems biology tools. In this review, we focus on shear stress-sensitive miRs, their differential regulation by athero-prone and athero-protective flows, and the functional responses in terms of endothelial health and function. We will also discuss integrated approaches combining in silico and experimental methods to be used for future study shear stress-induced miR responses as a network.
miRs, KLF2, and functional endothelium
A major step of miR regulation occurs at the transcriptional initiation of the miR genes or their parental genes [15]. Because multiple transcription factors are shear stress-inducible, it is expected that a myriad of miRs can be induced or repressed by shear stress-inducible transcription factors. One of these transcription factors is KLF2 [12, 13]. Functioning as a master regulator of endothelial lineage, KLF2 upregulates genes such as endothelial nitric oxide synthase (eNOS), thrombomodulin (TM), and nuclear factor erythroid 2-related factor 2 (Nrf2) that exert anti-inflammatory, anti-thrombotic, and anti-oxidative effects in ECs [16, 17]. KLF2 can also inhibit the expressions of vascular cell adhesion protein 1 (VCAM-1), E-selectin, plasminogen activator inhibitor-1 (PAI-1) and tissue factor in ECs, which adversely affect endothelial functions [16, 18]. KLF2 is predicted to transcriptionally regulate several miRs, including miR-126, miR-30a, miR-10a, miR-23b, miR-143, miR-145, and miR150 [15, 19, 20]. Among them, miR-126 can enhance vascular endothelial growth factor (VEGF) signaling [21] and seems to be endothelial-specific [19]. Nicoli et al. demonstrated that klf2a (zebrafish version of KLF2) regulates miR-126 to induce flow-stimulated angiogenesis in zebrafish [20]. Indeed, a putative KLF2 binding site is located -1 kb upstream of the miR-126 transcriptional start site in human [22].
In many instances, the shear stress-inducible transcriptional factors themselves are regulated at the transcriptional level. Athero-protective PS activates MEF5/ERK5/MEF2 and AMP-activated protein kinase (AMPK) pathways, which merge at the transcriptional up-regulation of KLF2 [23, 24]. In addition, KLF2 can be transcriptionally up-regulated through increased phosphorylation of histone deacetylase 5 (HDAC5) and hence HDAC dissociation from myocyte enhancer factor-2 (MEF2), which in turn leads to the enhanced MEF2 transactivation of KLF2 [25]. Interestingly, athero-prone oscillatory OS can up-regulate classes I and II HDACs resulting in nuclear accumulation to promote deacetylation of MEF2, leading to decreased KLF2 transactivation [26]. Importantly, KLF2 is also directly targeted and regulated by miRs. KLF2 and KLF4 mRNAs have been shown to be targeted by miR-92a, which is in line with the decreased expression of KLF2- and KLF4-regulated genes, such as eNOS, in ECs loaded with pre-miR-92a [12, 13]. OS, but not PS, elevates the level of miR-92a in ECs [12]. Given that KLF2 and KLF4 regulates EC functions, these results suggest that the functionally disturbed endothelium under the athero-prone flow is caused in part by the increased miR-92a targeting KLF2 and KLF4. Future study should be conducted to investigate whether the athero-prone regions in EC-specific miR-92a KO mice become resistant to atherogenesis. In addition to the experimentally validated miR-92a, bioinformatics analysis predicts that miR-32, miR-363, miR-25, miR-367, miR-101, miR-330-5p, miR-365, miR-326, miR-223, miR-185, miR-381, miR-300, miR-340, and miR-27 may also target KLF2 mRNA (microRNA.org) [27]. Experimental validation of these miRs that target KLF2, their correlation with flow conditions, and the consequent EC responses would be fruitful research topics.
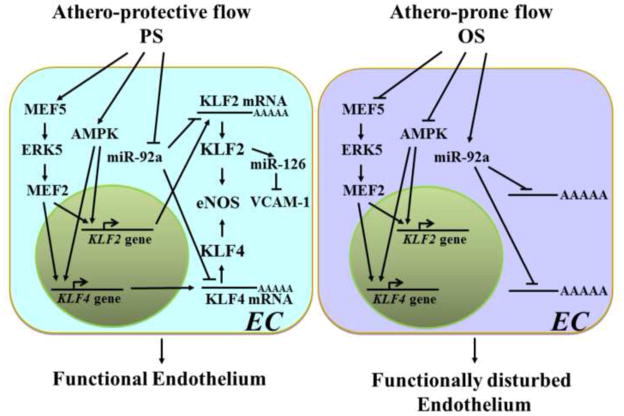
The advancement of miR biology makes it apparent that flow regulation of KLF2 occurs at multiple levels, including transcriptional, post-transcriptional, and post-translational (Fig. 1). Thus, a temporal regulation network including miRs, rather than chains of signaling events, should be considered for the study of KLF2 in EC responses to distinct flow patterns in relation to EC functional outcome.
Figure 1.
Different flow patterns determine distinct endothelial functional outcomes through multi-level regulation of KLF2 and KLF4. In response to athero-protective flow in vivo or PS in vitro, AMPK as well as MEF5/ERK5/MEF2 are activated to transactivate KLF2 and KLF4. Subsequent increase in the respective mRNA’s level leads to an increase in KLF2 and KLF4 proteins. This prompts KLF2 to transactivate miR-126 which targets VCAM-1. In parallel, KLF2 induces eNOS and increases NO bioavailability. Concurrently, KLF4 transactivates eNOS. These orchestrated pathways support the functional ECs. Under athero-prone flow or OS, the AMPK and the MEF5/ERK5/MEF2 pathways are repressed, resulting in down-regulation of KLF2 and KLF4. Athero-prone flow or OS also up-regulates miR-92a, which destabilizes KLF2 and KLF4 mRNA’s through post-transcriptional targeting. The coordinated modulation of these pathways mitigates the beneficial effects of both KLF2 and KLF4, leading to endothelial dysfunction.
Shear stress, EC redox state, and miRs
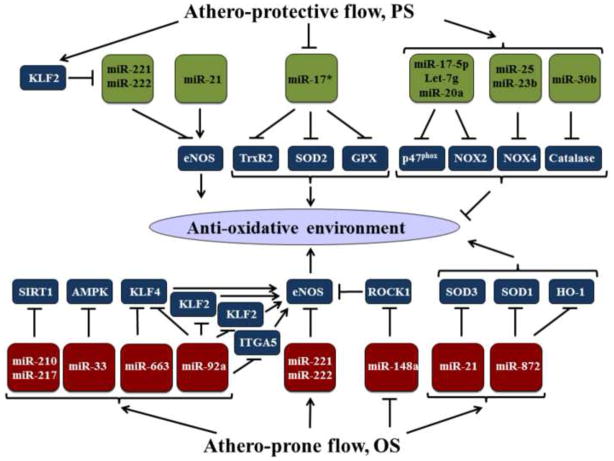
Athero-prone flow contributes to the production and accumulation of reactive oxygen species (ROS) in ECs, ultimately resulting in functionally disturbed endothelium and leading to the progression of vascular diseases such as atherosclerosis [28]. In ECs, the redox state is mediated in part by NADPH oxidase (NOX), eNOS, superoxide dismutase (SOD), glutathione peroxidase (GPx), thioredoxin-dependent peroxidase (TrxR2) and catalase [29, 30]. Ample evidence indicates that miRs are involved in the redox state of ECs exposed to distinct flow patterns (Fig. 2). Athero-prone flow patterns in vivo and OS in vitro may increase the expression of miRs that target anti-oxidant genes, while decreasing those that target pro-oxidant genes. Conversely, athero-protective flow patterns in vivo and PS in vitro have opposite effects on miR networks. Table 1 summarizes the PS- and OS- inducible miRs that probably contribute to the redox and inflammatory state of ECs.
Figure 2.
Proposed miR network that regulates redox status in ECs in response to flow. With the exception of miR-21, PS regulates a set of miRs that promote an anti-oxidative environment. This effect may be achieved by (1) KLF2 inhibition of eNOS-targeting miR-221/222; (2) inhibition of miR-17*, which targets anti-oxidative genes TrxR2, SOD2, GPX; and (3) induction of multiple miRs that repress pro-oxidative enzymes such as p47phox, Catalase, NOX4, and NOX2. In contrast, OS regulates an array of miRs that contributes to increased oxidative stress. These include OS-induced miRs that repress eNOS and/or its positive regulators (e.g. AMPK, SIRT1, KLF2, KLF4 and ITGA), OS-suppresses miRs that target eNOS negative regulators such as ROCK1, as well as OS-upregulated miRs that inhibit the anti-oxidative enzymes SOD3, SOD1, and HO-1.
Table 1.
Shear-inducible miRs and their role in endothelial oxidative stress and inflammation
| miRNAs | Physiological Stimulation | Target | Oxidative Stress | Inflammatory | Reference |
|---|---|---|---|---|---|
| miR-10a | PS | MAP3K7, βTRC | Anti- | [66] | |
| miR-17* | OS | SOD3, GPX2, TrxR2 | Pro- | [45] | |
| miR-20a | PS+ | CX43 | Anti- | [88] | |
| miR-23b | PS | E2F1, TAB2, TAB3, IKK-α | Anti- | Anti- | [11, 72] |
| miR-25 | PS+ | NOX4 | Anti- | [35] | |
| miR-30b | PS | Catalase | Pro- | Anti- | [44] |
| miR-126 | VCAM1, PAK1 | Anti- | [22, 73, 74, 93] | ||
| miR-148 | ROCK1 | [57] | |||
| miR-155 | PS | RHOA | Anti- | [70, 91] | |
| miR-143/145 | PS | ACE | Anti- | [14, 71] | |
| miR-214 | PS+ | eNOS | Pro- | [50] | |
| miR-33 | OS+ | AMPK1α | Pro- | [55] | |
| miR-92a | OS | KLF2, KLF4, ITGA5 | Pro- | [12, 13, 52, 77, 78] | |
| miR-217 | OS+ | SIRT1 | Pro- | [54] | |
| miR-221/222 | OS+ | Pro- | [9] | ||
| miR-633 | OS | Pro- | [77] | ||
| miR-21 | PS/OS | PPARA, SOD3 | Both | Pro- | [39–41] |
This table illustrates the functionally relevant miRs that are differentially expressed in endothelial cells in response to shear stress. The anti-atherogenic PS is associated with the functional phenotype characterized by low levels of ROS and inflammatory markers. On the other hand, the pro-atherogenic OS is associated with the dysfunctional phenotype manifested by high level of ROS and inflammatory markers.
Predicted to be regulated by the respective stimuli
NOX activation is a major source of ROS in ECs. Specifically, ECs express two major isoforms of the catalytic subunit of NOX, i.e., NOX2 (gp91phox) and NOX4 [31, 32]. Activation of NOX depends on the phosphorylation of NOX subunit p47phox, which is required for its translocation to the plasma membrane to prime its association with p67phox [33, 34]. Recent findings indicate that many NOX components are subject to regulation by miRs. NOX4 is a validated target of miR-25 [35] and putatively targeted by miR-23b. miR microarray profiling and flow channel experiments have revealed that PS induces miR-23b [11]. If NOX4 is a bona fide target of miR-25 and miR-23b in ECs, one can speculate that PS may down-regulate NOX4 by inducing miR-25 and miR-23b, contributing to anti-oxidative defense in vasculature. Similarly, NOX2 (gp91phox) may be targeted by miRs. As predicted by miRNA.org [27], miR-17-5p, Let-7g, and miR-20a are putative p47phox-targeting miRs. Collectively, shear stress may regulate a panel of miRs in ECs to fine tune the ROS production by targeting multiple NOX subunits.
SOD, GPx, TrxR2, and catalase are major antioxidant enzymes that serve essential roles in maintaining redox balance. SOD converts O2- to H2O2; GPx, TrxR2, and catalase reduce H2O2 to water and O2; GPx also reduces lipid hydroperoxides to alcohols [36–38]. Presumably, athero-prone flow decreases the expression of these anti-oxidant genes by up-regulating their respective miRs. However, the miR network is intricate in that PS and OS induce different but overlapping miR profiles that result in healthy versus pathophysiological phenotypes, respectively. For example, the promiscuous miR-21 has been shown to be increased in ECs under both PS and OS [39]. Under PS, miR-21 expression positively correlates with the phosphorylation and activity of eNOS [40]. Paradoxically, the OS-induced miR-21 may be pro-oxidative, since it directly targets SOD3 [41]. Another possible mechanism contributing to the pro-oxidative role of miR-21 is its attenuation of SOD2 by targeting TNF-α, a homeostatic mediator of SOD2 transcription [41, 42]. miR-30b, which has been shown to be increased under laminar shear stress [43], paradoxically inhibits catalase [44]. However, the regulation of some miRs by different flow patterns is more definitive. For example, PS down-regulates miR-17 [11]. Importantly, miR-17* targets SOD2, GPx2, and TrxR2 [11, 45]. In contrast, OS down-regulates SOD1 [28], possibly through the targeting by miR-872, a pro-oxidative stress miR that also targets heme oxygenase-1 [46, 47]. Additionally, thioredoxin (Trx), an oxidoreductase, and its thioredoxin-interacting protein (Trxnip) function in response to oxidative stress and may likely be subject to shear-responsive miR regulation. miR-17 and miR-373 target Txnip but have an anti-oxidative and anti-inflammatory effect [48, 49], which may serve a protective role in OS. Trx, on the other hand, serves an anti-oxidative role [50] but its regulation by miRs has not yet been reported. However, miRanda (micro.org) predicted that it might be targeted by hsa-miR-183, hsa-miR-129-5p, and hsa-miR-135a/b. Further study is warranted to delineate whether shear stress-mediated miR regulation of Trx and Txnip occurs, and if so, its ensuing effects on endothelium.
A decrease in eNOS-derived NO bioavailability results in impaired endothelial function and vascular tone [51]. The balance between NO and ROS is important in maintaining proper EC signaling and functions [52]. As shear stresses are important physiological and pathophysiological stimuli regulating eNOS, miRs distinctly regulated by PS and OS play an important role in eNOS expression and activity. For example, PS-induction of KLF2 decreases miR-214, which can directly target eNOS mRNA [53]. miR-221/222 have been shown to indirectly decrease eNOS expression [9, 54]. Thus, miR-221/222 are expected to be up-regulated during OS but down-regulated during PS. As mentioned previously, eNOS expression is also indirectly inhibited through miR-92a (17-92a cluster) targeting of integrin alpha-5 (ITGA5) and KLF2, both of which are necessary for eNOS expression [12, 55]. Shear stress further regulates eNOS at the post-translational level through a synergistic effect between AMPK phosphorylation and silent mating type information regulation 2 homolog 1 (SIRT1) deacetylation [56]. Given that miR-217 down-regulates SIRT1 [57], whilst miR-33 inhibits AMPK expression [58], these miRs may also take part in the negative effect of OS on eNOS. miR-148a can increase eNOS activity by targeting rho-associated, coiled-coil containing protein kinase 1 (ROCK1) [59, 60]. Considering that ROCK1 is highly expressed in atherosclerosis and that the Rho/ROCK pathway is pro-atherogenic by decreasing eNOS function [61], miR-148a is likely to be involved in OS-impairment of NO bioavailability.
Shear stress regulation of EC inflammatory state and miRs
In addition to the balanced redox state, a functional endothelium is manifested by its low inflammatory stress [2]. In line with this notion, athero-protective flow exerts an anti-inflammatory effect in ECs due largely to the down-regulation of pro-inflammatory and up-regulation of anti-inflammatory molecules, including miRs (Fig. 3). [62, 63]. In contrast, the athero-prone flow up-regulates pro-inflammatory and down-regulates anti-inflammatory molecules, causing a pro-inflammatory state in functionally disturbed endothelium. The inflammatory status of the endothelium is governed by the local flow patterns that activates or silences nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in ECs [64]. Activation of the NF-κB pathway prompts its nuclear translocation, resulting in transactivation of chemoattractants and adhesion molecules, including interleukin 6 (IL-6), IL-8, intercellular adhesion molecule 1 (ICAM-1), VCAM-1, and E-selectin [65–67]. These changes promote monocyte recruitment to endothelium and subsequent invasion into the subendothelial space [68]. miR profiling of athero-protective regions in vivo has shown that miR-10a is expressed under PS conditions [14]. This would result in miR-10a targeting the 3 UTR of mitogen-activated protein kinase kinase kinase 7 (MAP3K7) and β-transducin repeat containing E3 ubiquitin protein ligase (βTRC). Under PS, the down-regulation of these molecules is likely to prevent the degradation of IκB and the consequent nuclear translocation of NF-κB p50 and p65 subunits [69]. Both shear stress-sensitive miR-30b and miR-10a directly inhibit VCAM-1 and E-selectin [43, 69]. Additionally, the shear stress-sensitive miR-181b inhibits the NF-κB pathway by directly targeting importin-33 to decrease nuclear accumulation of p50 and p65 [70].
Figure 3.
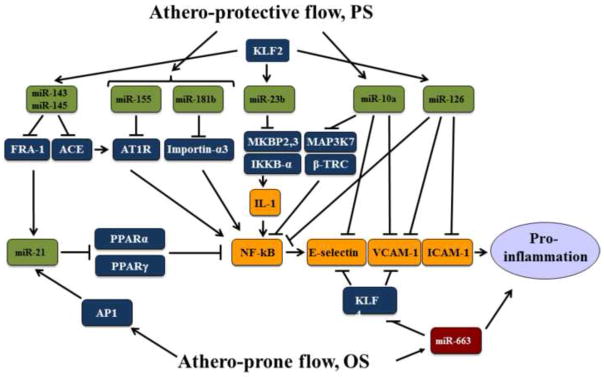
Putative miR network that regulates the pro-inflammatory NF- B pathway. PS activates the indicated miRs that result in the inhibition of NF- B by direct targeting and indirect regulation. Additionally, miR-10a and miR-126 directly inhibit the expression of E-selectin, VCAM-1, and ICAM-1. KLF2 plays a major role in transactivating many of the anti-inflammatory miRs, including miR-143, miR-145, miR-23b, and miR-126. In contrast, OS causes AP-1 activation and the consequent miR-21 induction. miR-21 inhibits PPARα and PPARγ, both negatively regulate NF-κB. OS also up-regulates miR-663 that targets KLF4 and therefore desuppresses E-selectin and VCAM-1. The overall outcome of this intricate network under OS is pro-inflammation.
Angiotensin II (Ang II) is a potent activator of the NF-κB pathway [71]. Although there is no clear indication whether Ang II-elicited signaling is suppressed under PS, miR-155 has been shown to be up-regulated by PS and targets Ang II receptor 1 [72, 73]. Additionally, PS increases miR-143/145 in ECs [14]. Because miR-143/145 has been shown to target angiotensin converting enzyme (ACE) [74], one would expect that under PS, Ang II level remains low because miR-143/145 would target ACE.
KLF2 is critical for functional endothelium, in part due to its anti-inflammatory effect. For example, KLF2-induced miR-23b exerts an anti-inflammatory effect by targeting the MAPK binding proteins 2 and 3 and IκB kinase-α (IKKB-α), which function to increase IL-1 mediated activation of NF-κB and MAPK8/JNK pathways [75]. KLF2-induced miR-126 is increased under PS, which would inhibit VCAM-1 and possibly ICAM-1 and NF-κB. [76, 77]. PPARα negatively regulates the expression of many pro-inflammatory genes by inhibiting the NF-κB and AP-1 pathways [78]. OS up-regulates the pro-inflammatory activity of miR-21 in an AP-1 dependent manner [39]. Subsequently, miR-21 targets PPARα and PPARγ [39]. Conversely, the KLF2-induced miR-143/145 may target FRA-1 to inhibit miR-21 transcription [79].
Ni et al. 2011 showed that miR-663 is up-regulated by OS and is necessary for monocyte binding to endothelium [80]. Although KLF4 mRNA does not have a direct binding site for miR-663, miR-663 knockdown significantly increases the transcription of KLF4 and decreases that of VCAM-1 and E-selectin [80]. Since KLF4 plays a positive role in enhancing endothelial functions, these miRs affecting the upstream signaling of KLF4 or transactivation of KLF4 may also have detrimental effect on endothelial functions [81].
Role of miRs in the shear stress regulation of EC Cell Cycle, Cytosleleton, and Gap junction
Distinctive EC phenotypes associated with different flow patterns also include proliferation or quiescence, altered endothelial permeability, and differential cytoskeletal organization [82–84]. For example, ECs under athero-protective flow are less proliferative [85]. This quiescence is maintained by preventing EC from entering into S phase. The shear stress-induced KLF2 is important for EC quiescence [86], which may be partly due to its regulation of miR-23b [14]. PS-sensitive miR-23b is correlated to hypophosphorylation of retinoblastoma (Rb) protein E2F1 [11]. The hypophosphorylated Rb increases its inhibitory association with E2F1 to prevent cell cycle progression from G1 to S phase. Because miR-23b does not directly target p53, p27, Cyclin D1, and CDK4, the mechanism by which miR-23b inhibits Rb phosphorylation needs to be investigated.
Shear stress modulates endothelial permeability and integrity, largely through its effects on intercellular junctional proteins, including connexins and vascular endothelial (VE)-cadherin [87]. Disturbed flow can up-regulate Cx43 in ECs [88] whereas VE-cadherin (VE-Cad) at EC borders is up-regulated in PS-predominant arterial regions [89]. Despite the lack of data on shear stress-regulation of junctional proteins through miRs, connexins and VE- cadherin are targeted by miRs [90, 91], and therefore may contribute to the shear stress-regulated endothelial permeability.
Different flow patterns also induce distinct morphologies in ECs [92]. A round-shaped EC with random and short actin filaments located mainly at the periphery of the cells are prominent morphological features induced by OS or disturbed flow. On the other hand, ECs exposed to PS are elongated, aligned in the direction of flow with organized and parallel actin stress fibers [93]. Shear stress-responsive miR-155 may mediate the effects of flow on endothelial cytoskeletal organization [73]. Overexpression of miR-155 decreased the expression of RhoA, a regulator of actin cytoskeleton organization [94]. p21-activated kinase (PAK), a Ser/Thr kinase that modulates small GTPases Rac and Cdc42-induced cytoskeletal remodeling, may be of interest in future investigations as its expression is known to be up-regulated by low shear stress [94, 95]. Because miR-126 represses PAK1 to regulate vascular integrity in zebrafish [96], it is possible that athero-prone flow, by repressing KLF2-induced miR-126, up-regulates PAK.
Future directions of research on shear stress regulation of miR in ECs
The current knowledge of shear stress regulation of miR and miR regulation of EC functions is mainly based on studies of mechanotransduction and/or gene expression events at one or a few time points. Furthermore, analysis of these signaling cascades has been performed in a linear, “snap shot” manner. In ECs, miR regulation networks are much more complicated because multiple mechanosensors and signaling cascades are engaged simultaneously or in a time-dependent manner. Additionally, several miRs can bind to a common target mRNA, multiple transcriptional factors/co-activators/co-suppressors can be involved in the induction of a given miR gene, and EC phenotype is determined by the synergism of an array of miR-regulated genes (e.g., pro-oxidant/anti-oxidant, pro-inflammatory/anti-inflammatory). In order to elucidate the interactions and causal effects of these complicated but coordinated events involving miRs, studies need to be extended to include time resolution and systems analysis.
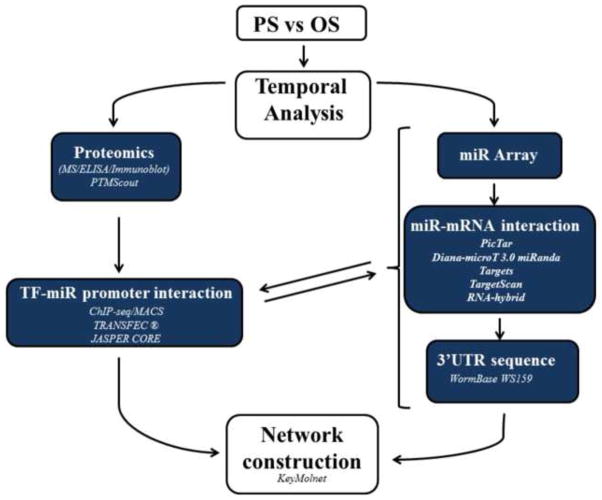
Many methods have been employed to construct relationships and networks from both experimental and predictive data sets [97]. Given that mechanotransduction responses in ECs are integrated and dynamic, investigations of signaling and gene expression regulatory networks that involve several steps require temporal and causal analysis to capture representative component relationships (Fig. 4). Mechanotransduction signaling pathways constitute post-translational modifications (PTM) to initiate transcription factor activation and miR expression. Predictive programs such as PTMScout (http://ptmscout.mit.edu) provide avenues for deriving the PTM pathways involved in transcriptional activation or repression of miR-induced regulatory networks [98]. Experimental evidence involving proteomic evaluation (mass spectroscopy, immunoblotting, enzyme-linked immunosorbant assay (ELISA) and gene expression and manipulation (microarrays, qPCR, expressional alteration, mutagenesis) is necessary to verify the effects a PTM has on miR expression and its downstream targets. Delineating the impact of a shear stress-regulated transcription factor in regulating miR networks involves the understanding of where does the TF bind in the genome. Both TRANSFAC® (http://www.gene-regulation.com/pub/databases.html) and JASPAR CORE databases (http://jaspar.genereg.net/) offer in silico approaches to investigate this issue by providing information on experimentally-proven transcription factor binding sites, consensus binding sequences, and regulated genes [99, 100]. Chromatin immunoprecipitation sequencing (ChIP-seq) applied to Model-based analysis of ChIP-seq (MACS, http://liulab.dfci.harvard.edu/MACS/) [101], can verify predicted binding sites or provide new information on transcription factors association with miR promoters. Understanding miR target specificity is also important for describing the influence of miRs in mediating flow-initiated phenotypes. 3′UTR sequences can be obtained from WormBase WS159, which can be cross-referenced to several programs used to predict miR targets, including: PicTar [102], Diana-microT 3.0 target prediction program (diana.cslab.ece.ntua.gr/microT) [103], miRanda Targets version 4 [104], TargetScan release 3.1 [105], and RNA-hybrid [106]. However, none of these databases directly addresses how many transcripts a particular miR can influence. Plaisier, et al. have developed a Framework for the Inference of Regulation by miRs (FIRM) to predict which miRs regulate a group of mRNAs [107]. This algorithm is based on the concept that genes regulated by the same miR can be identified by their correlated fluctuation in mRNA abundance accompanied by the presence of a conserved miR-binding site in their 3′UTRs. This algorithm also assesses which mRNAs are indirectly affected by a miR. Recently, Satoh et al. developed an effective global modeling scheme that integrated Ensembl IDs of miR respective target genes into KeyMolnet (http://www.immd.co.jp/en/product_1.html, KeyMolnet) [108]. The advantage of KeyMolnet is that it allows analysis of molecular interactions stemming from the traditional research.
Figure 4.
Network scheme for deriving miR-regulated networks and signaling pathways. Cells are subjected to shear stress for various time points and analyzed experimentally with proteomic tools to assess PTM-mediated regulation of signaling pathways. PTM Scout is integrated in this assessment, following which transcription factors regulating miR transactivation can be investigated utilizing experimental and bioinformatics approaches. This data are cross referenced with FIRM analysis, and can be utilized, with assistance from KeyMolnet, to construct miR-regulated networks.
The time-dependent data sets are necessary to integrate this information into a miR network featured in temporal correlation. This is particularly important because EC phenotypic outcomes at the distal end are a sum of the mechano-sensitive signaling and gene expression events in time-dependent manners. In silico construction of such miR networks based on wet experiments at the molecular level will increase the reliability for functional validations at cellular (i.e., EC) and organ (i.e., vessel) levels.
Highlights.
Pulsatile flow-induces miRs involved in anti-oxidant, anti-inflammatory networks.
Oscillatory flow-induced miRs involved in pro-oxidant, pro-inflammatory networks.
Krueppel-like factor 2 (KLF2) maintains redox balance via miRs.
Algorithms can be used to uncover temporal miR regulation of redox networks.
Acknowledgments
This work was supported in part by National Institutes of Health Grants HL106579 and HL108735 (S.C., S. S., J.S.), HL89940 (J.S).
Abbreviations
- 3′UTR
3′ untranslated region
- ACE
angiotensin converting enzyme
- AMPK
AMP-activated protein kinase
- βTRC
β-transducin repeat containing E3 ubiquitin protein ligase
- CDK4
cyclin-dependent kinase 4
- ChIP
chromatin immunoprecipitation
- EC
endothelial cell
- ELISA
enzyme-linked immunosorbent assay
- eNOS
endothelial nitric oxide syntase
- FIRM
Framework for the Inference of Regulation by MiRS
- GPx
glutathione peroxidase
- HDAC
histone deacetylase
- ICAM-1
intercellular adhesion molecule 1
- IL-6
interleukin 6
- IL-8
interleukin 8
- ITGA5
integrin alpha-5
- JNK
c-Jun N-terminal kinase
- KLF2
krueppel-like factor 2
- MAP3K7
mitogen-activated protein kinase kinase kinase 7
- MEF2
myocyte enhancer factor-2
- miR
micro RNA
- miRISC
miR-induced silencing complex
- NOX
NADPH oxidase
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- Nrf2
nuclear factor erythroid 2-related factor
- PAK
p21-activated kinase
- PS
pulsatile shear
- OS
oscillatory shear
- ROCK1
rho-associated, coiled-coil containing protein kinase 1
- ROS
reactive oxygen species
- SIRT1
silent mating type information regulation 2 homolog 1
- SOD
superoxide dismutase
- TM
thrombomodulin
- TrxR2
thioredoxin-dependent peroxidase
- VCAM-1
vascular cell adhesion protein 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fisher AB, Chien S, Barakat AI, Nerem RM. Endothelial cellular response to altered shear stress. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2001;281:L529–L533. doi: 10.1152/ajplung.2001.281.3.L529. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 3.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA: The Journal of the American Medical Association. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 4.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7–11. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 5.Chien S, Li S, Shyy JYJ. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 1998;31:162–169. doi: 10.1161/01.hyp.31.1.162. [DOI] [PubMed] [Google Scholar]
- 6.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 7.Lewis BP, Shih I, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 8.Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circulation Research. 2007;101:59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- 9.Suárez Y, Fernández-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circulation Research. 2007;100:1164–1173. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- 10.Qin X, Wang X, Wang Y, Tang Z, Cui Q, Xi J, Li YSJ, Chien S, Wang N. MicroRNA-19a mediates the suppressive effect of laminar flow on cyclin D1 expression in human umbilical vein endothelial cells. Proceedings of the National Academy of Sciences. 2010;107:3240–3244. doi: 10.1073/pnas.0914882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang KC, Garmire LX, Young A, Nguyen P, Trinh A, Subramaniam S, Wang N, Shyy JYJ, Li YS, Chien S. Role of microRNA-23b in flow-regulation of Rb phosphorylation and endothelial cell growth. Proceedings of the National Academy of Sciences. 2010;107:3234–3239. doi: 10.1073/pnas.0914825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu W, Xiao H, Laguna-Fernandez A, Villarreal G, Jr, Wang KC, Geary GG, Zhang Y, Wang WC, Huang HD, Zhou J. Flow-Dependent Regulation of Krüppel-Like Factor 2 Is Mediated by MicroRNA-92aClinical Perspective. Circulation. 2011;124:633–641. doi: 10.1161/CIRCULATIONAHA.110.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang Y, Davies PF. Site-Specific MicroRNA-92a Regulation of Krüppel-Like Factors 4 and 2 in Atherosusceptible Endothelium. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32:979–987. doi: 10.1161/ATVBAHA.111.244053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hergenreider E, Heydt S, Tréguer K, Boettger T, Horrevoets AJG, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nature Cell Biology. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 15.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 16.Lin Z, Kumar A, SenBanerjee S, Staniszewski K, Parmar K, Vaughan DE, Gimbrone MA, Balasubramanian V, García-Cardeña G, Jain MK. Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circulation Research. 2005;96:e48–e57. doi: 10.1161/01.RES.0000159707.05637.a1. [DOI] [PubMed] [Google Scholar]
- 17.Fledderus JO, Boon RA, Volger OL, Hurttila H, Ylä-Herttuala S, Pannekoek H, Levonen AL, Horrevoets AJG. KLF2 primes the antioxidant transcription factor Nrf2 for activation in endothelial cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28:1339–1346. doi: 10.1161/ATVBAHA.108.165811. [DOI] [PubMed] [Google Scholar]
- 18.SenBanerjee S, Lin Z, Atkins GB, Greif DM, Rao RM, Kumar A, Feinberg MW, Chen Z, Simon DI, Luscinskas FW. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. The Journal of Experimental Medicine. 2004;199:1305–1315. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wienholds E, Kloosterman WP, Miska E, Alvarez-Saavedra E, Berezikov E, de Bruijn E, Horvitz HR, Kauppinen S, Plasterk RHA. MicroRNA expression in zebrafish embryonic development. Science Signalling. 2005;309:310. doi: 10.1126/science.1114519. [DOI] [PubMed] [Google Scholar]
- 20.Nicoli S, Standley C, Walker P, Hurlstone A, Fogarty KE, Lawson ND. MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature. 2010;464:1196–1200. doi: 10.1038/nature08889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Developmental Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris TA, Yamakuchi M, Kondo M, Oettgen P, Lowenstein CJ. Ets-1 and Ets-2 regulate the expression of microRNA-126 in endothelial cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30:1990–1997. doi: 10.1161/ATVBAHA.110.211706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. Journal of Clinical Investigation. 2006;116:49. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young A, Wu W, Sun W, Larman HB, Wang N, Li YS, Shyy JY, Chien S, García-Cardeña G. Flow activation of AMP-activated protein kinase in vascular endothelium leads to Krüppel-like factor 2 expression. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29:1902–1908. doi: 10.1161/ATVBAHA.109.193540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang W, Ha CH, Jhun BS, Wong C, Jain MK, Jin ZG. Fluid shear stress stimulates phosphorylation-dependent nuclear export of HDAC5 and mediates expression of KLF2 and eNOS. Blood. 2010;115:2971–2979. doi: 10.1182/blood-2009-05-224824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee DY, Lee CI, Lin TE, Lim SH, Zhou J, Tseng YC, Chien S, Chiu JJ. Role of histone deacetylases in transcription factor regulation and cell cycle modulation in endothelial cells in response to disturbed flow. Proceedings of the National Academy of Sciences. 2012;109:1967–1972. doi: 10.1073/pnas.1121214109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Betel D, Wilson M, Gabow A, Marks DS, Sander C. The microRNA org resource: targets and expression. Nucleic Acids Research. 2008;36:D149–D153. doi: 10.1093/nar/gkm995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state role of a superoxide-producing NADH oxidase. Circulation Research. 1998;82:1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- 29.Harrison DG. Cellular and molecular mechanisms of endothelial cell dysfunction. Journal of Clinical Investigation. 1997;100:2153. doi: 10.1172/JCI119751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Topper JN, Cai J, Falb D, Gimbrone M., Jr Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proceedings of the National Academy of Sciences. 1996;93:10417–10422. doi: 10.1073/pnas.93.19.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones S, O’donnell V, Wood J, Broughton J, Hughes E, Jones O. Expression of phagocyte NADPH oxidase components in human endothelial cells. American Journal of Physiology-Heart and Circulatory Physiology. 1996;271:H1626–H1634. doi: 10.1152/ajpheart.1996.271.4.H1626. [DOI] [PubMed] [Google Scholar]
- 32.Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, Iida M. Nox4 as the major catalytic component of an endothelial NAD (P) H oxidase. Circulation. 2004;109:227–233. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- 33.Faust L, El Benna J, Babior BM, Chanock SJ. The phosphorylation targets of p47phox a subunit of the respiratory burst oxidase. Functions of the individual target serines as evaluated by site-directed mutagenesis. Journal of Clinical Investigation. 1995;96:1499. doi: 10.1172/JCI118187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raad H, Paclet MH, Boussetta T, Kroviarski Y, Morel F, Quinn MT, Gougerot-Pocidalo MA, Dang PMC, El-Benna J. Regulation of the phagocyte NADPH oxidase activity: phosphorylation of gp91phox/NOX2 by protein kinase C enhances its diaphorase activity and binding to Rac2, p67phox, and p47phox. The FASEB Journal. 2009;23:1011–1022. doi: 10.1096/fj.08-114553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu Y, Zhang Y, Wang Z, Wang L, Wei X, Zhang B, Wen Z, Fang H, Pang Q, Yi F. Regulation of NADPH oxidase activity is associated with miRNA-25-mediated NOX4 expression in experimental diabetic nephropathy. American Journal of Nephrology. 2010;32:581–589. doi: 10.1159/000322105. [DOI] [PubMed] [Google Scholar]
- 36.Macmillan-Crow LA, Cruthirds DL. Manganese superoxide dismutase in disease. Free Radical Research. 2001;34:325–336. doi: 10.1080/10715760100300281. [DOI] [PubMed] [Google Scholar]
- 37.Arthur J. The glutathione peroxidases. Cellular and Molecular Life Sciences. 2000;57:1825–1835. doi: 10.1007/PL00000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trachootham D, Lu W, Ogasawara MA, Valle NRD, Huang P. Redox regulation of cell survival. Antioxidants & Redox Signaling. 2008;10:1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou J, Wang KC, Wu W, Subramaniam S, Shyy JYJ, Chiu JJ, Li JYS, Chien S. MicroRNA-21 targets peroxisome proliferators-activated receptor-α in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proceedings of the National Academy of Sciences. 2011;108:10355–10360. doi: 10.1073/pnas.1107052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber M, Baker MB, Moore JP, Searles CD. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochemical and Biophysical Research Communications. 2010;393:643–648. doi: 10.1016/j.bbrc.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X, Ng WL, Wang P, Tian LL, Werner E, Wang H, Doetsch P, Wang Y. MicroRNA-21 Modulates the Levels of Reactive Oxygen Species by Targeting SOD3 and TNFα. Cancer Research. 2012;72:4707–4713. doi: 10.1158/0008-5472.CAN-12-0639. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 42.Fleissner F, Jazbutyte V, Fiedler J, Gupta SK, Yin X, Xu Q, Galuppo P, Kneitz S, Mayr M, Ertl G. Short communication: asymmetric dimethylarginine impairs angiogenic progenitor cell function in patients with coronary artery disease through a microRNA-21-dependent mechanism. Circulation Research. 2010;107:138–143. doi: 10.1161/CIRCRESAHA.110.216770. [DOI] [PubMed] [Google Scholar]
- 43.Doebele C, Hergenreider E, Boon RA, Reinfeld N, Zeiher AM, Dimmeler S. The MiR-30 Family is Regulated by Shear Stress and Affects the Expression of Inflammatory Cell-Cell Adhesion Molecules (abstract) Circulation. 2011;124:A15893. [Google Scholar]
- 44.Haque R, Chun E, Howell JC, Sengupta T, Chen D, Kim H. MicroRNA-30b-Mediated Regulation of Catalase Expression in Human ARPE-19 Cells. PLoS ONE. 2012;7:e42542. doi: 10.1371/journal.pone.0042542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Y, Fang F, Zhang J, Josson S, Clair WHS, Clair DKS. miR-17* suppresses tumorigenicity of prostate cancer by inhibiting mitochondrial antioxidant enzymes. PLoS ONE. 2010;5:e14356. doi: 10.1371/journal.pone.0014356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang CL, Au LC, Huang SW, Kwok CF, Ho LT, Juan CC. Insulin Up-Regulates Heme Oxygenase-1 Expression in 3T3-L1 Adipocytes via PI3-Kinase-and PKC- Dependent Pathways and Heme Oxygenase-1–Associated MicroRNA Downregulation. Endocrinology. 2011;152:384–393. doi: 10.1210/en.2010-0493. [DOI] [PubMed] [Google Scholar]
- 47.Papaioannou MD, Lagarrigue M, Vejnar CE, Rolland AD, Kühne F, Aubry F, Schaad O, Fort A, Descombes P, Neerman-Arbez M. Loss of Dicer in Sertoli cells has a major impact on the testicular proteome of mice. Molecular & Cellular Proteomics. 2011;10 doi: 10.1074/mcp.M900587-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lerner AG, Upton JP, Praveen PV, Ghosh R, Nakagawa Y, Igbaria A, Shen S, Nguyen V, Backes BJ, Heiman M, Heintz N, Greengard P, Hui S, Tang Q, Trusina A, Oakes SA, Papa FR. IRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metabolism. 2012;16:250–64. doi: 10.1016/j.cmet.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou J, Chng WJ. Roles of thioredoxin binding protein (TXNIP) in oxidative stress, apoptosis and cancer. Mitochondrion. 2012;12:00073–6. doi: 10.1016/j.mito.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Altschmied J, Haendeler J. Thioredoxin-1 and endothelial cell aging: role in cardiovascular diseases. Antioxid Redox Signal. 2009;11:1733–40. doi: 10.1089/ars.2008.2379. [DOI] [PubMed] [Google Scholar]
- 51.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circulation Research. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 52.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 53.Chan LS, Yue PYK, Mak NK, Wong RNS. Role of microRNA-214 in ginsenoside-Rg1-induced angiogenesis. European Journal of Pharmaceutical Sciences. 2009;38:370–377. doi: 10.1016/j.ejps.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 54.Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2010;65:1028. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science Signalling. 2009;324:1710. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 56.Chen Z, Peng IC, Cui X, Li YS, Chien S, Shyy JYJ. Shear stress, SIRT1, and vascular homeostasis. Proceedings of the National Academy of Sciences. 2010;107:10268–10273. doi: 10.1073/pnas.1003833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Menghini R, Casagrande V, Cardellini M, Martelli E, Terrinoni A, Amati F, Vasa-Nicotera M, Ippoliti A, Novelli G, Melino G. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation. 2009;120:1524–1532. doi: 10.1161/CIRCULATIONAHA.109.864629. [DOI] [PubMed] [Google Scholar]
- 58.Dávalos A, Goedeke L, Smibert P, Ramírez CM, Warrier NP, Andreo U, Cirera-Salinas D, Rayner K, Suresh U, Pastor-Pareja JC. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proceedings of the National Academy of Sciences. 2011;108:9232–9237. doi: 10.1073/pnas.1102281108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holliday-Ankeny CJ, Ankeny RF, Ferdous Z, Nerem RM, Jo H. Shear-and Side-dependent microRNAs and Messenger RNAs in Aortic Valvular Endothelium. QScience Proceedings. 2012;56 [Google Scholar]
- 60.Zhang J, Ying Z, Tang Z, Long L, Li K. MicroRNA-148a promotes myogenic differentiation by targeting the ROCK1 gene. Journal of Biological Chemistry. 2012;287:21093–21101. doi: 10.1074/jbc.M111.330381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mallat Z, Gojova A, Sauzeau V, Brun V, Silvestre JS, Esposito B, Merval R, Groux H, Loirand G, Tedgui A. Rho-associated protein kinase contributes to early atherosclerotic lesion formation in mice. Circulation Research. 2003;93:884–888. doi: 10.1161/01.RES.0000099062.55042.9A. [DOI] [PubMed] [Google Scholar]
- 62.World CJ, Garin G, Berk BC. Vascular shear stress and activation of inflammatory genes. Current Atherosclerosis Reports. 2006;8:240–244. doi: 10.1007/s11883-006-0079-8. [DOI] [PubMed] [Google Scholar]
- 63.Yamawaki H, Pan S, Lee RT, Berk BC. Fluid shear stress inhibits vascular inflammation by decreasing thioredoxin-interacting protein in endothelial cells. Journal of Clinical Investigation. 2005;115:733–738. doi: 10.1172/JCI200523001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mohan S, Mohan N, Sprague EA. Differential activation of NF-kappa B in human aortic endothelial cells conditioned to specific flow environments. American Journal of Physiology-Cell Physiology. 1997;273:C572–C578. doi: 10.1152/ajpcell.1997.273.2.C572. [DOI] [PubMed] [Google Scholar]
- 65.Manning A, Bell F, Rosenbloom C, Chosay J, Simmons C, Northrup J, Shebuski R, Dunn C, Anderson D. NF-kappa B is activated during acute inflammation in vivo in association with elevated endothelial cell adhesion molecule gene expression and leukocyte recruitment. Journal of Inflammation. 1995;45:283. [PubMed] [Google Scholar]
- 66.Chiu JJ, Lee PL, Chen CN, Lee CI, Chang SF, Chen LJ, Lien SC, Ko YC, Usami S, Chien S. Shear stress increases ICAM-1 and decreases VCAM-1 and E-selectin expressions induced by tumor necrosis factor-α in endothelial cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24:73–79. doi: 10.1161/01.ATV.0000106321.63667.24. [DOI] [PubMed] [Google Scholar]
- 67.Baeuerle PA, Henkel T. Function and activation of NF-kappaB in the immune system. Annual Review of Immunology. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 68.Hsiai TK, Cho SK, Wong PK, Ing M, Salazar A, Sevanian A, Navab M, Demer LL, Ho CM. Monocyte recruitment to endothelial cells in response to oscillatory shear stress. The FASEB Journal. 2003;17:1648–1657. doi: 10.1096/fj.02-1064com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fang Y, Shi C, Manduchi E, Civelek M, Davies PF. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proceedings of the National Academy of Sciences. 2010;107:13450–13455. doi: 10.1073/pnas.1002120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun X, Icli B, Wara AK, Belkin N, He S, Kobzik L, Hunninghake GM, Vera MP. MicroRNA-181b regulates NF-κB–mediated vascular inflammation. The Journal of Clinical Investigation. 2012;122:1973. doi: 10.1172/JCI61495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ruiz-Ortega M, Lorenzo O, Rupérez M, Suzuki Y, Egido J. Angiotensin II activates nuclear transcription factor-κB in aorta of normal rats and in vascular smooth muscle cells of AT1 knockout mice. Nephrology Dialysis Transplantation. 2001;16:27–33. doi: 10.1093/ndt/16.suppl_1.27. [DOI] [PubMed] [Google Scholar]
- 72.Zhu N, Zhang D, Chen S, Liu X, Lin L, Huang X, Guo Z, Liu J, Wang Y, Yuan W. Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation and migration. Atherosclerosis. 2011;215:286–293. doi: 10.1016/j.atherosclerosis.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 73.Weber M, Searles CD. Modulation of EC Function by miR-155, a Shear Stress-Responsive miRNA (abstract) Circulation. 2010;122:A21301. [Google Scholar]
- 74.Boettger T, Beetz N, Kostin S, Schneider J, Krüger M, Hein L, Braun T. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. The Journal of Clinical Investigation. 2009;119:2634. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu S, Pan W, Song X, Liu Y, Shao X, Tang Y, Liang D, He D, Wang H, Liu W. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-[alpha] Nature Medicine. 2012;18:1077–1086. doi: 10.1038/nm.2815. [DOI] [PubMed] [Google Scholar]
- 76.Angel-Morales G, Noratto G, Mertens-Talcott S. Red wine polyphenolics reduce the expression of inflammation markers in human colon-derived CCD-18Co myofibroblast cells: Potential role of microRNA-126. Food & Function. 2012;3:745–752. doi: 10.1039/c2fo10271d. [DOI] [PubMed] [Google Scholar]
- 77.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proceedings of the National Academy of Sciences. 2008;105:1516–1521. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Konstantinopoulos PA, Vandoros GP, Sotiropoulou-Bonikou G, Kominea A, Papavassiliou AG. NF-κB/PPARγ and/or AP-1/PPARγ ‘on/off’ switches and induction of CBP in colon adenocarcinomas: correlation with COX-2 expression. International Journal of Colorectal Disease. 2007;22:57–68. doi: 10.1007/s00384-006-0112-y. [DOI] [PubMed] [Google Scholar]
- 79.Horita HN, Simpson PA, Ostriker A, Furgeson S, Van Putten V, Weiser-Evans MCM, Nemenoff RA. Serum response factor regulates expression of phosphatase and tensin homolog through a MicroRNA network in vascular smooth muscle cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:2909–2919. doi: 10.1161/ATVBAHA.111.233585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ni CW, Qiu H, Jo H. MicroRNA-663 upregulated by oscillatory shear stress plays a role in inflammatory response of endothelial cells. American Journal of Physiology-Heart and Circulatory Physiology. 2011;300:H1762–H1769. doi: 10.1152/ajpheart.00829.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hamik A, Lin Z, Kumar A, Balcells M, Sinha S, Katz J, Feinberg MW, Gerszten RE, Edelman ER, Jain MK. Kruppel-like factor 4 regulates endothelial inflammation. Journal of Biological Chemistry. 2007;282:13769–13779. doi: 10.1074/jbc.M700078200. [DOI] [PubMed] [Google Scholar]
- 82.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiological Reviews. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 83.Galbraith C, Skalak R, Chien S. Shear stress induces spatial reorganization of the endothelial cell cytoskeleton. Cell Motility and the Cytoskeleton. 1998;40:317–330. doi: 10.1002/(SICI)1097-0169(1998)40:4<317::AID-CM1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 84.Tzima E, Del Pozo MA, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. The EMBO Journal. 2001;20:4639–4647. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Akimoto S, Mitsumata M, Sasaguri T, Yoshida Y. Laminar shear stress inhibits vascular endothelial cell proliferation by inducing cyclin-dependent kinase inhibitor p21Sdi1/Cip1/Waf1. Circulation Research. 2000;86:185–190. doi: 10.1161/01.res.86.2.185. [DOI] [PubMed] [Google Scholar]
- 86.Dekker RJ, Boon RA, Rondaij MG, Kragt A, Volger OL, Elderkamp YW, Meijers JCM, Voorberg J, Pannekoek H, Horrevoets AJG. KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood. 2006;107:4354–4363. doi: 10.1182/blood-2005-08-3465. [DOI] [PubMed] [Google Scholar]
- 87.Noria S, Cowan DB, Gotlieb AI, Langille BL. Transient and steady-state effects of shear stress on endothelial cell adherens junctions. Circulation Research. 1999;85:504–514. doi: 10.1161/01.res.85.6.504. [DOI] [PubMed] [Google Scholar]
- 88.Gabriels JE, Paul DL. Connexin43 is highly localized to sites of disturbed flow in rat aortic endothelium but connexin37 and connexin40 are more uniformly distributed. Circulation Research. 1998;83:636–643. doi: 10.1161/01.res.83.6.636. [DOI] [PubMed] [Google Scholar]
- 89.Miao H, Hu YL, Shiu YT, Yuan S, Zhao Y, Kaunas R, Wang Y, Jin G, Usami S, Chien S. Effects of flow patterns on the localization and expression of VE-cadherin at vascular endothelial cell junctions: in vivo and in vitro investigations. Journal of Vascular Research. 2005;42:77–89. doi: 10.1159/000083094. [DOI] [PubMed] [Google Scholar]
- 90.Muramatsu F, Kidoya H, Naito H, Sakimoto S, Takakura N. microRNA-125b inhibits tube formation of blood vessels through translational suppression of VE-cadherin. Oncogene. 2012 doi: 10.1038/onc.2012.68. In press. [DOI] [PubMed] [Google Scholar]
- 91.Li X, Pan JH, Song B, Xiong EQ, Chen ZW, Zhou ZS, Su YP. Suppression of CX43 expression by miR-20a in the progression of human prostate cancer. Cancer Biology & Therapy. 2012;13:890–898. doi: 10.4161/cbt.20841. [DOI] [PubMed] [Google Scholar]
- 92.Malek AM, Izumo S. Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. Journal of Cell Science. 1996;109:713–726. doi: 10.1242/jcs.109.4.713. [DOI] [PubMed] [Google Scholar]
- 93.Boon RA, Leyen TA, Fontijn RD, Fledderus JO, Baggen JMC, Volger OL, van Nieuw Amerongen GP, Horrevoets AJG. KLF2-induced actin shear fibers control both alignment to flow and JNK signaling in vascular endothelium. Blood. 2010;115:2533–2542. doi: 10.1182/blood-2009-06-228726. [DOI] [PubMed] [Google Scholar]
- 94.Kong W, Yang H, He L, Zhao J, Coppola D, Dalton WS, Cheng JQ. MicroRNA-155 is regulated by the transforming growth factor β/Smad pathway and contributes to epithelial cell plasticity by targeting RhoA. Molecular and Cellular Biology. 2008;28:6773–6784. doi: 10.1128/MCB.00941-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Orr AW, Hahn C, Blackman BR, Schwartz MA. P21-activated kinase signaling regulates oxidant-dependent NF-κB activation by flow. Circulation Research. 2008;103:671–679. doi: 10.1161/CIRCRESAHA.108.182097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zou J, Li WQ, Li Q, Li XQ, Zhang JT, Liu GQ, Chen J, Qiu XX, Tian FJ, Wang ZZ. Two Functional MicroRNA-126s Repress a Novel Target Gene p21-Activated Kinase 1 to Regulate Vascular Integrity in Zebrafish Novelty and Significance. Circulation Research. 2011;108:201–209. doi: 10.1161/CIRCRESAHA.110.225045. [DOI] [PubMed] [Google Scholar]
- 97.Lichtenstein I, Zomaya A, Gamble J, Vadas M. Approaches to Construction and Analysis of Microrna-Mediated Networks. Algorithms in Computational Molecular Biology: Techniques, Approaches and Applications. 2010:979–1006. [Google Scholar]
- 98.Naegle KM, Gymrek M, Joughin BA, Wagner JP, Welsch RE, Yaffe MB, Lauffenburger DA, White FM. PTMScout, a web resource for analysis of high throughput post-translational proteomics studies. Molecular & Cellular Proteomics. 2010;9:2558–2570. doi: 10.1074/mcp.M110.001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Matys V, Fricke E, Geffers R, Goessling E, Haubrock M, Hehl R, Hornischer K, Karas D, Kel AE, Kel-Margoulis OV. TRANSFAC®: transcriptional regulation, from patterns to profiles. Nucleic Acids Research. 2003;31:374–378. doi: 10.1093/nar/gkg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sandelin A, Alkema W, Engström P, Wasserman WW, Lenhard B. JASPAR: an open-access database for eukaryotic transcription factor binding profiles. Nucleic Acids Research. 2004;32:D91–D94. doi: 10.1093/nar/gkh012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nussbaum C, Myers RM, Brown M, Li W. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Krek A, Grün D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M. Combinatorial microRNA target predictions. Nature Genetics. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 103.Maragkakis M, Alexiou P, Papadopoulos GL, Reczko M, Dalamagas T, Giannopoulos G, Goumas G, Koukis E, Kourtis K, Simossis VA. Accurate microRNA target prediction correlates with protein repression levels. BMC Bioinformatics. 2009;10:295. doi: 10.1186/1471-2105-10-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Betel D, Koppal A, Agius P, Sander C, Leslie C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biology. 2010;11:R90. doi: 10.1186/gb-2010-11-8-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 106.Rehmsmeier M, Steffen P, Höchsmann M, Giegerich R. Fast and effective prediction of microRNA/target duplexes. RNA. 2004;10:1507–1517. doi: 10.1261/rna.5248604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Plaisier CL, Pan M, Baliga NS. A miRNA-regulatory network explains how dysregulated miRNAs perturb oncogenic processes across diverse cancers. Genome Research. 2012;22:2302–2314. doi: 10.1101/gr.133991.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sato H, Ishida S, Toda K, Matsuda R, Hayashi Y, Shigetaka M, Fukuda M, Wakamatsu Y, Itai A. New approaches to mechanism analysis for drug discovery using DNA microarray data combined with KeyMolnet. Current Drug Discovery Technologies. 2005;2:89–98. doi: 10.2174/1570163054064701. [DOI] [PubMed] [Google Scholar]